Removal Capacity and Mechanism of Modified Chitosan for Ochratoxin A Based on Rapid Magnetic Separation Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
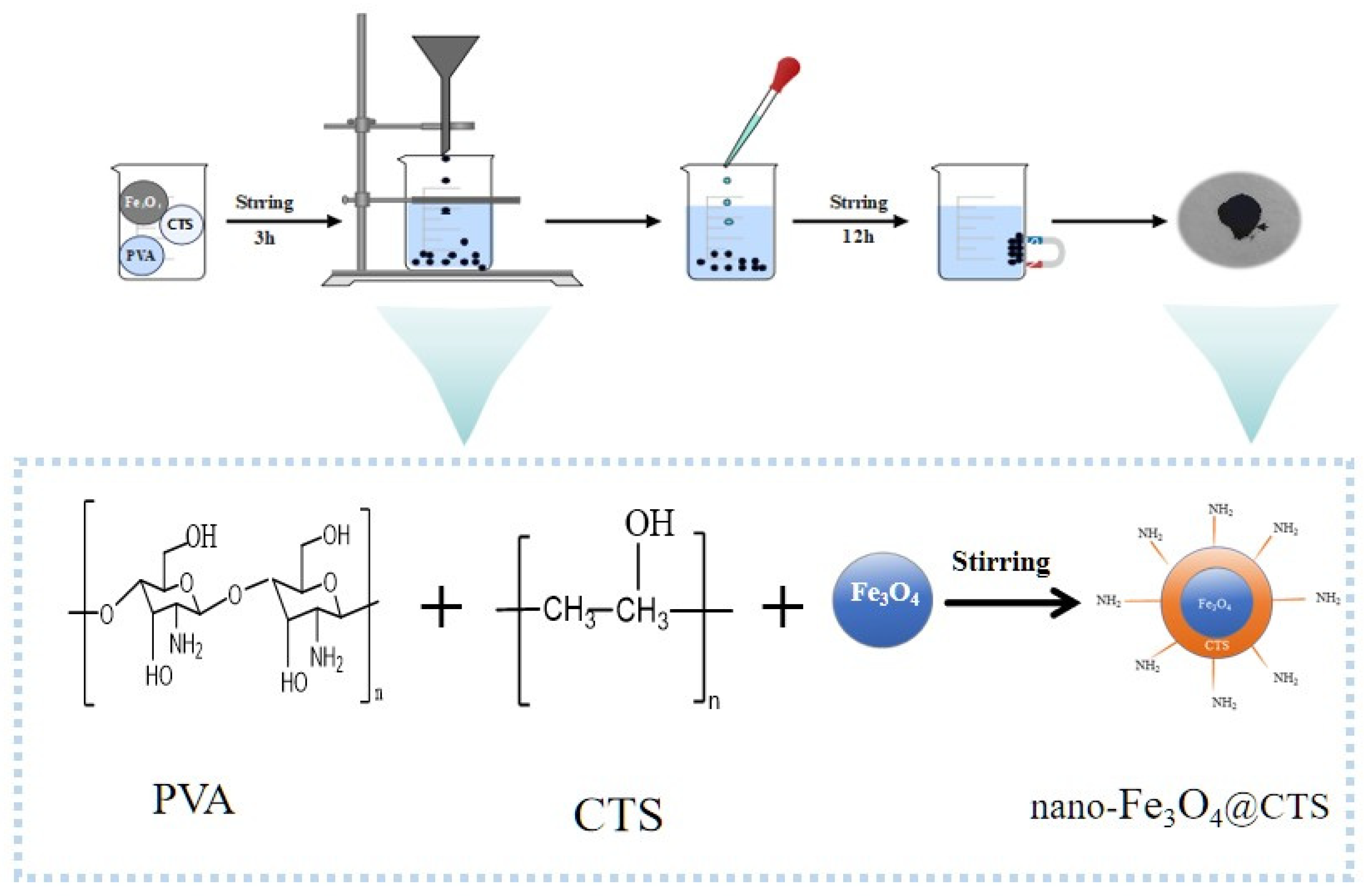
2.2. Preparation of Magnetic Nano-Fe3O4@CTS Adsorbent
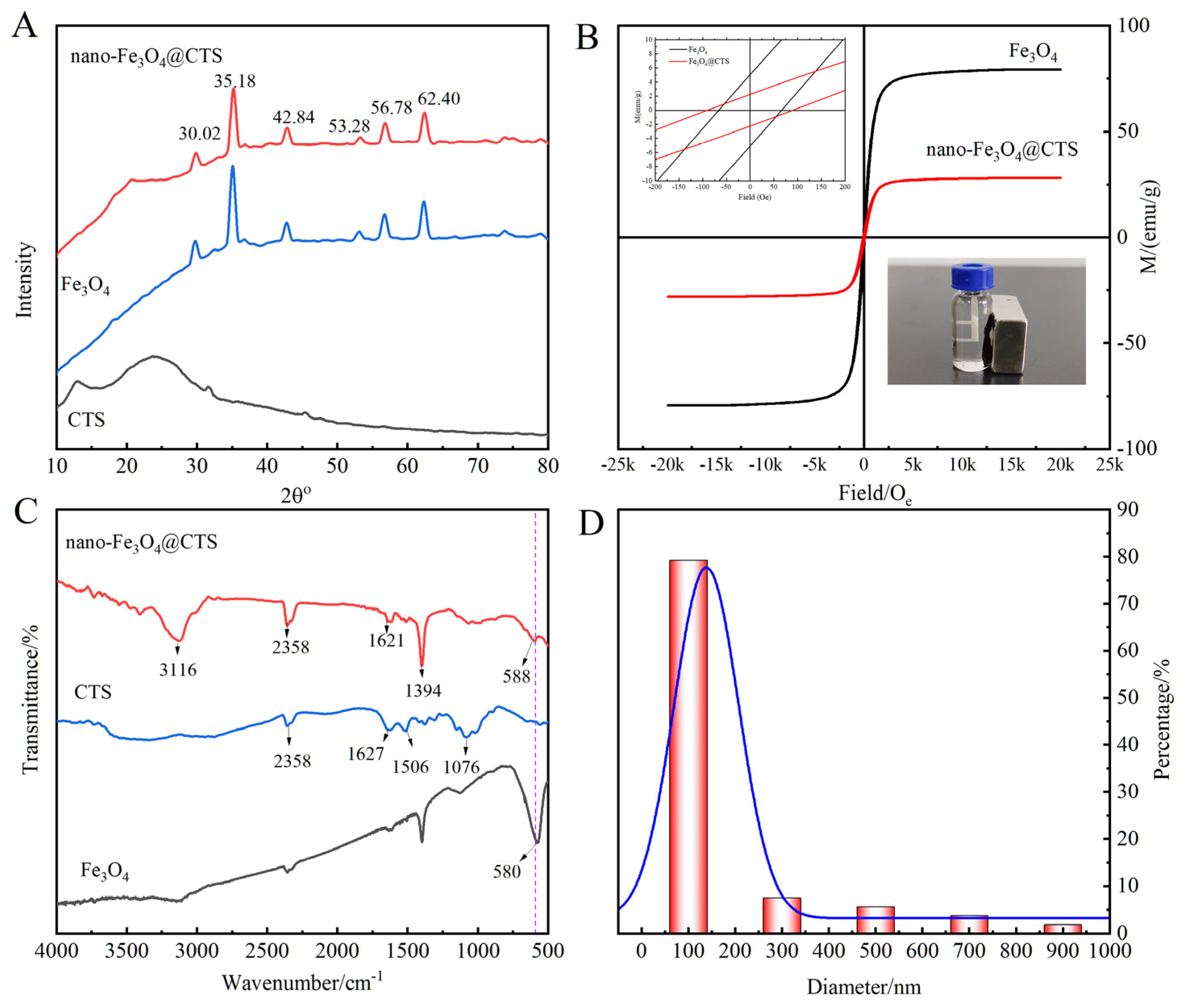
2.3. Characterization of Nano-Fe3O4@CTS Adsorbents
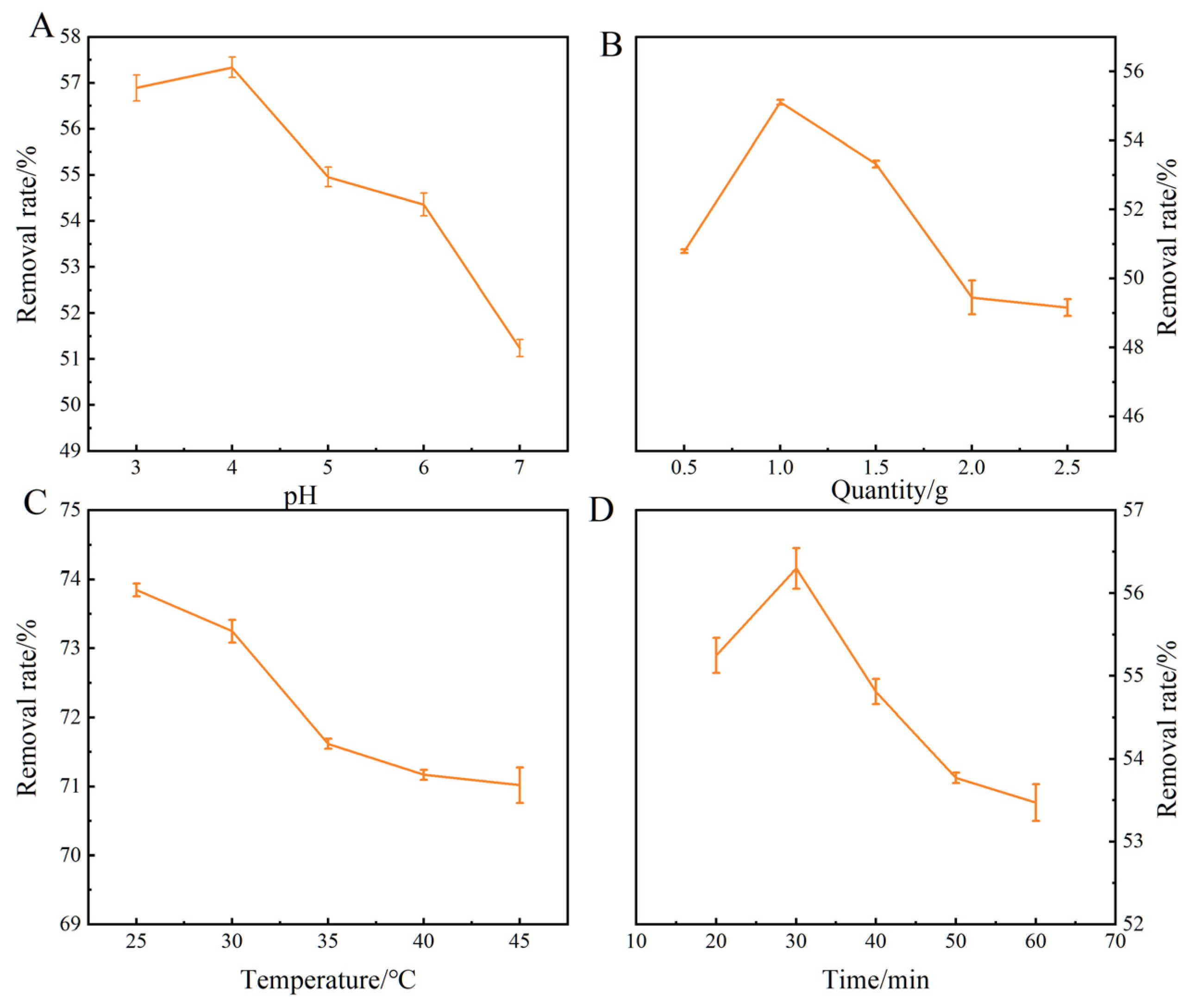
2.4. Screening of Optimization Conditions
2.5. Adsorption Kinetics
2.6. Adsorption Isotherms
2.7. Thermodynamic Parameters
2.8. HPLC-FLD Analysis
2.9. Removal of OTA from Wine
2.10. Wine Analysis
2.10.1. Measurement of Color Value
2.10.2. Measurement of Transmittance
2.10.3. Measurement of Total Soluble Solids
2.10.4. Measurement of Total Polyphenol
2.10.5. Measurement of Total Flavonoid Content
2.10.6. Measurement of Total Acidity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nano-Fe3O4@CTS
3.2. Adsorption Kinetics
3.3. Adsorption Isotherm
3.4. Thermodynamic Parameters
3.5. Extraction and Removal of OTA from Samples
3.6. Wine Analysis
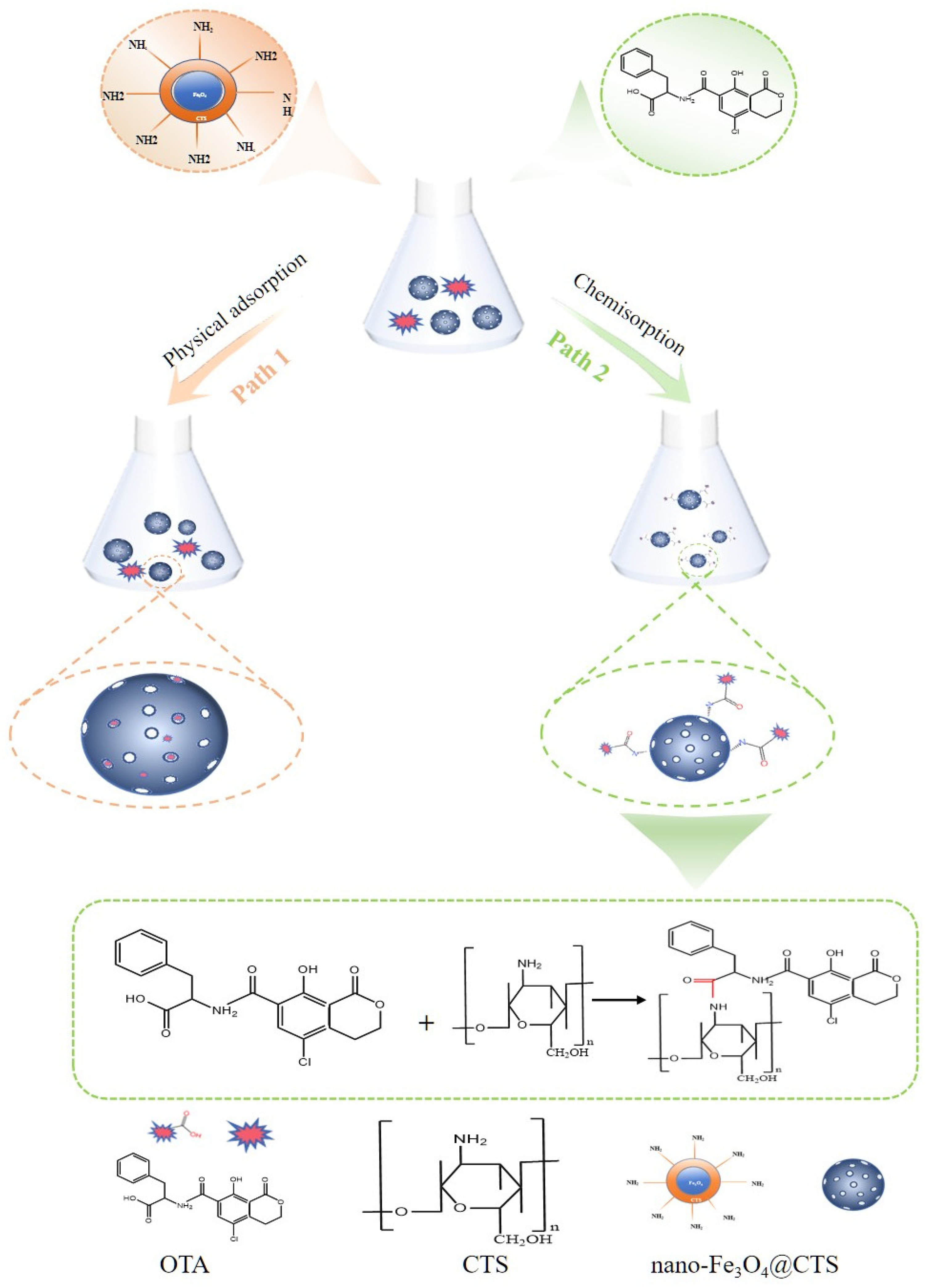
3.7. Removal Mechanism of OTA by Nano-Fe3O4@CTS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arroyo, M.N.; Campillo, N.; Lopez-Garcia, I.; Hernandez-Cordoba, M.; Vinas, P. High-resolution mass spectrometry for the determination of mycotoxins in biological samples. A review. Microchem. J. 2021, 166, 106197. [Google Scholar] [CrossRef]
- Nan, M.N.; Xue, H.L.; Bi, Y. Contamination, Detection and Control of Mycotoxins in Fruits and Vegetables. Toxins 2022, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Karami, O.R. Nanofluid extraction of Ochratoxin A in food. J. Food Compos. Anal. 2020, 87, 103425. [Google Scholar] [CrossRef]
