Effects of Barley (Hordeum vulgare L.) on Antioxidant Activities of Maillard Reaction Products and Consumer Acceptance of Barley Nurungji, Traditional Korean Snack
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Barley Nurungji
2.3. MRP Solution Preparation
2.4. Physicochemical Properties
2.5. Antioxidant Component Analysis and Antioxidant Activity Assay
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Barley Nurungji
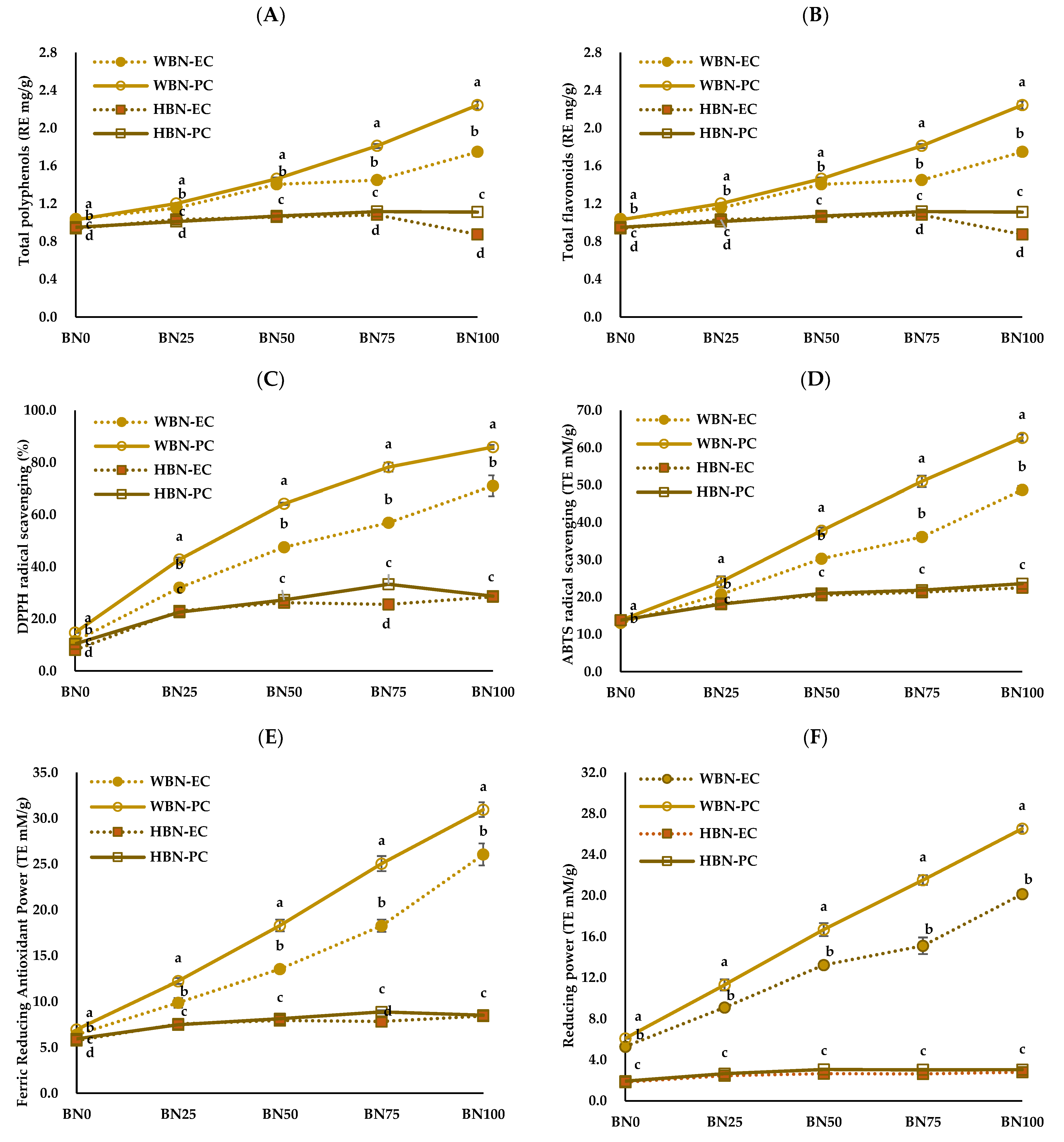
3.2. Antioxidant Contents and Antioxidant Activities of Barley Nurungji
3.3. Physicochemical Characteristics of the Model MRP Solution
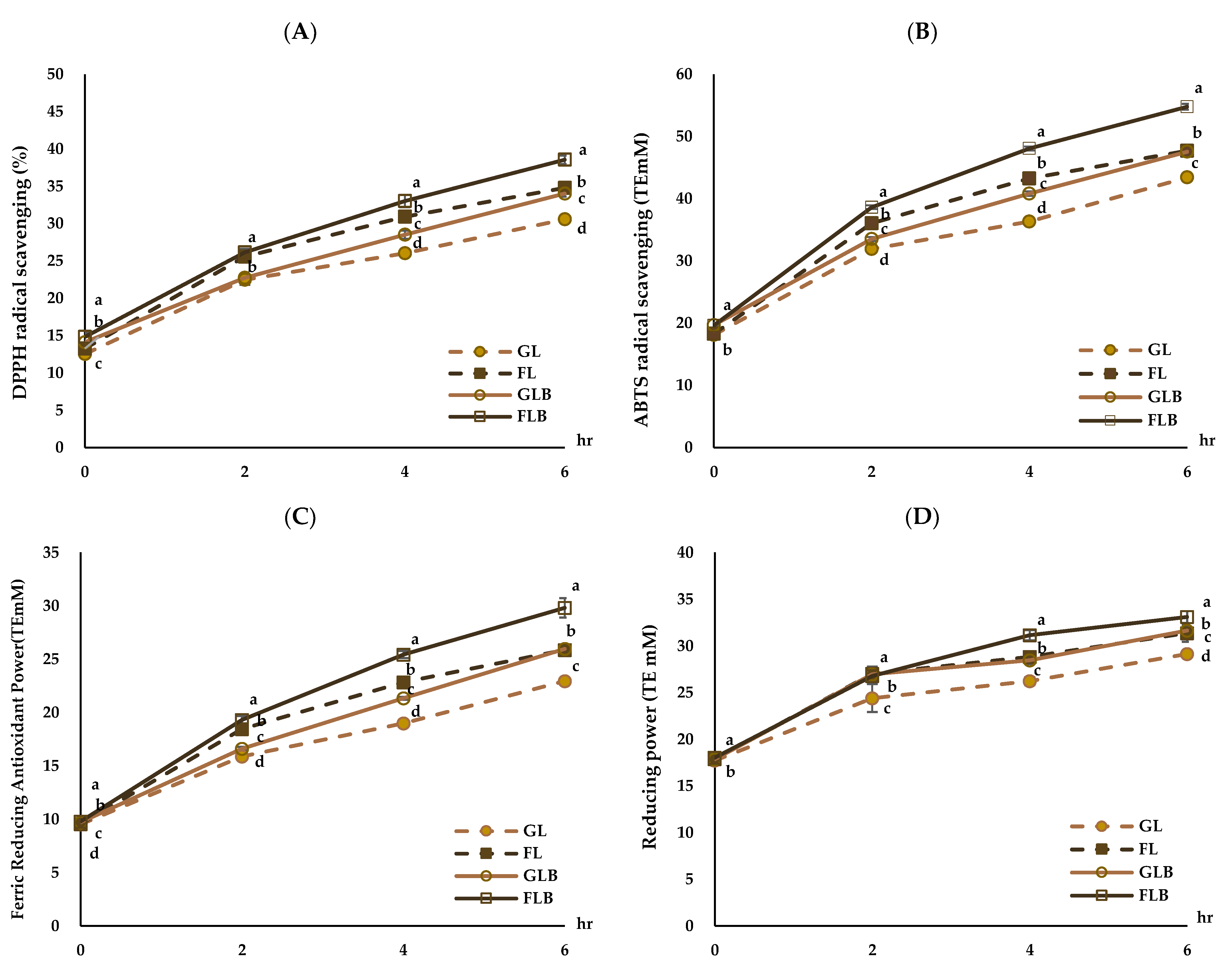
3.4. Antioxidant Activities of MRPs
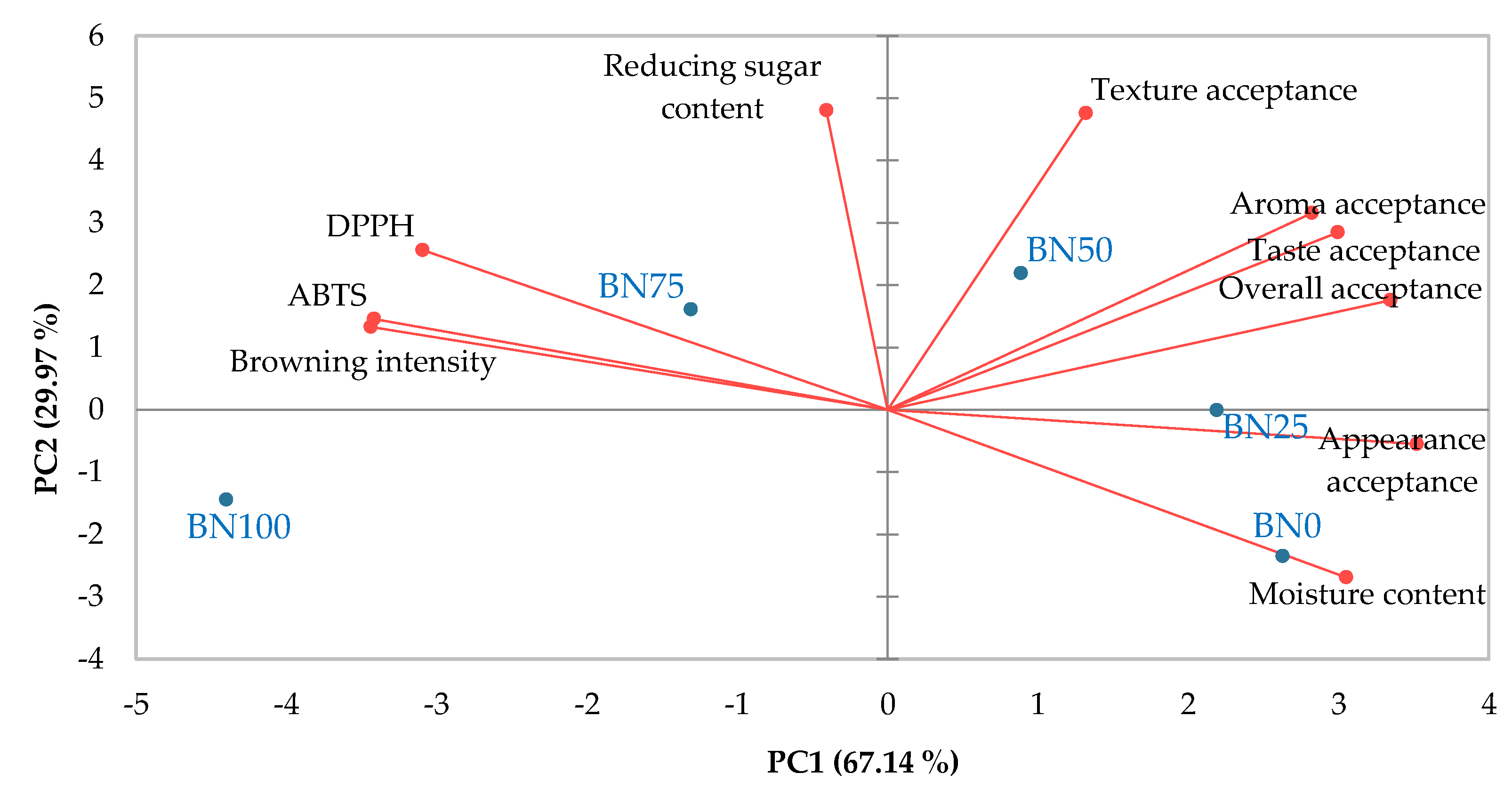
3.5. Consumer Acceptance of Barley Nurungji
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| ABTS+ | ABTS cation radical |
| DPPH | 2,2’-diphenyl-1-picryl-hydrazyl |
| DNS | 3,5-dinitrosalicylic acid |
| FL | fructose–lysine |
| FLB | fructose–lysine–β-glucan |
| FRAP | ferric-reducing antioxidant power |
| GL | glucose–lysine |
| GLB | glucose–lysine–β-glucan |
| MRP | Maillard reaction product |
| PCA | principal component analysis |
| L* | lightness |
| a* | redness |
| b* | yellowness |
| ΔE* | color differences |
| WBN-EC | waxy-barley nurungji cooked using an electric rice cooker |
| WBN-PC | waxy-barley nurungji cooked using an electric rice pressure cooker |
| HBN-EC | hulled-barley nurungji cooked using an electric rice cooker |
