Control of Listeria monocytogenes on Frankfurters by Surface Treatment with Olive Mill Wastewater Polyphenolic Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyphenolic Extract from Olive Mill Processing By-Products
2.2. Preliminary In Vitro Antimicrobial Activity Assay
2.3. Evaluation of PE Antimicrobial Activity on Frankfurters
2.3.1. Experimental Contamination and PE Treatment
2.3.2. Determination of LM
2.3.3. In Silico Evaluation of Growth Dynamics
2.4. Evaluation of PE Effect on Quality Characteristics of Uncontaminated Frankfurters
2.4.1. Physicochemical Determinations
2.4.2. Sensory Evaluation of Frankfurters
2.5. Statistical Analysis
3. Results and Discussions
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA FSIS, U.S. Department of Agriculture Food Safety Inspection Service FSIS Compliance Guideline: FSIS Compliance Guideline: Controlling Listeria Monocytogenes in Post-Lethality Exposed Ready-to-Eat Meat and Poultry Products. Available online: https://www.fsis.usda.gov/guidelines/2014-0001 (accessed on 20 October 2024).
- Kramer, B.; Mignard, C.; Warschat, D.; Gürbüz, S.; Aiglstorfer, P.; Muranyi, P. Inhibition of Listeria monocytogenes on bologna by a beta acid rich hop extract. Food Control 2021, 126, 108040. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- Zhang, H.; Wang, J.; Chang, Z.; Liu, X.; Chen, W.; Yu, Y.; Wang, X.; Dong, Q.; Ye, Y.; Zhang, X. Listeria monocytogenes contamination characteristics in two ready-to-eat meat plants from 2019 to 2020 in Shanghai. Front. Microbiol. 2021, 12, 729114. [Google Scholar] [CrossRef]
- Ravindhiran, R.; Sivarajan, K.; Sekar, J.N.; Murugesan, R.; Dhandapani, K. Listeria monocytogenes an emerging pathogen: A comprehensive overview on listeriosis, virulence determinants, detection, and anti-listerial interventions. Microb. Ecol. 2023, 86, 2231–2251. [Google Scholar] [CrossRef]
- Lagarde, J.; Feurer, C.; Denis, M.; Douarre, P.E.; Piveteau, P.; Roussel, S. Listeria monocytogenes prevalence and genomic diversity along the pig and pork production chain. Food Microbiol. 2023, 119, 104430. [Google Scholar] [CrossRef]
- Rohilla, A.; Kumar, V.; Ahire, J.J. Unveiling the persistent threat: Recent insights into Listeria monocytogenes adaptation, biofilm formation, and pathogenicity in foodborne infections. J. Food Sci. Technol. 2024, 61, 1428–1438. [Google Scholar] [CrossRef]
- Thévenot, D.; Delignette-Muller, M.L.; Christieans, S.; Vernozy-Rozand, C. Prevalence of Listeria monocytogenes in 13 dried sausage processing plants and their products. Int. J. Food Microbiol. 2005, 102, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.H.; Geveke, D.J.; Pulsfus, S.; Lemmenes, B. Inactivation of Listeria innocua on frankfurters by ultraviolet light and flash pasteurization. J. Food Sci. 2009, 74, M138–M141. [Google Scholar] [CrossRef] [PubMed]
- Aase, B.; Sundheim, G.; Langsrud, S.; Rørvik, L.M. Occurrence of and a possible mechanism for resistance to a quaternary ammonium compound in Listeria monocytogenes. Int. J. Food Microbiol. 2000, 62, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lavieri, N.A.; Sebranek, J.G.; Brehm-Stecher, B.F.; Cordray, J.C.; Dickson, J.S.; Horsch, A.M.; Jung, S.; Larson, E.M.; Manu, D.K.; Mendonca, A.F. Investigating the control of Listeria monocytogenes on alternatively-cured frankfurters using natural antimicrobial ingredients or post-lethality interventions. Meat Sci. 2014, 97, 568–574. [Google Scholar] [CrossRef]
