Effects of Aqua-Glycerol Uptake Facilitator Protein GlpF on Spore Germination of Bacillus subtilis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Construction
2.2. Sporulation and Purification of Spore
2.3. Spore Germination and Reduction in Optical Density (OD600) Assay
2.4. Spore Germination and DPA Measurements
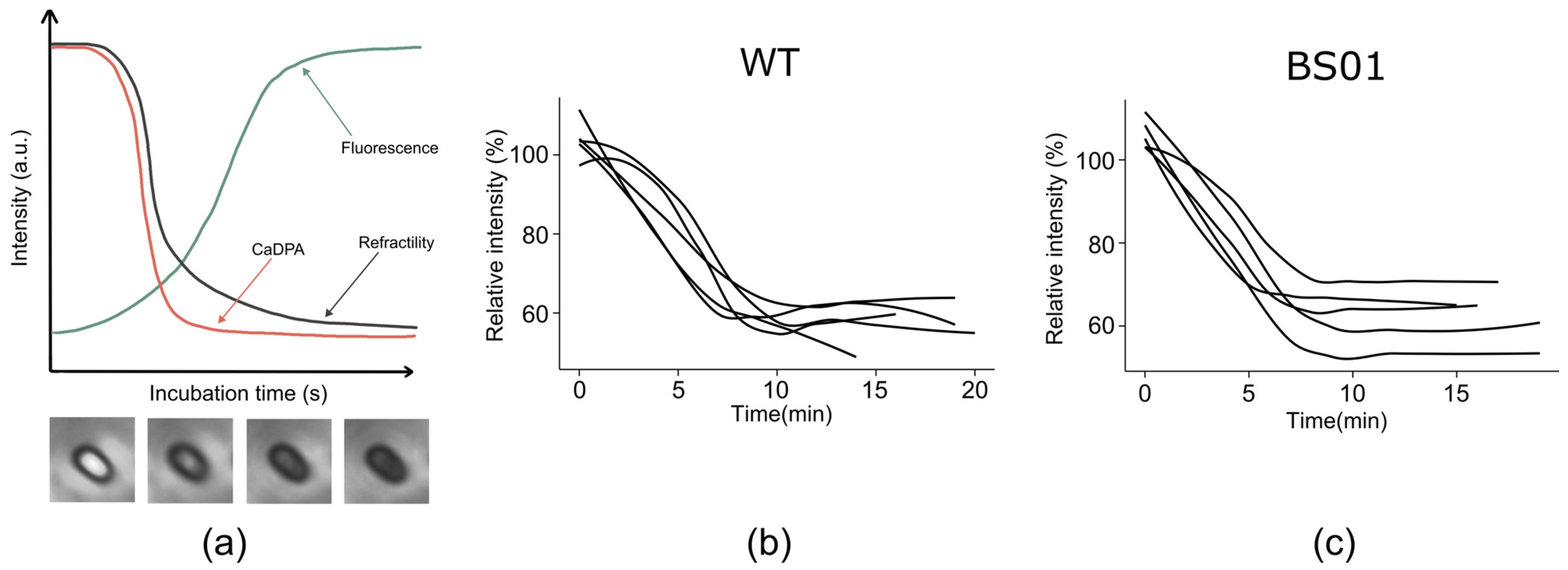
2.5. Microscopy
2.6. High-Pressure Treatment
2.7. Phylogenetic Analysis
2.8. Statistical Analysis
3. Results
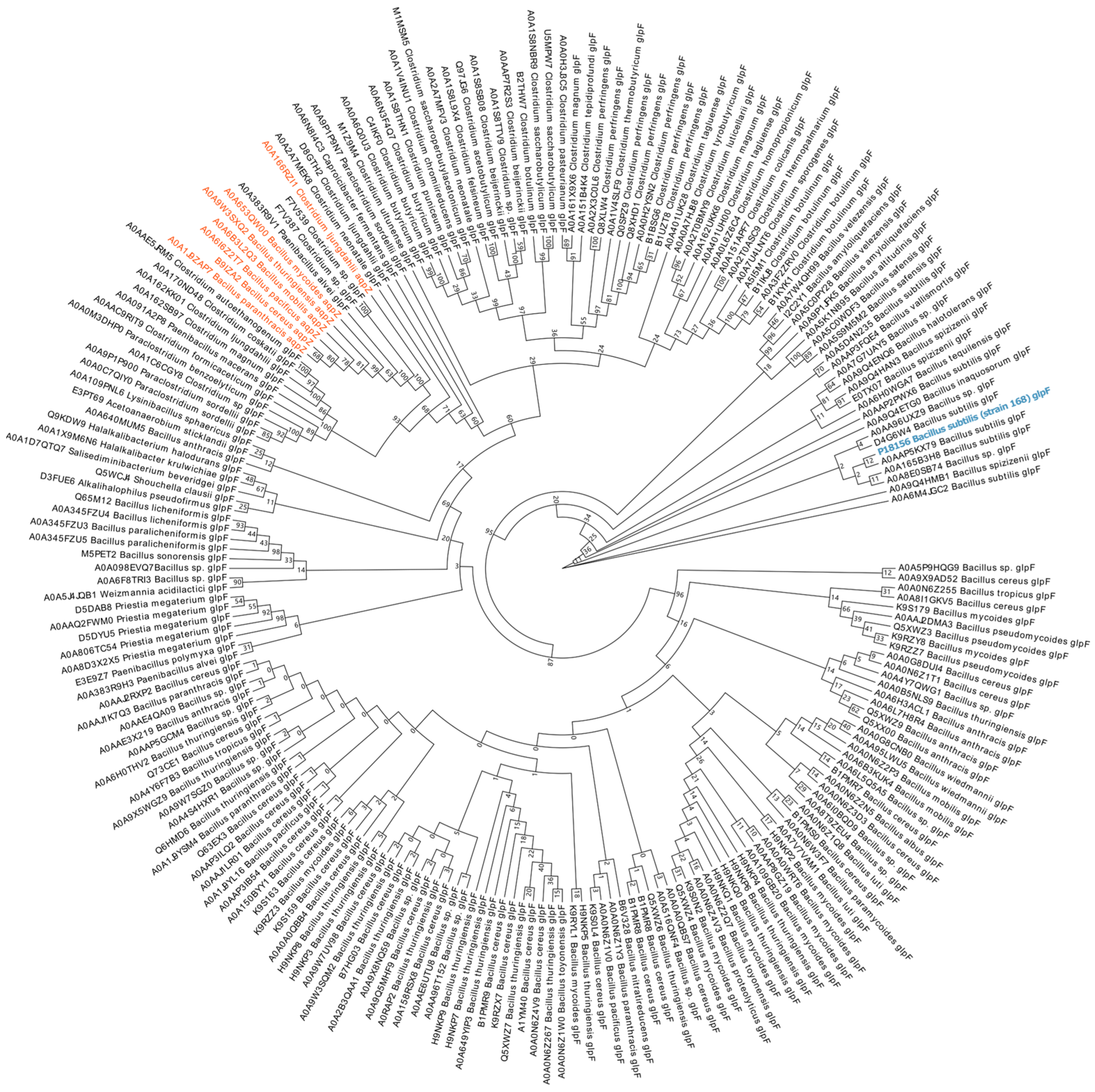
3.1. Phylogenetic Analysis of GlpF in Spore-Producing Bacteria
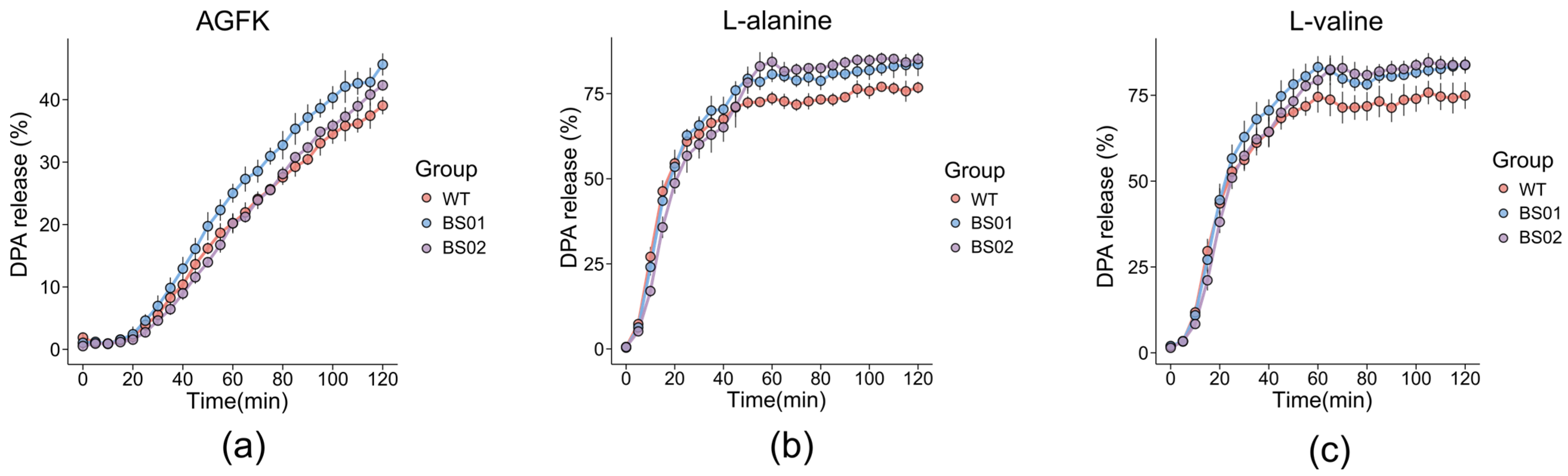
3.2. Impact of GlpF on Spore Germination and DPA Release in B. subtilis
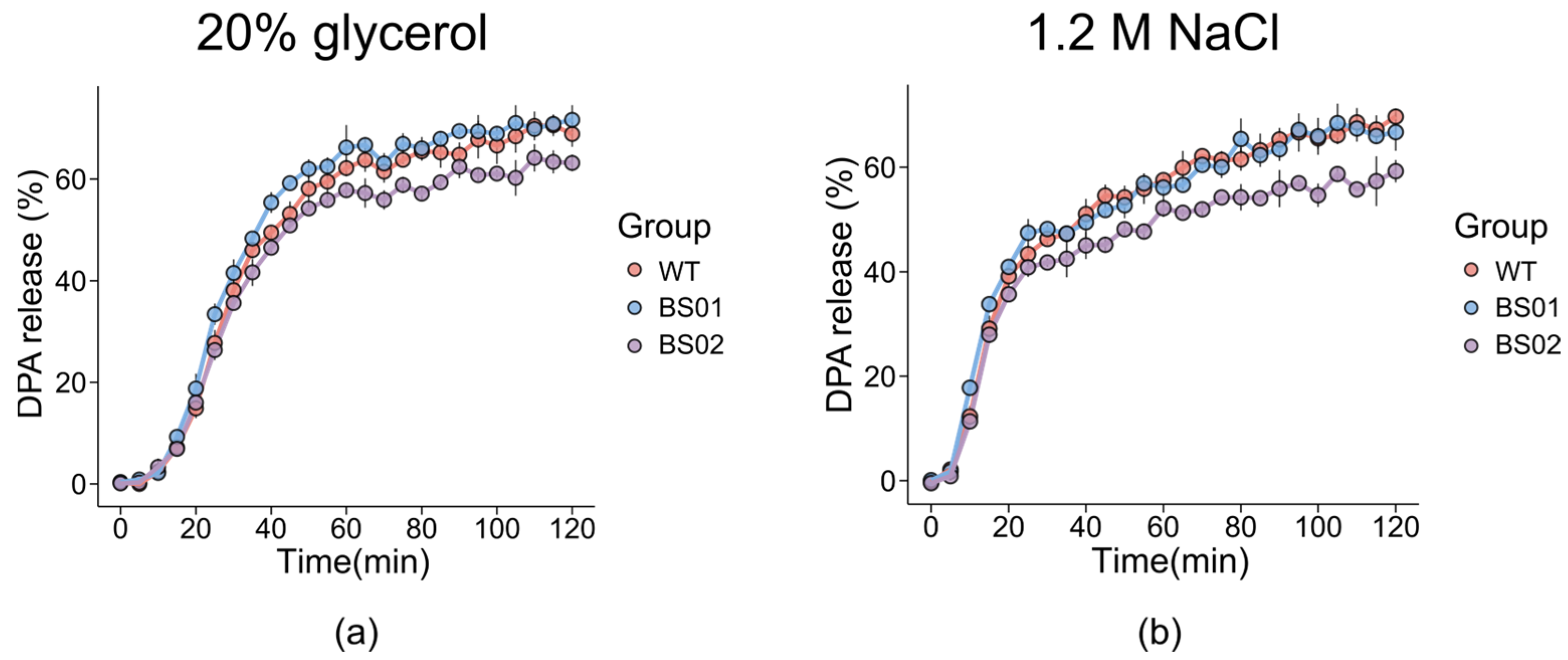
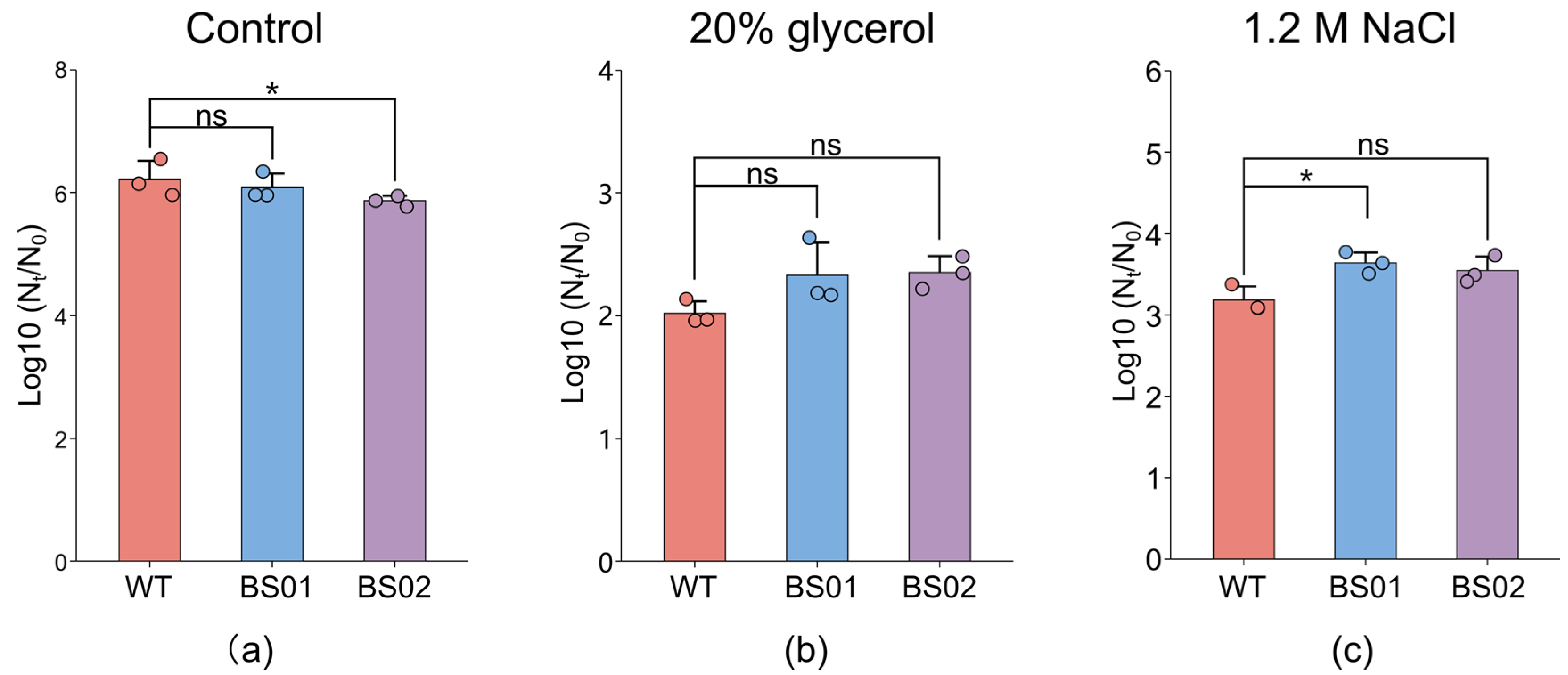
3.3. Effects of GlpF on DPA Release in Different Genotypes of B. subtilis with Osmotic Stress
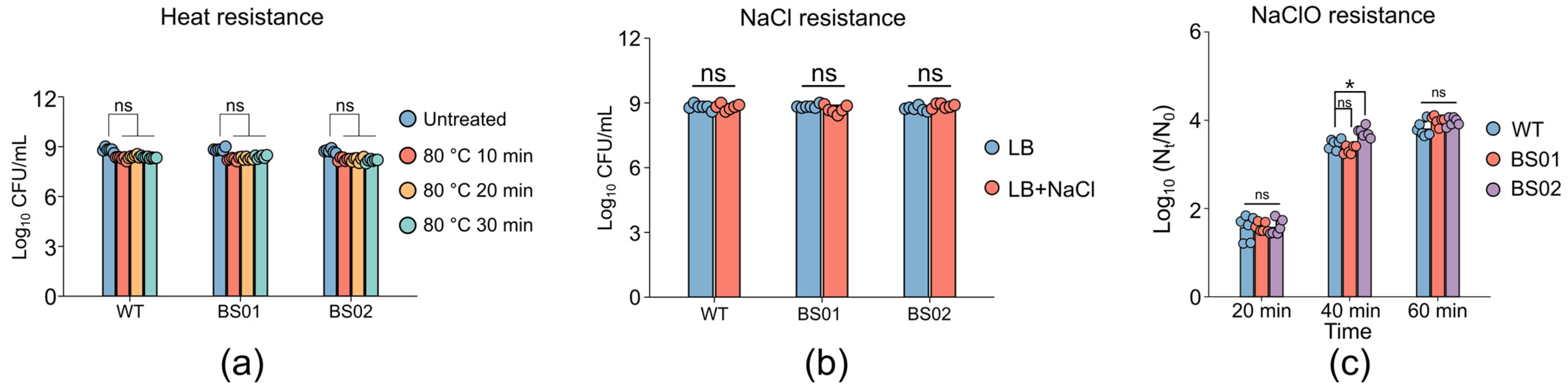
3.4. Impact of GlpF on Spore Resistance and Membrane Structure
3.5. Effect of Pressure–Temperature Treatment on Spore Inactivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, S.J.; Ramamurthi, K.S. Spore Formation in Bacillus subtilis. Environ. Microbiol. Rep. 2014, 6, 212–225. [Google Scholar] [CrossRef]
- Setlow, P.; Christie, G. New Thoughts on an Old Topic: Secrets of Bacterial Spore Resistance Slowly Being Revealed. Microbiol. Mol. Biol. Rev. 2023, 87, e0008022. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.I.L.; Chung, M.S. Bacillus Spores: A Review of Their Properties and Inactivation Processing Technologies. Food Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef]
- Setlow, P. Spore Resistance Properties. In The Bacterial Spore: From Molecules to Systems; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 201–215. [Google Scholar] [CrossRef]