| HBN-PC | hulled-barley nurungji cooked using an electric rice pressure cooker |
| BN0 | control group |
| BN25 | 25% barley ratio |
| BN50 | 50% barley ratio |
| BN75 | 75% barley ratio |
| BN100 | 100% barley ratio |
References
- Carcea, M. Value of Wholegrain Rice in a Healthy Human Nutrition. Agriculture 2021, 11, 720. [Google Scholar] [CrossRef]
- Rice|USDA Foreign Agricultural Service. Available online: https://www.fas.usda.gov/data/production/commodity/0422110 (accessed on 25 January 2015).
- Korea Agro-Fisheries; Food Trade Corporation. [Market Report] 2020 Rice Processed Food|Processed Food Segment Market Status Report. 2025. Available online: https://www.atfis.or.kr/home/board/FB0027.do?act=read&bpoId=3659 (accessed on 25 January 2015).
- Kostat. Results of the 2023 Grain Consumption Survey. 2025. Available online: https://kostat.go.kr/board.es?mid=a10301080900&bid=230&act=view&list_no=429221 (accessed on 25 January 2015).
- Korea Agro-Fisheries; Food Trade Corporation. 2020 Processed Food Segment Market Status: Rice Processed Food. 2025. Available online: https://www.atfis.or.kr/home/board/FB0003.do?act=read&subSkinYn=N&bpoId=3650&bcaId=0&pageIndex=3 (accessed on 25 January 2015).
- Lee, S.W. Cookery Cultural History of Korea; Gyomoon Inc.: Paju, Republic of Korea, 1985; pp. 74–124. [Google Scholar]
- Yang, J.; Choi, I.S. The Physicochemical characteristics and antioxidant properties of commercial Nurungji products in Korea. Korean J. Food Cook. Sci. 2016, 32, 575–584. [Google Scholar] [CrossRef]
