Authentication of EU-Authorized Edible Insect Species in Food Products by DNA Barcoding and High-Resolution Melting (HRM) Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction
2.3. PCR-HRM Analysis
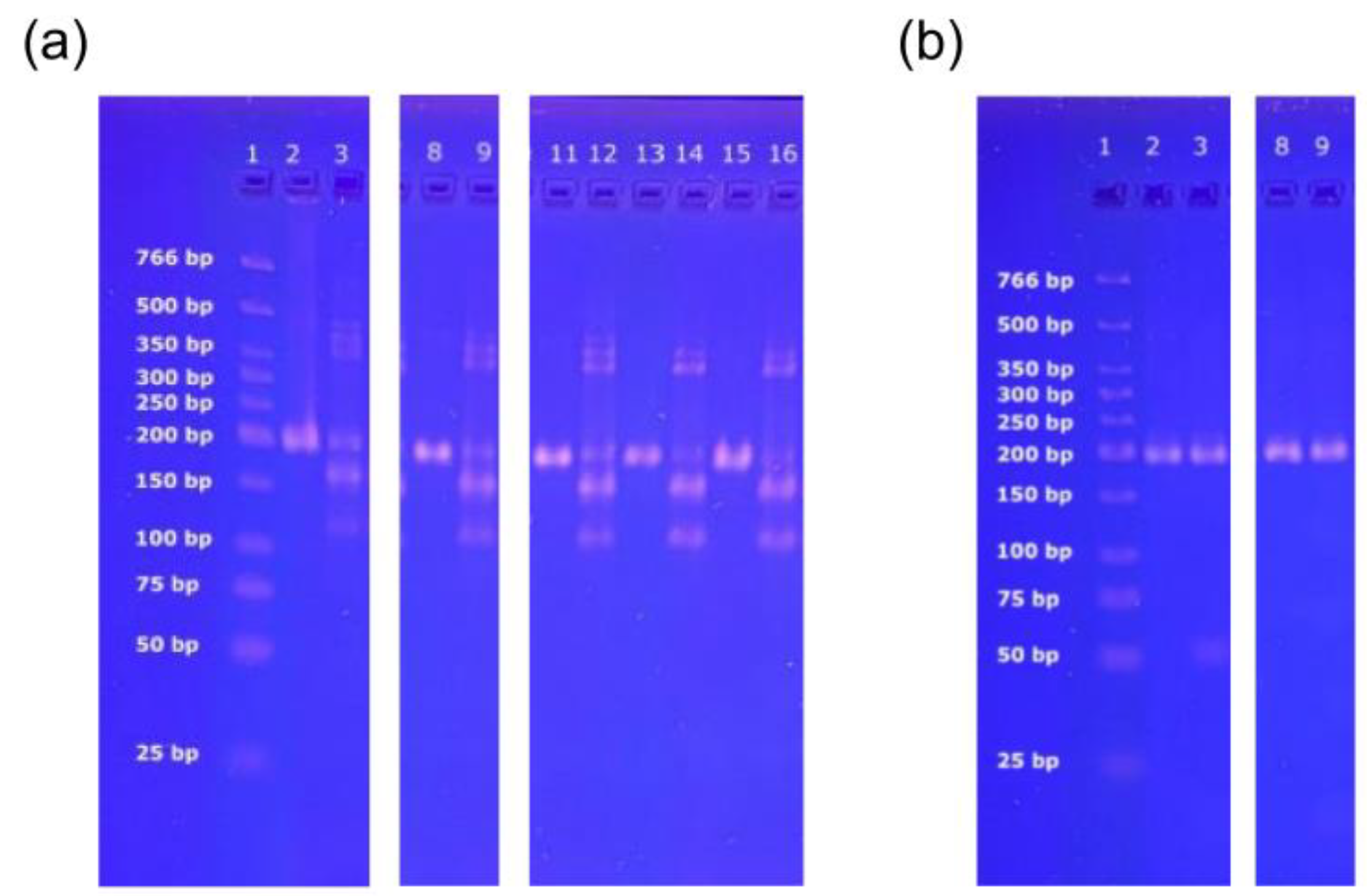
2.4. Agarose Gel Electrophoresis
3. Results and Discussion
3.1. Adaptation of PCR Conditions
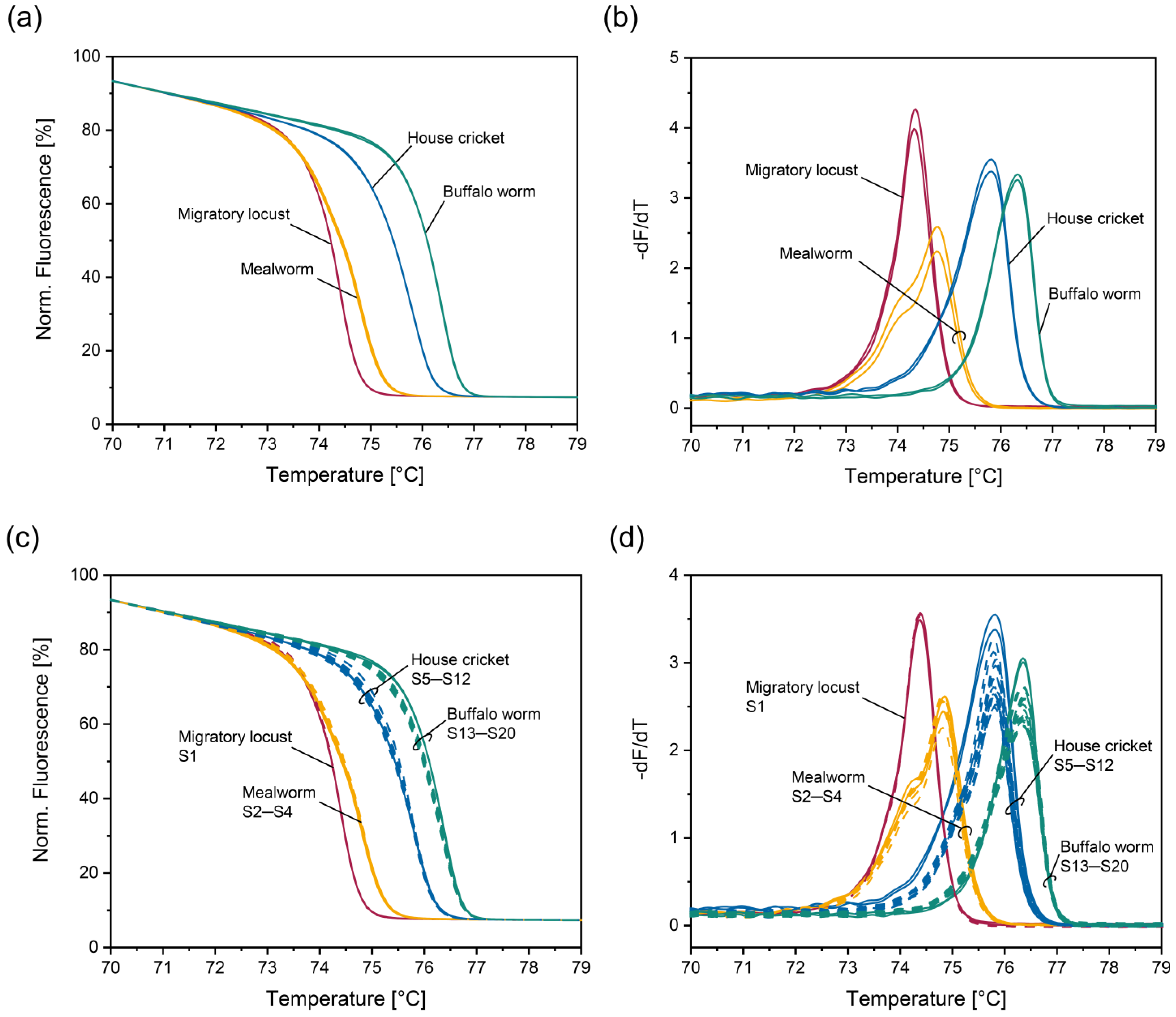
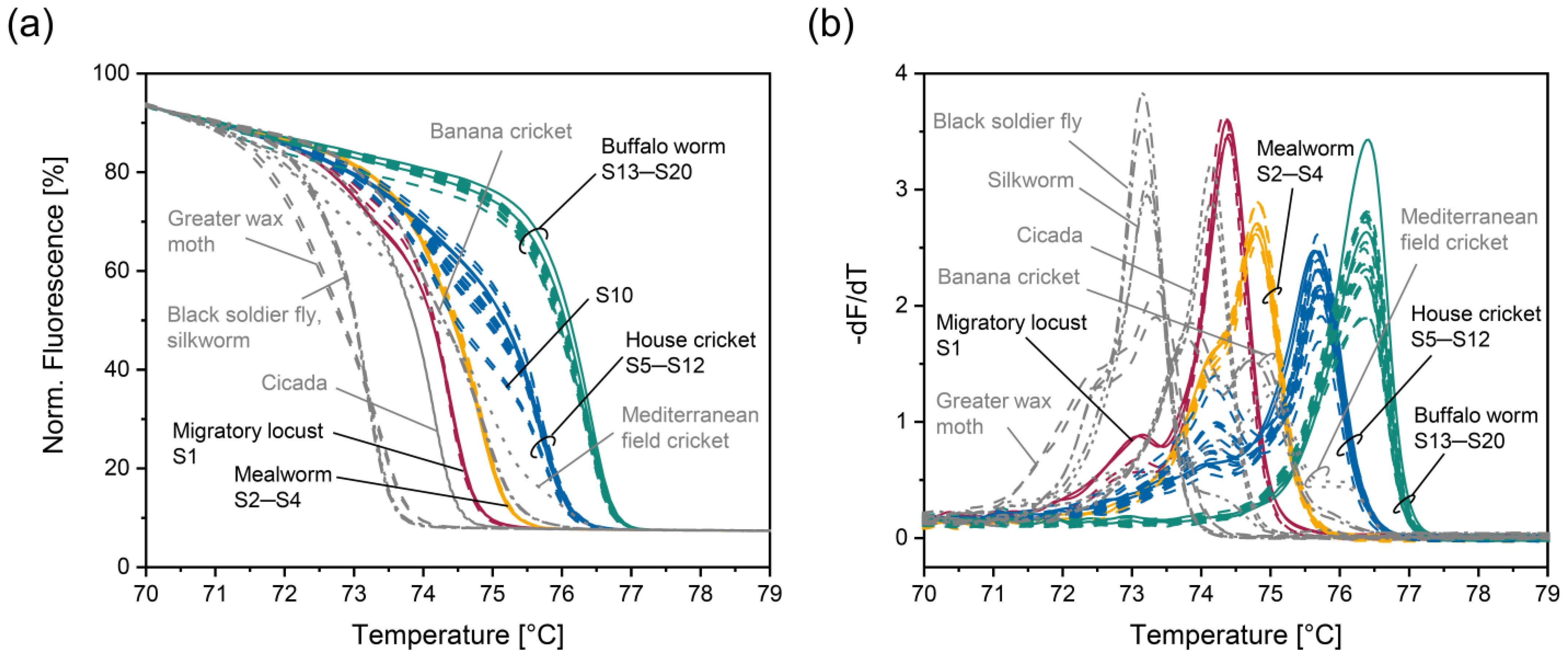
3.2. HRM Analysis of the Four Insect Species Authorized in the EU
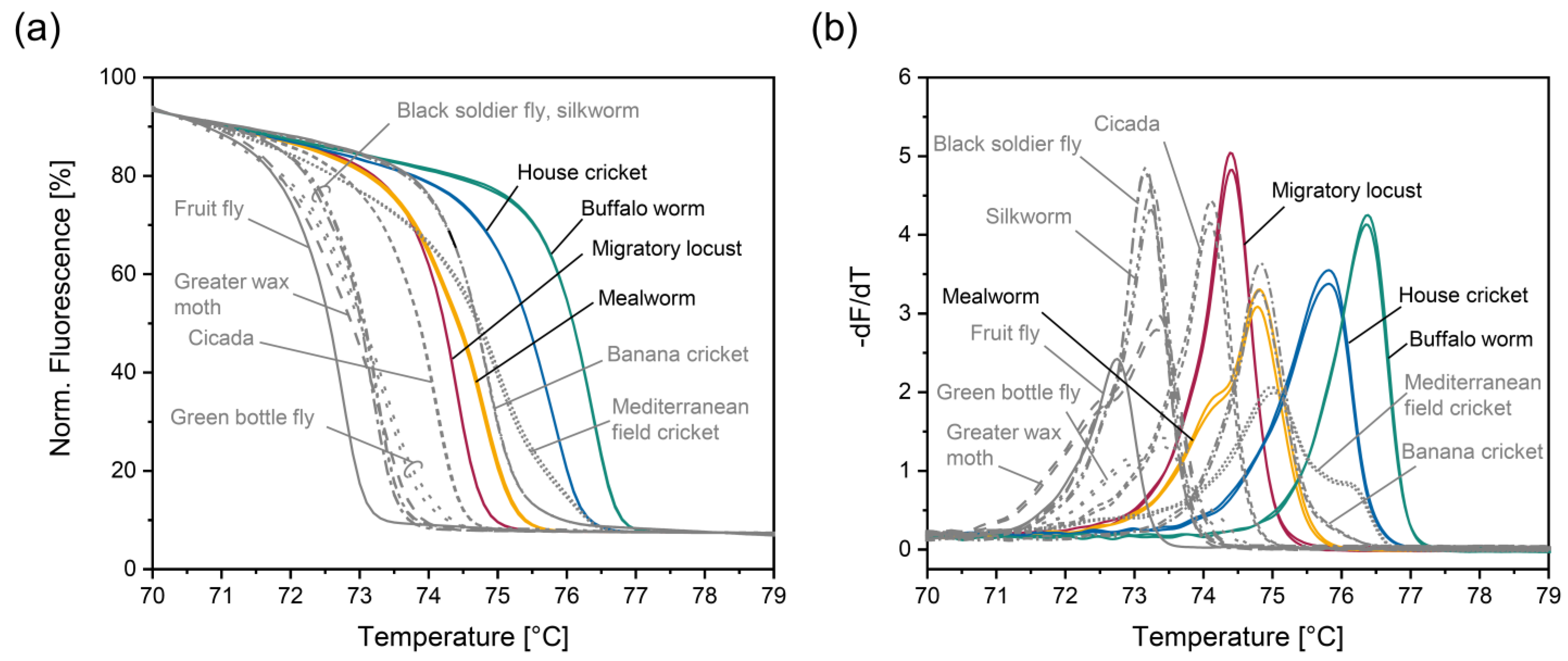
3.3. Analysis of Insect Species That Have Not Been Authorized in the EU
