Establishment and Application of Duplex Recombinase-Aided Amplification Combined with Lateral Flow Dipsticks for Rapid and Simultaneous Visual Detection of Klebsiella pneumoniae and Staphylococcus aureus in Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. DNA Extraction
2.3. Design of RAA Primers
2.4. The Duplex RAA–LFD Assay
2.5. Optimization of the Duplex RAA–LFD Reaction Conditions
2.6. Specificity of the Duplex RAA–LFD Assay
2.7. Sensitivity of the Duplex RAA–LFD Assay
2.8. Development of Selective Co-Growing Medium for S. aureus and K. pneumoniae
2.9. Analysis of the Growth Effect of S. aureus and K. pneumoniae in the SKII Medium
2.10. Simulated Sample Detection
2.11. PCR Assay
2.12. Detection of Actual Samples
2.13. Statistical Analysis
3. Results
3.1. Optimization of Primer Concentrations
3.2. Optimization of the Duplex RAA–LFD Detection Reaction Condition
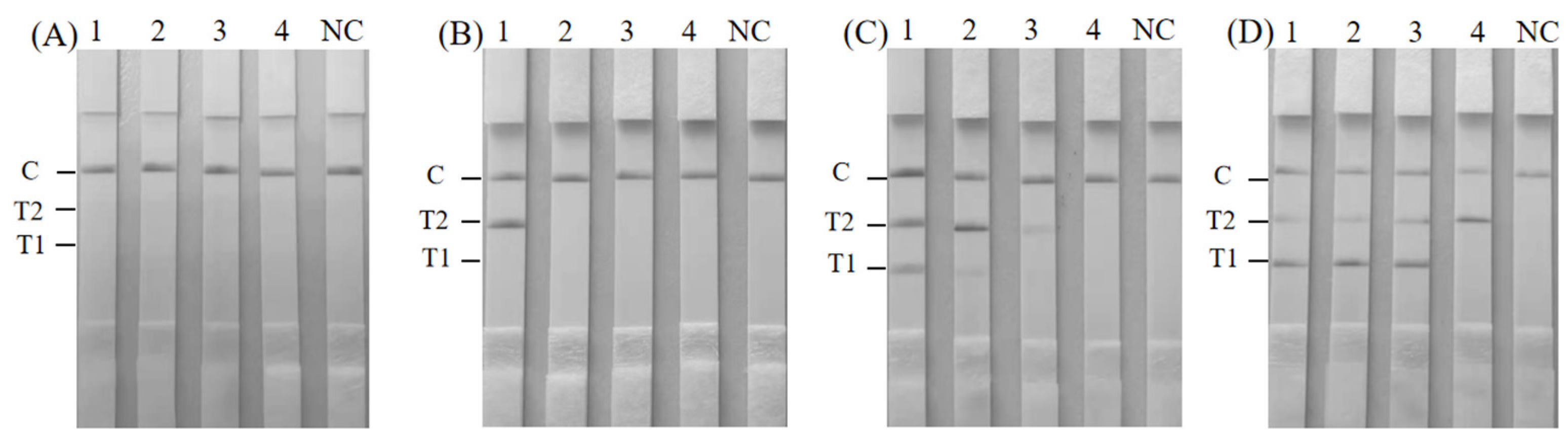
3.3. Specificity of the Duplex RAA–LFD Detection
3.4. Sensitivity of Duplex RAA–LFD Detection
3.5. Screening of Additives and Ratios in Co-Increasing Culture Medium
3.6. The Growth Effect of S. aureus and K. pneumoniae in the SKII Medium
3.7. Assay Performance in Artificially Contaminated Milk Samples
3.8. Detection in Actual Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Titouche, Y.; Akkou, M.; Djaoui, Y.; Mechoub, D.; Fatihi, A.; Campaña-Burguet, A.; Bouchez, P.; Bouhier, L.; Houali, K.; Torres, C.; et al. Nasal carriage of Staphylococcus aureus in healthy dairy cows in Algeria: Antibiotic resistance, enterotoxin genes and biofilm formation. BMC Vet. Res. 2024, 20, 247. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N.; Lozano, C.; Ruiz-Ripa, L.; Fernández-Fernández, R.; Zarazaga, M.; Torres, C. Ecology and Genetic Lineages of Nasal Staphylococcus aureus and MRSA Carriage in Healthy Persons with or without Animal-Related Occupational Risks of Colonization: A Review of Global Reports. PLoS Pathog. 2021, 10, 1000. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.Y.; Liu, S.Y.; Wang, J.F.; Nan, H.Z.; Liu, L.B.; Sun, X.X.; Li, D.Y.; Liu, M.; Wang, J.C.; Tan, K. Rapid Detection of Staphylococcus aureus in food using a Recombinase Polymerase Amplification-based assay. Food Anal. Methods. 2018, 11, 2847–2856. [Google Scholar] [CrossRef]
- Filipello, V.; Bonometti, E.; Campagnani, M.; Bertoletti, I.; Romano, A.; Zuccon, F.; Campanella, C.; Losio, M.N.; Finazzi, G. Investigation and Follow-Up of a Staphylococcal Food Poisoning Outbreak Linked to the Consumption of Traditional Hand-Crafted Alm Cheese. PLoS Pathog. 2020, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Macori, G.; Bellio, A.; Acutis, P.L.; Gallina, S.; Decastelli, L. Short communication: Characterization of Staphylococcus aureus isolated along the raw milk cheese production process in artisan dairies in Italy. J. Dairy. Sci. 2018, 101, 2915–2920. [Google Scholar] [CrossRef]
- Simeão do Carmo, L.; Souza Dias, R.; Roberto Linardi, V. Food poisoning due to enterotoxigenic strains of Staphylococcus present in Minas cheese and raw milk in Brazil. Food Microbiol. 2002, 19, 9–14. [Google Scholar] [CrossRef]