- Kim, M.K. Sensory profile of rice-based snack (Nuroongji) prepared from rice with different levels of milling degree. Foods 2020, 9, 685. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Lee, J.; Kim, M.K. Comparison of extraction methods for the flavor analysis of Nurungji. Korean J. Food Sci. Technol. 2024, 56, 126–129. [Google Scholar] [CrossRef]
- Suh, Y.K.; Park, Y.H.; Oh, Y.J. Cooking conditions for the production of instant Nurungji. J. Korean Soc. Nutr. Food Sci. 1996, 25, 58–62. Available online: https://www.dbpia.co.kr/journal/articleDetail?nodeId=NODE00639265 (accessed on 17 October 2024).
- Yong, J.E.; Kang, S.T. Changes in total polyphenol, flavonoid contents and antioxidant activity of Nurungji added with turmeric powder according to heating condition. Food Eng. Prog. 2022, 26, 228–233. [Google Scholar] [CrossRef]
- Jeong, A.L. Quality characteristics of pressurized Nurungji using green whole grain. Culin. Sci. Hosp. Res. 2018, 24, 29–37. [Google Scholar] [CrossRef]
- Yoo, J.S.; Baek, M.K.; Baek, S.H.; Park, H.S.; Cho, Y.C.; Kim, B.K.; Ha, K.Y. Comparison of characteristics of Nuroong-gi made from japonica rice cultivars. Food Eng. Prog. 2012, 16, 381–385. [Google Scholar] [CrossRef]
- Lee, H.S.; Kwon, K.H.; Kim, B.S.; Kim, J.H. Quality characteristics of instant Nuroong-gi to which Dioscorea japonica powder was added. Food Sci. Preserv. 2009, 16, 680–685. Available online: https://koreascience.kr/article/JAKO200908856869872.page (accessed on 26 September 2024).