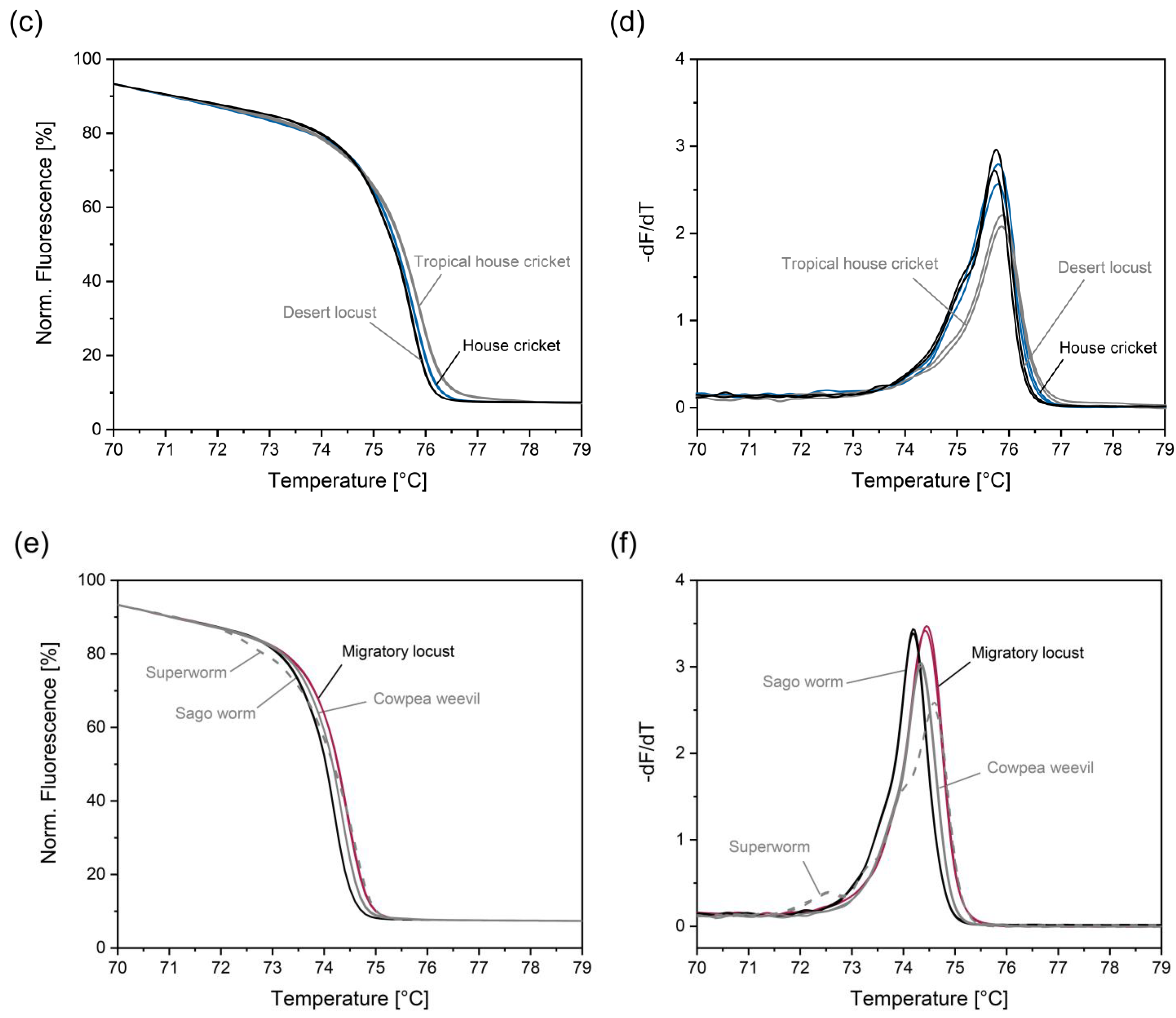
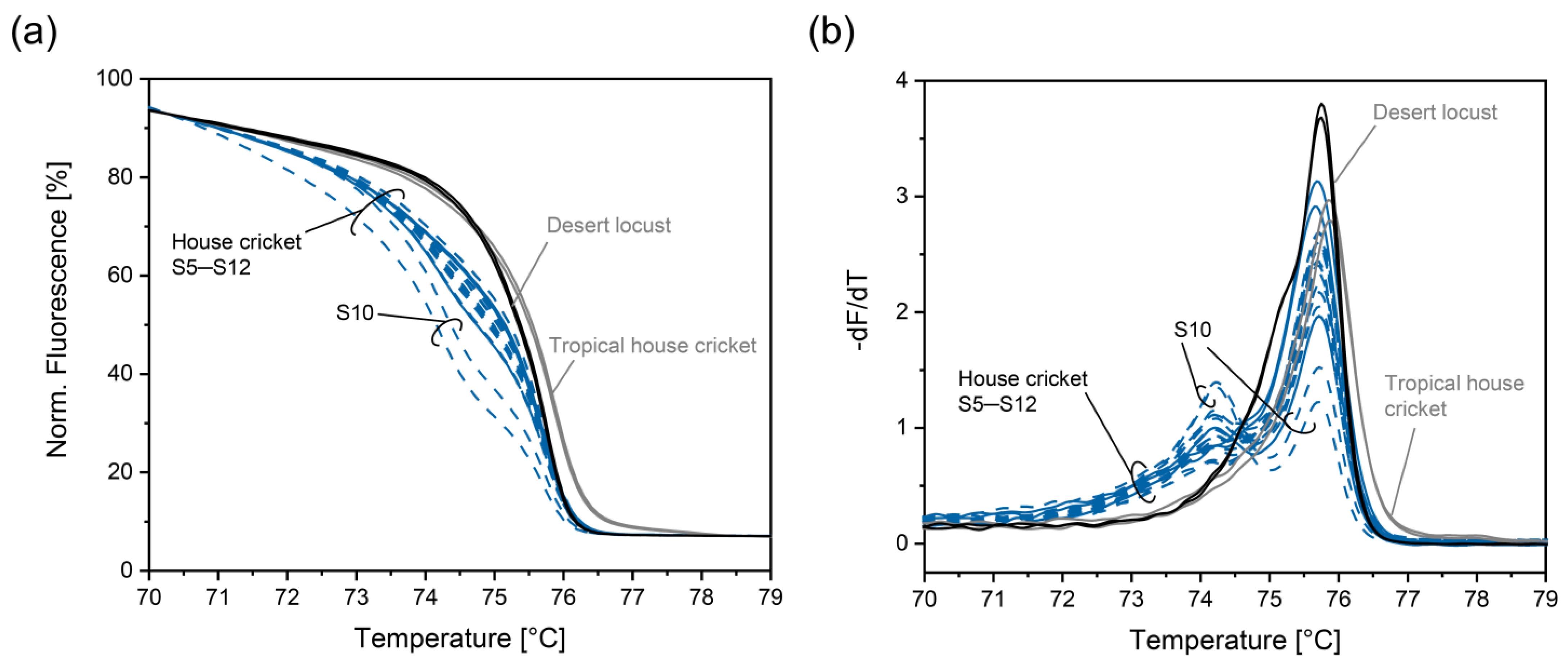
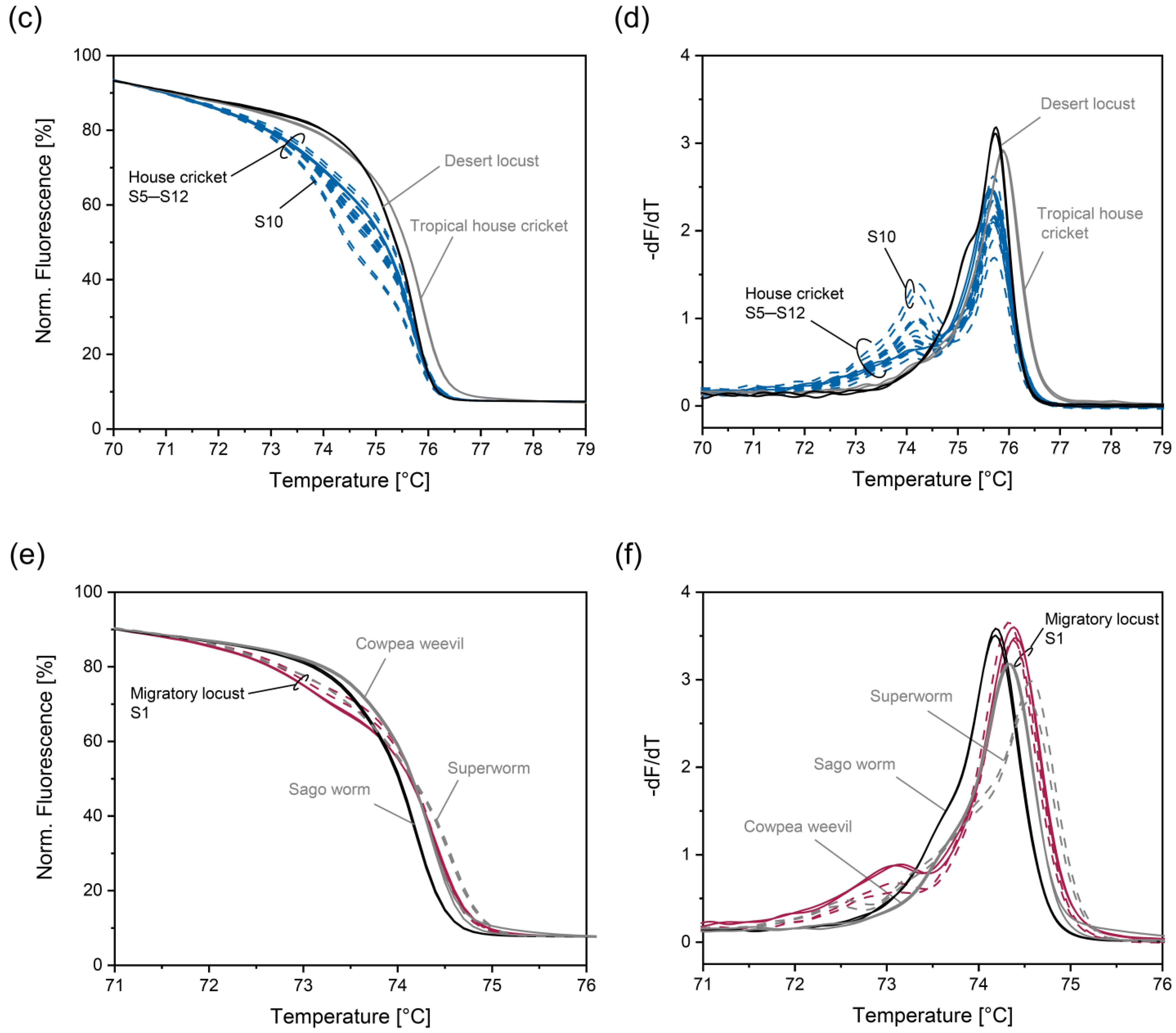
3.4. Improving Discrimination of House Cricket from Tropical House Cricket and Desert Locust
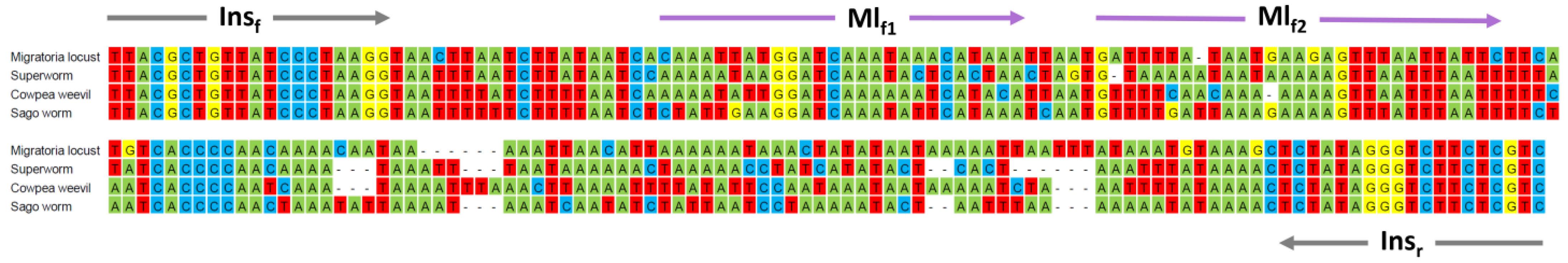
3.5. Improving Discrimination of Migratory Locust from Superworm, Cowpea Weevil, and Sago Worm
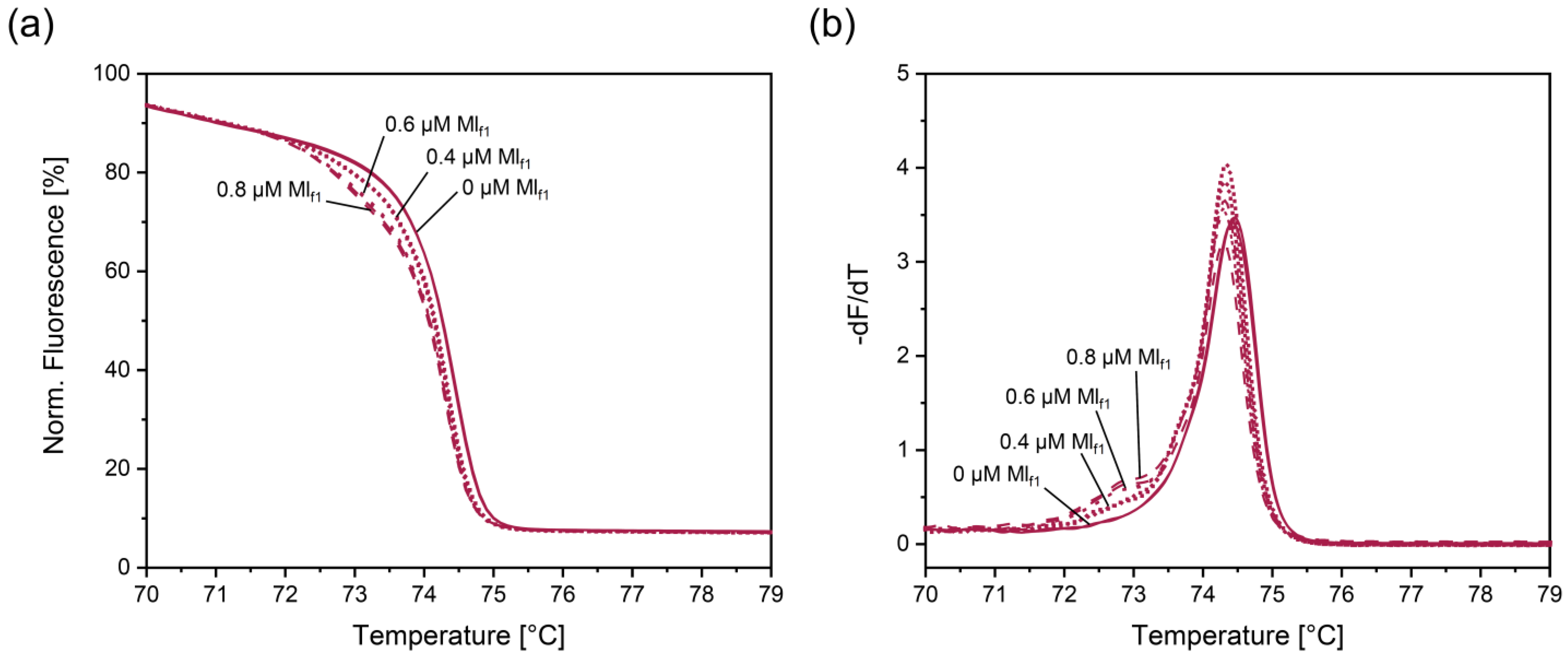
3.6. Optimized PCR-HRM Assay Involving Primers Insf, Insr, Hcf, Hcr1, and Mlf1
3.7. Strengths and Limitations of the Optimized PCR-HRM Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, 243. [Google Scholar] [CrossRef]
