Characterization and Anti-Aging Potency of Phenolic Compounds in Xianhu Tea Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Determination of Total Phenols, Polysaccharides, and Flavonoids
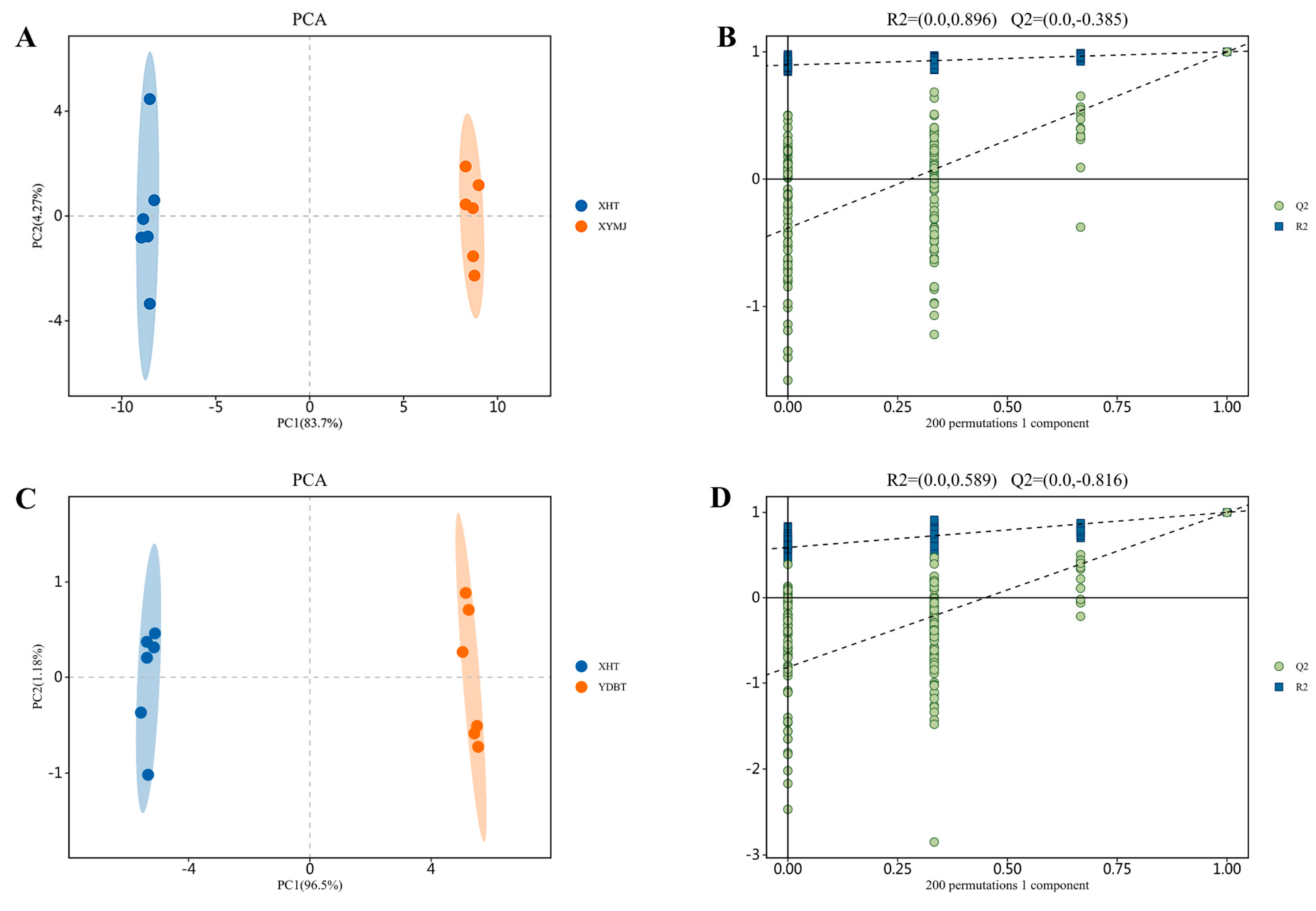
2.4. LC-MS/MS Analysis of Phenolic Compounds
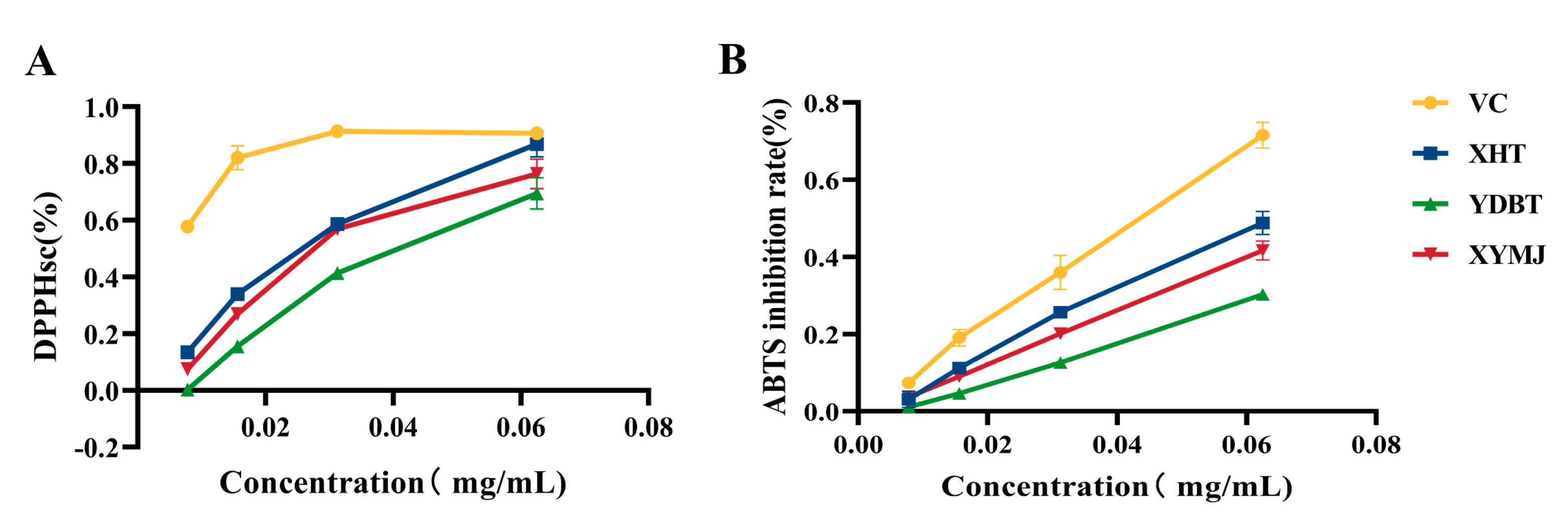
2.5. Determination of the DPPH Radical-Scavenging and ABTS Radical-Inhibitory Activities of XHT, YDBT, and XYMJ
2.6. Cultivation and Synchronization of Caenorhabditis elegans
2.7. Dietary Restriction
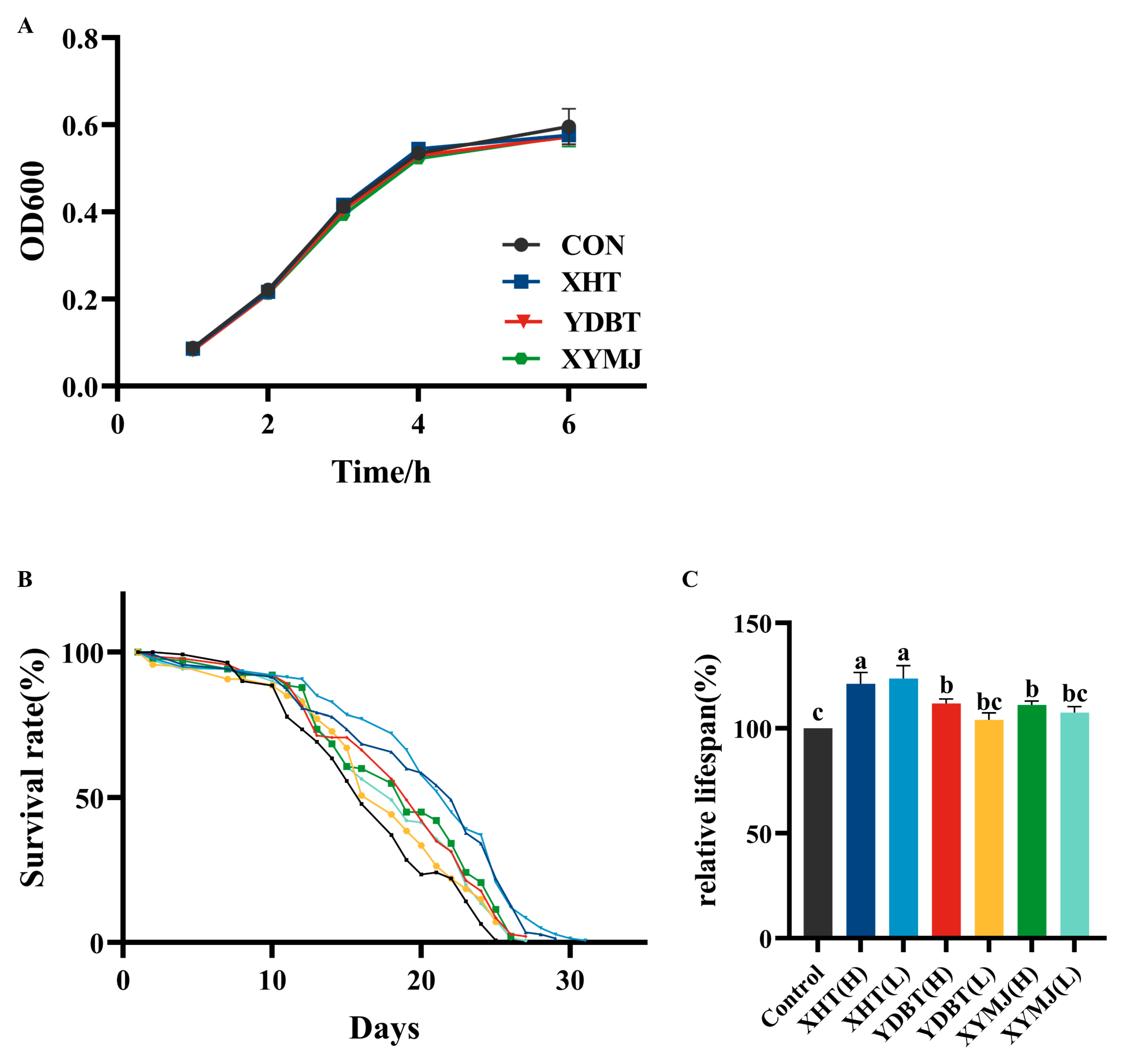
2.8. Determination of the Effect of XHT, YDBT, and XYMJ on Lifetime
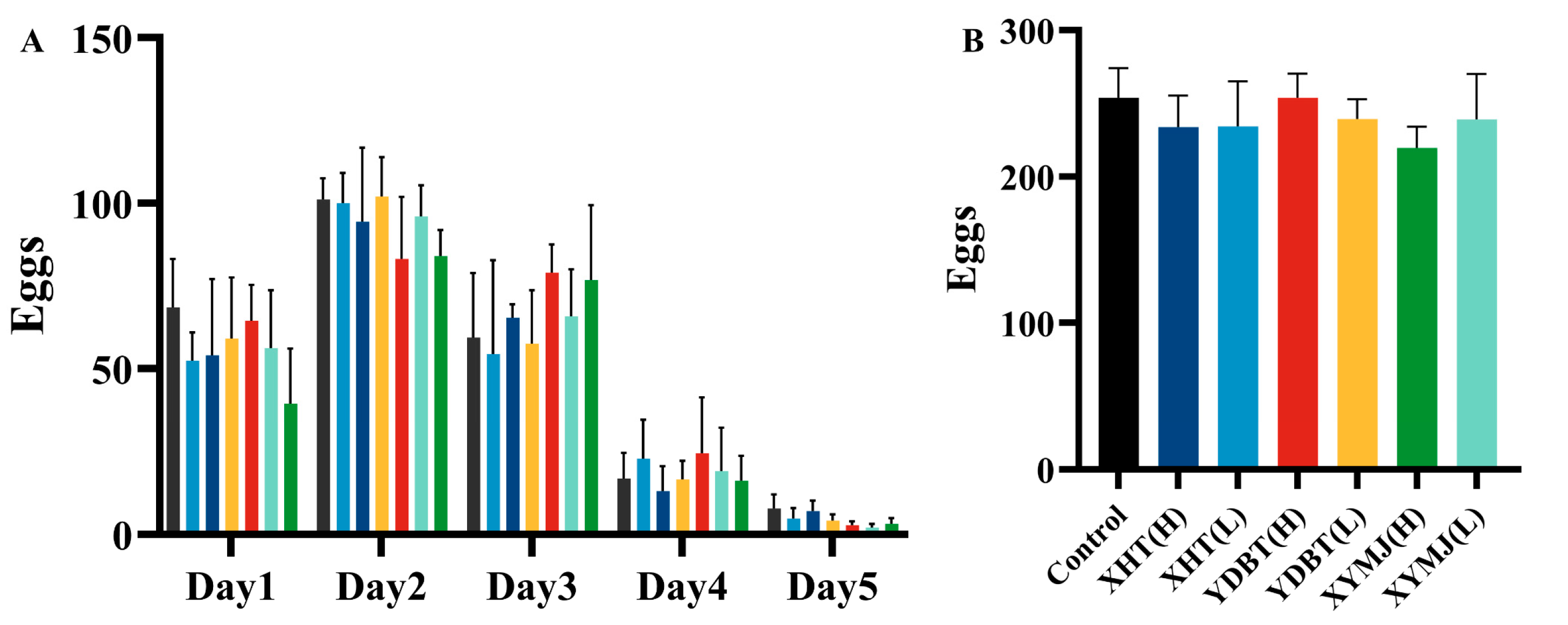
2.9. Fertility Experiment
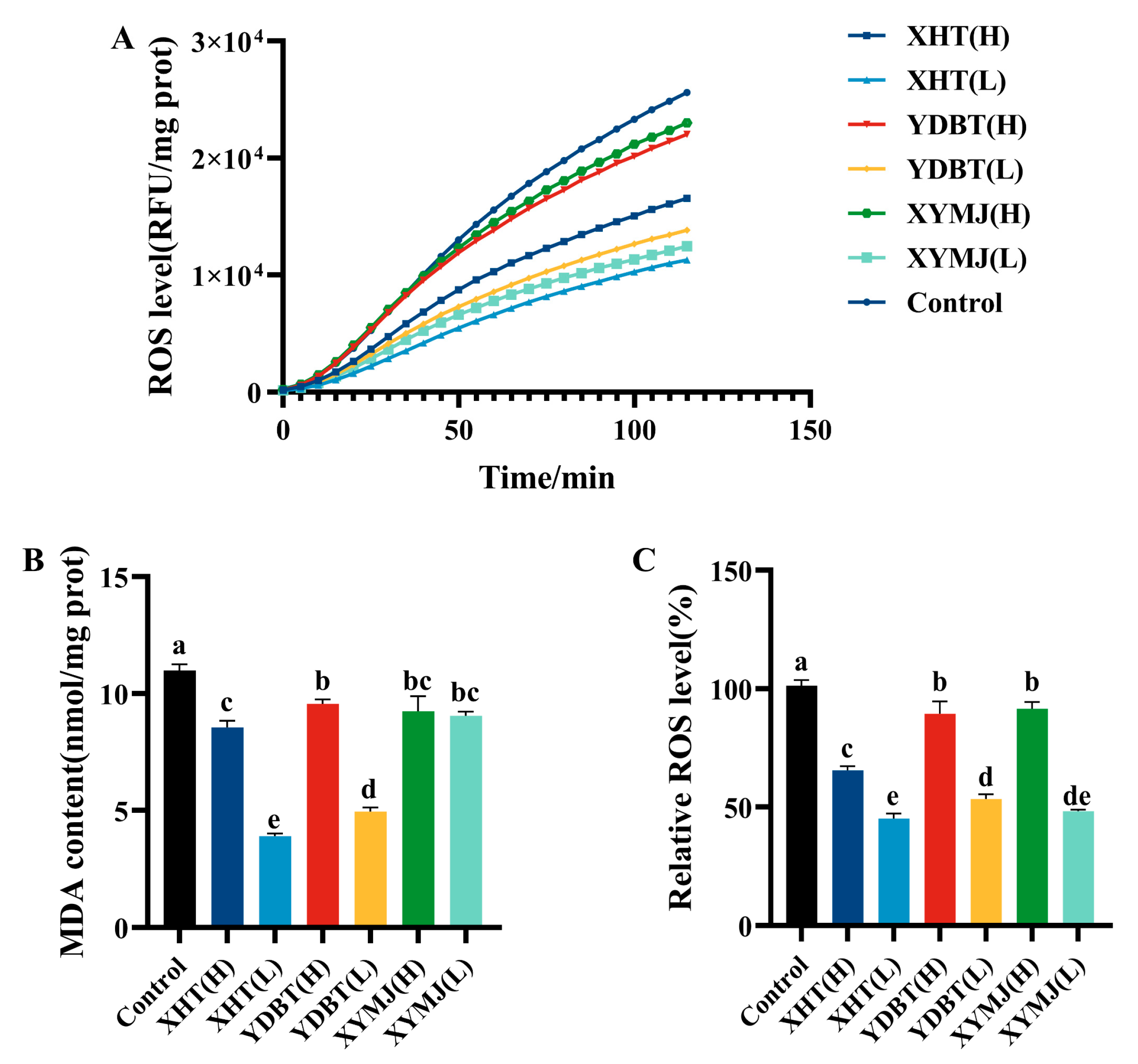
2.10. Measurement of ROS Levels
2.11. Measurement of Lipofuscin Levels
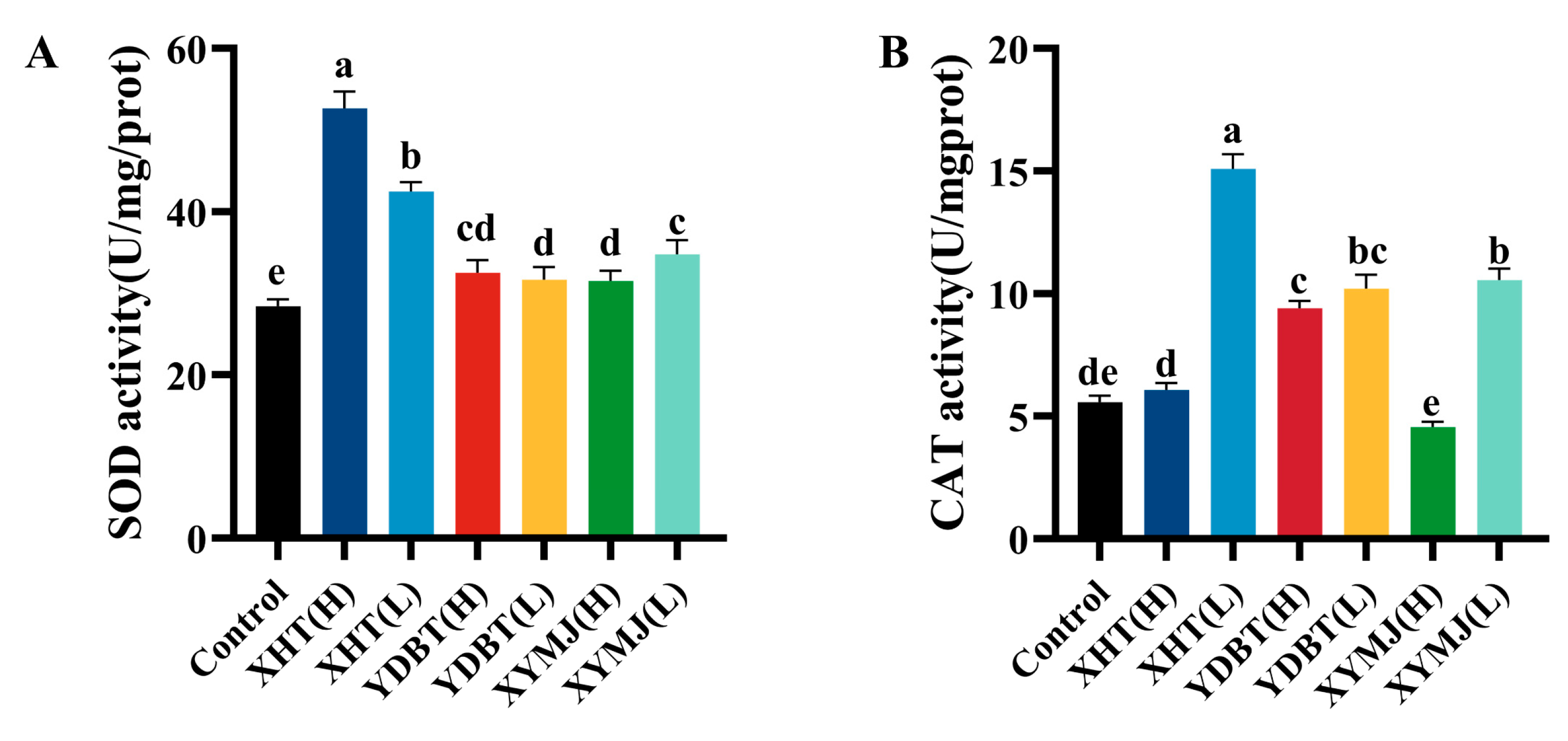
2.12. Determination of Antioxidant Enzyme Activity and MDA Levels
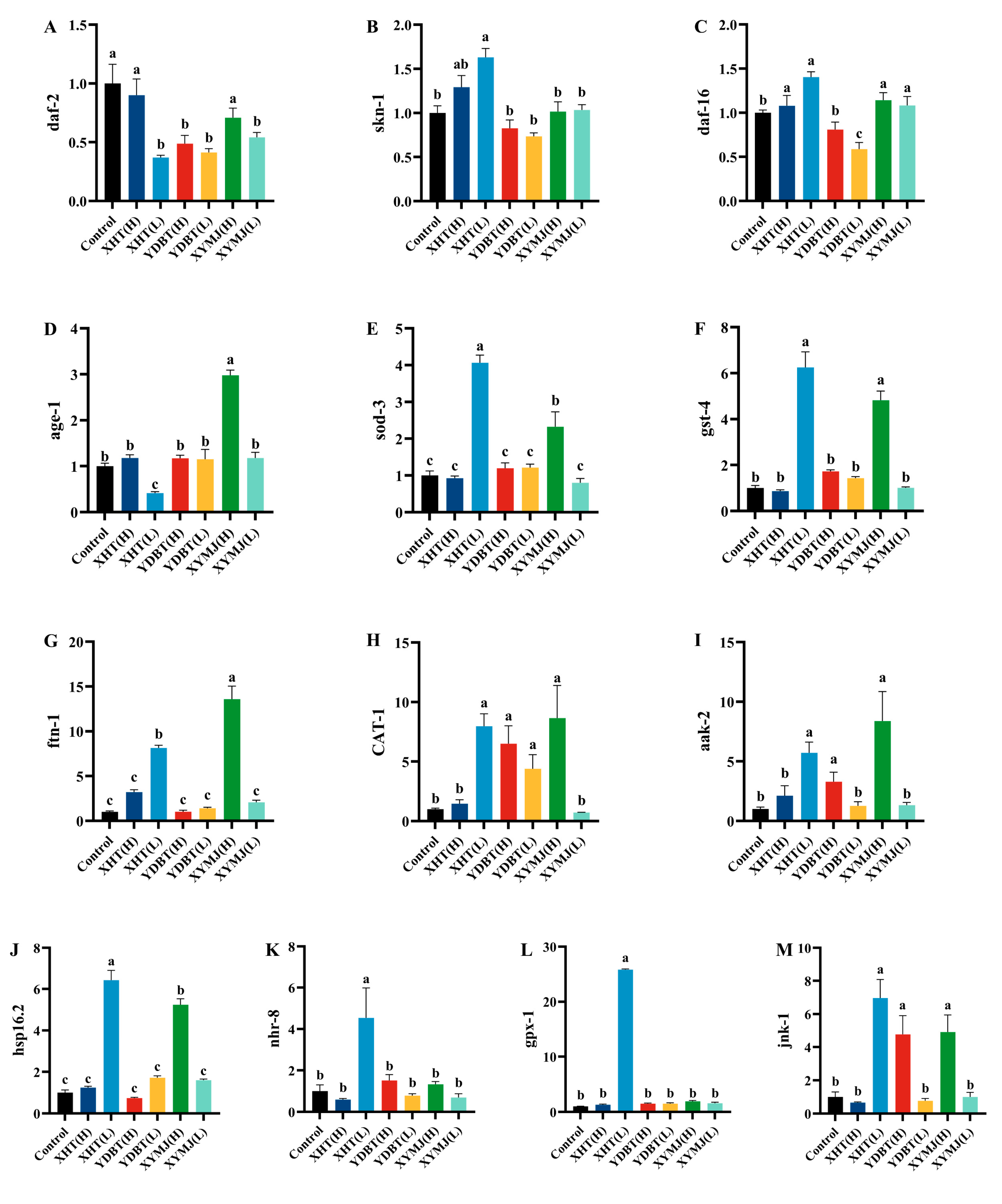
2.13. Expression of Senescence Genes in Nematodes
2.14. Statistics and Analysis
3. Results
3.1. Polysaccharide, Polyphenol, and Flavonoid Content of XHT Extracts
3.2. Quantitative Analysis of Polyphenolic Component in XHT Extracts
3.3. In Vitro Antioxidant Activity
3.4. ROS and MDA Levels in Nematodes
3.5. Determination of the Content and Activity of Antioxidant Enzymes in Nematodes
3.6. Effects on Lipofuscin Levels in Nematodes
3.7. Effects on Lifespan Extension and Reproductive Toxicity
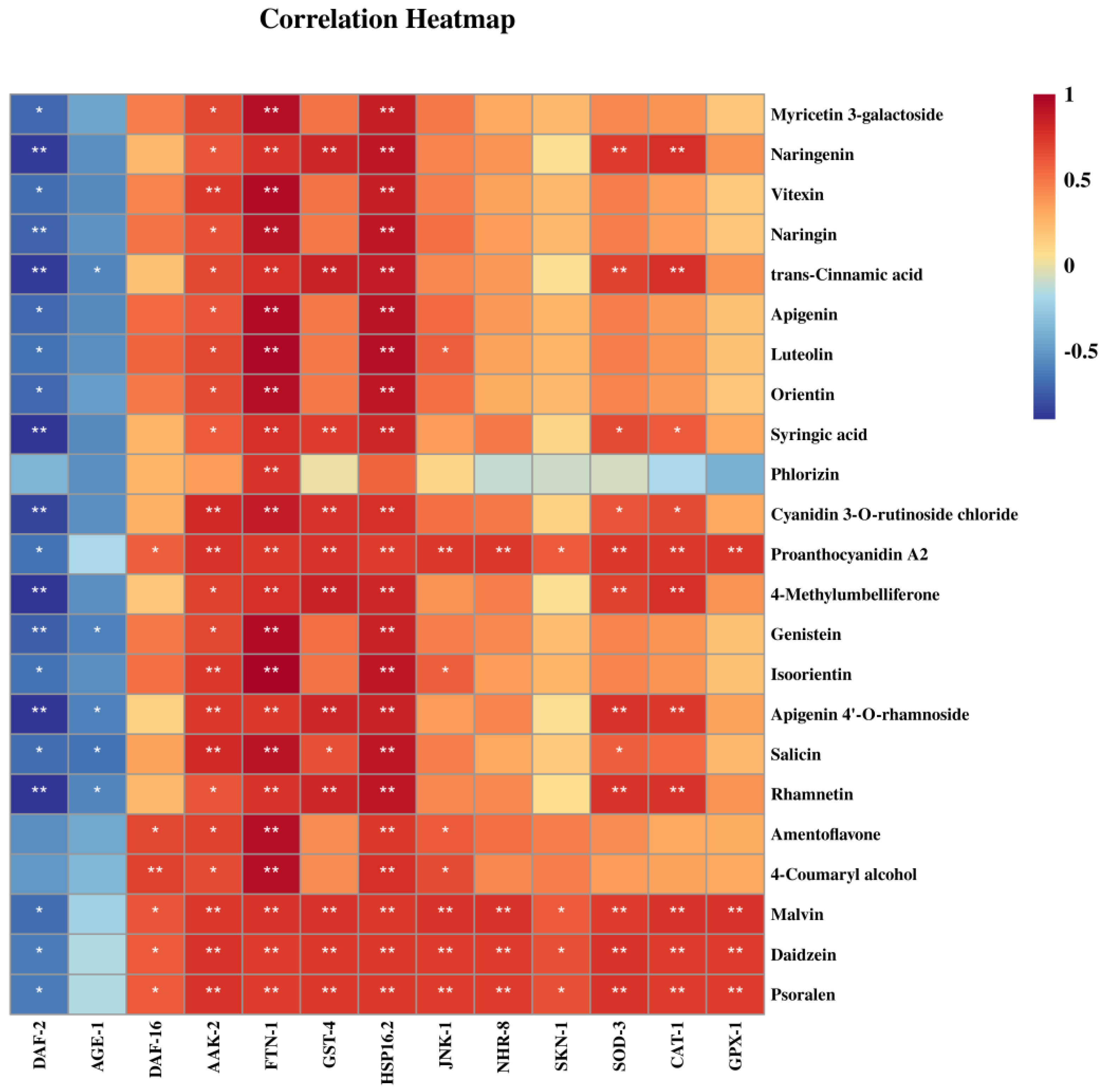
3.8. Effect of Phenolic Compounds in XHT Extracts on Anti-Aging Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| XHT | Xianhu tea water extract |
| XYMJ | Xinyang Maojian tea water extract |
| YDBT | Yingde black tea water extract |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) |
| C. elegans | Caenorhabditis elegans |
| 5-FUDR | 5-fluorouracil |
Appendix A
| Gene | Direction | Primer Sequences (5′-3′) |
|---|---|---|
| actin-1 | Forward | TCGGTATGGGACAGAAGGAC |
| Reverse | CATCCCAGTTGGTGACGATA | |
| ftn-1 | Forward | CGAGTGGGGAACTGTCCTTG |
| Reverse | TCATTGATCGAATGTACCTGCTCT | |