- Upadhyay, A.; Upadhyaya, I.; Karumathil, D.P.; Yin, H.B.; Nair, M.S.; Bhattaram, V.; Chen, C.; Flock, G.; Mooyottu, S.; Venkitanarayanan, K. Control of Listeria monocytogenes on skinless frankfurters by coating with phytochemicals. LWT 2015, 63, 37–42. [Google Scholar] [CrossRef]
- Castellano, P.; Peña, N.; Ibarreche, M.P.; Carduza, F.; Soteras, T.; Vignolo, G. Antilisterial efficacy of Lactobacillus bacteriocins and organic acids on frankfurters. Impact on sensory characteristics. J. Food Sci. Technol. 2018, 55, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Crim, S.M.; Runkle, A.; Henao, O.L.; Mahon, B.E.; Hoekstra, R.M.; Griffin, P.M. Bacterial enteric infections among older adults in the United States: Foodborne diseases active surveillance network, 1996–2012. Foodborne Pathog. Dis. 2015, 12, 492–499. [Google Scholar] [CrossRef]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef]
- Gadang, V.P.; Hettiarachchy, N.S.; Johnson, M.G.; Owens, C. Evaluation of antibacterial activity of whey protein isolate coating incorporated with nisin, grape seed extract, malic acid, and EDTA on a turkey frankfurter system. J. Food Sci. 2008, 73, M389–M394. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.; Dzuvor, C.K.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Ranucci, D.; Ortenzi, R.; Urbani, S.; Servili, M.; Valiani, A. Antimicrobial activity of olive mill wastewater extract against Pseudomonas fluorescens isolated from mozzarella cheese. J. Food Sci. Technol. 2016, 5, 5760. [Google Scholar] [CrossRef]
- Roila, R.; Primavilla, S.; Ranucci, D.; Galarini, R.; Paoletti, F.; Altissimi, C.; Valiani, A.; Branciari, R. The Effects of Encapsulation on the In Vitro Anti-Clostridial Activity of Olive Mill Wastewater Polyphenolic Extracts: A Promising Strategy to Limit Microbial Growth in Food Systems. Molecules 2024, 29, 1441. [Google Scholar] [CrossRef]
- Roila, R.; Stefanetti, V.; Carboni, F.; Altissimi, C.; Ranucci, D.; Valiani, A.; Branciari, R. Antilisterial activity of olive-derived polyphenols: An experimental study on meat preparations. J. Food Sci. Technol. 2024, 13, 12447. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for determining bactericidal activity of antimicrobial agents. In Approved Guideline; CLSI Document M26-A; CLSI: Wayne, PA, USA, 2023. [Google Scholar]
- European Union Reference Laboratory for Listeria monocytogenes. EURL Lm Technical Guidance Document for Conducting Shelf-Life Studies on Listeria monocytogenes in Ready-to-Eat Foods. 2021. Available online: https://food.ec.europa.eu/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf (accessed on 20 October 2024).
- ISO 11290; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 11290-2; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 2: Enumeration Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- CIE. CIE, Ed.; Colorimetry 15.Commission Internationale de l’Eclairage; CIE: Wien, Austria, 1976. [Google Scholar]
- ISO 4120; Sensory Analysis. Methodology. Triangle Test. International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 8587; Sensory Analysis: Methodology, Ranking. International Organization for Standardization: Geneva, Switzerland, 2006.
- JMP® 9 Basic Analysis and Graphing; SAS Institute Inc.: Cary, NC, USA, 2010.