- Park, Y.H.; Oh, Y.J. The physicochemical characteristics of instant Nuroong-gi. J. Korean Soc. Food Sci. Nutr. 1997, 26, 632–638. Available online: https://koreascience.kr/article/JAKO199711921349618.page (accessed on 18 July 2023).
- Cha, B.S. studies on processing conditions for Nooroong-gi powder by liquefaction and gellatinization of rice powder. Korean J. Korean Soc. Food Cook. Sci. 1999, 15, 469–474. Available online: https://www.dbpia.co.kr/journal/articleDetail?nodeId=NODE09605924 (accessed on 25 June 2024).
- Lee, H.S.; Kwon, K.H.; Kim, J.H.; Cha, H.S. Quality characteristics of instant Nuroong-gi prepared using a microwave. Food Sci. Preserv. 2009, 16, 669–674. Available online: https://koreascience.kr/article/JAKO200908856869869.page (accessed on 21 February 2023).
- Jeong, Y.R.; Choi, I.S. Comparison of quality characteristics between cooked rice and Nurungji prepared by different rice cooker type. Korean J. Hum. Ecol. 2024, 33, 127–137. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→3,1→4)-β-d-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Geng, L.; Li, M.; Zhang, G.; Ye, L. Barley: A potential cereal for producing healthy and functional foods. Food Qual. Saf. 2022, 6, 1–13. [Google Scholar] [CrossRef]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Drożdżyńska, A.; Gujska, E. Effect of boiling in water of barley and buckwheat groats on the antioxidant properties and dietary fiber composition. Plant Foods Hum. Nutr. 2014, 69, 276–282. [Google Scholar] [CrossRef]
- Holtekjølen, A.K.; Bævre, A.B.; Rødbotten, M.; Berg, H.; Knutsen, S.H. Antioxidant properties and sensory profiles of breads containing barley flour. Food Chem. 2008, 110, 414–421. [Google Scholar] [CrossRef]
- Kang, S.J.; Koo, N.G.; Park, M.J.; Nam, J.Y.; Lee, Y.T. Physicochemical and antioxidative properties of pigmented barley cultivars processed by germination and roasting. J. Korean Soc. Food Sci. Nutr. 2023, 52, 186–192. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.B. Extraction of nature pigment with antioxidant properties from sprout barley—Optimization using CCD-RSM. Appl. Chem. Eng. 2024, 35, 222–229. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Van Boekel, A.A.J.S. A kinetic model for the glucose/glycine Maillard reaction pathways. Food Chem. 2005, 90, 257–269. [Google Scholar] [CrossRef]
- The Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC: Washington DC, USA, 2020; pp. 33–36. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Canabady-Rochelle, L.L.S.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef]
- Biduski, B.; da Silva, W.M.F.; Colussi, R.; El Halal, S.L.D.M.; Lim, L.T.; Dias, Á.R.G.; da Rosa Zavareze, E. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Lukinac, J.; Jukić, M. Barley in the production of cereal-based products. Plants 2022, 11, 3519. [Google Scholar] [CrossRef]
- Girhammar, U.; Nair, B.M. Certain physical properties of water soluble non-starch polysaccharides from wheat, rye, triticale, barley and oats. Food Hydrocoll. 1992, 6, 329–343. [Google Scholar] [CrossRef]
- Boyd, L.; Storsley, J.; Ames, N. Effect of heat treatments on starch pasting, particle size, and color of whole-grain barley. Cereal Chem. 2017, 94, 325–332. [Google Scholar] [CrossRef]