| daf-16 | Forward | TCCGTCACAATCTGTCTCTTCATTC |
| Reverse | GTGTACGCCGTGGATTCCTTC | |
| age-1 | Forward | CCTGAACCGACTGCCAATC |
| Reverse | GTGCTTGACGAGATATGTGTATTG | |
| sod-3 | Forward | CAACTTGGCTAAGGATGGTGGAG |
| Reverse | AGCCTTGAACCGCAATAGTGATG | |
| skn-1 | Forward | GGTCTCCGTTGGCGTGATGATC |
| Reverse | CTGGTGGATGCTCGGTGAGTATTG | |
| aak-2 | Forward | ACCTCTCGCTCTTCCGCCATC |
| Reverse | TCAGCCGTCCGTGCTTAACAATG | |
| gst-4 | Forward | AGTTGTTGAACCAGCCCGTGATG |
| Reverse | GCCCAAGTCAATGAGTCTCCAACG | |
| nhr-8 | Forward | GTCTCGAAGGTCTGCTGTTCCAC |
| Reverse | CGCCTTACAAGATTCGCAAGTGAG | |
| hap16.2 | Forward | GTAGATGTTGGTGCAGTTGCTTCG |
| Reverse | CCTTGAACCGCTTCTTTCTTTGGC | |
| jnk-1 | Forward | ACACTCTGCTCGCATCCTCCTC |
| Reverse | CAGCCAATTCCCAACGGACTCG | |
| gpx-1 | Forward | GTCACTTTCGGATTACAAAGGAAA |
| Reverse | GGGAAGGCAAGAACTTCGAGA | |
| cat-1 | Forward | CCAGTTGGACGAAGTGAGAGGAG |
| Reverse | AGGTTTCTTGGCAGCAGGAGAC |
References
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Chowaniak, M.; Niemiec, M.; Zhu, Z.; Rashidov, N.; Gródek-Szostak, Z.; Szeląg-Sikora, A.; Sikora, J.; Kuboń, M.; Fayzullo, S.A.; Mahmadyorzoda, U.M.; et al. Quality Assessment of Wild and Cultivated Green Tea from Different Regions of China. Molecules 2021, 26, 3620. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-Q.; Li, Q.-S.; Xiang, L.-P.; Liang, Y.-R. Recent Advances in Volatiles of Teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, T.; Wang, Q.; LeCompte, J.; Harkess, R.L.; Bi, G. Screening Tea Cultivars for Novel Climates: Plant Growth and Leaf Quality of Camellia sinensis Cultivars Grown in Mississippi, United States. Front. Plant Sci. 2020, 11, 280. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Kang, J.; Chen, Y.; Hong, L.; Li, M.; Jia, Y.; Wang, Y.; Jia, X.; Wu, Z.; et al. Effects of Long-Term Use of Organic Fertilizer with Different Dosages on Soil Improvement, Nitrogen Transformation, Tea Yield and Quality in Acidified Tea Plantations. Plants 2022, 12, 122. [Google Scholar] [CrossRef]