- Nan, M.N.; Bi, Y.; Xue, H.L.; Xue, S.L.; Long, H.T.; Pu, L.M.; Fu, G.R. Rapid determination of ochratoxin A in grape and its commodities based on a label-free impedimetric aptasensor constructed by layer-by-layer self-assembly. Toxins 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, J.; Ma, T.; Li, Z.; Wang, L.; Ji, S.; Sun, M.; Liu, Y.; Hu, Z.; Liu, Q.; et al. Magnetic covalent organic framework for effective solid-phase extraction and HPLC determination of ochratoxin A in food. LWT 2023, 179, 114639. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Janda, K. Mycotoxins in red wine: Occurrence and risk assessment. Food Control 2021, 129, 108229. [Google Scholar] [CrossRef]
- Roi, A.; Yael, G.M. Bifunctional clay based sorbent for ‘Ochratoxin A’ removal and wine fining. Food Chem. 2023, 416, 135827. [Google Scholar]
- Jalili, M.; Jinap, S.; Son, R. The effect of chemical treatment on reduction of aflatoxins and ochratoxin A in black and white pepper during washing. Food Addit. Contam. A 2011, 28, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Nora, N.S.; Penteado Feltrin, A.C.; Marimon Sibaja, K.V.; Furlong, E.B.; Garda-Buffon, J. Ochratoxin A reduction by peroxidase in a model system and grape juice. Braz. J. Microbiol. 2019, 50, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, R.; Yue, T.; Yuan, Y.; Gao, Z.; Wang, Z. Assessment of traditional clarifiers on the adsorption of ochratoxin A in Cabernet Sauvignon red wine and their kinetics. Food Chem. 2022, 373, 131592. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Chang, S.; Guo, Q.; Yan, X.; Chen, H.; Yuan, Y.; Yue, T. Adsorption removal of ochratoxin A from milk by Tibetan kefir grains and its mechanism. LWT 2022, 169, 114024. [Google Scholar] [CrossRef]
- Cosme, F.; Inês, A.; Silva, D.; Filipe, R.L.; Abrunhosa, L.; Nunes, F.M. Elimination of ochratoxin A from white and red wines: Critical characteristics of activated carbons and impact on wine quality. LWT 2021, 140, 110838. [Google Scholar] [CrossRef]