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial activity and action approach of the olive oil polyphenol extract against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef]
- Liu, Y.; McKeever, L.C.; Malik, N.S. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Kalogianni, A.I.; Lazou, T.; Bossis, I.; Gelasakis, A.I. Natural phenolic compounds for the control of oxidation, bacterial spoilage, and foodborne pathogens in meat. Foods 2020, 9, 794. [Google Scholar] [CrossRef]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Amini, A.; Liu, M.; Ahmad, Z. Understanding the link between antimicrobial properties of dietary olive phenolics and bacterial ATP synthase. Int. J. Biol. Macromol. 2017, 101, 153–164. [Google Scholar] [CrossRef]
- Theivendran, S.; Hettiarachchy, N.S.; Johnson, M.G. Inhibition of Listeria monocytogenes by nisin combined with grape seed extract or green tea extract in soy protein film coated on turkey frankfurters. J. Food Sci. 2006, 71, M39–M44. [Google Scholar] [CrossRef]
- Bubonja-Sonje, M.; Giacometti, J.; Abram, M. Antioxidant and antilisterial activity of olive oil, cocoa and rosemary extract polyphenols. Food Chem. 2011, 127, 1821–1827. [Google Scholar] [CrossRef]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.S.; Rodríguez, M.R.; de Fernando, G.D.G. Effects of electron beam irradiation on the variability in survivor number and duration of lag phase of four food-borne organisms. Int. J. Food Microbiol. 2011, 149, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Branciari, R.; Ortenzi, R.; Roila, R.; Miraglia, D.; Ranucci, D.; Valiani, A. Listeria monocytogenes in soft spreadable salami: Study of the pathogen behavior and growth prediction during manufacturing process and shelf life. Appl. Sci. 2020, 10, 4438. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Sullivan, G.A.; Jackson, A.L.; Zhou, G.H.; Sebranek, J.G. Effects of natural antimicrobials on inhibition of Listeria monocytogenes and on chemical, physical and sensory attributes of naturally-cured frankfurters. Meat Sci. 2012, 90, 130–138. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No. 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union L 2015, 338, 1–26. [Google Scholar]
