Optimization and Comprehensive Characterization of the Microencapsulation Process for Taro Essence
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction of Taro Essence
2.3. Preparation of Taro Essence Microcapsules
2.4. Calculation of the Encapsulation Efficiency and Yield of Essence Microcapsules
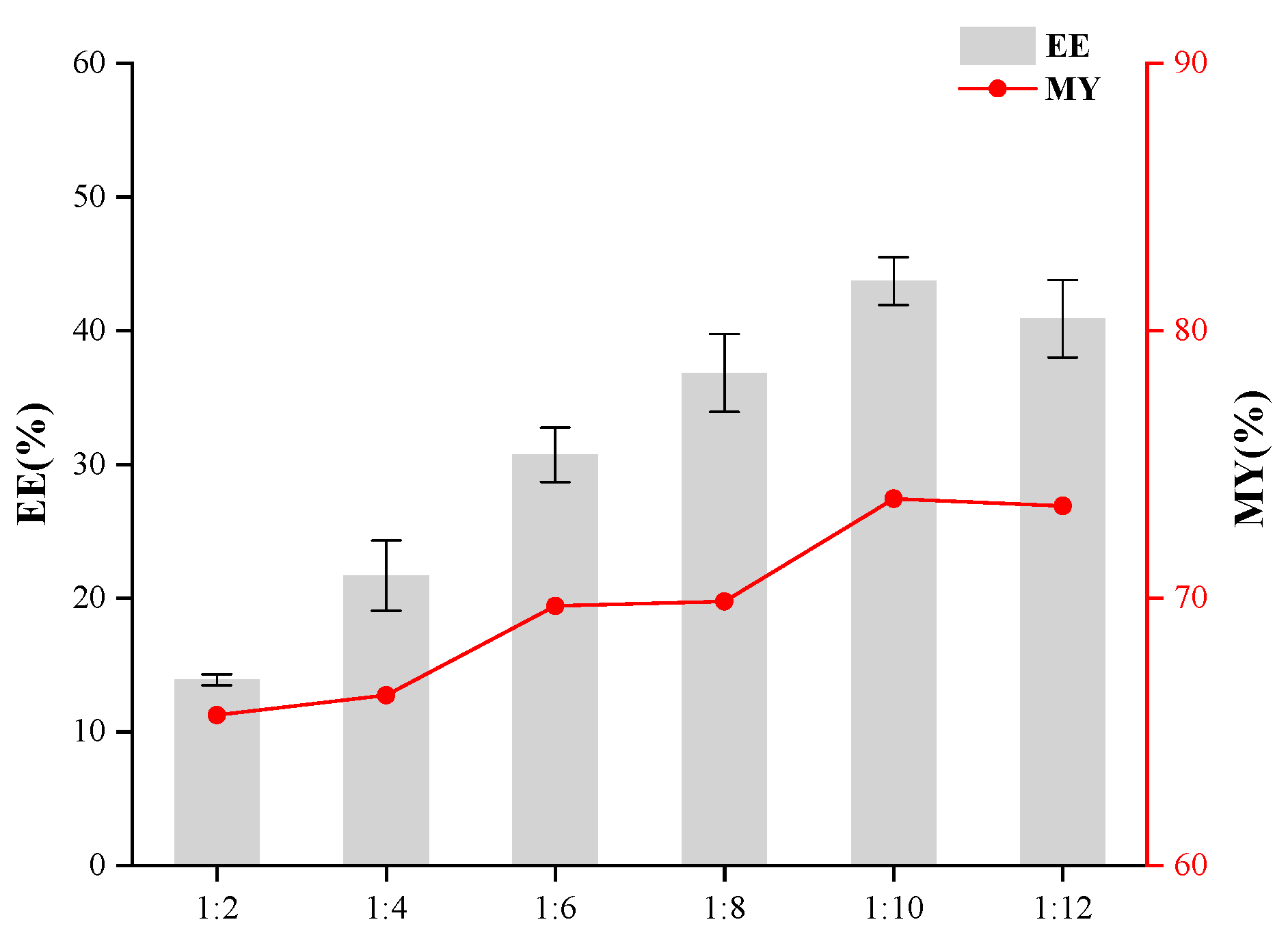
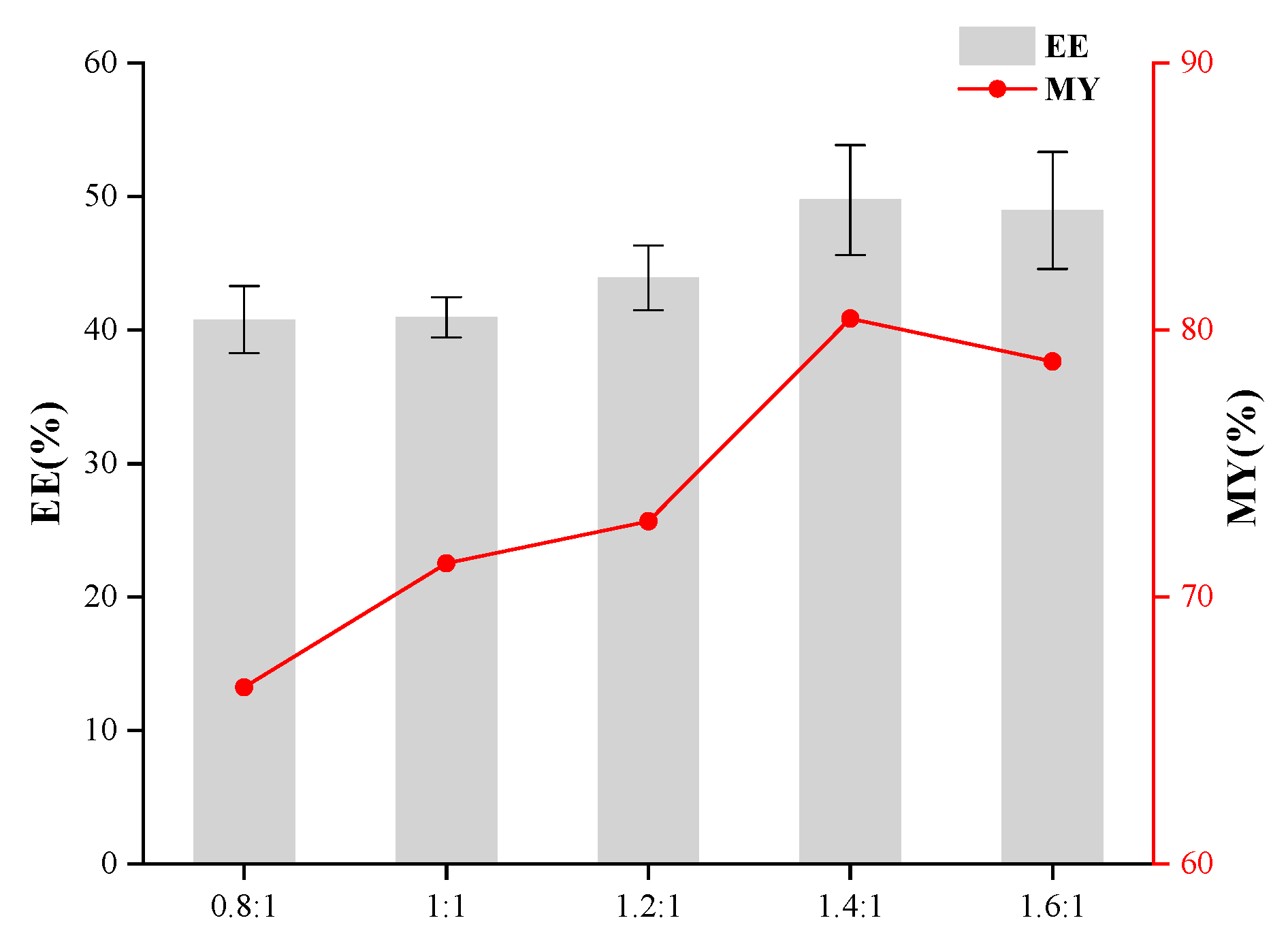
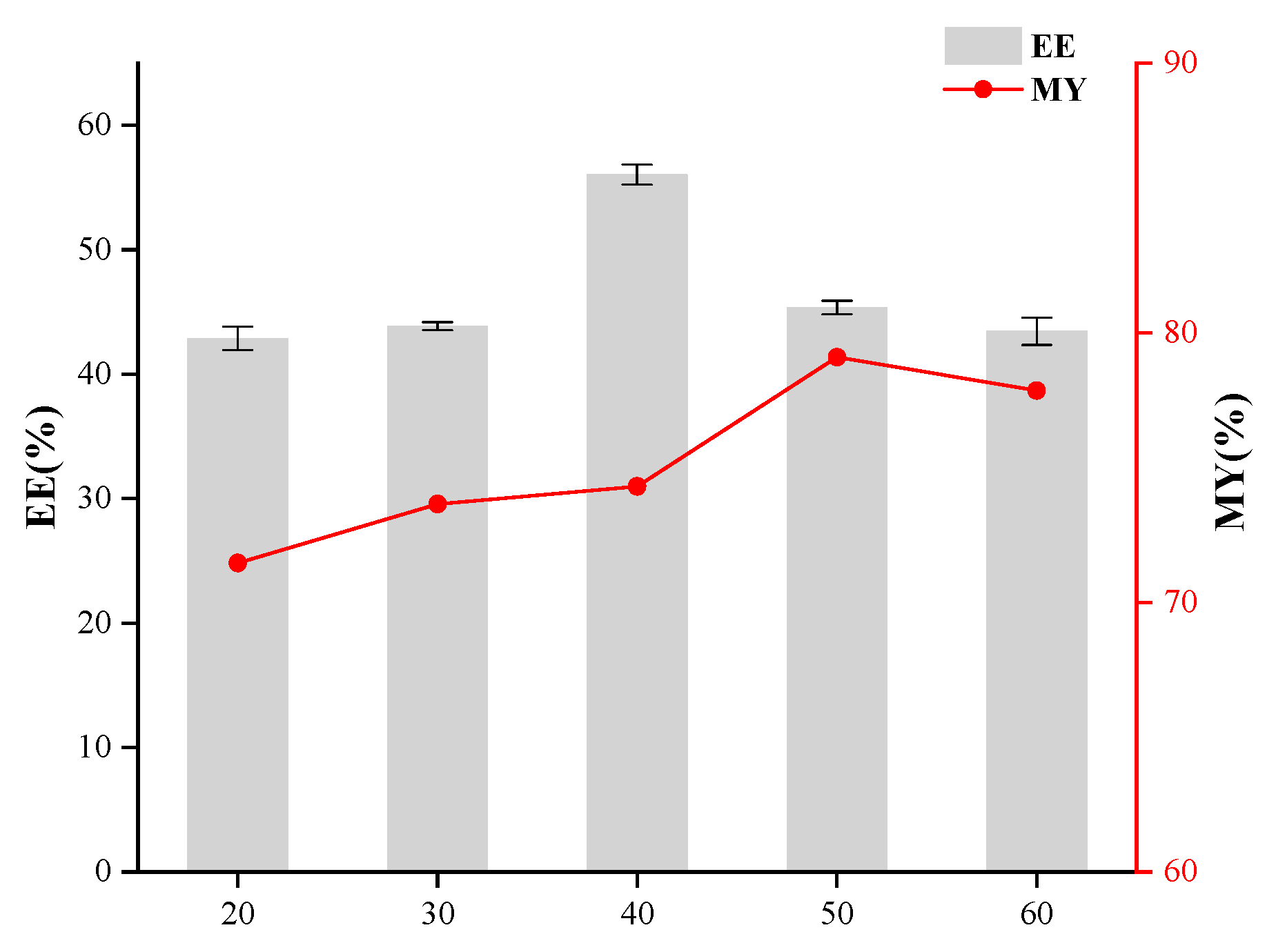
2.5. Single-Factor Experiments in the Preparation of Microcapsules
2.6. The Orthogonal Experiment for Microcapsule Preparation
2.7. Determination of Moisture Content
2.8. Measurement of Solubility
2.9. Measurement of the Angle of Repose
2.10. Measurement of the Bulk Density and Tapped Density
2.11. Measurement of the Flowability and Cohesiveness
2.12. Measurement of the Particle Size and Zeta Potential
2.13. Measurement of the Scanning Electron Microscopy (SEM)
2.14. Measurement of the Fourier Transform Infrared (FT-IR) Spectrum and X-Ray Diffraction (XRD) Patterns
2.15. Measurement of the Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
2.16. Analysis of Volatile Compounds by HS–SPME–GCMS
2.17. Statistical Analysis
3. Results and Discussion
3.1. Effects of Core-to-Wall Ratio, T-20/β-CD Mass Ratio, and Ultrasonic Time on Microcapsule Embedding Efficiency
3.2. Orthogonal Experimental Analysis
3.3. Analysis of Basic Physical Properties of Taro Essence Microcapsules
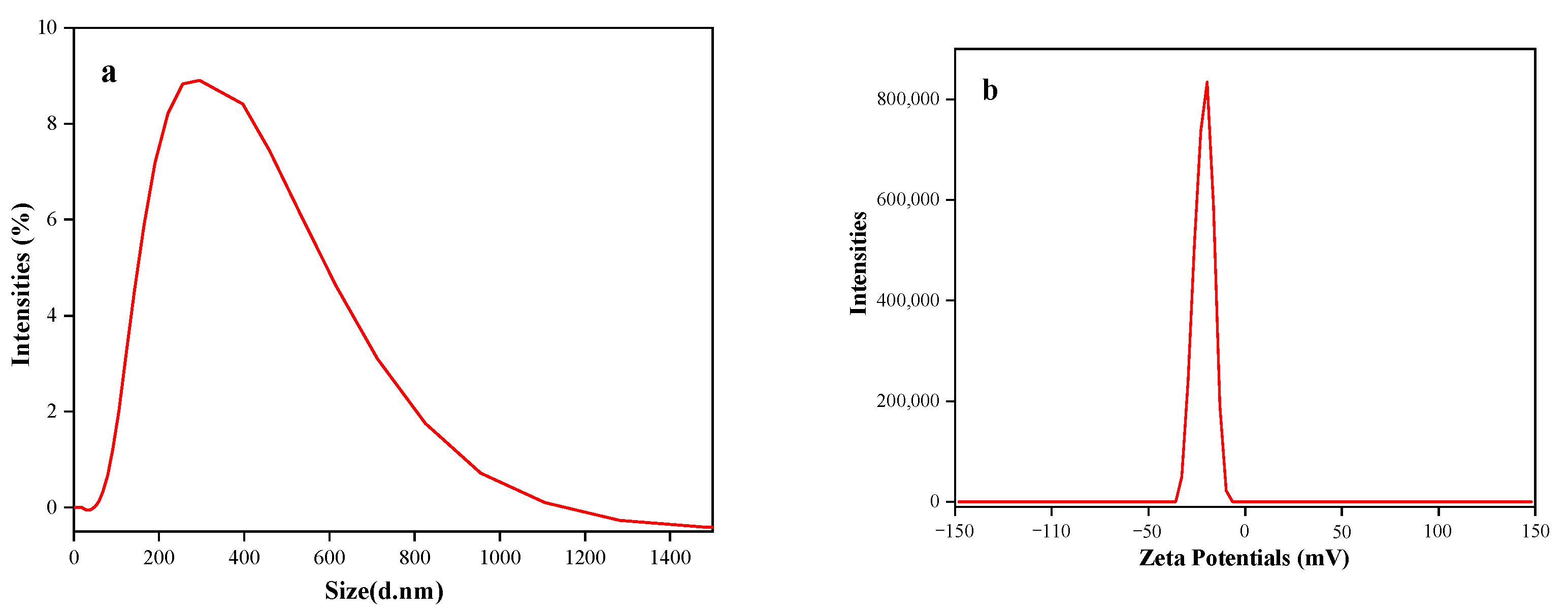
3.4. Analysis of Particle Size and Zeta Potential of Taro Essence Microcapsules
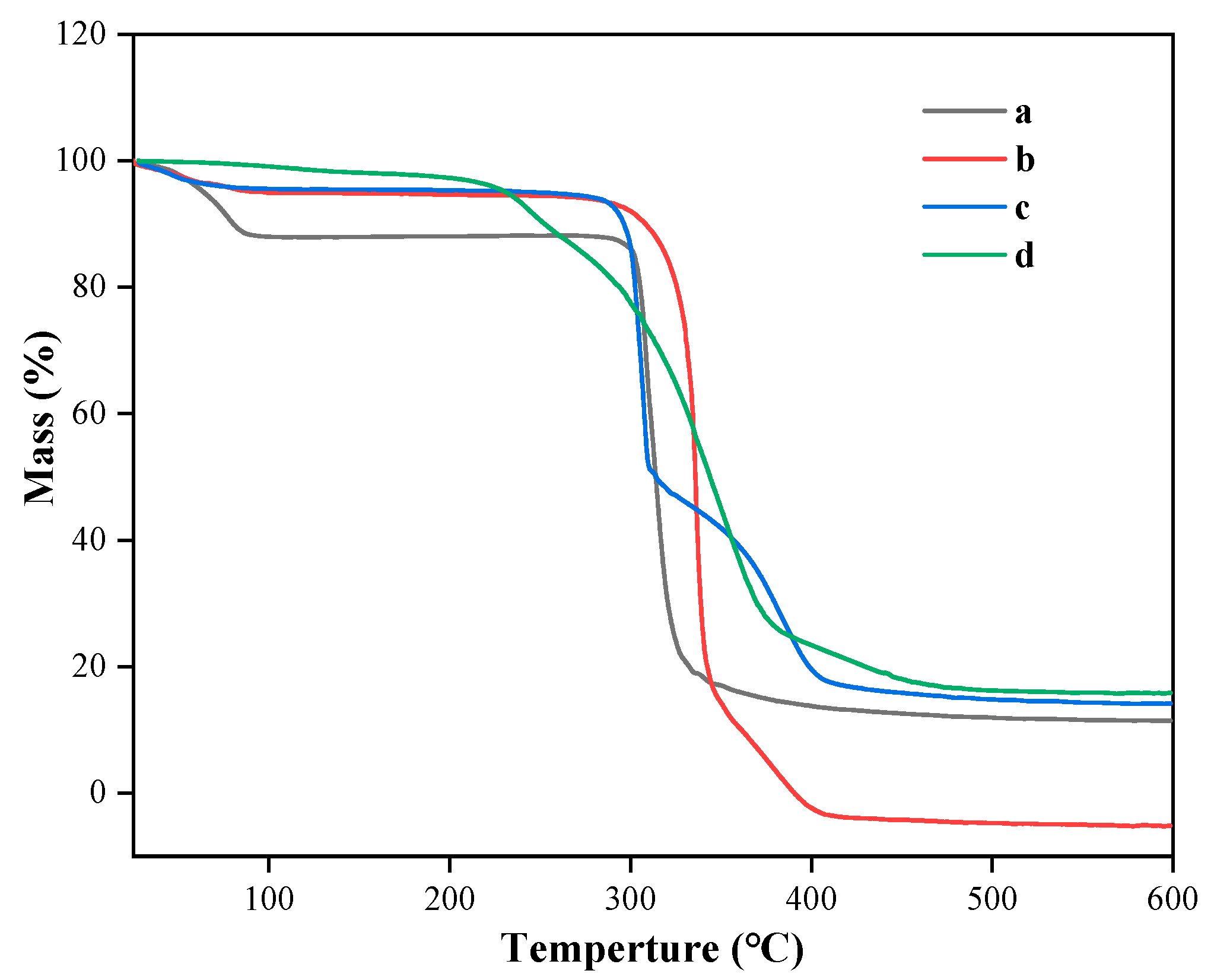
3.5. TGA Analysis of Taro Essence Microcapsules
3.6. DSC Analysis of Taro Essence Microcapsules
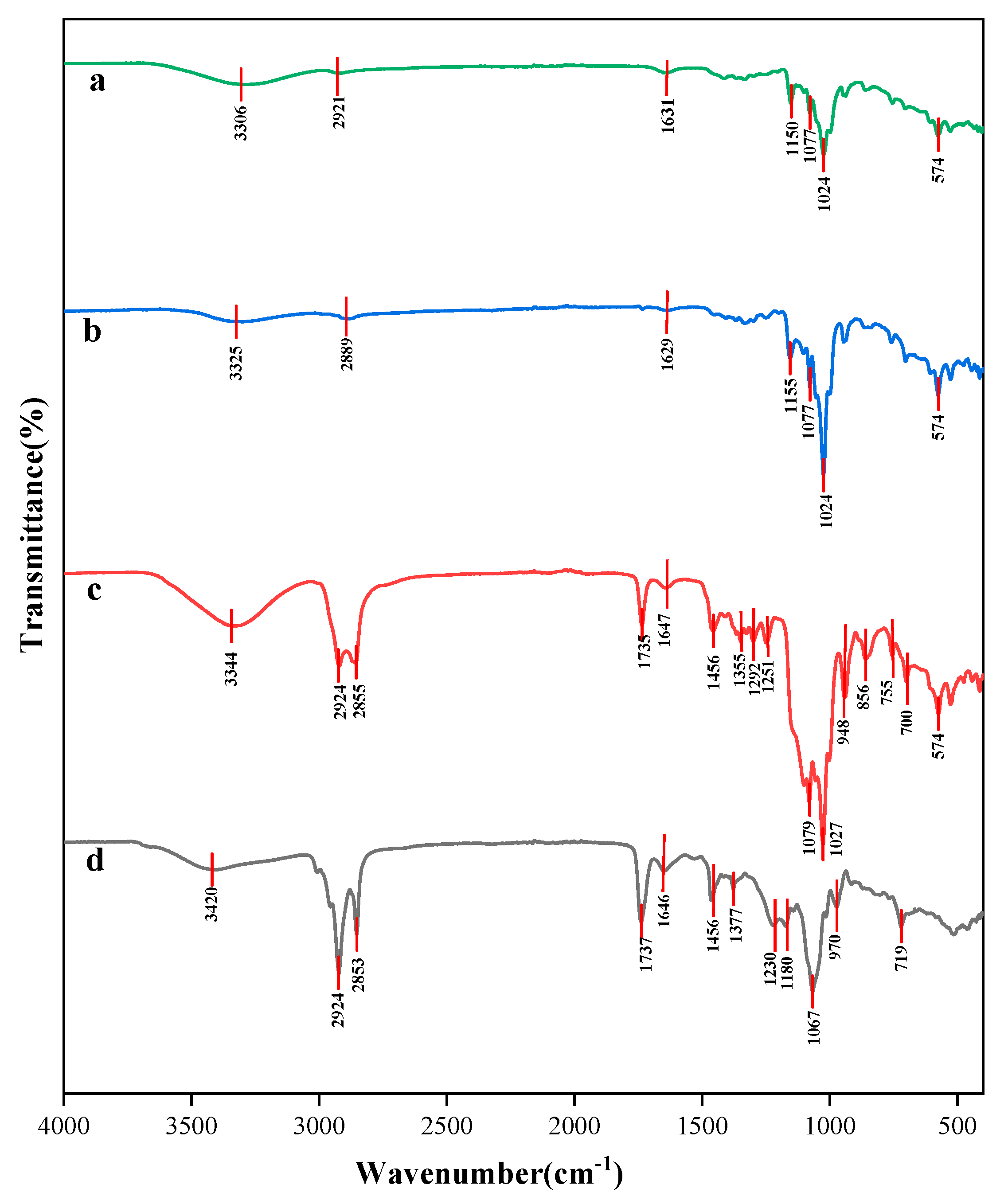
3.7. FT-IR Analysis of Taro Essence Microcapsules
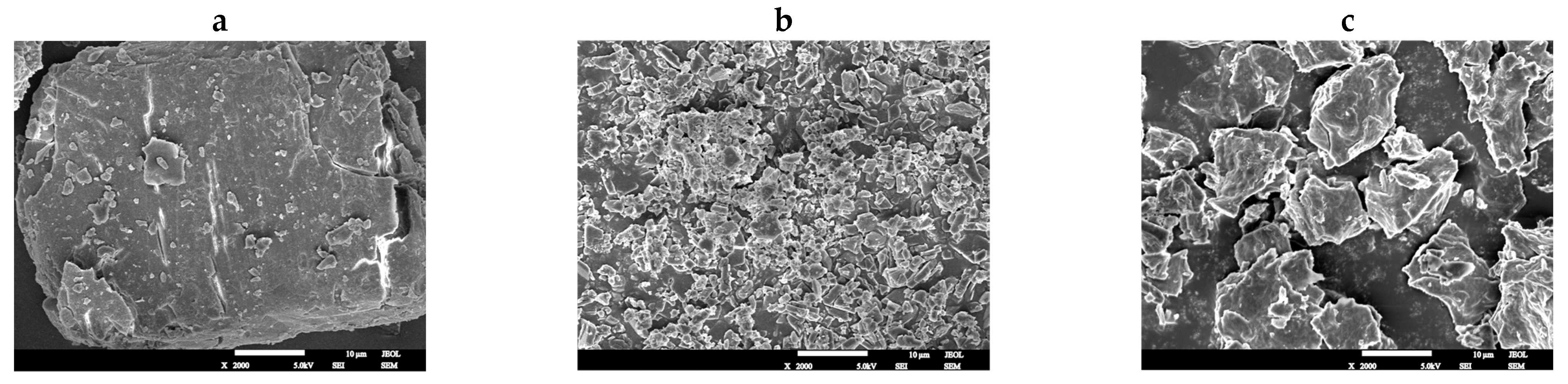
3.8. Morphological Analysis of Taro Essence Microcapsules by SEM
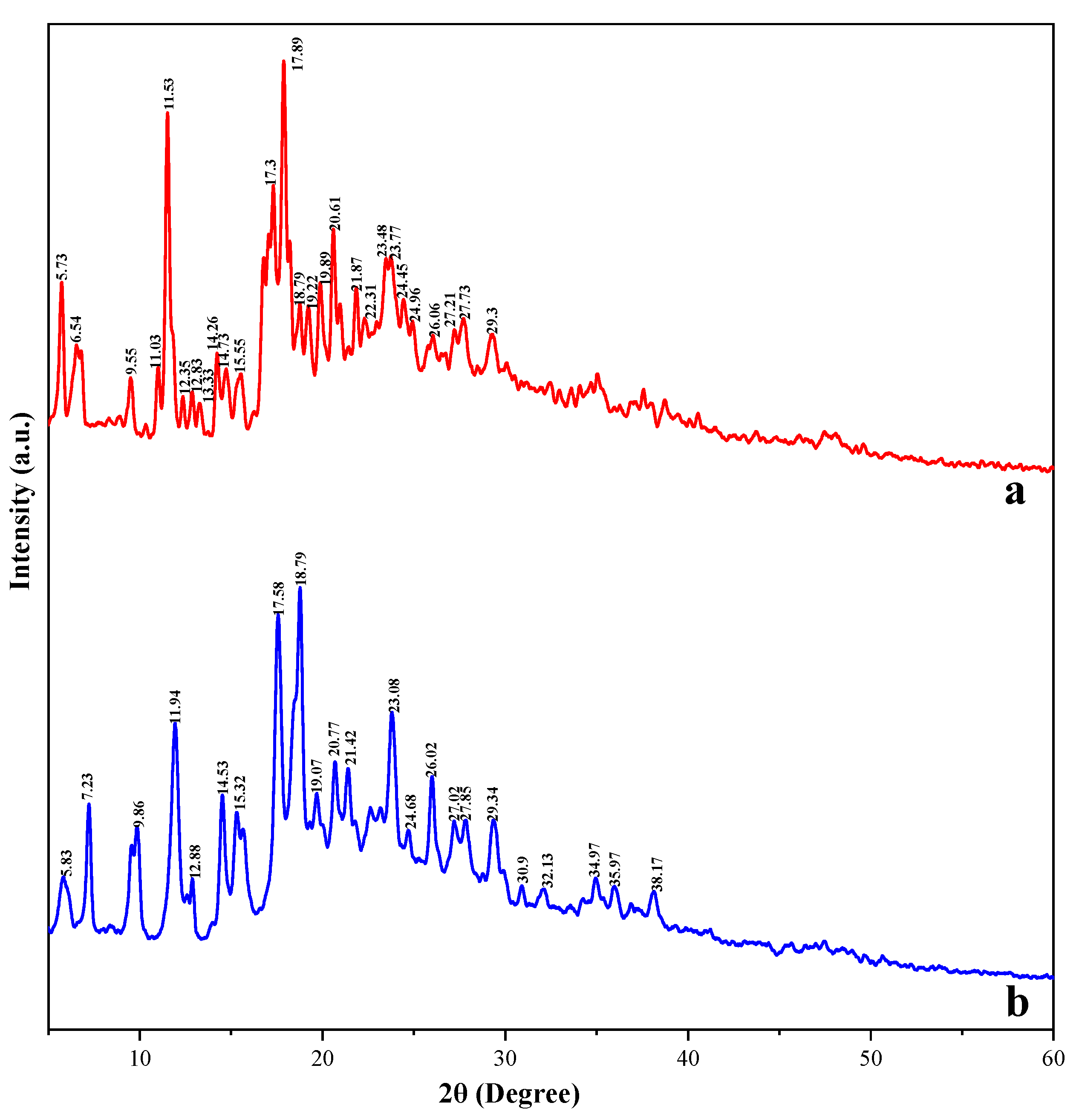
3.9. XRD Analysis of Taro Essence Microcapsules
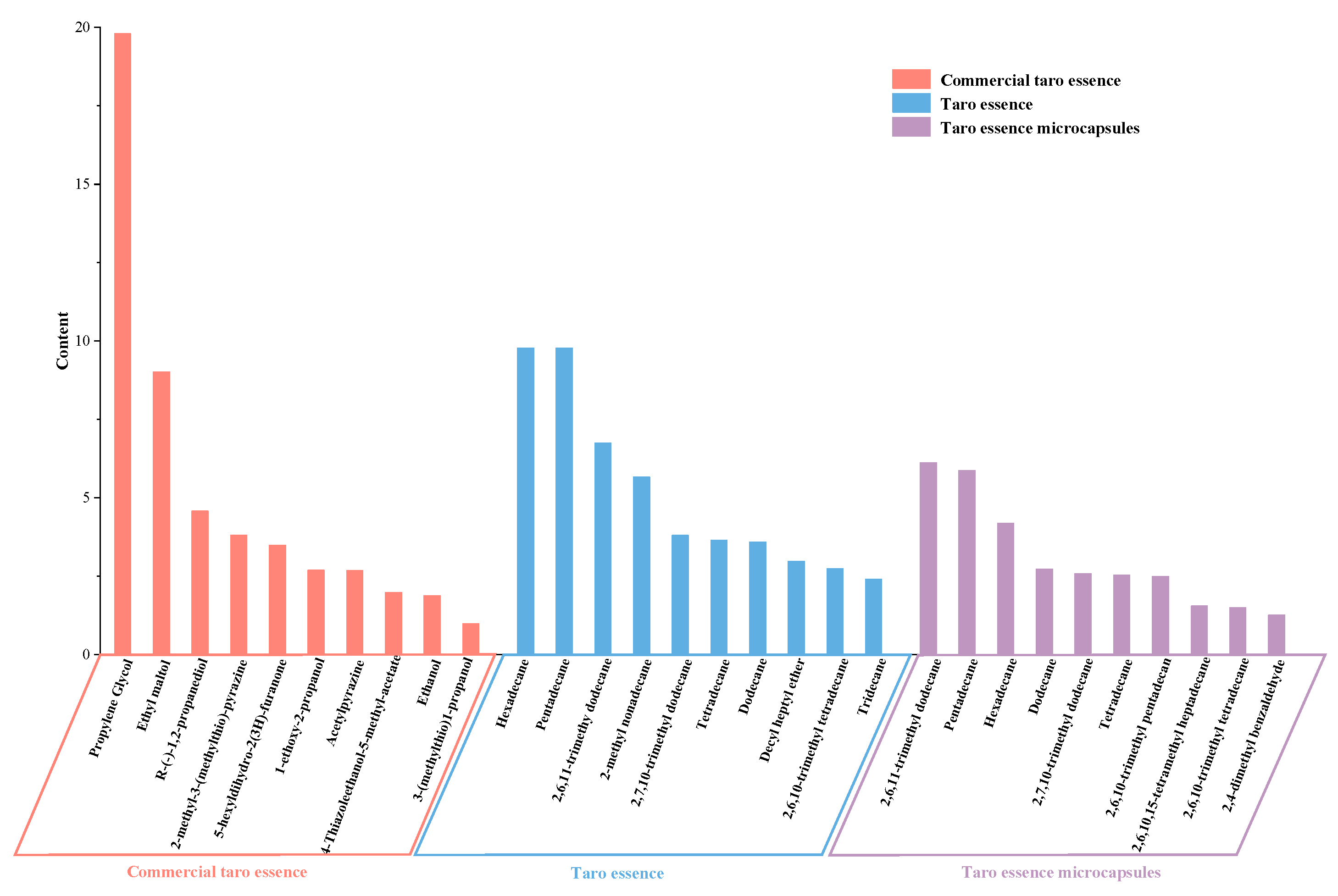
3.10. Analysis of Volatile Flavor Components in Taro Essence Microcapsules
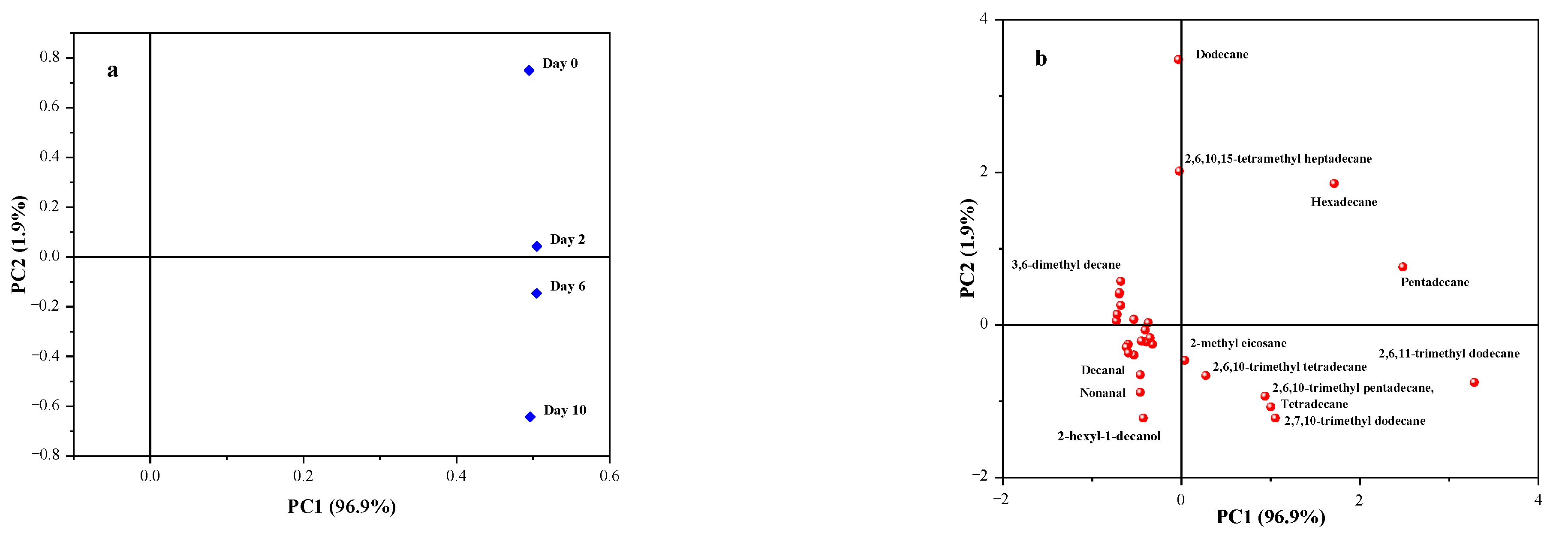
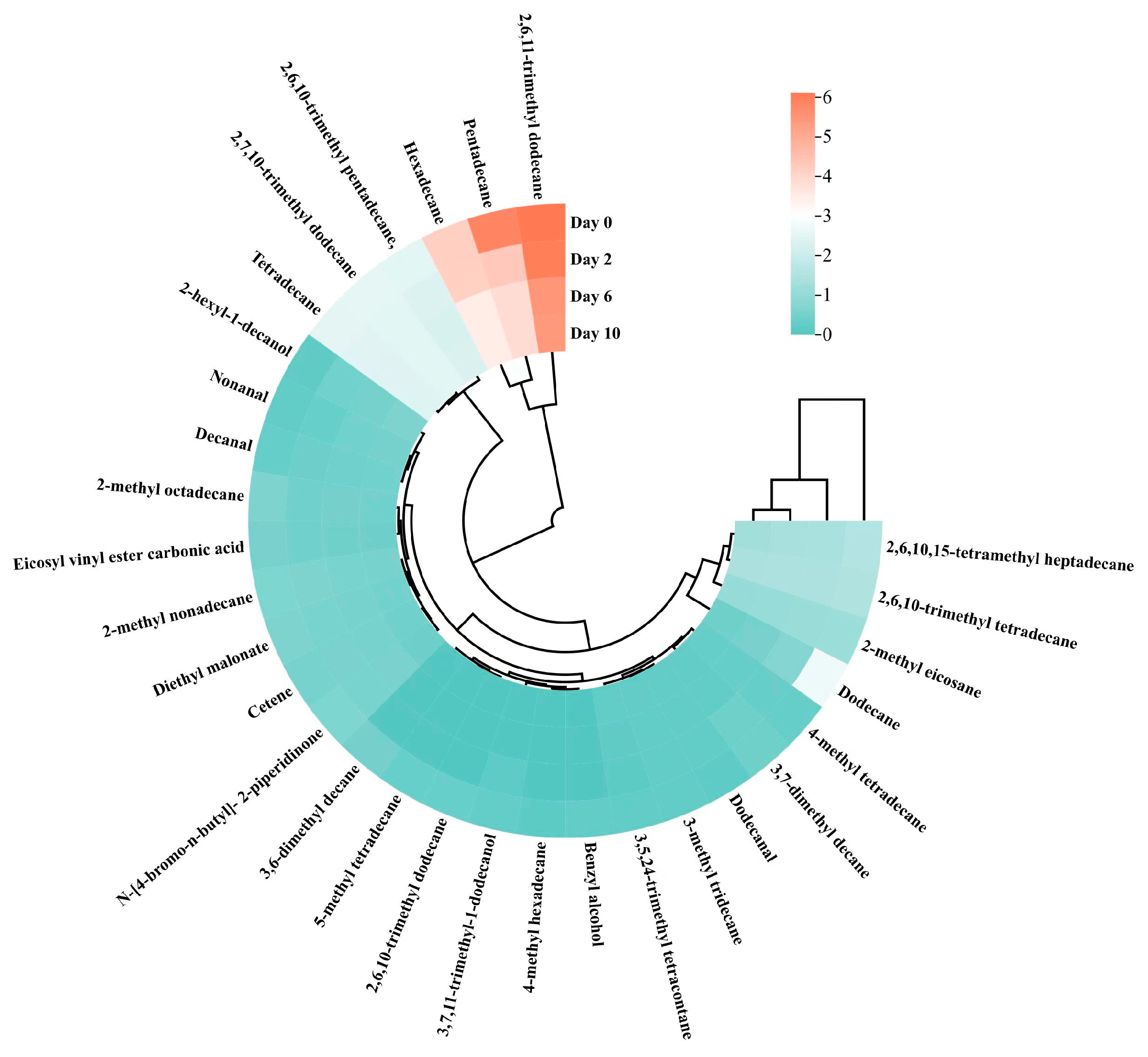
3.11. Analysis of Volatile Flavor Components in Taro Essence Microcapsules During Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, E.; Shen, W.; Jiang, W.; Li, W.; Wan, X.; Yu, X.; Xiong, F. Research Progress on the Bulb Expansion and Starch Enrichment in Taro (Colocasia esculenta (L). Schott). PeerJ 2023, 11, e15400. [Google Scholar] [CrossRef]