- Wan, Y.L.; Yang, L.; Li, Q.H.; Wang, X.W.; Zhou, T.; Chen, D.S.; Li, L.; Wang, Y.R.; Wang, X. Stability and emetic activity of enterotoxin like X (SElX) with high carrier rate of food poisoning Staphylococcus aureus. Int. J. Food Microbiol. 2023, 404, 110352. [Google Scholar] [CrossRef]
- Liang, D.; Arnold, L.M.; Stowe, C.J.; Harmon, R.J.; Bewley, J.M. Estimating US dairy clinical disease costs with a stochastic simulation model. J. Dairy. Sci. 2017, 100, 1472–1486. [Google Scholar] [CrossRef]
- Rollin, E.; Dhuyvetter, K.C.; Overton, M.W. The cost of clinical mastitis in the first 30 days of lactation: An economic modeling tool. Prev. Vet. Med. 2015, 122, 257–264. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wick, R.R.; Watts, S.C.; Cerdeira, L.T.; Wyres, K.L.; Hol, K.E. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat. Commun. 2021, 12, 4188. [Google Scholar] [CrossRef]
- Borer, A.; Saidel-Odes, L.; Riesenberg, K.; Eskira, S.; Peled, N.; Nativ, R.; Schlaeffer, F.; Sherf, M. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect. Control Hosp. Epidemiol. 2009, 30, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Ahmad, F.J.; Kar, S. Recent advances in loop-mediated isothermal amplification (LAMP) for rapid and efficient detection of pathogens. Curr. Res. Microb. Sci. 2022, 3, 100120. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.X.; Qiu, F.Z.; Shen, L.P.; Yan, T.F.; Zhao, M.C.; Qi, J.J.; Chen, C.; Zhao, L.; Wang, L.; Feng, Z.S.; et al. A rapid and sensitive recombinase aided amplification assay to detect hepatitis B virus without DNA extraction. BMC Infect. Dis. 2019, 19, 229. [Google Scholar] [CrossRef]
- Xue, G.H.; Li, S.L.; Zhao, H.Q.; Yan, C.; Feng, Y.L.; Cui, J.H.; Jiang, T.T.; Yuan, J. Use of a rapid recombinase-aided amplification assay for Mycoplasma pneumoniae detection. BMC Infect. Dis. 2020, 20, 79. [Google Scholar] [CrossRef]
- Zhang, S.S.; Duan, M.Y.; Li, S.; Hou, J.; Qin, T.; Teng, Z.W.; Hu, J.H.; Zhang, H.H.; Xia, X.J. Current status of recombinase polymerase amplification technologies for the detection of pathogenic microorganisms. Diagn. Microbiol. Infect. Dis. 2024, 108, 116097. [Google Scholar] [CrossRef]
- Bei, L.; Cheng, H.R.; Yan, Q.F.; Huang, Z.J.; Shen, G.F.; Zhang, Z.F.; Li, Y.N.; Deng, Z.X.; Lin, M. Recombinase-Aid Amplification:a novel technology of in vitro rapid nucleic acid amplification. Sci. Sin. 2010, 40, 983–988. [Google Scholar]
- Cordray, M.S.; Richards-Kortum, R.R. A paper and plastic device for the combined isothermal amplification and lateral flow detection of Plasmodium DNA. Malar. J. 2015, 14, 472. [Google Scholar] [CrossRef]
- Yongkiettrakul, S.; Kampeera, J.; Chareanchim, W.; Rattanajak, R.; Pornthanakasem, W.; Kiatpathomchai, W. Simple detection of single nucleotide polymorphism in Plasmodium falciparum by SNP-LAMP assay combined with lateral flow dipstick. Parasitol. Int. 2017, 66, 964–971. [Google Scholar] [CrossRef]