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. J. Agric. Food Chem. 2001, 49, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Rotter, R.G.; Frohlich, A.A.; Marquardt, R.R. Influence of dietary charcoal on ochratoxin A toxicity in Leghorn chicks. Can. Can. J. Vet. Res. 1989, 53, 449–453. [Google Scholar] [PubMed]
- Pirouz, A.A.; Karjiban, A.R.; Bakar, A.F. A Novel Adsorbent Magnetic Graphene Oxide Modified with Chitosan for the Simultaneous Reduction of Mycotoxins. Toxins 2018, 10, 361. [Google Scholar] [CrossRef]
- Bornet, A.; Teissedre, L.P. Chitosan, chitin-glucan and chitin effects on minerals (iron, lead, cadmium) and organic (ochratoxin A) contaminants in wines. Eur. Food Res. Technol. 2008, 226, 681–689. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, N.; Yang, L.; Wang, J.; Song, S.; Nie, D.; Yang, X.; Hou, J.; Wu, A. Cross-linked chitosan polymers as generic adsorbents for simultaneous adsorption of multiple mycotoxins. Food Control 2015, 57, 362–369. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2003, 38, 43–74. [Google Scholar] [CrossRef]
- Wang, J.; Kawde, A.N.; Erdem, A.; Salazar, M. Magnetic bead-based label-free electrochemical detection of DNA hybridization. Analyst 2002, 126, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Omidinasab, M.; Rahbar, N.; Ahmadi, M.; Kakavandi, B.; Ghanbari, F.; Kyzas, G.Z.; Martinez, S.S.; Jaafarzadeh, N. Removal of vanadium and palladium ions by adsorption onto magnetic chitosan nanoparticles. Environ. Sci. Pollut. R. 2018, 25, 34262–34276. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Capão, I.; Filipe-Ribeiro, L.; Bennett, R.; Mendes-Faia, A. Evaluating potential alternatives to potassium caseinate for white wine fining: Effects on physicochemical and sensory characteristics. LWT 2012, 46, 382–387. [Google Scholar] [CrossRef]