- Zubair, M.W.; Imran, A.; Islam, F.; Afzaal, M.; Saeed, F.; Zahra, S.M.; Akhtar, M.N.; Noman, M.; Ateeq, H.; Aslam, M.A.; et al. Functional Profile and Encapsulating Properties of Colocasia esculenta (Taro). Food Sci. Nutr. 2023, 11, 2440–2449. [Google Scholar] [CrossRef]
- Nip, W.-K. Taro. In Processing Vegetables; Routledge: Boca Raton, FL, USA, 2023; pp. 355–388. ISBN 978-0-203-74186-3. [Google Scholar]
- Muharja, M.; Darmayanti, R.F.; Khamil, A.I.; Prastika, A.; Rizalluddin, M.; Fadilah, S.N.; Sari, D.A.D. Evaluation of Dehydration Performance of Belitung Taro (Xanthosoma Sagittifolium) Using Tray Dryer. J. Technol. Sci. 2023, 34, 1. [Google Scholar] [CrossRef]
- Nurilmala, F.; Masnang, A.; Sonani, N. Pendampingan Budidaya Talas Varietas Baru Bagi KWT Sawargi Kelurahan Situgede Kecamatan Bogor Barat Kota Bogor. J. Abdimas Adpi Sos. Hum. 2023, 4, 588–593. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Comp. Rev. Food Sci. Food Safe 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Kłosowska, A.; Wawrzyńczak, A.; Feliczak-Guzik, A. Microencapsulation as a Route for Obtaining Encapsulated Flavors and Fragrances. Cosmetics 2023, 10, 26. [Google Scholar] [CrossRef]
- Russell, S.; Bruns, N. Encapsulation of Fragrances in Micro- and Nano-Capsules, Polymeric Micelles, and Polymersomes. Macromol. Rapid Commun. 2023, 44, 2300120. [Google Scholar] [CrossRef] [PubMed]
- El Kharraf, S.; Farah, A.; El-Guendouz, S.; Lourenço, J.P.; Rosa Costa, A.M.; El Hadrami, E.M.; Machado, A.M.; Tavares, C.S.; Figueiredo, A.C.; Miguel, M.G. β-Cyclodextrin Inclusion Complexes of Combined Moroccan Rosmarinus officinalis, Lavandula angustifolia and Citrus aurantium Volatile Oil: Production Optimization and Release Kinetics in Food Models. J. Essent. Oil Res. 2023, 35, 247–261. [Google Scholar] [CrossRef]
- Liu, H.-N.; Jiang, X.-X.; Naeem, A.; Chen, F.-C.; Wang, L.; Liu, Y.-X.; Li, Z.; Ming, L.-S. Fabrication and Characterization of β-Cyclodextrin/Mosla Chinensis Essential Oil Inclusion Complexes: Experimental Design and Molecular Modeling. Molecules 2022, 28, 37. [Google Scholar] [CrossRef]
- Iliadi, E.; Lamari, F. Encapsulation of Two Distinct Cistus Essential Oils in β- and γ-Cyclodextrins. Planta Medica 2022, 88, 1574. [Google Scholar]
