Design and Bioanalysis of Nanoliposome Loaded with Premium Red Palm Oil for Improved Nutritional Delivery and Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fabrication of RPO-Loaded Nanoliposomes
2.3. Nanoliposome Characterization
2.3.1. pH
2.3.2. Particle Size Distribution, Polydispersity Index (PDI), and Zeta Potential
2.3.3. EE and LC of β-Carotene
2.3.4. FTIR Spectra
2.3.5. TEM
2.4. Bioanalysis
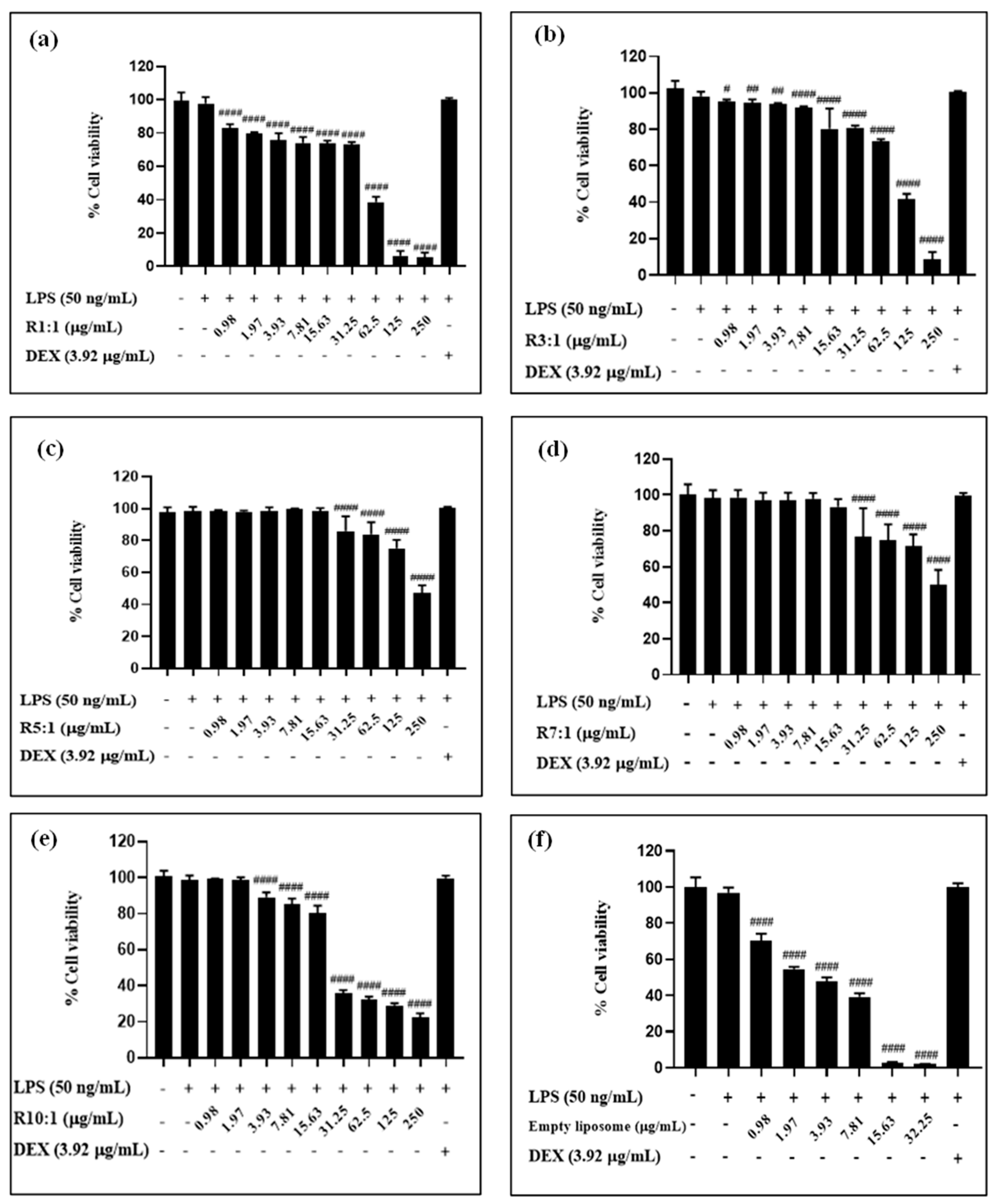
2.4.1. Cytotoxicity Assay
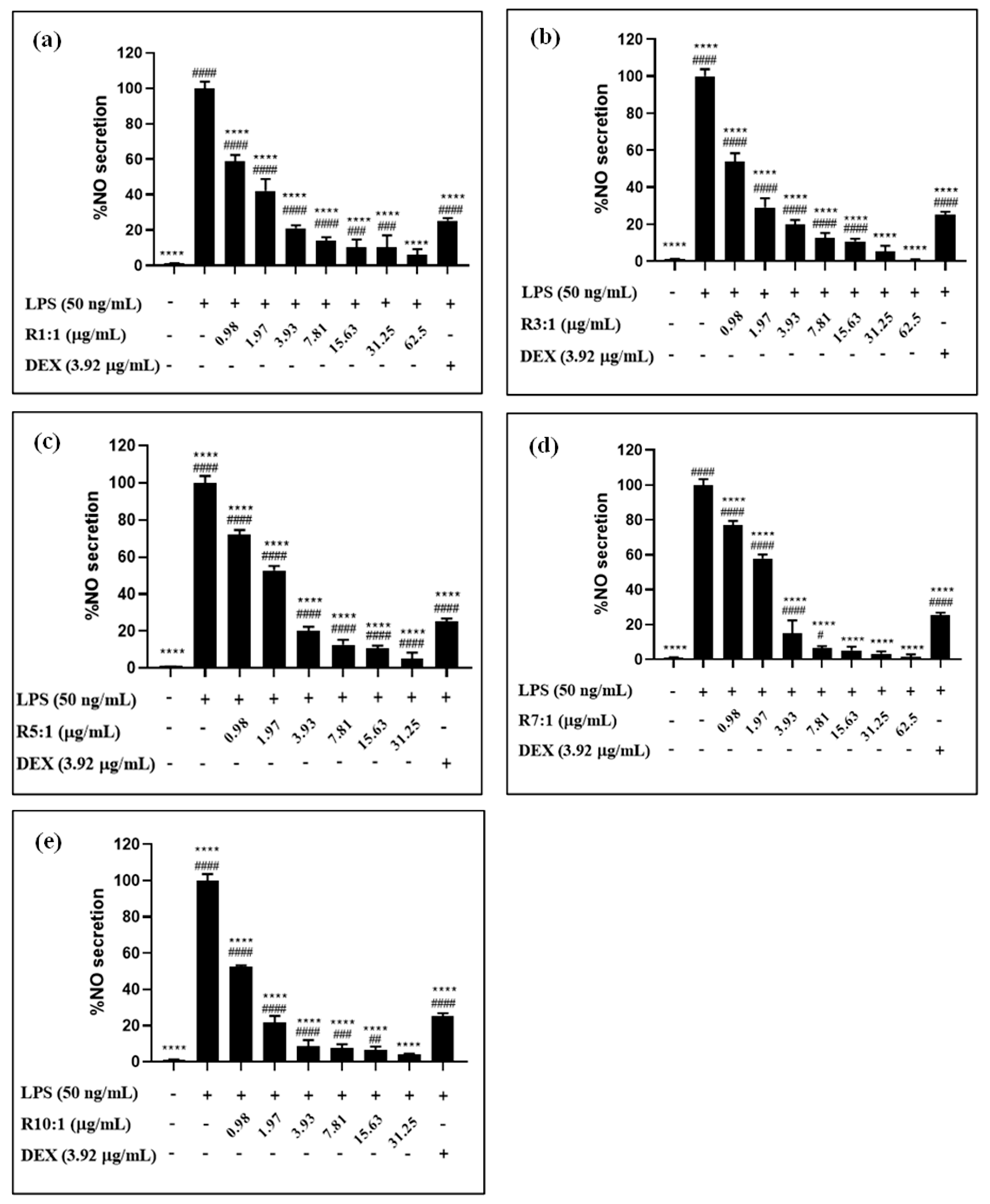
2.4.2. Nitric Oxide (NO) Inhibitory Activity
2.5. Storage Stability
2.5.1. β-Carotene Retention Rate
2.5.2. Free Radical Scavenging Activity
2.5.3. Lipid Oxidation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of RPO-Loaded Nanoliposomes
3.1.1. Particle Size, PDI, Zeta Potential, and pH
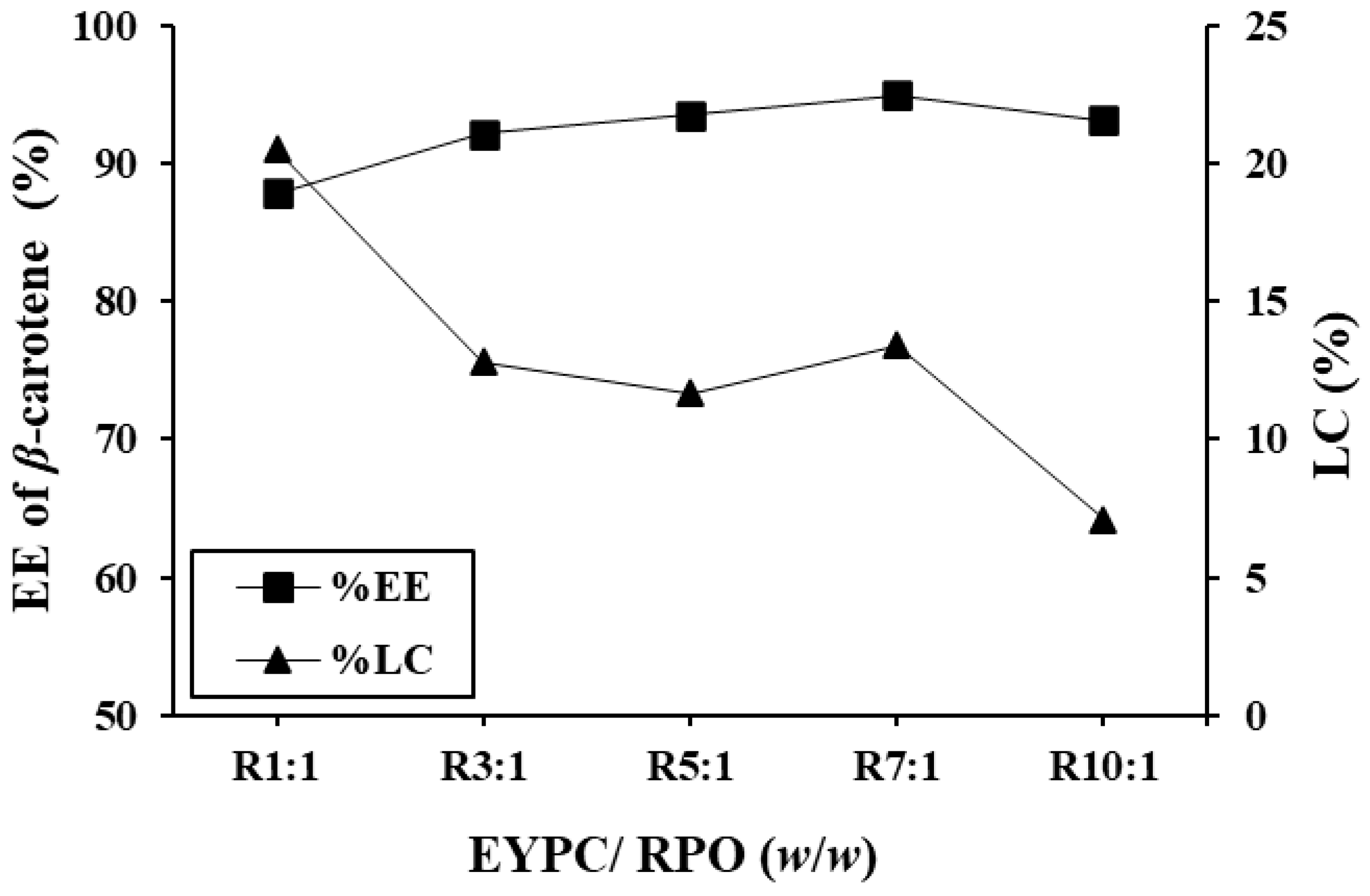
3.1.2. EE of β-Carotene and LC