- Kohyama, N.; Fujita, M.; Ono, H.; Ohnishi-Kameyama, M.; Matsunaka, H.; Takayama, T.; Murata, M. Effects of phenolic compounds on the browning of cooked barley. J. Agric. Food Chem. 2009, 57, 6402–6407. [Google Scholar] [CrossRef] [PubMed]
- Holtekjølen, A.K.; Knutsen, S.H. Chapter 32—Antioxidant activity and phenolics in breads with added barley flour. In Flour and Breads and Their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 355–363. [Google Scholar] [CrossRef]
- Kim, M.J.; Hyun, J.N.; Kim, J.A.; Park, J.C.; Kim, M.Y.; Kim, J.G.; Lee, S.J.; Chun, S.C.; Chung, I.M. Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J. Agric. Food Chem. 2007, 55, 4802–4809. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yoon, W.B.; Park, S.J.; Park, D.S.; Ahn, J. Effects of pressure level and processing time on the extraction of total phenols, flavonoids, and phenolic acids from Deodeok (Codonopsis lanceolata). Food Sci. Biotechnol. 2011, 20, 499–505. [Google Scholar] [CrossRef]
- Verardo, V.; Glicerina, V.; Cocci, E.; Frenich, A.G.; Romani, S.; Caboni, M.F. Determination of free and bound phenolic compounds and their antioxidant activity in buckwheat bread loaf, crust and crumb. LWT Food Sci. Technol. 2018, 87, 217–224. [Google Scholar] [CrossRef]
- Bai, Y.P.; Zhou, H.M.; Zhu, K.R.; Li, Q. Effect of thermal treatment on the physicochemical, ultrastructural and nutritional characteristics of whole grain highland barley. Food Chem. 2021, 346, 128657. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef]
- Luo, M.; Hou, F.; Dong, L.; Huang, F.; Zhang, R.; Su, D. Comparison of microwave and high-pressure processing on bound phenolic composition and antioxidant activities of sorghum hull. Int. J. Food Sci. Technol. 2020, 55, 3190–3202. [Google Scholar] [CrossRef]
- Kruma, Z.; Tomosone, L.; Galoburda, R.; Straumite, E.; Kronberga, A.; Åssveen, M. Total phenols and antioxidant capacity of hull-less barley and hull-less oats. Agron. Res. 2016, 14, 1361–1371. [Google Scholar]
- Kim, J.S.; Lee, Y.S. Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem. 2008, 108, 582–592. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Storsley, J.; Labossiere, D.; MacGregor, A.W.; Rossnagel, B.G. Variation in total and soluble β-glucan content in hulless barley: Effects of thermal, physical, and enzymic treatments. J. Agric. Food Chem. 2000, 48, 982–989. [Google Scholar] [CrossRef]
- Speck, J.C. The Lobry De Bruyn-Alberda Van Ekenstein Transformation. In Advances in Carbohydrate Chemistry; Wolfrom, M.L., Ed.; Academic Press: Cambridge, MA, USA, 1958; Volume 13, pp. 63–103. [Google Scholar] [CrossRef]
- Cui, H.P.; Yu, J.H.; Zhai, Y.; Feng, L.H.; Chen, P.S.; Hayat, K.; Xu, Y.; Zhang, X.M.; Ho, C.T. Formation and fate of Amadori rearrangement products in Maillard reaction. Trends Food Sci. Technol. 2021, 115, 391–408. [Google Scholar] [CrossRef]