- Farber, J.M.; Zwietering, M.; Wiedmann, M.; Schaffner, D.; Hedberg, C.W.; Harrison, M.A.; Harnett, E.; Chapman, B.; Donelly, C.W.; Goodburn, K.E.; et al. Alternative approaches to the risk management of Listeria monocytogenes in low risk foods. Food Control 2021, 123, 107601. [Google Scholar] [CrossRef]
- Dos Santos Alves, L.A.A.; Lorenzo, J.M.; Gonçalves, C.A.A.; Dos Santos, B.A.; Heck, R.T.; Cichoski, A.J.; Campagnol, P.C.B. Impact of lysine and liquid smoke as flavor enhancers on the quality of low-fat Bologna-type sausages with 50% replacement of NaCl by KCl. Meat Sci. 2017, 123, 50–56. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Color difference delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Wang, L.; McKeith, A.G.; Shen, C.; Carter, K.; Huff, A.; McKeith, R.; Zhang, X.; Chen, Z. Effect of hops beta acids on the survival of unstressed-or acid-stress-adapted Listeria monocytogenes and on the quality and sensory attributes of commercially cured ham slices. J. Food Sci. 2016, 81, M445–M453. [Google Scholar] [CrossRef]
- Gedikoğlu, A. The effect of Thymus vulgaris and Thymbra spicata essential oils and/or extracts in pectin edible coating on the preservation of sliced bolognas. Meat Sci. 2022, 184, 108697. [Google Scholar] [CrossRef]
- Morey, A.; Bowers, J.W.; Bauermeister, L.J.; Singh, M.; Huang, T.S.; McKee, S.R. Effect of salts of organic acids on Listeria monocytogenes, shelf life, meat quality, and consumer acceptability of beef frankfurters. J. Food Sci. 2014, 79, M54–M60. [Google Scholar] [CrossRef] [PubMed]
- Nunez de Gonzalez, M.T.; Keeton, J.T.; Ringer, L.J. Sensory and physicochemical characteristics of frankfurters containing lactate with antimicrobial surface treatments. J. Food Sci. 2004, 69, S221–S228. [Google Scholar] [CrossRef]