- Nie, J.; Wu, Y.; Li, H.; Wang, K.; Xu, G.; Yan, Z.; Wu, X. Evaluation System Established for Fresh Apple Juice Quality. Sci. Agric. Sin. 2013, 46, 1657–1667. [Google Scholar]
- Xin, X.; Nan, M.; Bi, Y.; Xue, H.; Zhang, Y.; Wang, J.; Lu, Z. Effects of Aspergillus niger Infection on the Quality of Jujube and Ochratoxin A Cumulative Effect. Toxins 2023, 15, 406. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Han, X.; Peng, Q.; Yang, H.; Hu, B.; Shen, C.; Tian, R. Influence of different carbohydrate sources on physicochemical properties and metabolites of fermented greengage (Prunus mume) wines. LWT 2020, 121, 108929. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, H.; Liu, C.; Fang, J.; Wang, M.; Chen, J.; Zhang, J.; Mao, C.; Xu, S. Preparation and characterization of Fe3O4/CdTe magnetic/fluorescent nanocomposites and their applications in immuno-labeling and fluorescent imaging of cancer cells. Langmuir 2009, 26, 1278–1284. [Google Scholar] [CrossRef]
- Reddy, K.H.D.; Lee, S. Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv. Colloid Interface Sci. 2013, 201–202, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Şakir, M.E. Synthesis and characterization of activated carbon supported magnetic nanoparticles (Fe3O4/AC@SiO2@Sulfanilamide) and its application in removal of toluene and benzene. Colloids Surf. A 2021, 617, 126231. [Google Scholar]
- Tian, M.; Liao, F.; Ke, Q.; Guo, Y.; Guo, Y. Synergetic effect of titanium dioxide ultralong nanofibers and activated carbon fibers on adsorption and photodegradation of toluene. Chem. Eng. J. 2017, 328, 962–976. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Sik, O.Y.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hussain, N.; Borah, J.D. Kinetics and Adsorption Behavior of the Methyl Blue at the Graphene Oxide/Reduced Graphene Oxide Nanosheet-Water Interface: A Comparative Study. J. Chem. Eng. Data 2013, 58, 3477–3488. [Google Scholar] [CrossRef]