- Abrantes, T.; Moura-Nunes, N.; Perrone, D. Gallic acid mitigates 5-Hydroxymethylfurfural formation while enhancing or preserving browning and antioxidant activity development in glucose/arginine and sucrose/arginine Maillard model systems. Molecules 2022, 27, 848. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Hu, C. Biological and chemical assessment of antioxidant activity of sugar-lysine model Maillard reaction products. Ann. N. Y. Acad. Sci. 2005, 1043, 501–512. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Huang, Z.; Elfalleh, W.; Qin, L.; Yu, D. Structure and flavor characteristics of Maillard reaction products derived from soybean meal hydrolysates-reducing sugars. LWT 2023, 185, 115097. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Biological activities of Maillard reaction products (MRPs) in a sugar–amino acid model system. Food Chem. 2011, 126, 221–227. [Google Scholar] [CrossRef]
- Kwak, E.J.; Lim, S.I. The effect of sugar, amino acid, metal ion, and NaCl on model Maillard reaction under pH control. Amino Acids 2004, 27, 85–90. [Google Scholar] [CrossRef]
- Liu, X.; Xia, B.; Hu, L.T.; Ni, Z.J.; Thakur, K.; Wei, Z.J. Maillard conjugates and their potential in food and nutritional industries: A review. Food Front. 2020, 1, 382–397. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.S. Antioxidant Activity of Melanoidins from Different Sugar/Amino Acid Model Systems: Influence of the Enantiomer Type. Food Sci. Technol. Int. 2009, 15, 291–297. [Google Scholar] [CrossRef]
- Chung, S.Y.; Han, S.H.; Lee, S.W.; Rhee, C. Effect of Maillard reaction products prepared from glucose–glycine model systems on starch digestibility. Starch Stärke 2012, 64, 657–664. [Google Scholar] [CrossRef]

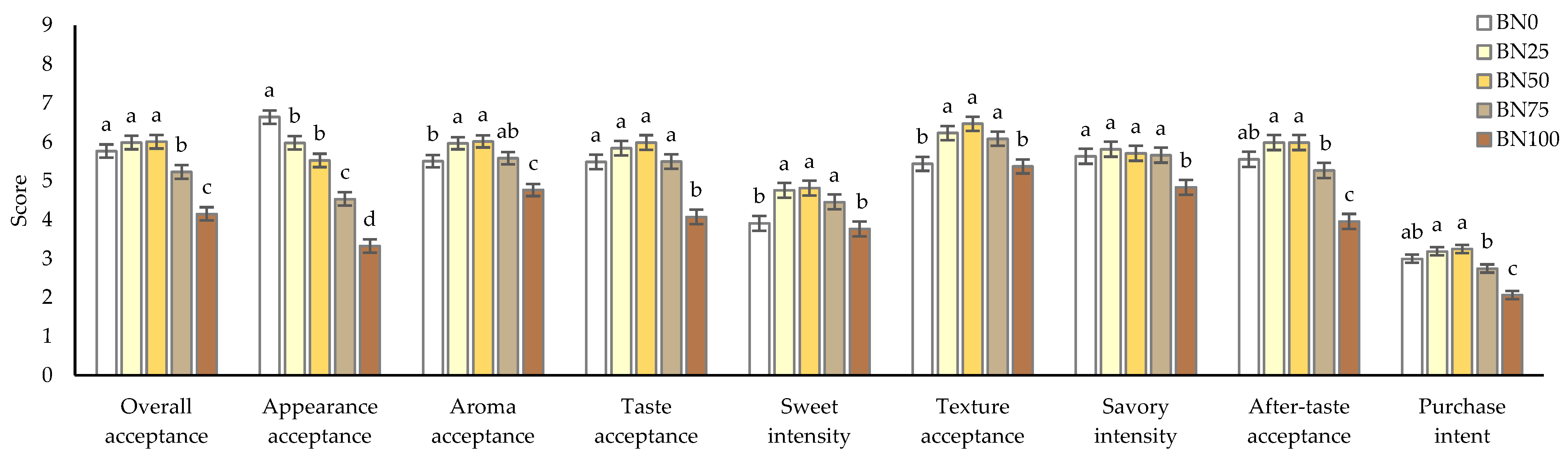
| Barley Ratio (%) | HBN-EC | HBN-PC | WBN-EC | WBN-PC | ||
|---|---|---|---|---|---|---|
| Moisture content (%) | BN0 | 4.16 ± 0.03 aC | 4.71 ± 0.04 aD | 6.96 ± 0.02 aA | 6.69 ± 0.03 aB | |
| BN25 | 3.89 ± 0.05 bD | 4.49 ± 0.08 bC | 6.02 ± 0.04 bA | 5.69 ± 0.03 bB | ||
| BN50 | 3.85 ± 0.04 bD | 4.36 ± 0.06 cB | 4.99 ± 0.04 cA | 4.20 ± 0.04 cC | ||
| BN75 | 4.11 ± 0.04 aB | 3.47 ± 0.03 dD | 4.31 ± 0.04 dA | 3.82 ± 0.02 dC | ||
| BN100 | 3.38 ± 0.21 cB | 3.41 ± 0.04 dB | 4.14 ± 0.04 eA | 3.44 ± 0.03 eB | ||
| Crude ash content (%) | BN0 | 0.25 ± 0.04 dA | 0.24 ± 0.02 dA | 0.16 ± 0.01 eB | 0.17 ± 0.02 eB | |
| BN25 | 0.39 ± 0.04 cA | 0.28 ± 0.05 dB | 0.32 ± 0.04 dB | 0.33 ± 0.03 dAB | ||
| BN50 | 0.53 ± 0.03 bA | 0.40 ± 0.01 cB | 0.54 ± 0.03 cA | 0.56 ± 0.01 cA | ||
| BN75 | 0.55 ± 0.04 bC | 0.55 ± 0.02 bC | 0.68 ± 0.02 bB | 0.85 ± 0.02 bA | ||
| BN100 | 0.77 ± 0.02 aC | 0.66 ± 0.04 aD | 0.88 ± 0.02 aB | 1.07 ± 0.01 aA | ||
| Total soluble solids (°Brix) | BN0 | 0.48 ± 0.05 cC | 0.68 ± 0.03 eA | 0.57 ± 0.05 dB | 0.60 ± 0.05 dB | |
| BN25 | 0.86 ± 0.05 bC | 0.88 ± 0.05 dC | 1.28 ± 0.05 cB | 1.60 ± 0.04 cA | ||
| BN50 | 1.03 ± 0.060 aC | 1.02 ± 0.03 cC | 1.88 ± 0.05 bB | 2.20 ± 0.06 bA | ||
| BN75 | 1.03 ± 0.04 aD | 1.22 ± 0.02 aC | 1.85 ± 0.10 bB | 2.20 ± 0.05 bA | ||
| BN100 | 1.03 ± 0.05 aC | 1.13 ± 0.01 bB | 2.28 ± 0.05 aA | 2.30 ± 0.09 aA | ||
| Reducing sugar (mg/g) | BN0 | 32.62 ± 0.17 bB | 32.41 ± 0.24 cB | 33.07 ± 0.05 eA | 32.89 ± 0.00 dA | |
| BN25 | 33.14 ± 0.24 aC | 32.72 ± 0.16 cC | 58.14 ± 0.12 dB | 76.55 ± 0.08 cA | ||
| BN50 | 33.22 ± 0.09 abD | 37.19 ± 0.28 bC | 87.30 ± 0.46 cB | 90.76 ± 0.16 bA | ||
| BN75 | 33.48 ± 0.28 aD | 38.38 ± 0.44 aC | 89.56 ± 0.25 bB | 90.76 ± 0.72 bA | ||