- Bohrer, B.M. Review: Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Biesalski, H.K. Meat as a component of a healthy diet—Are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Rust, N.A.; Ridding, L.; Ward, C.; Clark, B.; Kehoe, L.; Dora, M.; Whittingham, M.J.; McGowan, P.; Chaudhary, A.; Reynolds, C.J.; et al. How to transition to reduced-meat diets that benefit people and the planet. Sci. Total Environ. 2020, 718, 137208. [Google Scholar] [CrossRef]
- Lynch, J.; Cain, M.; Frame, D.; Pierrehumbert, R. Agriculture’s Contribution to Climate Change and Role in Mitigation Is Distinct from Predominantly Fossil CO2-Emitting Sectors. Front. Sustain. Food Syst. 2021, 4, 518039. [Google Scholar] [CrossRef]
- Pastrana-Pastrana, Á.J.; Rodríguez-Herrera, R.; Solanilla-Duque, J.F.; Flores-Gallegos, A.C. Plant proteins, insects, edible mushrooms and algae: More sustainable alternatives to conventional animal protein. J. Future Foods 2025, 5, 248–256. [Google Scholar] [CrossRef]
- van Huis, A. Edible insects: Challenges and prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Omuse, E.R.; Tonnang, H.E.Z.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Mohamed, S.F.; Kassie, M.; Subramanian, S.; Onditi, J.; et al. The global atlas of edible insects: Analysis of diversity and commonality contributing to food systems and sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Tarahi, M.; Aghababaei, F.; McClements, D.J.; Pignitter, M.; Hadidi, M. Bioactive peptides derived from insect proteins: Preparation, biological activities, potential applications, and safety issues. Food Chem. 2025, 465, 142113. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- de Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Moruzzo, R.; Riccioli, F.; Paci, G. European consumers’ readiness to adopt insects as food. A review. Food Res. Int. 2019, 122, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Giusti, A.; Spatola, G.; Mancini, S.; Nuvoloni, R.; Armani, A. Novel foods, old issues: Metabarcoding revealed mislabeling in insect-based products sold by e-commerce on the EU market. Food Res. Int. 2024, 184, 114268. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2015/2283; The European Parliament and the Council of 25 November 2015 on Novel Foods. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32015R2283 (accessed on 3 January 2025).