- André, S.; Vallaeys, T.; Planchon, S. Spore-Forming Bacteria Responsible for Food Spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 53–107. ISBN 9780128110324. [Google Scholar]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus Food Infection as Multifactorial Process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]
- Märtlbauer, E.; Granum, P.E. Bacillus cereus Toxins. Toxins 2021, 13, 295. [Google Scholar] [CrossRef]
- Brola, W.; Fudala, M.; Gacek, S.; Gruenpeter, P. Food-Borne Botulism: Still Actual Topic. BMJ Case Rep. 2013, 2013, 2012–2013. [Google Scholar] [CrossRef]
- Korza, G.; Goulet, M.; DeMarco, A.; Wicander, J.; Setlow, P. Role of Bacillus subtilis Spore Core Water Content and PH in the Accumulation and Utilization of Spores’ Large 3-Phosphoglyceric Acid Depot, and the Crucial Role of This Depot in Generating ATP Early during Spore Germination. Microorganisms 2023, 11, 195. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Barajas-Ornelas, R.D.C.; Amon, J.D.; Ramírez-Guadiana, F.H.; Alon, A.; Brock, K.P.; Marks, D.S.; Kruse, A.C.; Rudner, D.Z. The SpoVA Membrane Complex Is Required for Dipicolinic Acid Import during Sporulation and Export during Germination. Genes Dev. 2022, 36, 634646. [Google Scholar] [CrossRef] [PubMed]
- Paidhungat, M.; Setlow, B.; Driks, A.; Setlow, P. Characterization of Spores of Bacillus subtilis Which Lack Dipicolinic Acid. J. Bacteriol. 2000, 182, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.O.; Moran, C.P. Structure, Assembly, and Function of the Spore Surface Layers. Annu. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef] [PubMed]
- Christie, G.; Setlow, P. Bacillus Spore Germination: Knowns, Unknowns and What We Need to Learn. Cell. Signal. 2020, 74, 109729. [Google Scholar] [CrossRef] [PubMed]