- Kersting, S.; Rausch, V.; Bier, F.F.; Nickisch-Rosenegk, M. Multiplex isothermal solid-phase recombinase polymerase amplification for the specific and fast DNA-based detection of three bacterial pathogens. Mikrochim. Acta 2014, 181, 1715–1723. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, X.Z.; Wen, K.; Li, C.L.; Marti, G.M.; Jiang, H.Y.; Shi, W.M.; Shen, J.Z.; Wang, Z.H. Multiplex lateral flow immunoassays based on amorphous carbon nanoparticles for detecting three fusarium mycotoxins in maize. J. Agric. Food Chem. 2017, 65, 8063–8071. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Ge, J.Q. Development and application of a recombinase-aided amplification combined with a lateral flow dipstick assay for rapid visual detection of anguillid herpesvirus 1. J. Fish. Dis. 2024, 47, e13907. [Google Scholar] [CrossRef] [PubMed]
- Li, D.R.; Zhao, J.Y.; Lan, W.Q.; Zhao, Y.; Sun, X.H. Effect of food matrix on rapid detection of Vibrio parahaemolyticus in aquatic products based on toxR gene. World J. Microbiol. Biotechnol. 2023, 39, 188. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, H.H.; Huang, W.Z.; Wang, L.Z.; Zhao, Y.H.; Wang, J.Y.; Shao, H.H.; Tao, X.; Yong, B. Development of a new PCR assay and a recombinase-aided amplification based isothermal amplification coupled with lateral flow dipstick assay for potato late blight detection. Crop Prot. 2023, 168, 106235. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Li, G.X.; Li, X.N.; Shen, X.X.; Gao, Y.; Wang, L.; Fan, T.; Duan, Q.X.; Wang, Y.K.; Wang, J.; et al. A rapid and sensitive recombinase aided amplification assay incorporating competitive internal control to detect Bordetella pertussis using the DNA obtained by boiling. Int. J. Infect. Dis. 2019, 86, 108–113. [Google Scholar] [CrossRef]
- Hou, L.W.; Li, D.R.; Zhang, N.; Zhao, J.Y.; Zhao, Y.; Sun, X.H. Development of an isothermal recombinase-aided amplification assay for the rapid and visualized detection of Klebsiella pneumoniae. J. Sci. Food Agric. 2022, 102, 3879–3886. [Google Scholar] [CrossRef]
- Hou, L.W.; Li, D.R.; Deng, B.; Zhao, Y.; Pan, Y.J.; Feng, D.S.; Sun, X.H. Development and application of the RAA-LFD assays for rapid detection of Staphylococcus aureus. J. Food Sci. 2022, 43, 331–339. [Google Scholar]
- Graber, H.U.; Caset, M.G.; Naskova, J.; Steiner, A.; Schaeren, W. Development of a highly sensitive and specific assay to detect Staphylococcus aureus in bovine mastitic milk. J. Dairy Sci. 2007, 90, 4661–4669. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhao, P.P.; Dong, Y.; Chen, S.Q.; Shen, H.; Jiang, G.; Zhu, H.; Dong, J.Q.; Gao, S. An isothermal recombinase polymerase amplification and lateral flow strip combined method for rapid on-site detection of Vibrio vulnificus in raw seafood. Food Microbiol. 2021, 98, 103664. [Google Scholar] [CrossRef]
- GB4789.10-2016; State Food and Drug Administration, National Health and Family Planning Commission of the People’s Republic of China. Standards Press of China: Beijing, China, 2016.