- Commission Implementing Regulation (EU) 2021/1975 of 12 November 2021 authorising the placing on the market of frozen, dried and powder forms of Locusta migratoria as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2021. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/1975/oj/eng (accessed on 3 January 2025).
- Commission Implementing Regulation (EU) 2022/188 of 10 February 2022 authorising the placing on the market of frozen, dried and powder forms of Acheta domesticus as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2022. Available online: https://eur-lex.europa.eu/eli/reg_impl/2022/188/oj/eng (accessed on 3 January 2025).
- Commission Implementing Regulation (EU) 2021/882 of 1 June 2021 authorising the placing on the market of dried Tenebrio molitor larva as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2021. Available online: https://eur-lex.europa.eu/eli/reg_impl/2021/882/oj/eng (accessed on 3 January 2025).
- Commission Implementing Regulation (EU) 2023/58 of 5 January 2023 authorising the placing on the market of the frozen, paste, dried and powder forms of Alphitobius diaperinus larvae (lesser mealworm) as a novel food and amending Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2023. Available online: https://eur-lex.europa.eu/eli/reg_impl/2023/58/oj/eng (accessed on 3 January 2025).
- Palmer, L.K.; Marsh, J.T.; Lu, M.; Goodman, R.E.; Zeece, M.G.; Johnson, P.E. Shellfish Tropomyosin IgE Cross-Reactivity Differs Among Edible Insect Species. Mol. Nutr. Food Res. 2020, 64, 1900923. [Google Scholar] [CrossRef] [PubMed]
- Cichna-Markl, M.; Mafra, I. Techniques for Food Authentication: Trends and Emerging Approaches. Foods 2023, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Debode, F.; Marien, A.; Gérard, A.; Francis, F.; Fumière, O.; Berben, G. Development of real-time PCR tests for the detection of Tenebrio molitor in food and feed. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Garino, C.; Winter, R.; Broll, H.; Winkel, M.; Braeuning, A.; Reich, F.; Zagon, J. Development and validation of a novel real-time PCR protocol for the detection of buffalo worm (Alphitobius diaperinus) in food. Food Control 2022, 140, 109138. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.Y.; Jung, S.K.; Kim, M.Y.; Kim, H.Y. Development and validation of ultrafast PCR assays to detect six species of edible insects. Food Control 2019, 103, 21–26. [Google Scholar] [CrossRef]
- Köppel, R.; Schum, R.; Habermacher, M.; Sester, C.; Piller, L.E.; Meissner, S.; Pietsch, K. Multiplex real-time PCR for the detection of insect DNA and determination of contents of Tenebrio molitor, Locusta migratoria and Achaeta domestica in food. Eur. Food Res. Technol. 2019, 245, 559–567. [Google Scholar] [CrossRef]
- Hillinger, S.; Saeckler, J.; Domig, K.J.; Dobrovolny, S.; Hochegger, R. Development of a DNA Metabarcoding Method for the Identification of Insects in Food. Foods 2023, 12, 1086. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. DNA barcodes: Genes, genomics, and bioinformatics. Proc. Natl. Acad. Sci. USA 2008, 105, 2761–2762. [Google Scholar] [CrossRef]
- Druml, B.; Cichna-Markl, M. High resolution melting (HRM) analysis of DNA—Its role and potential in food analysis. Food Chem. 2014, 158, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Campos, B.; Amaral, J.S.; Nunes, M.E.; Oliveira, M.B.P.P.; Mafra, I. HRM analysis targeting ITS1 and matK loci as potential DNA mini-barcodes for the authentication of Hypericum perforatum and Hypericum androsaemum in herbal infusions. Food Control 2016, 61, 105–114. [Google Scholar] [CrossRef]
- Fernandes, T.J.R.; Amaral, J.S.; Mafra, I. DNA barcode markers applied to seafood authentication: An updated review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3904–3935. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Suokas, M.; Häggman, H. Novel approaches based on DNA barcoding and high-resolution melting of amplicons for authenticity analyses of berry species. Food Chem. 2010, 123, 494–500. [Google Scholar] [CrossRef]
- uMelt. uMelt. Available online: https://dna-utah.org/umelt/quartz/ (accessed on 3 January 2025).
- Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. Off. J. Eur. Union 2017. Available online: https://eur-lex.europa.eu/eli/reg/2017/893/oj/eng (accessed on 3 January 2025).
- Commission Regulation (EU) 2021/1925 of 5 November 2021 amending certain Annexes to Regulation (EU) No 142/2011 as regards the requirements for placing on the market of certain insect products and the adaptation of a containment method. Off. J. Eur. Union. 2021. Available online: https://eur-lex.europa.eu/eli/reg/2021/1925/oj (accessed on 3 January 2025).