- Laue, M.; Han, H.M.; Dittmann, C.; Setlow, P. Intracellular Membranes of Bacterial Endospores Are Reservoirs for Spore Core Membrane Expansion during Spore Germination. Sci. Rep. 2018, 8, 11388. [Google Scholar] [CrossRef] [PubMed]
- Kaieda, S.; Setlow, B.; Setlow, P.; Halle, B. Mobility of Core Water in Bacillus subtilis Spores by 2H NMR. Biophys. J. 2013, 105, 2016–2023. [Google Scholar] [CrossRef]
- Beaman, T.C.; Gerhardt, P. Heat Resistance of Bacterial Spores Correlated with Protoplast Dehydration, Mineralization, and Thermal Adaptation. Appl. Environ. Microbiol. 1986, 52, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Nakashio, S.; Gerhardt, P. Protoplast Dehydration Correlated with Heat Resistance of Bacterial Spores. J. Bacteriol. 1985, 162, 571–578. [Google Scholar] [CrossRef]
- Sunde, E.P.; Setlow, P.; Hederstedt, L.; Halle, B. The Physical State of Water in Bacterial Spores. Proc. Natl. Acad. Sci. USA 2009, 106, 19334–19339. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Setlow, P.; Li, Y.Q. Direct Analysis of Water Content and Movement in Single Dormant Bacterial Spores Using Confocal Raman Microspectroscopy and Raman Imaging. Anal. Chem. 2013, 85, 7094–7101. [Google Scholar] [CrossRef]
- Cowan, A.E.; Olivastro, E.M.; Koppel, D.E.; Loshon, C.A.; Setlow, B.; Setlow, P. Lipids in the Inner Membrane of Dormant Spores of Bacillus Species Are Largely Immobile. Proc. Natl. Acad. Sci. USA 2004, 101, 7733–7738. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, S.M.; Cermak, N.; Delgado, F.F.; Setlow, B.; Setlow, P.; Manalis, S.R. Water and Small-Molecule Permeation of Dormant Bacillus subtilis Spores. J. Bacteriol. 2016, 198, 168–177. [Google Scholar] [CrossRef]
- Wilking, J.N.; Zaburdaev, V.; De Volder, M.; Losick, R.; Brenner, M.P.; Weitz, D.A. Liquid Transport Facilitated by Channels in Bacillus subtilis Biofilms. Proc. Natl. Acad. Sci. USA 2013, 110, 848–852. [Google Scholar] [CrossRef]