- Albuquerque, P.M.; Azevedo, S.G.; De Andrade, C.P.; D’Ambros, N.C.D.S.; Pérez, M.T.M.; Manzato, L. Biotechnological Applications of Nanoencapsulated Essential Oils: A Review. Polymers 2022, 14, 5495. [Google Scholar] [CrossRef]
- Vasisht, N. Factors and Mechanisms in Microencapsulation. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 29–38. ISBN 978-0-12-821683-5. [Google Scholar]
- Tian, Y.; Luo, W.; Wang, Y.; Yu, Y.; Huang, W.; Tang, H.; Zheng, Y.; Liu, Z. Ultrasound-Assisted Fast Encapsulation of Metal Microparticles in SiO2 via an Interface-Confined Sol-Gel Method. Ultrason. Sonochem. 2021, 73, 105484. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Tian, J.; Chu, Z. Effect of a New Shell Material—Jackfruit Seed Starch on Novel Flavor Microcapsules Containing Vanilla Oil. Ind. Crops Prod. 2018, 112, 47–52. [Google Scholar] [CrossRef]
- Santos, F.S.D.; Figueirêdo, R.M.F.D.; Queiroz, A.J.D.M.; Paiva, Y.F.; Moura, H.V.; Silva, E.T.D.V.; Ferreira, J.P.D.L.; Melo, B.A.D.; Carvalho, A.J.D.B.A.; Lima, M.D.S.; et al. Influence of Dehydration Temperature on Obtaining Chia and Okra Powder Mucilage. Foods 2023, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Condori, S.; Saldaña, M.D.; Temelli, F. Microencapsulation of Flax Oil with Zein Using Spray and Freeze Drying. LWT 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Nishad, J.; Selvan, C.J.; Mir, S.A.; Bosco, S.J.D. Effect of Spray Drying on Physical Properties of Sugarcane Juice Powder (Saccharum officinarum L.). J. Food Sci. Technol. 2017, 54, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of Surfactant Surface Coverage on Formation of Solid Lipid Nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef]
- Sharma, R.; Borah, A.; Sharma, R.; Borah, A. Prospect of Microcapsules as a Delivery System in Food Technology: A Review. Pharma Innov. J. 2021, 10, 182–191. [Google Scholar] [CrossRef]
- da Dias, D.R.C.; Barros, Z.M.P.; de Carvalho, C.B.O.; Honorato, F.A.; Guerra, N.B.; Azoubel, P.M. Effect of Sonication on Soursop Juice Quality. LWT 2015, 62, 883–889. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Xu, Q.; Jin, H.; Hu, Q.; Han, D. Effective Two-Stage Heterotrophic Cultivation of the Unicellular Green Microalga Chromochloris Zofingiensis Enabled Ultrahigh Biomass and Astaxanthin Production. Front. Bioeng. Biotechnol. 2022, 10, 834230. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bi, J.; Jin, W.; Fan, B.; Qian, C. Determination of Polysaccharides Composition in Polygonatum Sibiricum and Polygonatum Odoratum by HPLC-FLD with Pre-Column Derivatization. Heliyon 2022, 8, e09363. [Google Scholar] [CrossRef]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Nolazco–Cama, D.; Sánchez-Contreras, A.; Tellez-Monzón, L.; Vargas-Delgado, L.; Condezo-Hoyos, L. Influence of Essential Oil: Cyclodextrin Ratio and Stirring Rate on Physicochemical Characteristics of Orange Essential Oil: β-Cyclodextrin Microparticles. CyTA-J. Food 2023, 21, 366–373. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Microencapsulation of Betaxanthin Pigments from Pitahaya (Hylocereus megalanthus) by-Products: Characterization, Food Application, Stability, and in Vitro Gastrointestinal Digestion. Foods 2023, 12, 2700. [Google Scholar] [CrossRef] [PubMed]
- Yeddes, W.; Mejri, I.; Wannes, W.A.; Affes, T.G.; Khammassi, S.; Hammami, M.; Tounsi, M.S. Effect of Emulsifiers and Wall Materials on Particle Size Distribution and Stability of the Blended Essential Oils Nanoemulsions. J. Sustain. Mater. Process. Manag. 2022, 2, 24–31. [Google Scholar] [CrossRef]
- Ravindran Maniam, M.M.; Loong, Y.H.; Samsudin, H. Understanding the Formation of β-Cyclodextrin Inclusion Complexes and Their Use in Active Packaging Systems. Starch Stärke 2022, 74, 2100304. [Google Scholar] [CrossRef]
- Lad, S.; Narkhede, S.; Luhar, S.; Prajapati, A. Review on Moisture Content: A Stability Problem in Pharmaceuticals. EPRA Int. J. Res. Dev. (IJRD) 2022, 7, 27–33. [Google Scholar]
- Biyani, M. HPMC Capsules for Moisture Sensitive and Hygroscopic Products. Pharm. Sci. Technol. 2021, 5, 50. [Google Scholar] [CrossRef]
- Sukri, N.; Annisa, D.S.; Djali, M.; Cahyana, Y.; Mahani, M.; Huda, S. Effect of Whey Protein Isolate (WPI)–Pectin Ratio on Phenolic Content Stability of Propolis Microcapsules. Preprints 2023. [Google Scholar] [CrossRef]
- Nasr, A.M.; Elhady, S.S.; Swidan, S.A.; Badawi, N.M. Celecoxib Loaded In-Situ Provesicular Powder and Its In-Vitro Cytotoxic Effect for Cancer Therapy: Fabrication, Characterization, Optimization and Pharmacokinetic Evaluation. Pharmaceutics 2020, 12, 1157. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Sahoo, N.; Sahoo, R.K.; Biswas, N.; Guha, A.; Kuotsu, K. Recent Advancement of Gelatin Nanoparticles in Drug and Vaccine Delivery. Int. J. Biol. Macromol. 2015, 81, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, M.; Yusof, Y.A.; Chin, N.L.; Aziz, M.G. Physicochemical and Flow Properties of Fruit Powder and Their Effect on the Dissolution of Fast Dissolving Fruit Powder Tablets. Powder Technol. 2016, 301, 396–404. [Google Scholar] [CrossRef]