- Weissman, D.B.; Gray, D.A.; Thi Pham, H.; Tijssen, P. Billions and billions sold: Pet-feeder crickets (Orthoptera: Gryllidae), commercial cricket farms, an epizootic densovirus, and government regulations make for a potential disaster. Zootaxa 2012, 3504, 67–88. [Google Scholar] [CrossRef]
- Preckel, L.; Brünen-Nieweler, C.; Denay, G.; Petersen, H.; Cichna-Markl, M.; Dobrovolny, S.; Hochegger, R. Identification of mammalian and poultry species in food and pet food samples using 16s rdna metabarcoding. Foods 2021, 10, 2875. [Google Scholar] [CrossRef]


| Insect Species ID | Scientific Name | Commercial Name |
|---|---|---|
| I1 | Locusta migratoria | migratory locust |
| I2 | Tenebrio molitor | mealworm |
| I3 | Acheta domesticus | house cricket |
| I4 | Alphitobius diaperinus | buffalo worm |
| I5 | Drosophila hydei | fruit fly |
| I6 | Galleria mellonella | greater wax moth |
| I7 | Lucilia sericata | green bottle fly |
| I8 | Hermetia illucens | black soldier fly |
| I9 | Bombyx mori | silkworm moth |
| I10 | Cicadae | cicada |
| I11 | Rhynchophorus ferrugineus | sago worm |
| I12 | Zophobas atratus | superworm |
| I13 | Callosobruchus maculatus | cowpea weevil |
| I14 | Musca domestica | terfly |
| I15 | Gryllus bimaculatus | Mediterranean field cricket |
| I16 | Gryllus locorojo | banana cricket |
| I17 | Gryllodes sigillatus | tropical house cricket |
| I18 | Schistocerca gregaria | desert locust |
| Sample ID | Product | Insect Species Declared 1 |
|---|---|---|
| S1 | locust, blanched, freeze-dried | migratory locust |
| S2 | mealworms | mealworm |
| S3 | dark chocolate with roasted mealworms | mealworm (2%) |
| S4 | whole milk chocolate with roasted mealworms | mealworm (2%) |
| S5 | crickets | cricket 2 |
| S6 | fusilli with cricket flour | cricket 2 |
| S7 | fried crickets | cricket 2 |
| S8 | seasoned crickets (tomato) | house cricket |
| S9 | seasoned crickets (smoked) | house cricket |
| S10 | cricket crackers (tomato, oregano) | house cricket (15%) |
| S11 | cricket crackers (rosemary, thyme) | house cricket (16%) |
| S12 | cricket cracker (curcuma, smoked pepper) | house cricket (15%) |
| S13 | ready-to-mix beetroot risotto with insect protein | buffalo worm (5.7%) |
| S14 | ready-to-mix brownie cake with insect protein | buffalo worm meal (5.5%) |
| S15 | ready-to-mix oat patty with insect protein | buffalo worm (12%) |
| S16 | raw bar sour cherry with insect protein | buffalo worm (12%) |
| S17 | raw bar apple strudel with insect protein | buffalo worm (12%) |
| S18 | raw bar apricot with insect protein | buffalo worm (13%) |
| S19 | protein shake with buffalo worm (strawberry flavor) | buffalo worm (50%) |
| S20 | peanut cream with buffalo worm | buffalo worm (17%) |
| Primer ID | Sequence (5′ → 3′) | Target Species | Reference |
|---|---|---|---|
| Insf | TWACGCTGTTATCCCTAAGG | insects | [28] |
| Insr | GACGAGAAGACCCTATAGA | insects | [28] |
| Hcf | CAGGATCAATTAACCAATCATC | house cricket | this work |
| Hcr1 | TTGAAATTTATGTTTGGTGGTTTT | house cricket | this work |
| Hcr2 | TTATGTTTGGTGGTTTTTTATAGAT | house cricket | this work |
| Mlf1 | CAAATTATGGATCAAATAAACATAAA | migratory locust | this work |
| Mlf2 | GATTTTATAATGAAGAGTTTAATTATTC | migratory locust | this work |
| Insect Species | Length [bp] | Number of Bases | GC Content [%] | |||
|---|---|---|---|---|---|---|
| A | C | G | T | |||
| migratory locust | 198 | 86 | 28 | 17 | 67 | 22.7 |
| mealworm | 197 | 98 | 29 | 15 | 55 | 22.3 |
| house cricket | 196 | 77 | 40 | 15 | 64 | 28.1 |
| buffalo worm | 198 | 93 | 34 | 18 | 53 | 26.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wildbacher, M.; Andronache, J.; Pühringer, K.; Dobrovolny, S.; Hochegger, R.; Cichna-Markl, M. Authentication of EU-Authorized Edible Insect Species in Food Products by DNA Barcoding and High-Resolution Melting (HRM) Analysis. Foods 2025, 14, 751. https://doi.org/10.3390/foods14050751
Wildbacher M, Andronache J, Pühringer K, Dobrovolny S, Hochegger R, Cichna-Markl M. Authentication of EU-Authorized Edible Insect Species in Food Products by DNA Barcoding and High-Resolution Melting (HRM) Analysis. Foods. 2025; 14(5):751. https://doi.org/10.3390/foods14050751
Chicago/Turabian StyleWildbacher, Michaela, Julia Andronache, Katharina Pühringer, Stefanie Dobrovolny, Rupert Hochegger, and Margit Cichna-Markl. 2025. "Authentication of EU-Authorized Edible Insect Species in Food Products by DNA Barcoding and High-Resolution Melting (HRM) Analysis" Foods 14, no. 5: 751. https://doi.org/10.3390/foods14050751
APA StyleWildbacher, M., Andronache, J., Pühringer, K., Dobrovolny, S., Hochegger, R., & Cichna-Markl, M. (2025). Authentication of EU-Authorized Edible Insect Species in Food Products by DNA Barcoding and High-Resolution Melting (HRM) Analysis. Foods, 14(5), 751. https://doi.org/10.3390/foods14050751