- Tong, H.; Hu, Q.; Zhu, L.; Dong, X. Prokaryotic Aquaporins. Cells 2019, 8, 1316. [Google Scholar] [CrossRef]
- Stroud, R.M.; Nollert, P.; Miercke, L. The Glycerol Facilitator GlpF, Its Aquaporin Family of Channels, and Their Selectivity. Adv. Protein Chem. 2003, 63, 291–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, H.; Wang, L.; Shen, Y.; Chen, W.; Song, B.; Zhang, Z.; Zheng, A.; Lin, Q.; Fu, R.; et al. Gating Mechanism of Aquaporin Z in Synthetic Bilayers and Native Membranes Revealed by Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2018, 140, 7885–7895. [Google Scholar] [CrossRef] [PubMed]
- Pluhackova, K.; Schittny, V.; Bürkner, P.C.; Siligan, C.; Horner, A. Multiple Pore Lining Residues Modulate Water Permeability of GlpF. Protein Sci. 2022, 31, e4431. [Google Scholar] [CrossRef] [PubMed]
- Saparov, S.M.; Tsunoda, S.P.; Pohl, P. Proton Exclusion by an Aquaglyceroprotein: A Voltage Clamp Study. Biol. Cell 2005, 97, 545–550. [Google Scholar] [CrossRef]
- Borgnia, M.J.; Agre, P. Reconstitution and Functional Comparison of Purified GlpF and AqpZ, the Glycerol and Water Channels from Escherichia Coli. Proc. Natl. Acad. Sci. USA 2001, 98, 2888–2893. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F.; O’Connell, J.D.; Miercke, L.J.W.; Finer-Moore, J.; Stroud, R.M. Structural Context Shapes the Aquaporin Selectivity Filter. Proc. Natl. Acad. Sci. USA 2010, 107, 17164–17169. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.W.; Simmons, L.A. Complete Genome Sequence of Bacillus subtilis Strain PY79. Genome Announc. 2013, 1, 2164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Semanjski, M.; Orlovetskie, N.; Bhattacharya, S.; Alon, S.; Argaman, L.; Jarrous, N.; Zhang, Y.; Macek, B.; Sinai, L.; et al. Arginine Dephosphorylation Propels Spore Germination in Bacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 14228–14237. [Google Scholar] [CrossRef]