- Benyerbah, N.; Ispas-Szabo, P.; Sakeer, K.; Chapdelaine, D.; Mateescu, M.A. Ampholytic and Polyelectrolytic Starch as Matrices for Controlled Drug Delivery. Pharmaceutics 2019, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, E.A.; Bendas, E.R.; Mohamed, M.I. Preparation and Evaluation of Self-Nanoemulsifying Tablets of Carvedilol. AAPS PharmSciTech 2009, 10, 183–192. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Y.; Que, F.; Zhang, H. Recent Advances in Fabrication of Edible Polymer Oleogels for Food Applications. Curr. Opin. Food Sci. 2022, 43, 114–119. [Google Scholar] [CrossRef]
- Tomsone, L.; Galoburda, R.; Kruma, Z.; Durrieu, V.; Cinkmanis, I. Microencapsulation of Horseradish (Armoracia rusticana L.) Juice Using Spray-Drying. Foods 2020, 9, 1332. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, S.; Tu, Z.; Wang, H.; Li, R.; Zhang, L.; Huang, T.; Su, T.; Li, C. Quality Evaluation of Peony Seed Oil Spray-Dried in Different Combinations of Wall Materials during Encapsulation and Storage. J. Food Sci. Technol. 2016, 53, 2597–2605. [Google Scholar] [CrossRef]
- Jornada, D.S.; Fiel, L.A.; Bueno, K.; Gerent, J.F.; Petzhold, C.L.; Beck, R.C.; Guterres, S.S.; Pohlmann, A.R. Lipid-Core Nanocapsules: Mechanism of Self-Assembly, Control of Size and Loading Capacity. Soft Matter 2012, 8, 6646–6655. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Seladorai, R.; Masdar, M.S.; Mohd Sofian, N.; Wan Isahak, W.N.R. Core Shell Nanostructure: Impregnated Activated Carbon as Adsorbent for Hydrogen Sulfide Adsorption. Molecules 2022, 27, 1145. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Chen, H.; Wei, Q. The Role of Roller Rotation Pattern in the Spreading Process of Polymer/Short-Fiber Composite Powder in Selective Laser Sintering. Polymers 2022, 14, 2345. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gao, Z.; Zhong, W.; Fu, F.; Li, G.; Guo, J.; Shan, Y. Preparation, Characterization, and Antioxidant Activity of Nanoemulsions Incorporating Lemon Essential Oil. Antioxidants 2022, 11, 650. [Google Scholar] [CrossRef]