- Mine, H.; Zehra, B.; Melek, M.; Kadir, Y. Reduction of ochratoxin a level in red wine by bentonite, modified bentonites, and chitosan. J. Agric. Food Chem. 2008, 56, 2541–2545. [Google Scholar] [CrossRef]
- Annadurai, G.; Ling, L.; Lee, J.F. Adsorption of reactive dye from an aqueous solution by chitosan: Isotherm, kinetic and thermodynamic analysis. J. Hazard. Mater. 2008, 152, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Johari, N.; Gnanasundaram, A.; Appusamy, M. Thanabalan, Impact of surface modification on adsorptive removal of BTX onto activated carbon. J. Mol. Liq. 2019, 280, 238–251. [Google Scholar] [CrossRef]
- Ziegler, T. Approximate density functional theory as a practical tool in molecular energetics and dynamics. Chem. Rev. 1991, 91, 651–667. [Google Scholar] [CrossRef]
- Yue, T.; Guo, C.; Yuan, Y.; Wang, Z.; Luo, Y.; Wang, L. Adsorptive removal of patulin from apple juice using Ca-alginate-activated carbon beads. J. Food Sci. 2013, 78, T1629–T1635. [Google Scholar] [CrossRef] [PubMed]
- Djeribi, R.; Hamdaoui, O. Sorption of copper (II) from aqueous solution by cedar sawdust and crushed brick. Desalination 2008, 225, 95–112. [Google Scholar] [CrossRef]
- Altundag, H.; Dundar, M.; Dogancı, S.; Celik, M.; Tuzen, M. The use of a sequential extraction procedure for heavy metal analysis of house dusts by atomic absorption spectrometry. J. AOAC Int. 2013, 96, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Ofomaja, A. Kinetics and thermodynamics of lead ion sorption on palm kernel fibre from aqueous solution. Process Biochem. 2005, 40, 3455–3461. [Google Scholar] [CrossRef]