- Gao, Y.; Amon, J.D.; Artzi, L.; Ramírez-Guadiana, F.H.; Brock, K.P.; Cofsky, J.C.; Marks, D.S.; Kruse, A.C.; Rudner, D.Z. Bacterial Spore Germination Receptors Are Nutrient-Gated Ion Channels. Science 2023, 380, 387–391. [Google Scholar] [CrossRef]
- Wen, J.; Smelt, J.P.P.M.; Vischer, N.O.E.; de Vos, A.L.; Setlow, P.; Brul, S. Heat Activation and Inactivation of Bacterial Spores: Is There an Overlap? Appl. Environ. Microbiol. 2022, 88, e0232421. [Google Scholar] [CrossRef]
- Yi, X.; Setlow, P. Studies of the Commitment Step in the Germination of Spores of Bacillus Species. J. Bacteriol. 2010, 192, 3424–3433. [Google Scholar] [CrossRef] [PubMed]
- Sinai, L.; Rosenberg, A.; Smith, Y.; Segev, E.; Ben-Yehuda, S. The Molecular Timeline of a Reviving Bacterial Spore. Mol. Cell 2015, 57, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wu, R.; Cui, T.; Zhang, Z.; Dong, L.; Chen, F.; Hu, X. Proteomic Response of Bacillus subtilis Spores under High Pressure Combined with Moderate Temperature and Random Peptide Mixture LK Treatment. Foods 2022, 11, 1123. [Google Scholar] [CrossRef]
- Griffiths, K.K.; Setlow, P. Effects of Modification of Membrane Lipid Composition on Bacillus subtilis Sporulation and Spore Properties. J. Appl. Microbiol. 2009, 106, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Abhyankar, W.; Ouwerling, N.; Dekker, H.L.; Van Veen, H.; Van Der Wel, N.N.; Roseboom, W.; De Koning, L.J.; Brul, S.; De Koster, C.G. Bacillus subtilis Spore Inner Membrane Proteome. J. Proteome Res. 2016, 15, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Mckenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis Endospore: Assembly and Functions of the Multilayered Coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Rutberg, L. The GlpP and GlpF Genes of the Glycerol Regulon in Bacillus subtilis. J. Gen. Microbiol. 1993, 139, 349–359. [Google Scholar]