- Ge, S.; Wang, Y.; Shen, K.; Wang, Q.; Ahammed, G.J.; Han, W.; Jin, Z.; Li, X.; Shi, Y. Effects of Differential Shading on Summer Tea Quality and Tea Garden Microenvironment. Plants 2024, 13, 202. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, Age-Related Diseases and Oxidative Stress: What to Do Next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Liu, L.; Guo, P.; Wang, P.; Zheng, S.; Qu, Z.; Liu, N. The Review of Anti-Aging Mechanism of Polyphenols on Caenorhabditis elegans. Front. Bioeng. Biotechnol. 2021, 9, 635768. [Google Scholar] [CrossRef]
- Duan, H.; Pan, J.; Guo, M.; Li, J.; Yu, L.; Fan, L. Dietary Strategies with Anti-Aging Potential: Dietary Patterns and Supplements. Food Res. Int. 2022, 158, 111501. [Google Scholar] [CrossRef]
- Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients 2022, 14, 857. [Google Scholar] [CrossRef]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A Living Model for Obesity and Aging Research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, C.; Cao, Y.; Chen, Y. Caenorhabditis elegans as an in Vivo Model for the Identification of Natural Antioxidants with Anti-Aging Actions. Biomed. Pharmacother. 2023, 167, 115594. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- He, M.; Zeng, J.; Zhai, L.; Liu, Y.; Wu, H.; Zhang, R.; Li, Z.; Xia, E. Effect of in Vitro Simulated Gastrointestinal Digestion on Polyphenol and Polysaccharide Content and Their Biological Activities among 22 Fruit Juices. Food Res. Int. 2017, 102, 156–162. [Google Scholar] [CrossRef]
- Tian, Z.-X.; Li, Y.-F.; Long, M.-X.; Liang, Q.; Chen, X.; Huang, D.-M.; Ran, Y.-Q. Effects of Six Different Microbial Strains on Polyphenol Profiles, Antioxidant Activity, and Bioaccessibility of Blueberry Pomace with Solid-State Fermentation. Front. Nutr. 2023, 10, 1282438. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Berger, S.; Oesterle, I.; Ayeni, K.I.; Ezekiel, C.N.; Rompel, A.; Warth, B. Polyphenol Exposure of Mothers and Infants Assessed by LC-MS/MS Based Biomonitoring in Breast Milk. Anal. Bioanal. Chem. 2024, 416, 1759–1774. [Google Scholar] [CrossRef]