- Fernandes, A.N.; Almedia, C.A.P.; Debacher, N.A.; Sierra, M.D.S. Isotherm and thermodynamic data of adsorption of methylene blue from aqueous solution onto peat. J. Mol. Struct. 2010, 982, 62–65. [Google Scholar] [CrossRef]
- Seki, Y.; Yurdakoc, K. Adsorption of promethazine hydrochloride with KSF montmorillonite. Adsorption 2006, 12, 89–100. [Google Scholar] [CrossRef]
- Ai, L.; Li, M.; Li, M. Adsorption of Methylene Blue from Aqueous Solution with Activated Carbon/Cobalt Ferrite/Alginate Composite Beads: Kinetics, Isotherms, and Thermodynamics. J. Chem. Eng. Data 2011, 56, 3475–3483. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methyleneblue from aqueous so lution by graphene. Colloids Surf. B 2012, 90, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Marín, M.; Prete, D.V.; Garcia-Moruno, E.; Garcia-Moruno, C.; Fernandez-Gonzalez, C.; Macias-García, A.; Gomez-Serrano, V. The development of an activated carbon from cherry stones and its use in the removal of ochratoxin A from red wine. Food Control 2008, 20, 298–303. [Google Scholar] [CrossRef]
- Shi, M.; Gao, S.; Dong, R.; Li, F.; Qiao, D.; Liu, M.; Xue, F.; Wei, D.; Zhang, Z. Evaluation of different clarifiers for in vitro adsorption of ochratoxin A in wine. Food Ferment. Ind. 2023, 50, 135–141. [Google Scholar]
- Hufnagel, J.C.; Hofmann, T. Orosensory-directed identification of astringent mouthfeel and bitter-tasting compounds in red wine. J. Agric. Food Chem. 2008, 56, 1376–1386. [Google Scholar] [CrossRef]
- Phisut, N.; Nathathai, J.; Titada, Y.Y. Influence of clarifying agents on the quality of pasteurised palmyra palm sap (Borassus flabellifer Linn.). Int. J. Food Sci. Tech. 2014, 49, 1175–1183. [Google Scholar]
- Lee, S.; Lee, D.E.; Singh, D.; Lee, C.H. Metabolomics reveal optimal grain preprocessing (milling) toward rice koji fermentation. J. Agric. Food Chem. 2018, 66, 2694–2703. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, F.; Zhang, J.; Ma, Y.; Zhao, Q.; Gao, H. Study on Adsorption of Montmorillonite and Attapulgite to Mycotoxins. China Anim. Husb. Vet. Med. 2014, 41, 133–138. [Google Scholar]