- Luu, S.; Setlow, P. Analysis of the Loss in Heat and Acid Resistance during Germination of Spores of Bacillus Species. J. Bacteriol. 2014, 196, 1733–1740. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, P.; Wang, G.; Yu, J.; Setlow, P.; Li, Y. Characterization of Bacterial Spore Germination Using Phase-Contrast and Fluorescence Microscopy, Raman Spectroscopy and Optical Tweezers. Nat. Protoc. 2011, 6, 625–639. [Google Scholar] [CrossRef]
- Wang, D.; Weng, J.; Wang, W. Glycerol Transport through the Aquaglyceroporin GlpF: Bridging Dynamics and Kinetics with Atomic Simulation. Chem. Sci. 2019, 10, 6957–6965. [Google Scholar] [CrossRef]
- Liu, X.D.; Wei, Y.; Zhou, X.Y.; Pei, X.; Zhang, S.H. Aspergillus Glaucus Aquaglyceroporin Gene GlpF Confers High Osmosis Tolerance in Heterologous Organisms. Appl. Environ. Microbiol. 2015, 81, 6926–6937. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Feeherry, F.E.; Ghosh, S.; Liao, X.; Lin, X.; Zhang, P.; Li, Y.; Doona, C.J.; Setlow, P. Effects of Lowering Water Activity by Various Humectants on Germination of Spores of Bacillus Species with Different Germinants. Food Microbiol. 2018, 72, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hou, F.; Muhammad, A.I.; Ruiling, L.V.; Watharkar, R.B.; Guo, M.; Ding, T.; Liu, D. Synergistic Inactivation and Mechanism of Thermal and Ultrasound Treatments against Bacillus subtilis Spores. Food Res. Int. 2019, 116, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Germination of Spores of Bacillus Species: What We Know and Do Not Know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Eijlander, R.T.; Abee, T.; Kuipers, O.P. Bacterial Spores in Food: How Phenotypic Variability Complicates Prediction of Spore Properties and Bacterial Behavior. Curr. Opin. Biotechnol. 