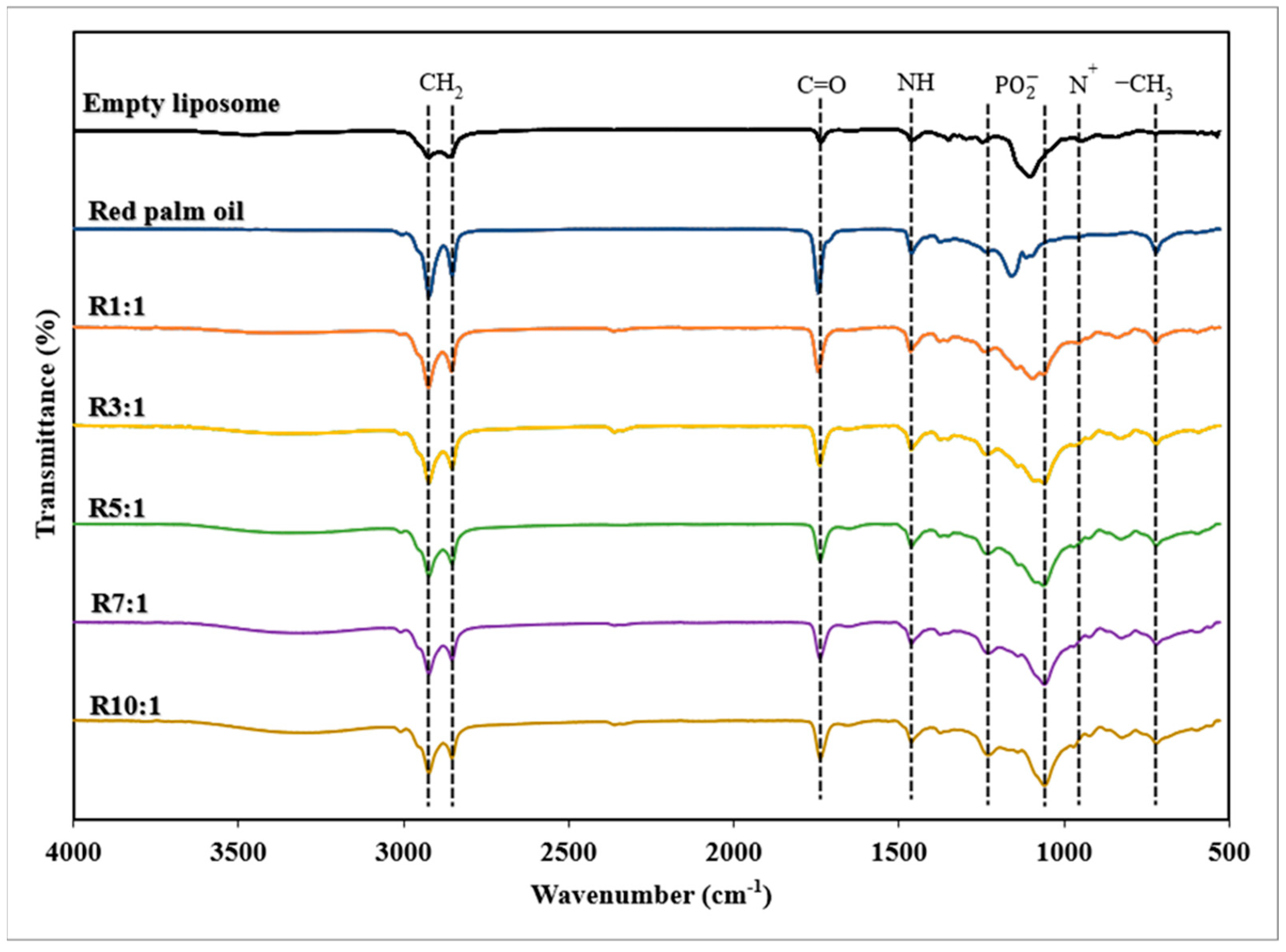
3.1.3. FTIR Spectra
3.2. Morphological Structure
3.3. Storage Stability
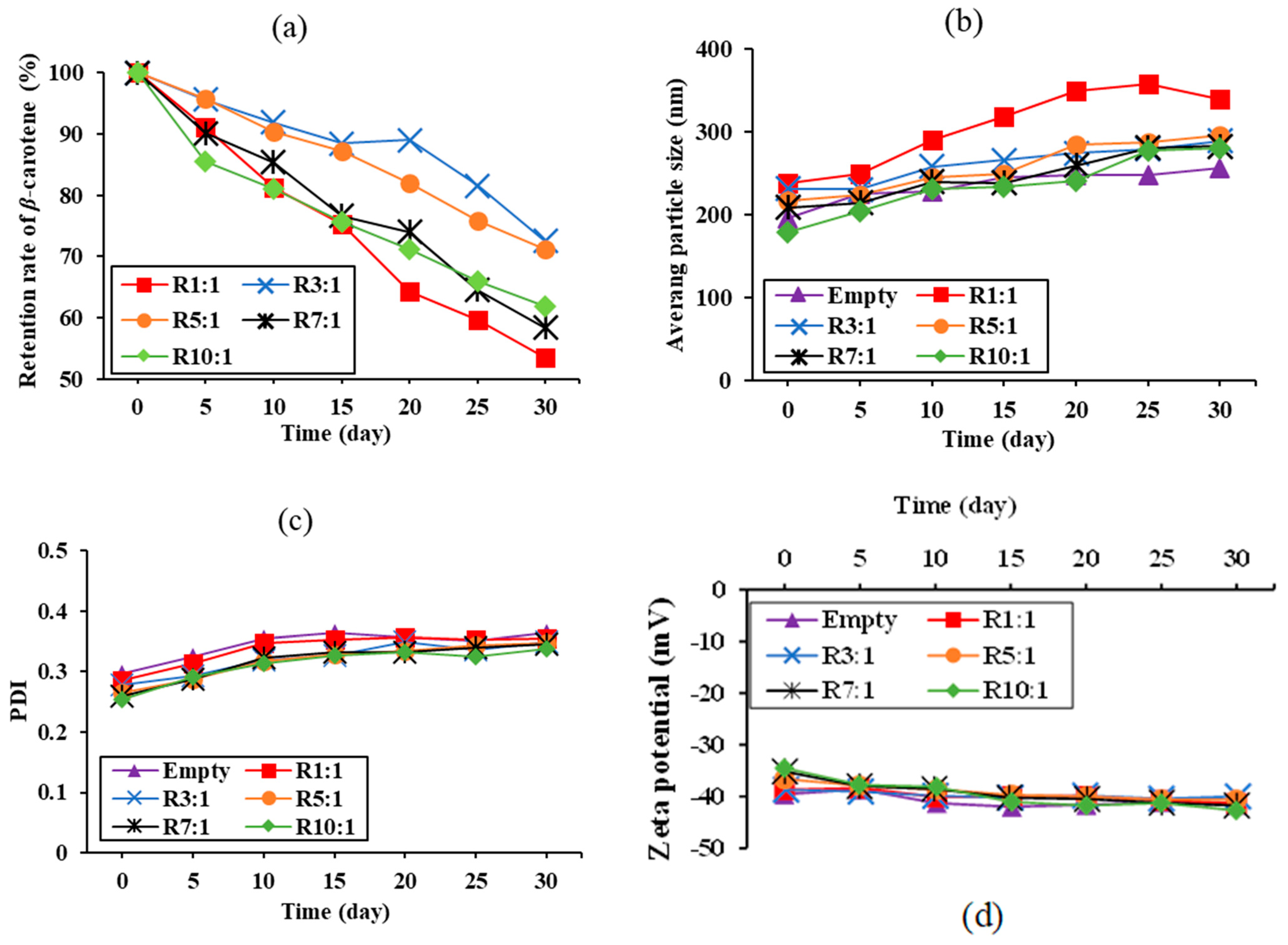
3.3.1. Retention of β-Carotene
3.3.2. Vesicle Sizes, PDI, and Zeta Potential
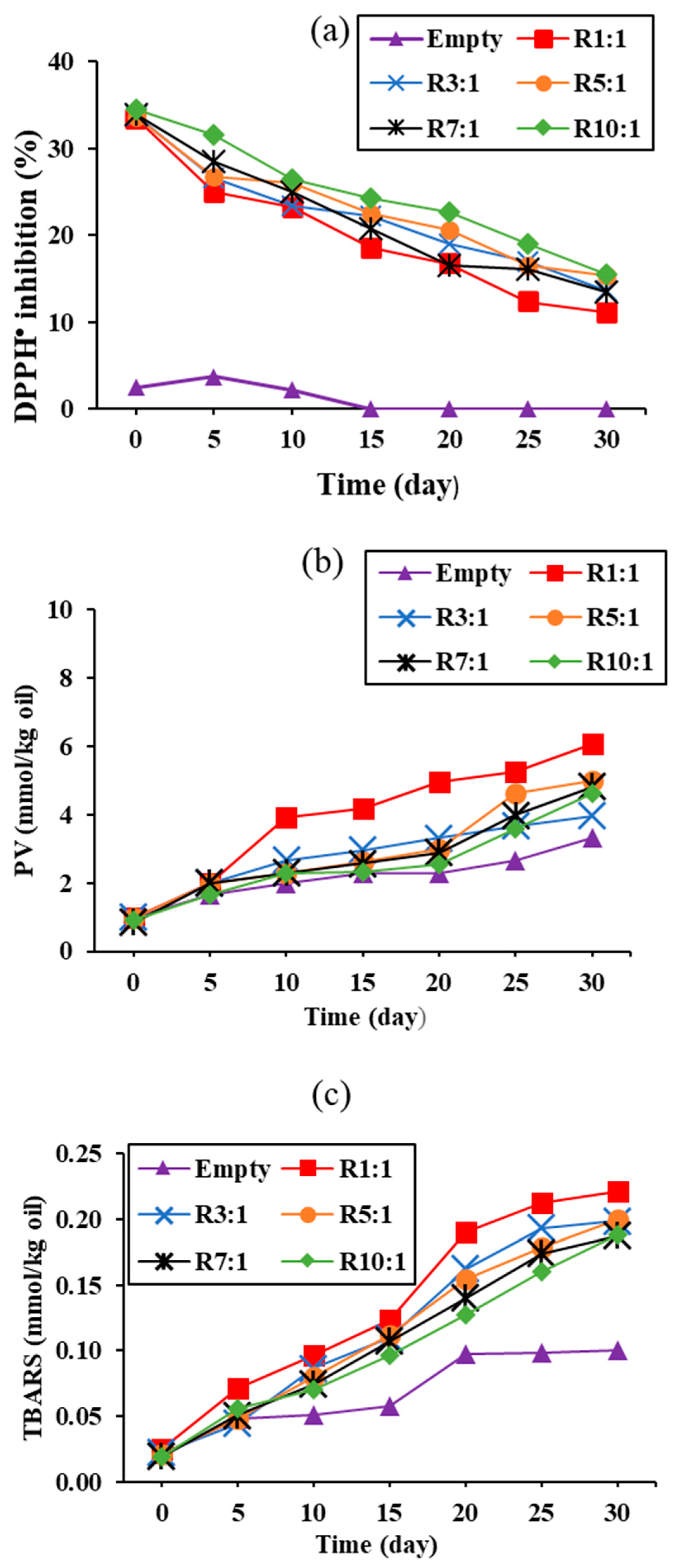
3.3.3. DPPH• Scavenging Activity and Lipid Oxidation
3.4. Biocompatibility and In Vitro Anti-Inflammatory Activity of Nanoliposomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wondi, M.H.; Haris, N.I.N.; Shamsudin, R.; Yunus, R.; Ali, M.M.; Iswardi, A.H. Development and testing of an oil palm (Elaeis guineensis Jacq.) fruit digester process for kernel free in crude palm oil production. Ind. Crop. Prod. 2024, 208, 117755. [Google Scholar] [CrossRef]
- Tan, C.H.; Lee, C.J.; Tan, S.N.; Poon, D.T.S.; Chong, C.Y.E.; Pui, L.P. Red palm oil: A review on processing, health benefits and its application in food. J. Oleo Sci. 2021, 70, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, R.; Subramaniam, K.M.; Radhakrishnan, A.K.; Choo, Y.M.; Teng, K.T. Health-promoting effects of red palm oil: Evidence from animal and human studies. Nutr. Rev. 2017, 75, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Nisoa, M.; Rachpibul, A. Simulation and design of microwave heating oven for interaction study of microwave and oil palm fruits. Thai Sci. Technol. J. 2022, 30, 15–31. [Google Scholar]
- Sarah, M.; Ramadhan, M.R.; Zahra, A.; Madinah, I.; Maulina, S.; Misran, E. Sterilization of oil palm fruit utilizing continuous microwave sterilizer. Case Stud. Therm. Eng. 2023, 52, 103698. [Google Scholar] [CrossRef]
- Cheng, S.F.; Chuah, C.H. Microwave pretreatment: A clean and dry method for palm oil production. Ind. Crop. Prod. 2011, 34, 967–971. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry–A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Listiarini, H.; Chandra, D.A.; Azizah, F.H.; Kurniadi, N.; Lubis, R.F.; Budijanto, S.; Prangdimurti, E.; Armetha, V.; Purwanti, N.; Sitanggang, A.B. Characterization and stability of zinc oxide nanoparticles stabilized multiphase system composed of red palm oil and water. Food Bioprod. Process. 2024, 147, 115–123. [Google Scholar] [CrossRef]
- Huang, R.; Song, H.; Li, S.; Guan, X. Selection strategy for encapsulation of hydrophilic and hydrophobic ingredients with food-grade materials: A systematic review and analysis. Food Chem. X 2025, 25, 102149. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Song, F.F.; Tian, S.J.; Yang, G.L.; Sun, X.Y. Effect of phospholipid/flaxseed oil ratio on characteristics, structure change, and storage stability of liposomes. LWT 2022, 157, 113040. [Google Scholar] [CrossRef]