- Gulzar, S.; Nilsuwan, K.; Raju, N.; Benjakul, S. Whole Wheat Crackers Fortified with Mixed Shrimp Oil and Tea Seed Oil Microcapsules Prepared from Mung Bean Protein Isolate and Sodium Alginate. Foods 2022, 11, 202. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, L.; Chen, X.; Guo, J.; Yang, F.; Wang, J.; Zheng, Y.; Hu, Y. Hypophosphite/Graphitic Carbon Nitride Hybrids: Preparation and Flame-Retardant Application in Thermoplastic Polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.J.; Caira, M.R.; Hunter, R.; Bourne, S.A. Characterization of (E)- and (Z)-Ajoene Inclusion Complexes with α-, β-, γ- and Permethylated-β-Cyclodextrin Using PXRD, Single-Crystal X-Ray Diffraction and Thermal Analysis. Acta Crystallogr. Sect. A 2004, 60, s217. [Google Scholar] [CrossRef]
- Dos Santos, C.; Buera, M.P.; Mazzobre, M.F. Phase Solubility Studies and Stability of Cholesterol/Β-cyclodextrin Inclusion Complexes. J. Sci. Food Agric. 2011, 91, 2551–2557. [Google Scholar] [CrossRef]
- Su, J.; Chen, J.; Li, L.; Li, B.; Shi, L.; Chen, L.; Xu, Z. Formation of β-Cyclodextrin Inclusion Enhances the Stability and Aqueous Solubility of Natural Borneol. J. Food Sci. 2012, 77, C658–C664. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and Release Characterisation of Garlic Oil-β-Cyclodextrin Inclusion Complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Ruellan, S.; Fourmentin, S. Cyclodextrin, an Efficient Tool for Trans-Anethole Encapsulation: Chromatographic, Spectroscopic, Thermal and Structural Studies. Food Chem. 2014, 164, 454–461. [Google Scholar] [CrossRef]
- Li, J.; Hou, X.; Jiang, L.; Xia, D.; Chen, A.; Li, S.; Li, Q.; Gu, X.; Mo, X.; Zhang, Z. Optimization and Characterization of Sichuan Pepper (Zanthoxylum Bungeanum Maxim) Resin Microcapsule Encapsulated with β-Cyclodextrin. LWT 2022, 171, 114120. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yu, T.; Yuan, L.; Rao, G.; Li, D.; Mu, C. Preparation, Physicochemical Characterization and Release Behavior of the Inclusion Complex of Trans-Anethole and β-Cyclodextrin. Food Res. Int. 2015, 74, 55–62. [Google Scholar] [CrossRef]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in the Solid State: A Review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhao, X.; Wang, C.; Fang, Q.; Zhong, L.; Wei, Q. Preparation, Optimization, and Characterization of Inclusion Complexes of Cinnamomum Longepaniculatum Essential Oil in β-Cyclodextrin. Sustainability 2022, 14, 9513. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; He, Y.; Cao, J.; Wang, K.; Lin, H.; Qu, C.; Miao, J. Regulatory Effects of Functional Soluble Dietary Fiber from Saccharina Japonica Byproduct on the Liver of Obese Mice with Type 2 Diabetes Mellitus. Mar. Drugs 2022, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Topper, T.P.; Guo, J.; Clausen, S.; Skovsted, C.B.; Zhang, Z. Reply to ‘Re-Evaluating the Phylogenetic Position of the Enigmatic Early Cambrian Deuterostome Yanjiahella’. Nat. Commun. 2020, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.-N.; Park, H.-M. Recent Progress on Optical Tomographic Technology for Measurements and Inspections of Film Structures. Micromachines 2022, 13, 1074. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liao, H.; Pan, Z.; Cheng, F. Insights into Coproduction of Silica Gel via Desulfurization of Steel Slag and Silica Gel Adsorption Performance. ACS Omega 2022, 7, 21062–21074. [Google Scholar] [CrossRef] [PubMed]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing an Essential Oil Component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Su, X.; Cadwallader, K.R. Testing Tools and Physical, Chemical, and Microbiological Characterization of Microencapsulated Systems. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 367–400. ISBN 978-0-12-821683-5. [Google Scholar]
- Saroch, P.; Naman, S.; Baldi, A. Delivery Systems for Flavors and Fragrances: Quality by Design-Based Considerations. In ACS Symposium Series; Balakrishnan, P., Gopi, S., Eds.; American Chemical Society: Washington, DC, USA, 2022; Volume 1433, pp. 245–297. ISBN 978-0-8412-9731-9. [Google Scholar]
- Marzban, C.; Yurtsever, U.; Richman, M. Principal Component Analysis for Equation Discovery. arXiv 2024, arXiv:2401.04797. [Google Scholar]
- Li, C.; Al-Dalali, S.; Wang, Z.; Xu, B.; Zhou, H. Investigation of Volatile Flavor Compounds and Characterization of Aroma-Active Compounds of Water-Boiled Salted Duck Using GC–MS–O, GC–IMS, and E-Nose. Food Chem. 2022, 386, 132728. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Al-Dalali, S.; Zhou, H.; Wang, Z.; Xu, B. Influence of Mixture of Spices on Phospholipid Molecules during Water-Boiled Salted Duck Processing Based on Shotgun Lipidomics. Food Res. Int. 2021, 149, 110651. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chen, J.; Li, X.; Qi, Y.; Jiang, R. Flavor Components Detection and Discrimination of Isomers in Huaguo Tea Using Headspace-Gas Chromatography-Ion Mobility Spectrometry and Multivariate Statistical Analysis. Anal. Chim. Acta 2023, 1243, 340842. [Google Scholar] [CrossRef] [PubMed]