- SN/T2552.9-2010; People’s Republic of China Entry-Exit Inspection and Quarantine Industry Standard. Standards Press of China: Beijing, China, 2010.
- Nakano, R.; Nakano, A.; Ishii, Y.; Ubagai, T.; Kikuchi-Ueda, T.; Kikuchi, H.; Tansho-Nagakawa, S.; Kamoshida, G.; Mu, X.Q.; Ono, Y. Rapid detection of the Klebsiella pneumoniae carbapenemase (KPC) gene by loop-mediated isothermal amplification (LAMP). J. Infect. Chemother. 2015, 21, 202–206. [Google Scholar] [CrossRef]
- Sheet, O.H.; Grabowski, N.T.; Klein, G.; Abdulmawjood, A. Development and validation of a loop mediated isothermal amplification (LAMP) assay for the detection of Staphylococcus aureus in bovine mastitis milk samples. Mol. Cell. Probes. 2016, 30, 320–325. [Google Scholar] [CrossRef]
- Raja, B.; Goux, H.J.; Marapadaga, A.; Rajagopalan, S.; Kourentzi, K.; Willson, R.C. Development of a panel of recombinase polymerase amplification assays for detection of common bacterial urinary tract infection pathogens. J. Appl. Microbiol. 2017, 123, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, D.; Li, H.; Pang, J.; Guo, H.; Qiu, J. Establishment and application of multiplex PCR for simultaneously detecting Escherichia coli, Salmonella, Klebsiella pneumoniae and Staphylococcus aureus in minks. Front. Vet. Sci. 2020, 17, 588173. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.T.N.; Phat, V.V.; Vinh, C.; Lan, N.P.H.; Phuong, N.L.N.; Ngan, L.T.Q.; Thwaites, G.; Thwaites, L.; Rabaa, M.; Nguyen, A.T.K.; et al. Development and validation of multiplex real-time PCR for simultaneous detection of six bacterial pathogens causing lower respiratory tract infections and antimicrobial resistance genes. BMC Infect. Dis. 2024, 24, 164. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.T.; Ye, Q.H.; Wu, Q.P.; Pang, R.; Zhou, B.Q.; Wang, C.F.; Xiang, X.R.; Li, F.; Wang, J.; Zhang, Y.Z. PCR and multiplex PCR assays for the detection of Cronobacter species using specific targets obtained by a bioinformatics approach. Food Control. 2021, 125, 107896. [Google Scholar] [CrossRef]
- Wang, P.; Liao, L.; Ma, C.; Zhang, X.; Yu, J.; Yi, L.; Liu, X.; Shen, H.; Gao, S.; Lu, Q. Duplex on-site detection of Vibrio cholerae and Vibrio vulnificus by recombinase polymerase amplification and three-segment lateral flow strips. Biosensors 2021, 11, 151. [Google Scholar] [CrossRef]
- Feng, X.Y.; Zhou, D.G.; Xie, G.Y.; Liu, J.; Xiong, Q.; Xu, H.Y. A novel photoreactive DNA-binding dye for detecting viable Klebsiella pneumoniae in powdered infant formula. J. Dairy Sci. 2022, 105, 4895–4902. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; Von Stetten, F. Review: A comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst 2018, 144, 31–67. [Google Scholar]
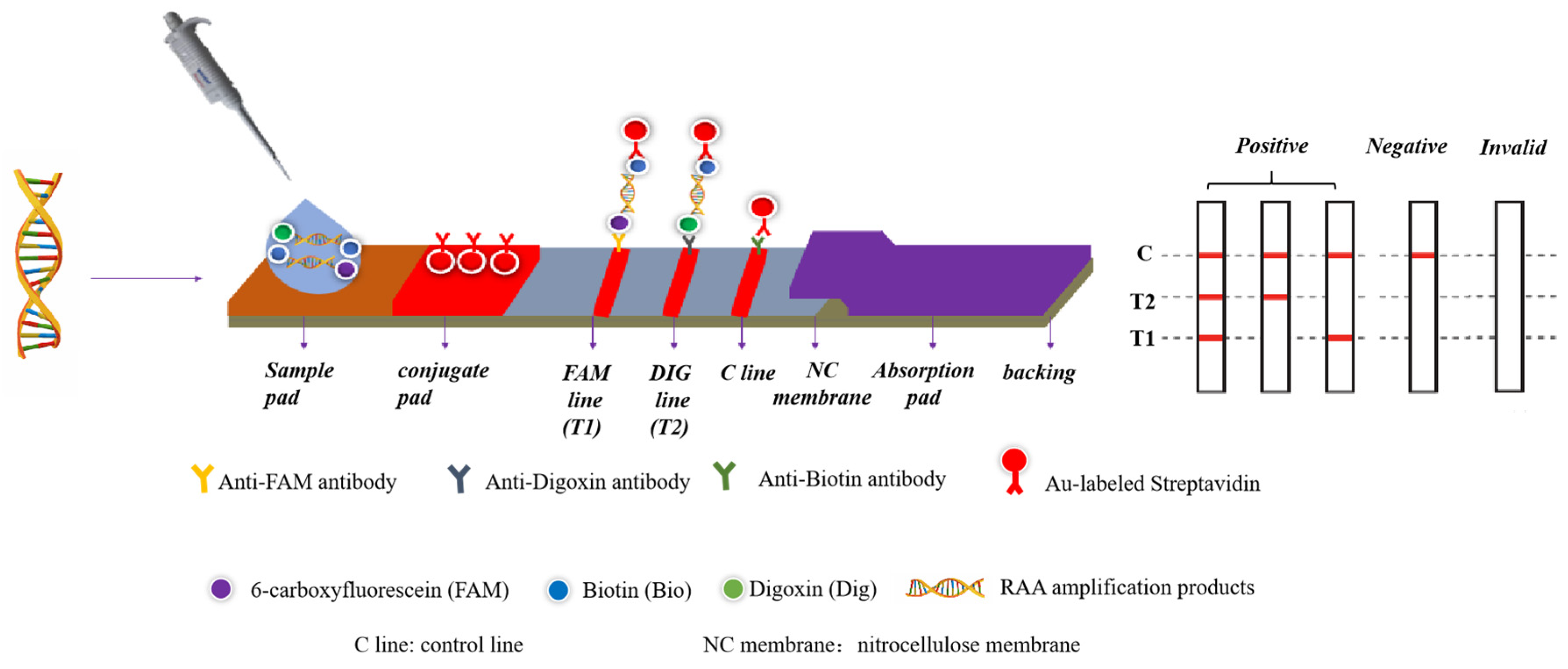
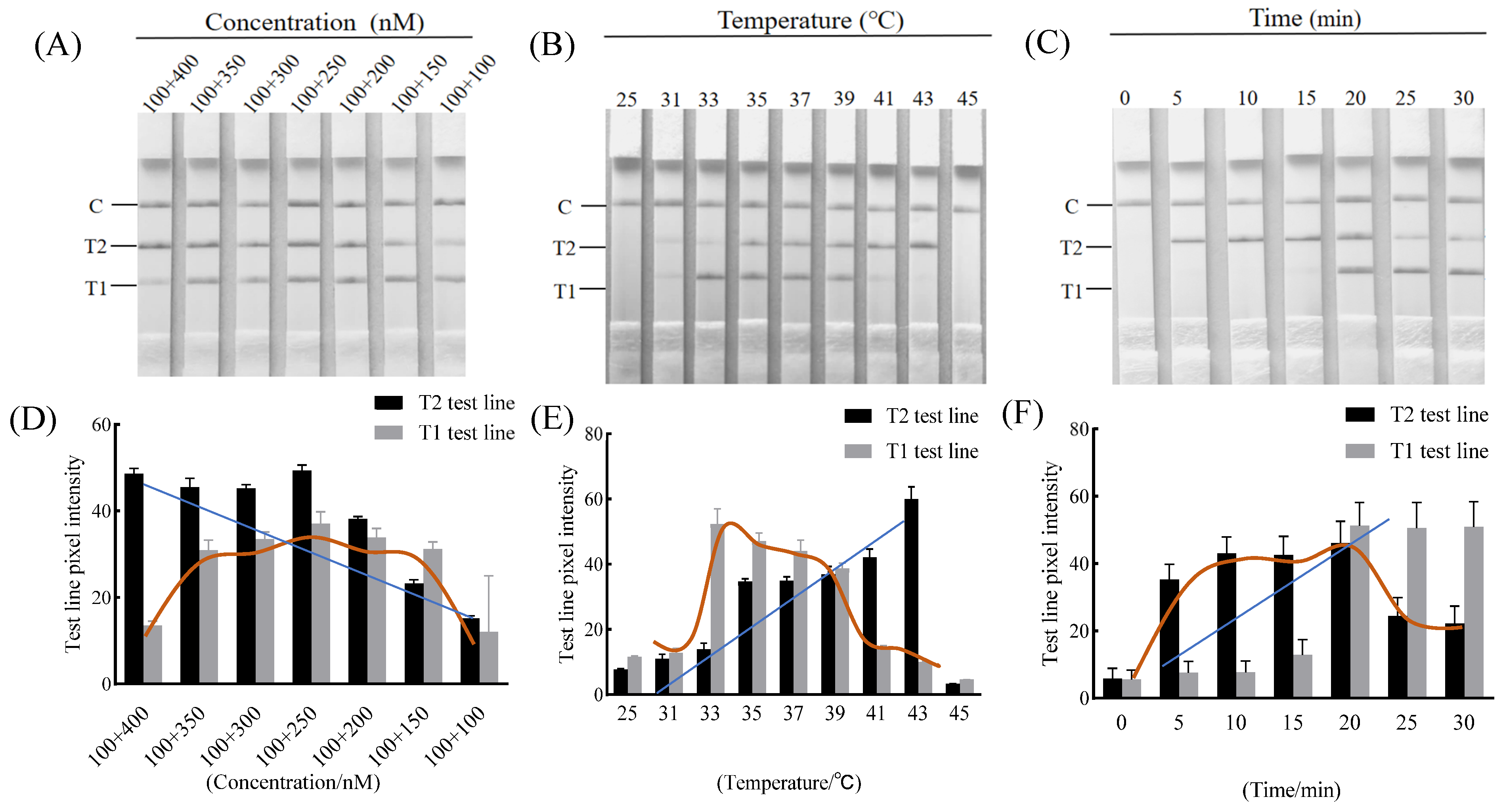
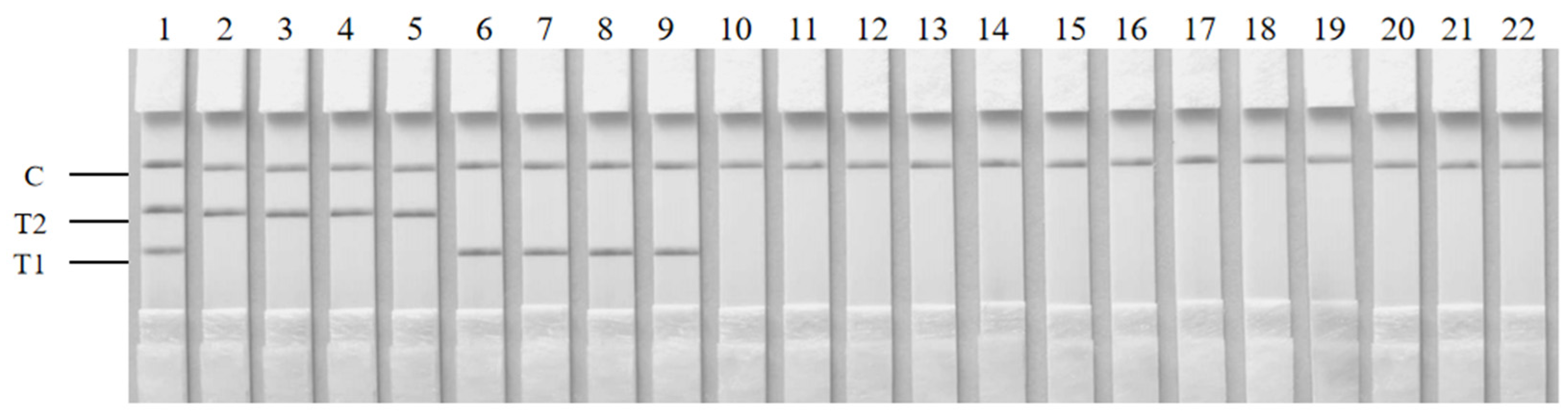

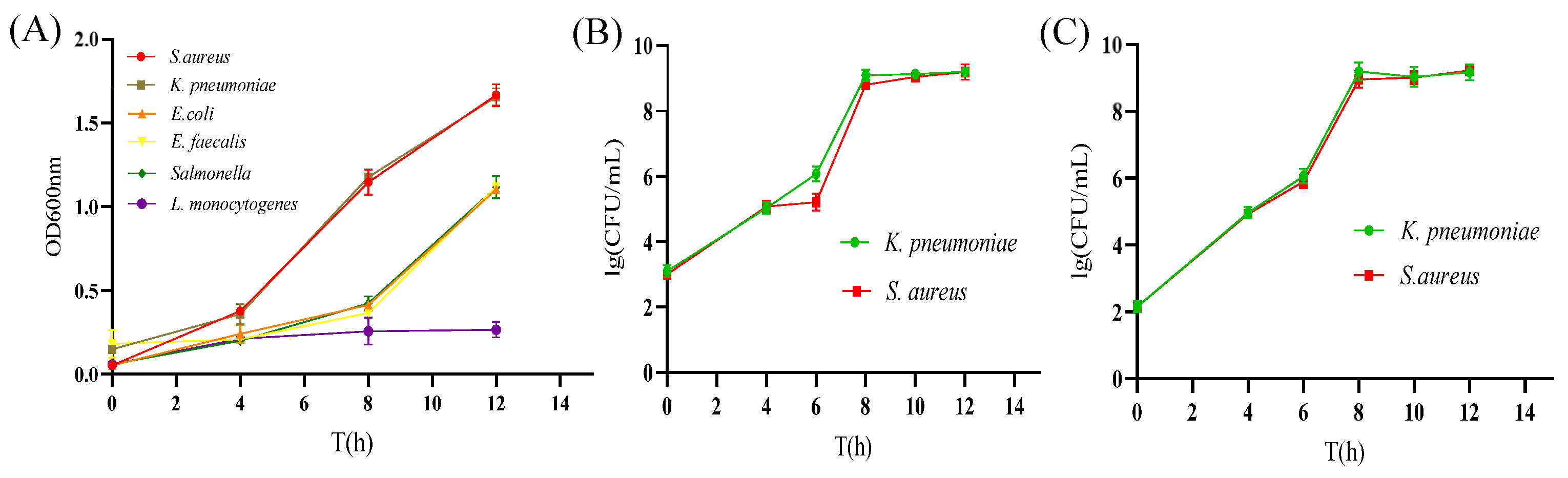
| Species | Strain | Source |
|---|---|---|