2011, 22, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Atluri, S.; Setlow, B.; Setlow, P. Mechanisms of Killing of Bacillus subtilis Spores by Hypochlorite and Chlorine Dioxide. J. Appl. Microbiol. 2006, 101, 1161–1168. [Google Scholar] [CrossRef]
- Klobutcher, L.A.; Ragkousi, K.; Setlow, P. The Bacillus subtilis Spore Coat Provides “Eat Resistance” during Phagocytic Predation by the Protozoan Tetrahymena Thermophila. Proc. Natl. Acad. Sci. USA 2006, 103, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Bressuire-Isoard, C.; Broussolle, V.; Carlin, F. Sporulation Environment Influences Spore Properties in Bacillus: Evidence and Insights on Underlying Molecular and Physiological Mechanisms. FEMS Microbiol. Rev. 2018, 42, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Delbrück, A.I.; Zhang, Y.; Heydenreich, R.; Mathys, A. Bacillus Spore Germination at Moderate High Pressure: A Review on Underlying Mechanisms, Influencing Factors, and Its Comparison with Nutrient Germination. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4159–4181. [Google Scholar] [CrossRef] [PubMed]
- Paidhungat, M.; Setlow, P. Role of Ger Proteins in Nutrient and Nonnutrient Triggering of Spore Germination in Bacillus subtilis. J. Bacteriol. 2000, 182, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Black, E.P.; Koziol-dube, K.; Guan, D.; Wei, J.; Setlow, B.; Cortezzo, D.E.; Hoover, D.G.; Setlow, P.; Moir, A. Factors Influencing Germination of Bacillus subtilis Spores via Activation of Nutrient Receptors by High Pressure. Appl. Environ. Microbiol. 2005, 71, 5879–5887. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Alon, S.; Rao, L.; Sinai, L.; Ben-Yehuda, S. Reviving the View: Evidence That Macromolecule Synthesis Fuels Bacterial Spore Germination. microLife 2022, 3, uqac004. [Google Scholar] [CrossRef]