- García, A.; González Alriols, M.; Spigno, G.; Labidi, J. Lignin as Natural Radical Scavenger. Effect of the Obtaining and Purification Processes on the Antioxidant Behaviour of Lignin. Biochem. Eng. J. 2012, 67, 173–185. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.-H. Ultrasound-Assisted Polysaccharide Extraction from Cercis chinensis and Properites, Antioxidant Activity of Polysaccharide. Ultrason. Sonochem 2023, 96, 106422. [Google Scholar] [CrossRef]
- Xiao, H.; Jedrychowski, M.P.; Schweppe, D.K.; Huttlin, E.L.; Yu, Q.; Heppner, D.E.; Li, J.; Long, J.; Mills, E.L.; Szpyt, J.; et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell 2020, 180, 968–983.e24. [Google Scholar] [CrossRef]
- Zhang, C.; Song, X.; Cui, W.; Yang, Q. Antioxidant and Anti-Ageing Effects of Enzymatic Polysaccharide from Pleurotus eryngii Residue. Int. J. Biol. Macromol. 2021, 173, 341–350. [Google Scholar] [CrossRef]
- Wu, H.; Kong, Y.; Zhao, W.; Wang, F. Measurement of Cellular MDA Content through MTBE-Extraction Based TBA Assay by Eliminating Cellular Interferences. J. Pharm. Biomed. Anal. 2024, 248, 116332. [Google Scholar] [CrossRef]
- Zhao, D.; Yan, M.; Xu, H.; Liang, H.; Zhang, J.; Li, M.; Wang, C. Antioxidant and Antiaging Activity of Fermented Coix Seed Polysaccharides on Caenorhabditis elegans. Nutrients 2023, 15, 2474. [Google Scholar] [CrossRef]
- Chen, J.-C.; Wang, R.; Wei, C.-C. Anti-Aging Effects of Dietary Phytochemicals: From Caenorhabditis elegans, Drosophila melanogaster, Rodents to Clinical Studies. Crit. Rev. Food Sci. Nutr. 2023, 64, 5958–5983. [Google Scholar] [CrossRef]
- Lin, Q.; Song, B.; Zhong, Y.; Yin, H.; Li, Z.; Wang, Z.; Cheong, K.-L.; Huang, R.; Zhong, S. Effect of Sodium Hyaluronate on Antioxidant and Anti-Ageing Activities in Caenorhabditis elegans. Foods 2023, 12, 1400. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Luo, J.; Peng, X. Anti-Aging Potency Correlates with Metabolites from in Vitro Fermentation of Edible Fungal Polysaccharides Using Human Fecal Intestinal Microflora. Food Funct. 2022, 13, 11592–11603. [Google Scholar] [CrossRef]