| BN100 | 33.07 ± 0.12 bC | 32.74 ± 0.20 cC | 91.51 ± 0.08 aB | 95.29 ± 0.05 aA | ||
| pH | BN0 | 5.30 ± 0.04 bD | 5.56 ± 0.04 aB | 5.67 ± 0.02 aA | 5.49 ± 0.02 aC | |
| BN25 | 5.32 ± 0.03 bB | 5.31 ± 0.01 cBC | 5.40 ± 0.02 bA | 5.29 ± 0.01 bC | ||
| BN50 | 5.30 ± 0.01 bC | 5.34 ± 0.01 bcB | 5.36 ± 0.01 cA | 5.18 ± 0.00 cD | ||
| BN75 | 5.37 ± 0.01 aA | 5.34 ± 0.01 bA | 5.19 ± 0.02 dB | 5.06 ± 0.01 dC | ||
| BN100 | 5.39 ± 0.01 aA | 5.36 ± 0.01 bB | 5.16 ± 0.02 eC | 5.07 ± 0.01 dD | ||
| Color | L* value | BN0 | 86.68 ± 0.15 aA | 86.90 ± 0.24 aA | 83.03 ± 0.24 aB | 82.90 ± 0.29 aB |
| BN25 | 76.73 ± 0.46 bB | 77.43 ± 0.15 bA | 74.35 ± 0.24 bC | 72.93 ± 0.43 bD | ||
| BN50 | 74.68 ± 0.59 dA | 75.20 ± 0.28 cA | 71.08 ± 1.28 cB | 66.10 ± 0.96 cC | ||
| BN75 | 75.70 ± 0.70 cA | 73.35 ± 0.24 dB | 67.33 ± 0.46 dC | 61.10 ± 0.36 dD | ||
| BN100 | 73.03 ± 0.62 eB | 75.03 ± 0.49 cA | 63.88 ± 0.42 eC | 59.73 ± 0.79 eD | ||
| a* value | BN0 | 4.73 ± 0.13 dB | 4.73 ± 0.13 dB | 5.95 ± 0.10 cA | 6.08 ± 0.13 dA | |
| BN25 | 7.60 ± 0.22 abA | 7.08 ± 0.10 bcB | 7.13 ± 0.22 bB | 6.73 ± 0.17 cC | ||
| BN50 | 7.75 ± 0.19 aA | 7.18 ± 0.21 bB | 7.28 ± 0.36 bB | 7.98 ± 0.21 aA | ||
| BN75 | 6.95 ± 0.39 cC | 7.50 ± 0.16 aD | 7.90 ± 0.24 aA | 7.95 ± 0.10 abA | ||
| BN100 | 7.35 ± 0.30 bC | 6.87 ± 0.06 cD | 8.03 ± 0.05 aA | 7.73 ± 0.13 bB | ||
| b* value | BN0 | 14.95 ± 0.45 aB | 15.53 ± 0.29 cA | 14.90 ± 0.14 cB | 14.85 ± 0.17 bB | |
| BN25 | 17.40 ± 0.39 aA | 16.43 ± 0.36 bB | 15.38 ± 0.29 abC | 14.15 ± 0.24 cD | ||
| BN50 | 17.43 ± 0.33 aA | 16.43 ± 0.64 bA | 15.35 ± 0.13 bA | 15.28 ± 0.28 aA | ||
| BN75 | 17.13 ± 0.62 aA | 17.28 ± 0.43 aA | 15.68 ± 0.32 aB | 14.23 ± 0.15 cC | ||
| BN100 | 17.28 ± 0.28 aA | 17.33 ± 0.24 aA | 15.50 ± 0.08 abB | 14.15 ± 0.41 cC | ||
| ΔE* | BN0 | 17.07 ± 0.40 cB | 17.29 ± 0.37 eB | 20.13 ± 0.19 eA | 20.24 ± 0.35 eA | |
| BN25 | 26.94 ± 0.61 bC | 25.73 ± 0.17 dD | 27.88 ± 0.37 dB | 28.55 ± 0.46 dA | ||
| BN50 | 27.87 ± 0.57 bC | 27.62 ± 0.29 cC | 30.79 ± 1.27 cB | 35.44 ± 0.88 cA | ||
| BN75 | 27.46 ± 0.98 bD | 29.66 ± 0.32 aC | 34.43 ± 0.54 bB | 39.77 ± 0.34 bA | ||
| BN100 | 29.89 ± 0.73 aC | 28.09 ± 0.36 bD | 37.56 ± 0.37 aB | 41.00 ± 0.63 aA | ||
| Browning intensity | BN0 | 0.11 ± 0.01 dB | 0.14 ± 0.01 dA | 0.10 ± 0.00 eC | 0.10 ± 0.00 eC | |
| BN25 | 0.15 ± 0.03 cC | 0.15 ± 0.02 bD | 0.23 ± 0.01 dB | 0.35 ± 0.00 dA | ||
| BN50 | 0.17 ± 0.02 bC | 0.15 ± 0.01 cD | 0.29 ± 0.01 cB | 0.69 ± 0.01 cA | ||
| BN75 | 0.15 ± 0.01 cD | 0.19 ± 0.00 aC | 0.36 ± 0.00 bB | 0.96 ± 0.01 bA | ||
| BN100 | 0.19 ± 0.03 aC | 0.19 ± 0.02 aC | 0.76 ± 0.02 aB | 1.31 ± 0.00 aA | ||
| Heating Time (h) | GL | FL | GLB | FLB | ||
|---|---|---|---|---|---|---|
| Total soluble solids (°Brix) | 0 | 1.10 ± 0.03 aA | 1.10 ± 0.03 aA | 1.10 ± 0.03 cA | 1.10 ± 0.03 cA | |
| 2 | 1.10 ± 0.02 aB | 1.10 ± 0.02 aB | 1.20 ± 0.02 bA | 1.20 ± 0.02 bA | ||
| 4 | 1.10 ± 0.03 aB | 1.10 ± 0.03 aB | 1.20 ± 0.03 bA | 1.20 ± 0.03 bA | ||
| 6 | 1.10 ± 0.03 aB | 1.10 ± 0.03 aB | 1.30 ± 0.03 aA | 1.30 ± 0.03 aA | ||
| Reducing sugar (mg/g) | 0 | 44.55 ± 0.65 aD | 47.54 ± 0.28 aC | 50.06 ± 1.01 aB | 53.67 ± 1.29 aA | |
| 2 | 38.07 ± 0.83 bB | 36.36 ± 0.74 bC | 43.91 ± 1.95 bA | 42.17 ± 2.03 bA | ||