| Klebsiella pneumoniae | G412 | Isolated from dairy farms |
| Klebsiella pneumoniae | G304 | Isolated from dairy farms |
| Klebsiella pneumoniae | G305 | Isolated from dairy farms |
| Klebsiella pneumoniae | G413 | Isolated from dairy farms |
| Staphylococcus aureus | AB91093 | CDC |
| Staphylococcus aureus | G404 | Isolated from dairy farms |
| Staphylococcus aureus | G109 | Isolated from dairy farms |
| Staphylococcus aureus | G115 | Isolated from dairy farms |
| Klebsiella oxytoca | G414 | Isolated from dairy farms |
| Staphylococcus saprophyticus | ATCC BAA-750 | ATCC |
| Staphylococcus epidermidis | ATCC1228 | ATCC |
| Vibrio cholerae | C6067 | Preserved in our laboratory |
| Listeria monocytogenes | G12 | Isolated from dairy farms |
| Listeria monocytogenes | ATCC19115 | ATCC |
| Pseudomonas aeruginosa | H012 | Preserved in our laboratory |
| Escherichia coli O157:H7 | ATCC43889 | ATCC |
| Vibrio parahaemolyticus | ATCC17802 | ATCC |
| Vibrio harveyi | ATCC33842 | ATCC |
| Enterococcus faecalis | G401 | Isolated from dairy farms |
| Salmonella Enteritidis | CMCC50041 | CMCC |
| Method | Pathogen | Primers | Sequence (5′–3′) | Product Size (bp) | Cite |
|---|---|---|---|---|---|
| RAA–LFD | Klebsiella pneumoniae | rcsA-LF3 | DIG-TGTATTTTCTTTTTAATATTGCCTTTATGCG | 137 | [25] |
| rcsA-LR3 | Biotin-TTGTCACTGAGTAAAACAGAATCAAATATGC | ||||
| Staphylococcus aureus | nuc-LF2 | 6-FAM-GACAAAGGTCAAAGAACTGATAAATATGGA | 159 | [26] | |
| nuc-LR2 | Biotin-TTCACTTTTTCTTAAAAGTTGTTCATGTGT | ||||
