Effects of Dietary Pretreatment with All-trans Lycopene on Lipopolysaccharide-Induced Jejunal Inflammation: A Multi-Pathway Phenomenon
Abstract
1. Introduction
2. Materials and Methods
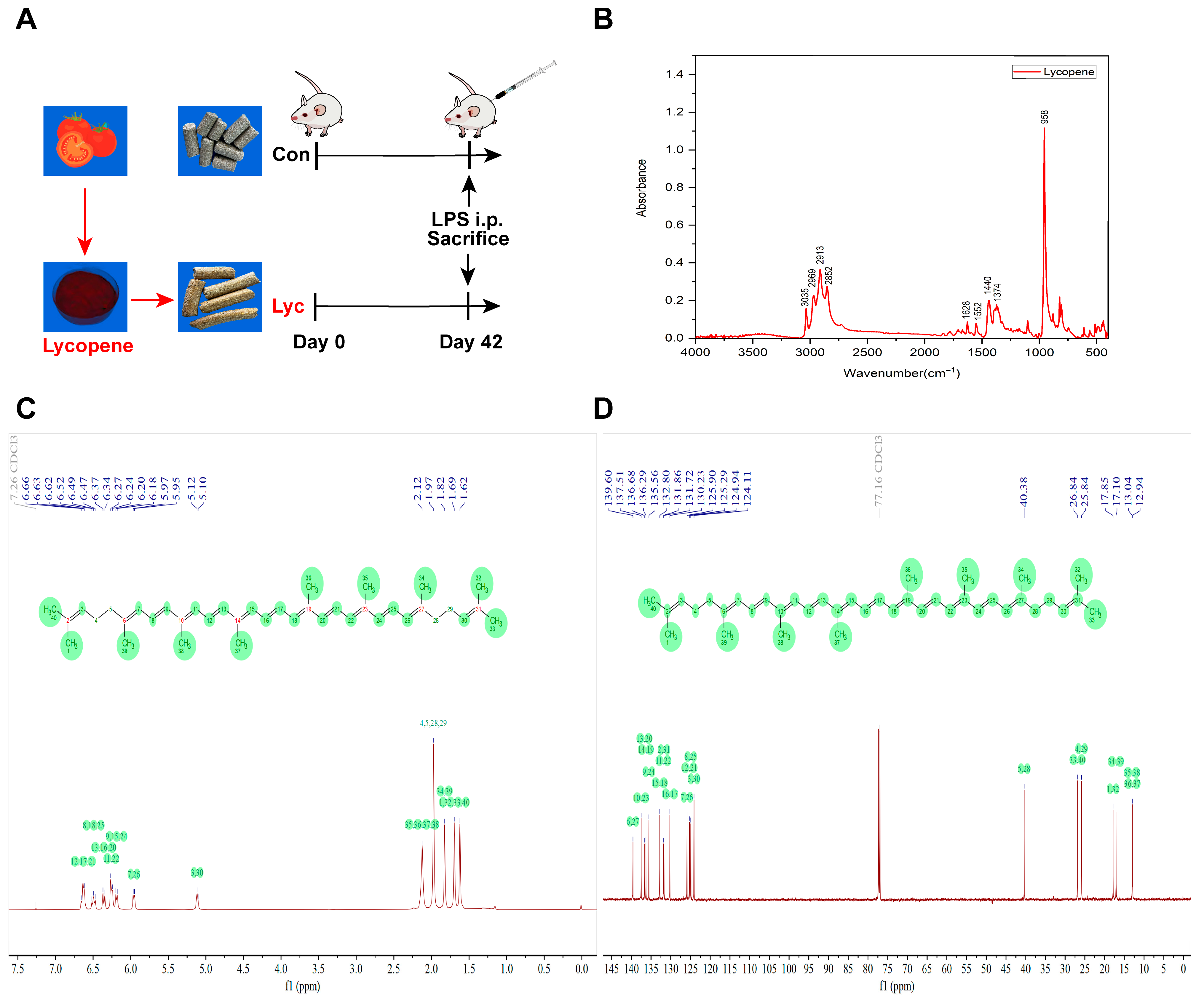
2.1. Animals
2.2. Escherichia coli Lipopolysaccharide Challenge
2.3. FTIR Analysis
2.4. NMR Analysis
2.5. LC-MS/MS Profiling of Serum Untargeted Metabolomics
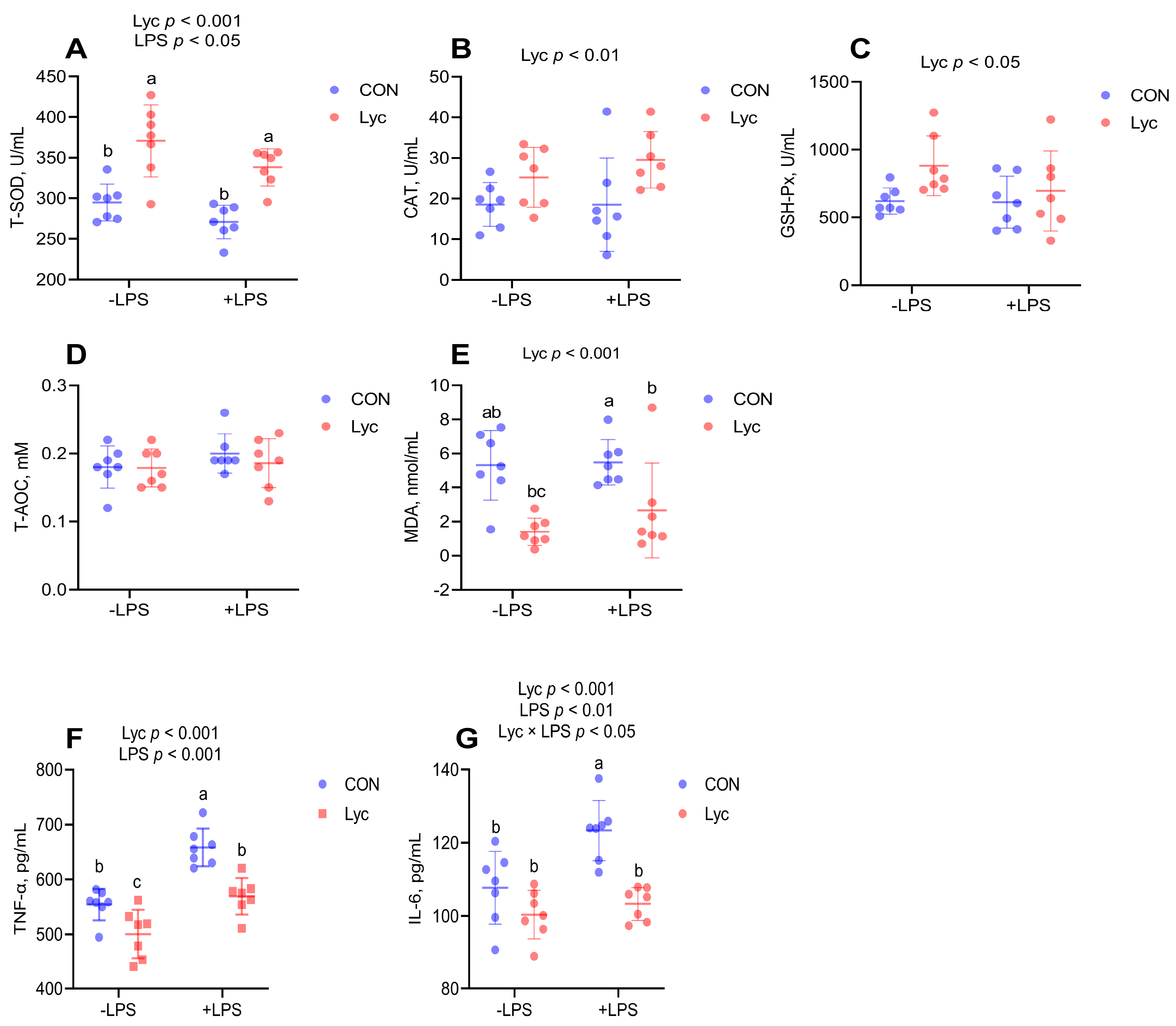
2.6. Enzyme Activity Assay
2.7. Serum TNF-α and IL-6 Analysis
2.8. Real-Time Quantitative PCR
2.9. Immunohistochemistry
2.10. NF-κB p65 Activity Assay
2.11. Immunofluorescence
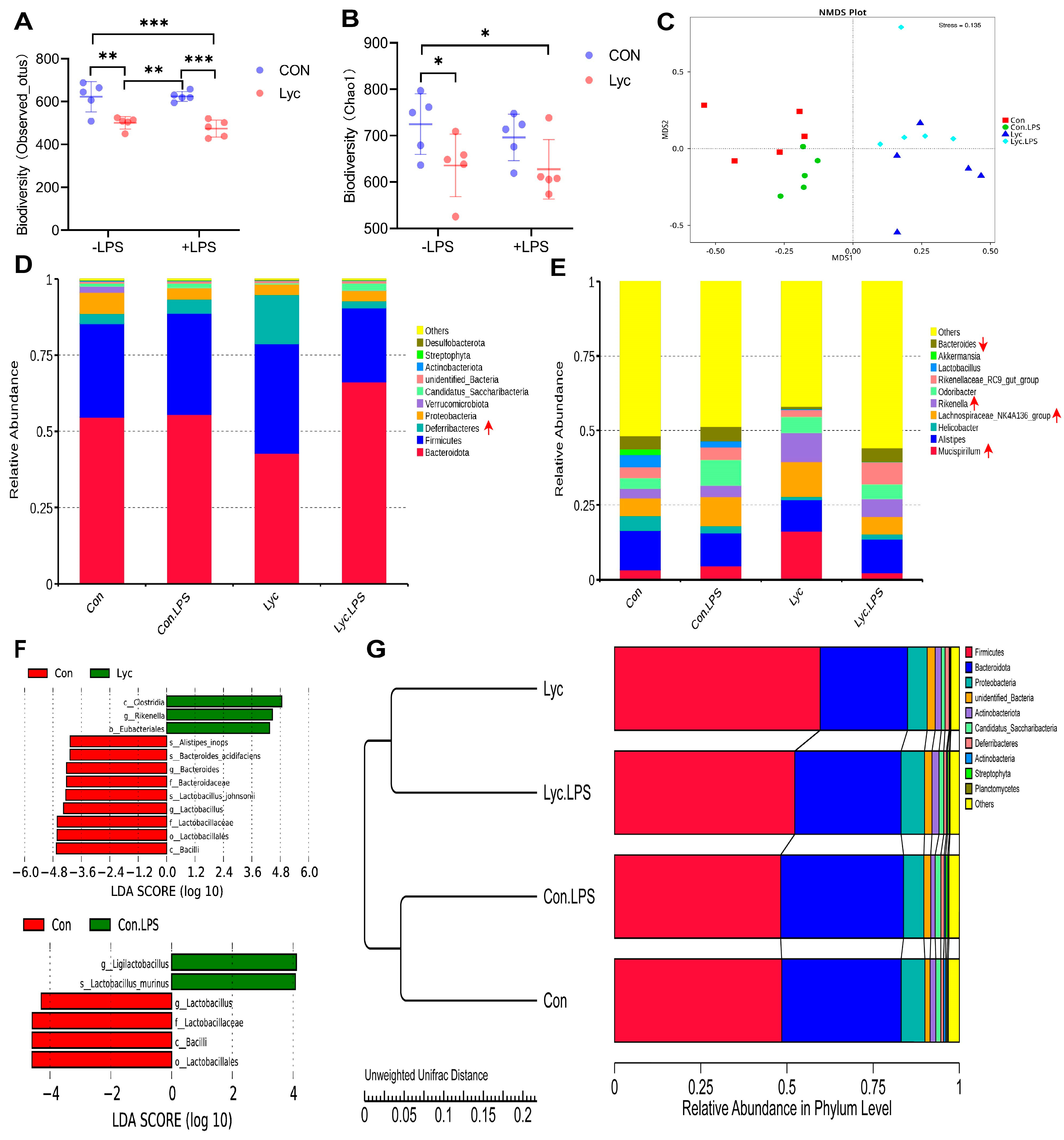
2.12. Microbial Analyses
2.13. Statistical Analyses
3. Results
3.1. FTIR and NMR Spectra of Lycopene
3.2. Effect of Lycopene on Metabolomics of Mice Serum
3.3. Effect of Lycopene on the Serum Antioxidant Capacity and Inflammatory Factor Concentrations of Mice
3.4. Effect of Lycopene on the Jejunal Antioxidant Capacity of Mice
3.5. The Potential Mechanism of Lycopene on Inflammation of the Jejunum
3.6. Effect of Lycopene on the Jejunal Inflammatory Factor and ZO-1 Protein Expression of Mice
3.7. Effect of Lycopene Supplementation on Gut Microbiota in Mice
3.8. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mou, D.; Ding, D.; Yang, M.; Jiang, X.; Zhao, L.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Zhuo, Y.; et al. Maternal organic selenium supplementation during gestation improves the antioxidant capacity and reduces the inflammation level in the intestine of offspring through the NF-κB and ERK/Beclin-1 pathways. Food Funct. 2021, 12, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Mou, D.; Zhu, H.; Jiang, X.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Zhuo, Y.; Li, J.; et al. Maternal Organic Selenium Supplementation Relieves Intestinal Endoplasmic Reticulum Stress in Piglets by Enhancing the Expression of Glutathione Peroxidase 4 and Selenoprotein S. Front. Nutr. 2022, 9, 900421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mou, D.; Hu, L.; Zhen, J.