| 4 | 33.41 ± 0.65 cB | 30.26 ± 0.61 cC | 37.99 ± 0.73 cA | 37.16 ± 1.09 cA | ||
| 6 | 29.78 ± 0.79 dC | 28.69 ± 0.77 dD | 36.93 ± 1.71 cA | 34.43 ± 1.72 dB | ||
| pH | 0 | 10.13 ± 0.02 aB | 10.12 ± 0.03 aB | 10.26 ± 0.02 aA | 10.28 ± 0.02 aA | |
| 2 | 9.22 ± 0.07 bC | 9.22 ± 0.02 bBC | 9.43 ± 0.03 bA | 9.28 ± 0.03 bB | ||
| 4 | 8.70 ± 0.05 cB | 8.68 ± 0.01 cB | 8.90 ± 0.01 cA | 8.73 ± 0.07 cB | ||
| 6 | 8.31 ± 0.02 dB | 8.15 ± 0.02 dD | 8.52 ± 0.01 dA | 8.25 ± 0.01 dC | ||
| Color | L* value | 0 | 58.23 ± 0.13 aA | 58.20 ± 0.18 aA | 57.35 ± 0.37 aB | 57.55 ± 0.26 aB |
| 2 | 53.53 ± 0.33 bA | 51.63 ± 0.46 bC | 52.28 ± 0.32 bB | 50.30 ± 0.47 bD | ||
| 4 | 49.98 ± 0.30 cA | 47.63 ± 0.46 cC | 48.50 ± 0.63 cB | 47.65 ± 0.99 cBC | ||
| 6 | 47.88 ± 0.64 dA | 46.93 ± 0.13 dB | 45.33 ± 0.78 dC | 43.88 ± 0.57 dD | ||
| a* value | 0 | −0.60 ± 0.03 dA | −0.60 ± 0.03 dA | −0.60 ± 0.03 dA | −0.60 ± 0.03 dA | |
| 2 | 0.90 ± 0.18 cD | 2.23 ± 0.15 cB | 1.63 ± 0.26 cC | 3.15 ± 0.06 cA | ||
| 4 | 3.48 ± 0.05 bD | 5.00 ± 0.00 bB | 4.18 ± 0.35 bC | 5.45 ± 0.40 bA | ||
| 6 | 5.38 ± 0.33 aC | 6.55 ± 0.13 aB | 6.85 ± 0.30 aB | 7.95 ± 0.19 aA | ||
| b* value | 0 | −2.50 ± 0.08 dC | −1.68 ± 0.26 dA | −1.65 ± 0.33 dA | −2.10 ± 0.20 dB | |
| 2 | 12.80 ± 0.47 cC | 15.00 ± 0.35 cB | 13.23 ± 0.78 cC | 16.53 ± 0.13 cA | ||
| 4 | 16.85 ± 0.40 bC | 17.58 ± 0.15 bB | 16.75 ± 0.31 bC | 17.93 ± 0.05 aA | ||
| 6 | 18.55 ± 0.13 aB | 18.85 ± 0.13 aA | 18.13 ± 0.05 aC | 17.53 ± 0.38 bD | ||
| ΔE* | 0 | 32.59 ± 0.13 dB | 32.69 ± 0.21 dB | 33.54 ± 0.40 dA | 33.30 ± 0.28 dA | |
| 2 | 41.32 ± 0.49 cD | 44.06 ± 0.27 cB | 42.6 6 ± 0.64 cC | 45.99 ± 0.44 cA | ||
| 4 | 46.46 ± 0.19 bC | 49.00 ± 0.35 bA | 47.77 ± 0.73 bB | 49.20 ± 0.92 bA | ||
| 6 | 49.29 ± 0.61 aD | 50.40 ± 0.16 aC | 51.51 ± 0.69 aB | 52.68 ± 0.39 aA | ||
| Browning intensity | 0 | 0.00 ± 0.00 dC | 0.00 ± 0.00 dC | 0.01 ± 0.00 dB | 0.01 ± 0.00 dA | |
| 2 | 0.20 ± 0.01 cD | 0.27 ± 0.00 cB | 0.23 ± 0.02 cC | 0.31 ± 0.02 cA | ||
| 4 | 0.34 ± 0.01 bC | 0.42 ± 0.01 bB | 0.41 ± 0.03 bB | 0.46 ± 0.03 bA | ||
| 6 | 0.48 ± 0.02 aD | 0.52 ± 0.01 aC | 0.57 ± 0.03 aB | 0.67 ± 0.02 aA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Choi, I.S. Effects of Barley (Hordeum vulgare L.) on Antioxidant Activities of Maillard Reaction Products and Consumer Acceptance of Barley Nurungji, Traditional Korean Snack. Foods 2025, 14, 655. https://doi.org/10.3390/foods14040655
Jeong Y, Choi IS. Effects of Barley (Hordeum vulgare L.) on Antioxidant Activities of Maillard Reaction Products and Consumer Acceptance of Barley Nurungji, Traditional Korean Snack. Foods. 2025; 14(4):655. https://doi.org/10.3390/foods14040655
Chicago/Turabian StyleJeong, Yerim, and Il Sook Choi. 2025. "Effects of Barley (Hordeum vulgare L.) on Antioxidant Activities of Maillard Reaction Products and Consumer Acceptance of Barley Nurungji, Traditional Korean Snack" Foods 14, no. 4: 655. https://doi.org/10.3390/foods14040655
APA StyleJeong, Y., & Choi, I. S. (2025). Effects of Barley (Hordeum vulgare L.) on Antioxidant Activities of Maillard Reaction Products and Consumer Acceptance of Barley Nurungji, Traditional Korean Snack. Foods, 14(4), 655. https://doi.org/10.3390/foods14040655