- Yen, C.A.; Curran, S.P. Gene-Diet Interactions and Aging in C. elegans. Exp. Gerontol. 2016, 86, 106–112. [Google Scholar] [CrossRef]
- Sharma, G.; Shin, E.-J.; Sharma, N.; Nah, S.-Y.; Mai, H.N.; Nguyen, B.T.; Jeong, J.H.; Lei, X.G.; Kim, H.-C. Glutathione Peroxidase-1 and Neuromodulation: Novel Potentials of an Old Enzyme. Food Chem. Toxicol. 2021, 148, 111945. [Google Scholar] [CrossRef]
- Lee, H.-B.; Kim, E.-K.; Park, S.-J.; Bang, S.-G.; Kim, T.G.; Chung, D.-W. Isolation and Characterization of Nicotiflorin Obtained by Enzymatic Hydrolysis of Two Precursors in Tea Seed Extract. J. Agric. Food Chem. 2010, 58, 4808–4813. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Zhang, Y.-N.; Chen, J.-X.; Wang, F.; Du, Q.-Z.; Yin, J.-F. Quantitative Analyses of the Bitterness and Astringency of Catechins from Green Tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef]
- Huang, Y.; He, M.; Zhang, J.; Cheng, S.; Cheng, X.; Chen, H.; Wu, G.; Wang, F.; Zeng, S. White Tea Aqueous Extract: A Potential Anti-Aging Agent Against High-Fat Diet-Induced Senescence in Drosophila melanogaster. Foods 2024, 13, 4034. [Google Scholar] [CrossRef]
- Chen, T.; Luo, S.; Wang, X.; Zhou, Y.; Dai, Y.; Zhou, L.; Feng, S.; Yuan, M.; Ding, C. Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway. Antioxidants 2021, 10, 1744. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (‒)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Zhao, X.; Song, J.-L.; Yi, R.; Li, G.; Sun, P.; Park, K.-Y.; Suo, H. Comparison of Antioxidative Effects of Insect Tea and Its Raw Tea (Kuding Tea) Polyphenols in Kunming Mice. Molecules 2018, 23, 204. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Li, W.-H.; Shi, Y.-C.; Yen, P.-L.; Lin, H.-Y.; Liao, V.H.-C.; Chang, S.-T. Antioxidant Activity and Delayed Aging Effects of Hot Water Extract from Chamaecyparis obtusa var. formosana leaves. J. Agric. Food Chem. 2014, 62, 4159–4165. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Drouet, S.; Hano, C. Flavonoids from Sacred Lotus Stamen Extract Slows Chronological Aging in Yeast Model by Reducing Oxidative Stress and Maintaining Cellular Metabolism. Cells 2022, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A Multifunctional Flavonol in Biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Büchter, C.; Ackermann, D.; Honnen, S.; Arnold, N.; Havermann, S.; Koch, K.; Wätjen, W. Methylated Derivatives of Myricetin Enhance Life Span in Caenorhabditis elegans Dependent on the Transcription Factor DAF-16. Food Funct. 2015, 6, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, A.N.; Clayton, Z.S.; Wahl, D.; Hutton, D.A.; McEntee, C.M.; Seals, D.R.; LaRocca, T.J. Protective Effects of Apigenin on the Brain Transcriptome with Aging. Mech. Ageing Dev. 2024, 217, 111889. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.S.; Hutton, D.A.; Brunt, V.E.; VanDongen, N.S.; Ziemba, B.P.; Casso, A.G.; Greenberg, N.T.; Mercer, A.N.; Rossman, M.J.; Campisi, J.; et al. Apigenin Restores Endothelial Function by Ameliorating Oxidative Stress, Reverses Aortic Stiffening, and Mitigates Vascular Inflammation with Aging. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H185–H196. [Google Scholar] [CrossRef]
- Kramer, D.J.; Johnson, A.A. Apigenin: A Natural Molecule at the Intersection of Sleep and Aging. Front. Nutr. 2024, 11, 1359176. [Google Scholar] [CrossRef]
- Zumerle, S.; Sarill, M.; Saponaro, M.; Colucci, M.; Contu, L.; Lazzarini, E.; Sartori, R.; Pezzini, C.; Rinaldi, A.; Scanu, A.; et al. Targeting Senescence Induced by Age or Chemotherapy with a Polyphenol-Rich Natural Extract Improves Longevity and Healthspan in Mice. Nat. Aging 2024, 4, 1231–1248. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef]
- Guo, P.; Wang, P.; Liu, L.; Wang, P.; Lin, G.; Qu, Z.; Yu, Z.; Liu, N. Naringin Alleviates Glucose-Induced Aging by Reducing Fat Accumulation and Promoting Autophagy in Caenorhabditis elegans. Nutrients 2023, 15, 907. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, H.; Wang, J.; Liu, G.; Cui, S.W.; Kang, J.; Jiang, Y.; Wang, H. Naringenin Prolongs Lifespan and Delays Aging Mediated by IIS and MAPK in Caenorhabditis elegans. Food Funct. 2021, 12, 12127–12141. [Google Scholar] [CrossRef]