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Feng, B.; Li, J.; et al. Effects of Maternal Low-Energy Diet during Gestation on Intestinal Morphology, Disaccharidase Activity, and Immune Response to Lipopolysaccharide Challenge in Pig Offspring. Nutrients 2017, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, L.M.; Jones, G.R.; Bain, C.C. Macrophages in intestinal homeostasis and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Tulkens, J.; Vergauwen, G.; Van Deun, J.; Geeurickx, E.; Dhondt, B.; Lippens, L.; De Scheerder, M.A.; Miinalainen, I.; Rappu, P.; De Geest, B.G.; et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut 2020, 69, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Perez, S.; Talens-Visconti, R.; Rius-Perez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radical. Bio Med. 2017, 104, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Yue, Y.; Shi, M.; Song, X.; Ma, C.; Li, D.; Hu, X.; Chen, F. Lycopene Ameliorated DSS-Induced Colitis by Improving Epithelial Barrier Functions and Inhibiting the Escherichia coli Adhesion in Mice. J. Agric. Food Chem. 2024, 72, 5784–5796. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.; Hu, L. Recent technological strategies for enhancing the stability of lycopene in processing and production. Food Chem. 2023, 405, 134799. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.W.; He, J.; Zheng, P.; Yu, J.; Luo, Y.; Yan, H.; Yu, B. Lycopene promotes a fast-to-slow fiber type transformation through Akt/FoxO1 signaling pathway and miR-22-3p. J. Funct. Foods 2021, 80, 104430. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Luo, Y.; Yan, H.; Yu, J. Lycopene increases the proportion of slow-twitch muscle fiber by AMPK signaling to improve muscle anti-fatigue ability. J. Nutr. Biochem. 2021, 94, 108750. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; He, J.; Luo, Y.; Yan, H.; Chen, H.; Zheng, P.; et al. Dietary lycopene supplementation improves meat quality, antioxidant capacity and skeletal muscle fiber type transformation in finishing pigs. Anim. Nutr. 2022, 8, 256–264. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Li, J.; Bai, Y.; Luo, Y.; Ji, B.; Xia, B.; Liu, Z.; Tan, X.; Lv, J.; et al. Lycopene Alleviates DSS-Induced Colitis and Behavioral Disorders via Mediating Microbes-Gut-Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 3963–3975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, X.; Yin, M.; Li, C.; Han, L. Preventive Effect of Lycopene in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice through the Regulation of TLR4/TRIF/NF-κB Signaling Pathway and Tight Junctions. J. Agric. Food Chem. 2021, 69, 13500–13509. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.T.; Wan, X.; Yang, H.; Wang, Z. Dietary Lycopene Supplementation Could Alleviate Aflatoxin B(1) Induced Intestinal Damage through Improving Immune Function and Anti-Oxidant Capacity in Broilers. Animals 2021, 11, 3165. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, S.; Xie, J.; Ji, F.; Peng, W.; Qian, J.; Shen, Q.; Hou, G. Effects of Dietary Lycopene on the Growth Performance, Antioxidant Capacity, Meat Quality, Intestine Histomorphology, and Cecal Microbiota in Broiler Chickens. Animals 2024, 14, 203. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Li, J.; Shi, B.; Ma, Q.; Shan, A. Lycopene Affects Intestinal Barrier Function and the Gut Microbiota in Weaned Piglets via Antioxidant Signaling Regulation. J. Nutr. 2022, 152, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, F.; Gu, S.; Cao, C.; Xiao, Y.; Bao, W.; Wang, H. Lycopene alleviates Deoxynivalenol-induced toxicity in Porcine intestinal epithelial cells by mediating mitochondrial function. Toxicology 2024, 506, 153880. [Google Scholar] [CrossRef]
- Tu, T.; Liu, H.; Liu, Z.; Liang, Y.; Tan, C.; Feng, D.; Zou, J. Amelioration of Atherosclerosis by lycopene is linked to the modulation of gut microbiota dysbiosis and related gut-heart axis activation in high-fat diet-fed ApoE(-/-) mice. Nutr. Metab. 2023, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zuo, C.; Liang, T.; Huang, Y.; Kang, P.; Xiao, K.; Liu, Y. Lycopene alleviates multiple-mycotoxin-induced toxicity by inhibiting mitochondrial damage and ferroptosis in the mouse jejunum. Food Funct. 2022, 13, 11532–11542. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.A.; Liang, S.J.; Wang, X.Q.; Yan, H.C. Lycopene Protects Intestinal Epithelium from Deoxynivalenol-Induced Oxidative Damage via Regulating Keap1/Nrf2 Signaling. Antioxidants 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Niu, X.; Li, Y.; Yao, Y.; Han, L. Preventive Mechanism of Lycopene on Intestinal Toxicity Caused by Cyclophosphamide Chemotherapy in Mice by Regulating TLR4-MyD88/TRIF-TRAF6 Signaling Pathway and Gut-Liver Axis. Nutrients 2022, 14, 4467. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Bucknall, M.P.; Arcot, J. Interferences of anthocyanins with the uptake of lycopene in Caco-2 cells, and their interactive effects on anti-oxidation and anti-inflammation in vitro and ex vivo. Food Chem. 2019, 276, 402–409. [Google Scholar] [CrossRef]
- Shen, R.; Jiang, X.; Ye, W.; Song, X.; Liu, L.; Lao, X.; Wu, C. A novel and practical synthetic route for the total synthesis of lycopene. Tetrahedron 2011, 67, 5610–5614. [Google Scholar] [CrossRef]
- Mou, D.; Wang, J.; Liu, H.; Chen, Y.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Feng, B.; Li, J.; et al. Maternal methyl donor supplementation during gestation counteracts bisphenol A-induced oxidative stress in sows and offspring. Nutrition 2018, 45, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Mou, D.; Ding, D.; Yan, H.; Qin, B.; Dong, Y.; Li, Z.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; et al. Maternal supplementation of organic selenium during gestation improves sows and offspring antioxidant capacity and inflammatory status and promotes embryo survival. Food Funct. 2020, 11, 7748–7761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yan, H.; Zhang, Y.; Qi, R.; Zhang, H.; Liu, J. Growth performance, bile acid profile, fecal microbiome and serum metabolomics of growing-finishing pigs fed diets with bile acids supplementation. J. Anim. Sci. 2023, 101, skad393. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, X.; Ma, Z.; Raza, H.; Li, X.; Jin, L. Oil-based Z-isomer-rich lycopene: Efficient production in dual-media and stability evaluation. LWT 2024, 199, 116146. [Google Scholar] [CrossRef]
- Halim, Y.; Schwartz, S.J.; Francis, D.; Baldauf, N.A.; Rodriguez-Saona, L.E. Direct determination of lycopene content in tomatoes (Lycopersicon esculentum) by attenuated total reflectance infrared spectroscopy and multivariate analysis. J. AOAC Int. 2006, 89, 1257–1262. [Google Scholar] [CrossRef]