- Singham, P.; Birwal, P.; Yadav, B.K. Importance of objective and subjective measurement of food quality and their inter-relationship. J. Food Process Technol. 2015, 6, 488. [Google Scholar]
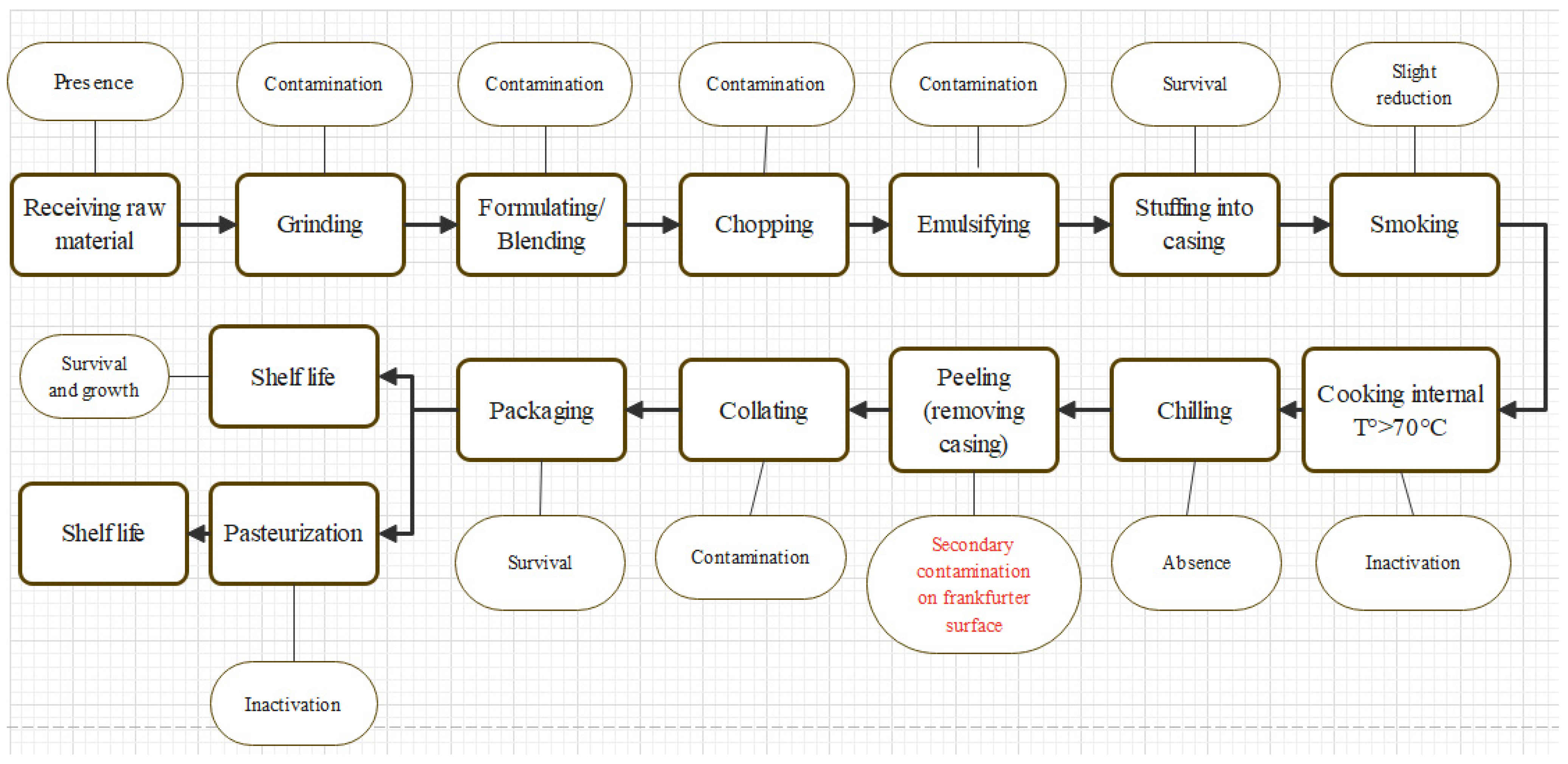
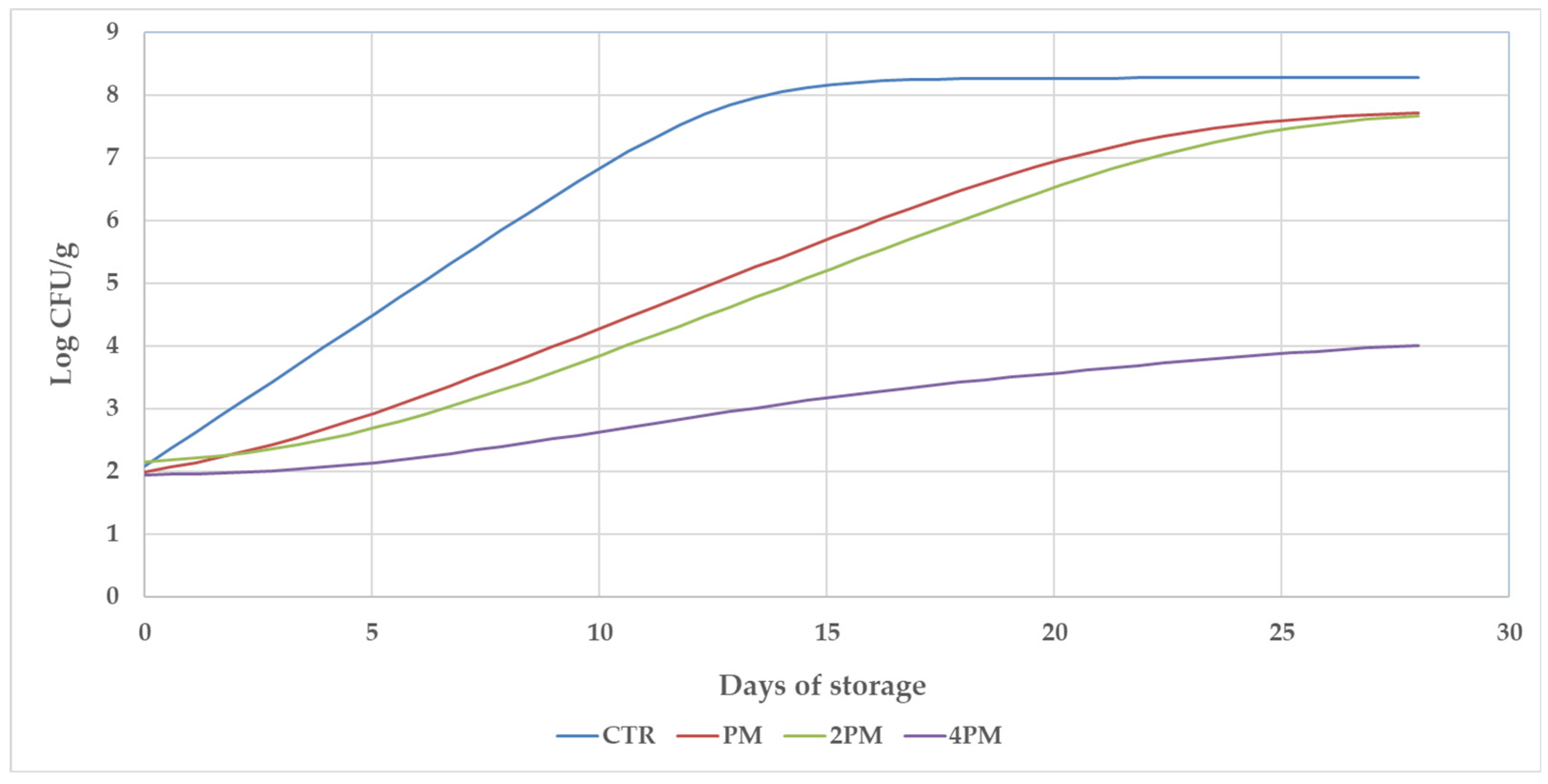
| Days of Storage | SEM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 0.150 | S | T | SXT | |
| CTR | 2.07 a | 5.5 bC | 7.95 cD | 8.21 cC | 8.39 cC | <0.001 | <0.001 | <0.001 | |
| PM | 2.00 a | 3.43 bB | 5.43 cC | 7.07 dB | 7.74 eB | ||||
| 2PM | 2.15 a | 3.10 bB | 4.91 cB | 6.77 dB | 7.66 eB | ||||
| 4PM | 1.94 a | 2.31 bA | 3.09 cA | 3.62 cdA | 4.01 dA | ||||
| CTR | PM | 2PM | 4PM | SEM | p-Value | |
|---|---|---|---|---|---|---|
| Initial value(Log CFU/g) | 2.085 | 1.997 | 2.152 | 1.940 | 0.047 | 0.177 |
| λ (h) | n.d. | 52.059 a | 97.079 b | 99.741 b | 15.469 | <0.001 |
| µmax (Log CFU/g/h) | 0.020 c | 0.012 b | 0.012 b | 0.005 a | 0.003 | <0.001 |
| Final value(Log CFU/g) | 8.268 b | 7.763 b | 7.764 b | 4.158 a | 0.951 | <0.001 |
| Days of Storage | SEM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | 0 | 7 | 14 | 21 | 28 | T | S | TxS | ||
| pH | CTR | 6.09 b | 6.00 ab | 5.97 ab | 5.94 ab | 5.90 a | 0.038 | 0.006 | 0.323 | 0.925 |
| PM | 5.97 | 5.97 | 5.95 | 5.93 | 5.91 | |||||
| 2PM | 6.05 | 6.00 | 5.98 | 5.96 | 5.93 | |||||
| 4PM | 5.98 | 5.97 | 5.95 | 5.95 | 5.93 | |||||
| aw | CTR | 0.978 c | 0.975 bc | 0.973 b | 0.972 ab | 0.968 a | 0.001 | <0.01 | 0.042 | 0.554 |
| PM | 0.978 b | 0.976 b | 0.974 ab | 0.972 a | 0.971 a | |||||
| 2PM | 0.976 b | 0.974 b | 0.973 b | 0.973 b | 0.967 a | |||||
| 4PM | 0.979 c | 0.975 bc | 0.974 b | 0.973 ab | 0.970 a | |||||
| Days of Storage | Samples | SEM | T | S | TxS | ||||
|---|---|---|---|---|---|---|---|---|---|
| CTR | PM | 2PM | 4PM | ||||||
| L* | 0 | 55.82 | 55.27 | 55.18 | 54.76 | 0.417 | 0.112 | 0.850 | 0.337 |
| 28 | 54.50 | 54.97 | 54.59 | 54.99 | |||||
| a* | 0 | 15.30 B | 15.09 | 15.02 | 14.91 | 0.184 | 0.001 | 0.480 | 0.099 |
| 28 | 14.20 A | 14.91 | 14.63 | 14.57 | |||||
| b* | 0 | 11.87 | 12.18 | 12.44 | 12.79 | 0.447 | 0.479 | 0.858 | 0.600 |