- Güngör Ertuğral, T.; Danışman, M.; Oral, A. Microencapsulation of N-Tridecane/n-Tetradecane Eutectic Mixture with Poly (Methyl Methacrylate) Shell for Candidate for Food Packaging Thermal Energy Storage Material. Polym.-Plast. Technol. Mater. 2023, 62, 554–562. [Google Scholar] [CrossRef]

| Title 1 | Factors | ||
|---|---|---|---|
| A (Taro Flavor/β-CD) | B (T-20/β-CD) | C (Ultrasound Time) | |
| 1 | 1:8 | 1.2:1 | 30 |
| 2 | 1:10 | 1.4:1 | 40 |
| 3 | 1:12 | 1.6:1 | 50 |
| NO. | A | B | C | EE (%) |
|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 36.38 ± 0.03 |
| 2 | 1 | 2 | 2 | 46.70 ± 0.01 |
| 3 | 1 | 3 | 3 | 48.92 ± 0.05 |
| 4 | 2 | 1 | 2 | 55.94 ± 0.01 |
| 5 | 2 | 2 | 3 | 52.29 ± 0.04 |
| 6 | 2 | 3 | 1 | 49.84 ± 0.01 |
| 7 | 3 | 1 | 3 | 49.37 ± 0.01 |
| 8 | 3 | 2 | 1 | 52.45 ± 0.01 |
| 9 | 3 | 3 | 2 | 54.72 ± 0.01 |
| K1 | 44.030 | 47.230 | 46.223 | |
| K2 | 52.690 | 50.510 | 52.483 | |
| K3 | 52.180 | 51.160 | 50.193 | |
| R | 8.660 | 3.930 | 6.260 | |
| Significance order of factors | ACB | |||
| Optimal preparation condition | A2B3C2 | |||
| NO. | Physical Propezrties | Unit | Value |
|---|---|---|---|
| 1 | Moisture content | % | 4.26 ± 0.04 |
| 2 | Solubility | % | 61.50 ± 0.05 |
| 3 | Angle of repose | ° | 40.49 ± 0.34 |
| 4 | ρa | mg/mL | 0.04 ± 0.004 |
| 5 | ρb | mg/mL | 0.04 ± 0.006 |
| 6 | CI | - | 12.72 ± 0.40 |
| 7 | HR | - | 0.88 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Gu, Y.; Ren, A.; Li, X.; Wu, S.; Gong, Y.; Luo, Y. Optimization and Comprehensive Characterization of the Microencapsulation Process for Taro Essence. Foods 2025, 14, 754. https://doi.org/10.3390/foods14050754
Song Y, Gu Y, Ren A, Li X, Wu S, Gong Y, Luo Y. Optimization and Comprehensive Characterization of the Microencapsulation Process for Taro Essence. Foods. 2025; 14(5):754. https://doi.org/10.3390/foods14050754
Chicago/Turabian StyleSong, Yongxin, Yipeng Gu, Aiqing Ren, Xiaochun Li, Shujie Wu, Yuwen Gong, and Yanghe Luo. 2025. "Optimization and Comprehensive Characterization of the Microencapsulation Process for Taro Essence" Foods 14, no. 5: 754. https://doi.org/10.3390/foods14050754
APA StyleSong, Y., Gu, Y., Ren, A., Li, X., Wu, S., Gong, Y., & Luo, Y. (2025). Optimization and Comprehensive Characterization of the Microencapsulation Process for Taro Essence. Foods, 14(5), 754. https://doi.org/10.3390/foods14050754