- De Nardo, T.; Shiroma-Kian, C.; Halim, Y.; Francis, D.; Rodriguez-Saona, L.E. Rapid and simultaneous determination of lycopene and beta-carotene contents in tomato juice by infrared spectroscopy. J. Agric. Food Chem. 2009, 57, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ni, Y.; Nagata, N.; Zhuge, F.; Xu, L.; Nagashimada, M.; Yamamoto, S.; Ushida, Y.; Fuke, N.; Suganuma, H.; et al. Lycopene Alleviates Obesity-Induced Inflammation and Insulin Resistance by Regulating M1/M2 Status of Macrophages. Mol. Nutr. Food Res. 2019, 63, e1900602. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Tocmo, R.; Parkin, K. S-Alk(en)ylmercaptocysteine suppresses LPS-induced pro-inflammatory responses in murine macrophages through inhibition of NF-κB pathway and modulation of thiol redox status. Free Radic. Biol. Med. 2018, 129, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, F.; Gong, P.; Qiu, Y.; Zhou, W.; Cui, Y.; Li, J.; Chen, H. Subneurotoxic copper(II)-induced NF-κB-dependent microglial activation is associated with mitochondrial ROS. Toxicol. Appl. Pharmacol. 2014, 276, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Freitas, V.; Almeida, L.; Laranjinha, J. Red wine extract preserves tight junctions in intestinal epithelial cells under inflammatory conditions: Implications for intestinal inflammation. Food Funct. 2019, 10, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, A. Risk and protection in the gut. Nat. Rev. Microbiol. 2019, 17, 397. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, H.; Bolotnikov, G.; Krämer, M.; Gotzmann, N.; Knippschild, U.; Kissmann, A.K.; Rosenau, F. Enriched Aptamer Libraries in Fluorescence-Based Assays for Rikenella microfusus-Specific Gut Microbiome Analyses. Microorganisms 2023, 11, 2266. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Petito, V.; Graziani, C.; Schiavoni, E.; Paroni Sterbini, F.; Poscia, A.; Gaetani, E.; Franceschi, F.; Cammarota, G.; Sanguinetti, M.; et al. Gut Microbiota in Health, Diverticular Disease, Irritable Bowel Syndrome, and Inflammatory Bowel Diseases: Time for Microbial Marker of Gastrointestinal Disorders. Dig. Dis. 2018, 36, 56–65. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, J.; Cui, Z.; Ma, K.; Wu, D.; Luo, J.; Li, F.; Xiong, W.; Rao, S.; Xiang, Q.; et al. Lachnospiraceae-derived butyrate mediates protection of high fermentable fiber against placental inflammation in gestational diabetes mellitus. Sci. Adv. 2023, 9, eadi7337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, H.; Song, N.; Zhang, L.; Cao, Y.; Tai, J. Long-term hexavalent chromium exposure facilitates colorectal cancer in mice associated with changes in gut microbiota composition. Food Chem. Toxicol. 