- Setlow, P. Summer Meeting 2013—When the Sleepers Wake: The Germination of Spores of Bacillus Species. J. Appl. Microbiol. 2013, 115, 1251–1268. [Google Scholar] [CrossRef]

| Bacterial Strains | Genetype | Description | Reference |
|---|---|---|---|
| B. subtilis PY79 | Wild type | Laboratory collection | |
| BS01 | glpF::kan | PY79 strain transformed with the DNA fragment containing kanR and the upstream and downstream homologous arms of glpF. | This study |
| BS02 | amyE::PIPTG-glpF spc | PY79 strain transformed with the pBS1 plasmid. | This study |
| Plasmids | Genotype | Description | Reference |
| pDR111 | amyE::PIPTG spc | Laboratory collection | |
| pBS1 | amyE::PIPTG-glpF spc | pDR111 derivative plasmid encoding glpF from Phyper-spank | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, T.; Zhang, Z.; Mu, K.; Shi, Y.; Chen, F.; Dong, L.; Hu, X. Effects of Aqua-Glycerol Uptake Facilitator Protein GlpF on Spore Germination of Bacillus subtilis. Foods 2025, 14, 750. https://doi.org/10.3390/foods14050750
Cui T, Zhang Z, Mu K, Shi Y, Chen F, Dong L, Hu X. Effects of Aqua-Glycerol Uptake Facilitator Protein GlpF on Spore Germination of Bacillus subtilis. Foods. 2025; 14(5):750. https://doi.org/10.3390/foods14050750
Chicago/Turabian StyleCui, Tianlin, Zequn Zhang, Kangyi Mu, Yicong Shi, Fang Chen, Li Dong, and Xiaosong Hu. 2025. "Effects of Aqua-Glycerol Uptake Facilitator Protein GlpF on Spore Germination of Bacillus subtilis" Foods 14, no. 5: 750. https://doi.org/10.3390/foods14050750
APA StyleCui, T., Zhang, Z., Mu, K., Shi, Y., Chen, F., Dong, L., & Hu, X. (2025). Effects of Aqua-Glycerol Uptake Facilitator Protein GlpF on Spore Germination of Bacillus subtilis. Foods, 14(5), 750. https://doi.org/10.3390/foods14050750