- Wang, R.; Ding, A.; Wang, J.; Wang, J.; Zhou, Y.; Chen, M.; Ju, S.; Tan, M.; Xiang, Z. Astragalin from Thesium Chinense: A Novel Anti-Aging and Antioxidant Agent Targeting IGFR/CD38/Sirtuins. Antioxidants 2024, 13, 859. [Google Scholar] [CrossRef] [PubMed]
- Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkötter, A.; Wätjen, W. Myricetin-Mediated Lifespan Extension in Caenorhabditis elegans Is Modulated by DAF-16. Int. J. Mol. Sci. 2013, 14, 11895–11914. [Google Scholar] [CrossRef]
- Tao, M.; Li, R.; Zhang, Z.; Wu, T.; Xu, T.; Zogona, D.; Huang, Y.; Pan, S.; Xu, X. Vitexin and Isovitexin Act through Inhibition of Insulin Receptor to Promote Longevity and Fitness in Caenorhabditis elegans. Mol. Nutr. Food Res. 2022, 66, e2100845. [Google Scholar] [CrossRef] [PubMed]
- Lashmanova, E.; Zemskaya, N.; Proshkina, E.; Kudryavtseva, A.; Volosnikova, M.; Marusich, E.; Leonov, S.; Zhavoronkov, A.; Moskalev, A. The Evaluation of Geroprotective Effects of Selected Flavonoids in Drosophila melanogaster and Caenorhabditis elegans. Front. Pharmacol. 2017, 8, 884. [Google Scholar] [CrossRef] [PubMed]



| Group | Polysaccharide Content (mg/g Dry Tea ± SD) | Polyphenol Content (mg/g Dry Tea ± SD) | Flavonoid Content (mg/g Dry Tea ± SD) |
|---|---|---|---|
| XHT | 23.0497 ± 0.3204 a | 206.1709 ± 0.9456 a | 5.1354 ± 0.2393 a |
| YDBT | 19.6990 ± 0.5782 b | 133.3794 ± 2.0122 c | 4.0187 ± 0.1303 c |
| XYMJ | 22.4382 ± 0.1394 a | 166.9787 ± 1.1612 b | 4.6300 ± 0.7343 b |
| Metabolites (Number) | XHT (ng/mL) | XYMJ (ng/mL) | YDBT (ng/mL) |
|---|---|---|---|
| Luteolin (79) | 3343.57 ± 130.62 a | 1469.33 ± 100.78 b | 592.86 ± 117.75 c |
| Orientin (93) | 2911.33 ± 135.48 a | 1261.73 ± 85.38 b | 376.61 ± 24.80 c |
| Isoorientin (71) | 329.39 ± 56.91 a | 106.47 ± 15.37 b | 86.04 ± 19.08 b |
| Apigenin (30) | 5248.16 ± 186.58 a | 1675.53 ± 243.92 b | 573.83 ± 40.22 c |
| Vitexin (130) | 14,242.06 ± 1027.54 a | 3039.50 ± 312.81 b | 1518.14 ± 101.19 c |
| Amentoflavone (29) | 26.01 ± 11.26 a | 5.16 ± 1.53 b | 0 b |
| Myricetin 3-galactoside (85) | 66,786.83 ± 3798.76 a | 22,854.64 ± 1430.50 b | 8251.01 ± 1317.31 c |