| PCR | Klebsiella pneumoniae | khe-F | TGATTGCATTCGCCACTGG | 428 | [26] |
| khe-R | GGTCAACCCAACGATCCTG | ||||
| Staphylococcus aureus | nuc-F | CTGGCATATGTATGGCAATTGTT | 664 | [27] | |
| nuc-R | TATTGACCTGAATCAGCGTTGTCT |
| Additive | Dose (g/L) | Inhibition Rate (%) | |
|---|---|---|---|
| S. aureus | K. pneumoniae | ||
| Sodium pyruvate | 1.0 | −8.45 ± 0.011 a | −39.30 ± 0.027 a |
| 2.0 | −2.12 ± 0.006 b | −37.46 ± 0.045 a | |
| 4.0 | −3.54 ± 0.005 b | 15.33 ± 0.007 b | |
| Glucose | 2.0 | −20.81 ± 0.195 a | −31.22 ± 0.009 a |
| 4.0 | −16.24 ± 0.035 a | −37.92 ± 0.024 ab | |
| 6.0 | −21.41 ± 0.101 a | −34.35 ± 0.004 b | |
| Lithium chloride | 0.5 | −1.17 ± 0.006 a | −11.45 ± 0.046 ab |
| 1.0 | −1.49 ± 0.005 a | −8.30 ± 0.029 a | |
| 2.0 | 5.73 ± 0.006 b | −21.20 ± 0.074 b | |
| Brilliant green | 0.005 | 58.10 ± 0.097 a | 35.86 ± 0.043 a |
| 0.01 | 38.78 ± 0.009 b | 15.33 ± 0.007 b | |
| 0.02 | 21.13 ± 0.030 b | 16.73 ± 0.024 c | |
| Strains | Klebsiella pneumoniae | Staphylococcus aureus | ||
|---|---|---|---|---|
| Duplex RAA–LFD /SN/T 2552.9–2010 | Duplex RAA–LFD/PCR | Duplex RAA–LFD /GB 4789.10–2016 | Duplex RAA–LFD /PCR | |
| Raw milk (57) | 34/30 | 34/32 | 4/3 | 4/3 |
| TP | 30 | 32 | 3 | 3 |
| TN | 23 | 23 | 53 | 53 |
| FP | 4 | 2 | 1 | 1 |
| FN | 0 | 0 | 0 | 0 |
| PPV (%) | 88.24 | 94.12 | 75.00 | 75.00 |
| NPV (%) | 100.0 | 100.0 | 100.0 | 100.0 |
| Sensitivity (%) | 100.0 | 100.0 | 100.0 | 100.0 |
| Specificity (%) | 85.19 | 92.00 | 98.15 | 98.15 |