2020, 138, 111237. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Huang, S.H.; Ding, H.F.; Kwek, E.; Liu, J.H.; Chen, Z.X.; Ma, K.Y.; Chen, Z.Y. Adverse effect of oxidized cholesterol exposure on colitis is mediated by modulation of gut microbiota. J. Hazard. Mater. 2023, 459, 132057. [Google Scholar] [CrossRef]
- Herp, S.; Brugiroux, S.; Garzetti, D.; Ring, D.; Jochum, L.M.; Beutler, M.; Eberl, C.; Hussain, S.; Walter, S.; Gerlach, R.G.; et al. Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe 2019, 25, 681–694.e8. [Google Scholar] [CrossRef] [PubMed]
- Bloom, S.M.; Bijanki, V.N.; Nava, G.M.; Sun, L.; Malvin, N.P.; Donermeyer, D.L.; Dunne, W.M., Jr.; Allen, P.M.; Stappenbeck, T.S. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe 2011, 9, 390–403. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mou, D.; Ding, D.; Pu, J.; Zhou, P.; Cao, E.; Zhang, X.; Lan, J.; Ye, L.; Wen, W. Effects of Dietary Pretreatment with All-trans Lycopene on Lipopolysaccharide-Induced Jejunal Inflammation: A Multi-Pathway Phenomenon. Foods 2025, 14, 794. https://doi.org/10.3390/foods14050794
Mou D, Ding D, Pu J, Zhou P, Cao E, Zhang X, Lan J, Ye L, Wen W. Effects of Dietary Pretreatment with All-trans Lycopene on Lipopolysaccharide-Induced Jejunal Inflammation: A Multi-Pathway Phenomenon. Foods. 2025; 14(5):794. https://doi.org/10.3390/foods14050794
Chicago/Turabian StyleMou, Daolin, Dajiang Ding, Junning Pu, Pan Zhou, Enming Cao, Xueyan Zhang, Junrong Lan, Lu Ye, and Wanxue Wen. 2025. "Effects of Dietary Pretreatment with All-trans Lycopene on Lipopolysaccharide-Induced Jejunal Inflammation: A Multi-Pathway Phenomenon" Foods 14, no. 5: 794. https://doi.org/10.3390/foods14050794
APA StyleMou, D., Ding, D., Pu, J., Zhou, P., Cao, E., Zhang, X., Lan, J., Ye, L., & Wen, W. (2025). Effects of Dietary Pretreatment with All-trans Lycopene on Lipopolysaccharide-Induced Jejunal Inflammation: A Multi-Pathway Phenomenon. Foods, 14(5), 794. https://doi.org/10.3390/foods14050794