| Kinetic Models | Fitting Equation | Kinetic Parameters | ||
|---|---|---|---|---|
| Pseudo-first order equation | Ln (177.86 − qt) = 5.181 − 3.57 × 10−2t | R12 0.5920 | K1(1/min) 3.57 × 10−2 | qe (ng/g) 177.86 |
| Pseudo-second order equation | t/qt = t/2259.16 + 3.929 × 10−4 | R22 0.9991 | k2 (g/ng/min) 4.832 × 10−4 | qe (ng/g) 2259.16 |
| Weber-Morris equation | qt = 27.77t0.5 + 2035.17 | R32 0.3305 | k3(ng/g/min0.5) 27.77 | C (ng/g) 2035.17 |
| Model | Temperature/°C | Fitting Equation | Parameters | ||
|---|---|---|---|---|---|
| KL | qm | R2 | |||
| Langmuir model | 25 | 2.7910 | 5018.07 | 0.9754 | |
| 35 | 2.5312 | 4484.30 | 0.9575 | ||
| 45 | 2.6557 | 3988.83 | 0.9970 | ||
| Kf | n | R2 | |||
| Freundlich model | 25 | lnqe = 3.8077lnce + 7.9156 | 2.7397 | 0.2626 | 0.9735 |
| 35 | lnqe = 3.8858lnce + 7.5510 | 1.9025 | 0.2573 | 0.9591 | |
| 45 | lnqe = 4.3133lnce + 8.1607 | 3.5007 | 0.2318 | 0.9552 | |
| KT | β | R2 | |||
| Temkin model | 25 | qe = 68.4555lnce + 112.9230 | 5.2049 | 36.2107 | 0.8415 |
| 35 | qe = 67.9942lnce + 104.1987 | 4.6297 | 37.6791 | 0.8681 | |
| 45 | qe = 72.5497lnce + 111.4258 | 4.6455 | 36.4591 | 0.7382 | |
| Concentration (µg/mL) | ΔH° (KJ/mol) | ΔG° (KJ/mol) | ΔS° (KJ/(mol*K)) | ||||
|---|---|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | ||
| 0.5 | −4.195 | −0.651 | −0.660 | −0.613 | −0.0118 | −0.0115 | −0.0113 |
| 1.0 | −3.981 | −0.0112 | −0.0107 | −0.0106 | |||
| 1.5 | −4.391 | −0.0125 | −0.0121 | −0.0119 | |||
| 2.0 | −3.419 | −0.0095 | −0.0090 | −0.0088 | |||
| 2.5 | −1.483 | −0.0028 | −0.0027 | −0.0027 | |||
| Sample | Initial Concentration (ng/mL) | Final Concentration (ng/mL) | Removal Rate (%) |
|---|---|---|---|