| 28 | 12.54 | 12.75 | 12.55 | 12.36 | |||||
| SEM | p-value | ||||||||
| ΔE | 1.99 b | 1.62 ab | 1.89 b | 1.23 a | 0.170 | 0.016 | |||
| Session | Compared Samples | Number of Untrained Assessors | Correctly Identified | Correctly Identified % | Significance Differences (α = 0.05; β = 0.05) |
|---|---|---|---|---|---|
| 1 | CTR vs. PM | 30 | 5 | 16.7 | no |
| 2 | CTR vs. 2PM | 30 | 8 | 26.7 | no |
| 3 | CTR vs. 4PM | 30 | 11 | 36.7 | no |
| 4 | PM vs. 2PM | 30 | 4 | 13.3 | no |
| 5 | PM vs. 4PM | 30 | 5 | 16.7 | no |
| 6 | 2PM vs. 4PM | 30 | 7 | 23.3 | no |
| Rank Sum | χ2 | p-Value | ||||
|---|---|---|---|---|---|---|
| CTR | PM | 2PM | 4PM | |||
| 1 day of storage | 33 | 31 | 26 | 30 | 1.3 | 0.729 |
| 28 days of storage | 28 | 26 | 34 | 32 | 2 | 0.572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roila, R.; Valiani, A.; Servili, M.; Ranucci, D.; Galarini, R.; Ortenzi, R.; Primavilla, S.; Branciari, R. Control of Listeria monocytogenes on Frankfurters by Surface Treatment with Olive Mill Wastewater Polyphenolic Extract. Foods 2025, 14, 774. https://doi.org/10.3390/foods14050774
Roila R, Valiani A, Servili M, Ranucci D, Galarini R, Ortenzi R, Primavilla S, Branciari R. Control of Listeria monocytogenes on Frankfurters by Surface Treatment with Olive Mill Wastewater Polyphenolic Extract. Foods. 2025; 14(5):774. https://doi.org/10.3390/foods14050774
Chicago/Turabian StyleRoila, Rossana, Andrea Valiani, Maurizio Servili, David Ranucci, Roberta Galarini, Roberta Ortenzi, Sara Primavilla, and Raffaella Branciari. 2025. "Control of Listeria monocytogenes on Frankfurters by Surface Treatment with Olive Mill Wastewater Polyphenolic Extract" Foods 14, no. 5: 774. https://doi.org/10.3390/foods14050774
APA StyleRoila, R., Valiani, A., Servili, M., Ranucci, D., Galarini, R., Ortenzi, R., Primavilla, S., & Branciari, R. (2025). Control of Listeria monocytogenes on Frankfurters by Surface Treatment with Olive Mill Wastewater Polyphenolic Extract. Foods, 14(5), 774. https://doi.org/10.3390/foods14050774