| Rhamnetin (112) | 175.72 ± 13.23 a | 54.61 ± 1.64 b | 60.16 ± 1.29 b |
| Apigenin 4′-O-rhamnoside (31) | 2020.02 ± 5.76 a | 22.12 ± 8.02 b | 28.98 ± 1.11 b |
| Genistein (65) | 449.71 ± 48.76 a | 68.13 ± 9.11 b | 41.45 ± 7.47 b |
| Daidzein (50) | 5.36 ± 2.12 a | 0 b | 0 b |
| Naringenin (88) | 18,631.95 ± 672.69 a | 7762.99 ± 618.52 b | 8990.59 ± 565.74 b |
| Naringin (89) | 13,505.05 ± 608.34 a | 5510.38 ± 151.54 b | 1731.10 ± 83.88 c |
| Cyanidin 3-O-rutinoside chloride (48) | 1393.81 ± 67.91 a | 450.03 ± 65.09 b | 598.77 ± 200.29 b |
| Malvin (80) | 12.76 ± 1.69 a | 0 b | 0 b |
| Phlorizin (98) | 1676.33 ± 28.19 a | 4369.75 ± 103.26 b | 704.02 ± 21.95 c |
| Proanthocyanidin A2 (99) | 1271.09 ± 134.51 a | 0 b | 0 b |
| 4-Coumaryl alcohol (18) | 20.40 ± 1.46 a | 9.82 ± 0.78 b | 0 b |
| 4-Methylumbelliferone (21) | 573.53 ± 21.92 a | 213.59 ± 7.19 b | 262.79 ± 34.21 b |
| Psoralen (105) | 1.25 ± 0.55 a | 0 b | 0 b |
| Trans-cinnamic acid (124) | 8406.17 ± 295.10 a | 2376.58 ± 117.05 b | 3927.76 ± 881.13 c |
| Salicin (115) | 167.18 ± 7.46 a | 45.26 ± 4.99 b | 44.07 ± 5.60 b |
| Syringic acid (121) | 1440.59 ± 209.19 a | 115.81 ± 9.91 b | 110.58 ± 10.92 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Jiang, W.; Hu, Q.; Luo, J.; Peng, X. Characterization and Anti-Aging Potency of Phenolic Compounds in Xianhu Tea Extracts. Foods 2025, 14, 737. https://doi.org/10.3390/foods14050737
Zhang G, Jiang W, Hu Q, Luo J, Peng X. Characterization and Anti-Aging Potency of Phenolic Compounds in Xianhu Tea Extracts. Foods. 2025; 14(5):737. https://doi.org/10.3390/foods14050737
Chicago/Turabian StyleZhang, Guangwen, Wenwen Jiang, Qing Hu, Jianming Luo, and Xichun Peng. 2025. "Characterization and Anti-Aging Potency of Phenolic Compounds in Xianhu Tea Extracts" Foods 14, no. 5: 737. https://doi.org/10.3390/foods14050737
APA StyleZhang, G., Jiang, W., Hu, Q., Luo, J., & Peng, X. (2025). Characterization and Anti-Aging Potency of Phenolic Compounds in Xianhu Tea Extracts. Foods, 14(5), 737. https://doi.org/10.3390/foods14050737