| 10 | 3.2 | 68.4 | |
| Red wine | 20 | 5.2 | 74.2 |
| 30 | 6.2 | 79.4 | |
| 10 | 5.4 | 46.0 | |
| White wine | 20 | 7.8 | 61.1 |
| 30 | 11.0 | 63.3 |
| Color Vale | Total Phenol | TSS | Transmittance | Total Flavonoid | Total Acid | ||
|---|---|---|---|---|---|---|---|
| Red wine | Before | 2.606 ± 0.0014 | 11.118 ± 0.0182 | 7.803 ± 0.0205 | 0.938 ± 0.0043 | 52.741 ± 0.5014 | 5.766 ± 0.5340 |
| After | 0.995 ± 0.0017 | 5.807 ± 0.0232 | 7.177 ± 0.0205 | 0.467 ± 0.0012 | 31.162 ± 0.8684 | 0.546 ± 0.1370 | |
| White wine | Before | 0.134 ± 0.0012 | 3.350 ± 0.0168 | 6.697 ± 0.0047 | 0.007 ± 0.0005 | 7.665 ± 0.3762 | 4.442 ± 0.0430 |
| Before | 0.245 ± 0.0026 | 0.975 ± 0.0019 | 6.683 ± 0.0624 | 0.143 ± 0.0026 | 3.493 ± 0.4978 | 1.352 ± 0.1673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, X.; Nan, M.; Bi, Y.; Xue, H.; Lyu, L.; Jiang, D.; Chen, H.; Luo, Q. Removal Capacity and Mechanism of Modified Chitosan for Ochratoxin A Based on Rapid Magnetic Separation Technology. Foods 2025, 14, 666. https://doi.org/10.3390/foods14040666
Xin X, Nan M, Bi Y, Xue H, Lyu L, Jiang D, Chen H, Luo Q. Removal Capacity and Mechanism of Modified Chitosan for Ochratoxin A Based on Rapid Magnetic Separation Technology. Foods. 2025; 14(4):666. https://doi.org/10.3390/foods14040666
Chicago/Turabian StyleXin, Xueyan, Mina Nan, Yang Bi, Huali Xue, Liang Lyu, Daiwei Jiang, Hongjuan Chen, and Qifang Luo. 2025. "Removal Capacity and Mechanism of Modified Chitosan for Ochratoxin A Based on Rapid Magnetic Separation Technology" Foods 14, no. 4: 666. https://doi.org/10.3390/foods14040666
APA StyleXin, X., Nan, M., Bi, Y., Xue, H., Lyu, L., Jiang, D., Chen, H., & Luo, Q. (2025). Removal Capacity and Mechanism of Modified Chitosan for Ochratoxin A Based on Rapid Magnetic Separation Technology. Foods, 14(4), 666. https://doi.org/10.3390/foods14040666