| TCR (%) | 92.98 | 96.49 | 98.25 | 98.25 |
| K | 0.86 | 0.93 | 0.85 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Hou, L.; Li, D.; Lan, W.; Zhao, Y.; Sun, X. Establishment and Application of Duplex Recombinase-Aided Amplification Combined with Lateral Flow Dipsticks for Rapid and Simultaneous Visual Detection of Klebsiella pneumoniae and Staphylococcus aureus in Milk. Foods 2025, 14, 573. https://doi.org/10.3390/foods14040573
Zhang N, Hou L, Li D, Lan W, Zhao Y, Sun X. Establishment and Application of Duplex Recombinase-Aided Amplification Combined with Lateral Flow Dipsticks for Rapid and Simultaneous Visual Detection of Klebsiella pneumoniae and Staphylococcus aureus in Milk. Foods. 2025; 14(4):573. https://doi.org/10.3390/foods14040573
Chicago/Turabian StyleZhang, Ni, Laiwang Hou, Darong Li, Weiqing Lan, Yong Zhao, and Xiaohong Sun. 2025. "Establishment and Application of Duplex Recombinase-Aided Amplification Combined with Lateral Flow Dipsticks for Rapid and Simultaneous Visual Detection of Klebsiella pneumoniae and Staphylococcus aureus in Milk" Foods 14, no. 4: 573. https://doi.org/10.3390/foods14040573
APA StyleZhang, N., Hou, L., Li, D., Lan, W., Zhao, Y., & Sun, X. (2025). Establishment and Application of Duplex Recombinase-Aided Amplification Combined with Lateral Flow Dipsticks for Rapid and Simultaneous Visual Detection of Klebsiella pneumoniae and Staphylococcus aureus in Milk. Foods, 14(4), 573. https://doi.org/10.3390/foods14040573