- Song, F.; Chen, J.; Zhang, Z.; Tian, S. Preparation, characterization, and evaluation of flaxseed oil liposomes coated with chitosan and pea protein isolate hydrolysates. Food Chem. 2023, 404, 134547. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Caddeo, C.; Lubrano, V.; Valenti, D.; Pucci, L. Encapsulation of bioactive fermented wheat (Lisosan G) in Eudragit-liposomes. LWT 2022, 156, 113044. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 1, 591325. [Google Scholar] [CrossRef]
- Yamasaki, S.; Kurita, S.; Ochiai, A.; Hashimoto, M.; Sueki, K.; Utsunomiya, S. Calcium molybdate nanoparticles formation in egg phosphatidyl choline-based liposome caused by liposome fusion. J. Colloid Interface Sci. 2018, 530, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ravald, H.; Wiedmer, S.K. Potential of liposomes and lipid membranes for the separation of β-blockers by capillary electromigration and liquid chromatographic techniques. J. Chromatogr. A 2023, 1706, 464265. [Google Scholar] [CrossRef] [PubMed]
- Montagner, G.E.; Ribeiro, M.F.; Cadoná, F.C.; Franco, C.; Gomes, P. Liposomes loading grape seed extract: A nanotechnological solution to reduce wine-making waste and obtain health-promoting products. Future Foods 2022, 5, 100144. [Google Scholar] [CrossRef]
- Amalia, E.; Sopyan, I.; Putriana, N.A.; Sriwidodo, S. Preparation and molecular interaction of organic solvent-free piperine pro-liposome from soy lecithin. Heliyon 2023, 9, e16674. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of vitamin C and β-carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, Y.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. Preparation and characterization of tea tree oil solid liposomes to control brown rot and improve quality in peach fruit. LWT 2022, 162, 113442. [Google Scholar] [CrossRef]
- Pu, C.; Tang, W.; Li, X.; Li, M.; Sun, Q. Stability enhancement efficiency of surface decoration on curcumin-loaded liposomes: Comparison of guar gum and its cationic counterpart. Food Hydrocoll. 2019, 87, 29–37. [Google Scholar] [CrossRef]
- Pengjam, Y.; Syazwani, N.; Inchai, J.; Numit, A.; Yodthong, T.; Pitakpornpreecha, T.; Panichayupakaranant, P. High water-soluble curcuminoids-rich extract regulates osteogenic differentiation of MC3T3-E1 cells: Involvement of Wnt/β-catenin and BMP signaling pathway. Chin. Herb. Med. 2021, 13, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Rodsamai, T.; Chaijan, M.; Nisoa, M.; Donlao, N.; Rawdkuen, S.; Chunglok, W.; Cheong, L.Z.; Panpipat, W. Improved curcumin recovery and in vitro biological activity of turmeric extracts using nipa palm syrup–and nipa palm vinegar–based Natural Deep Eutectic Solvent (NADES) hybridized with microwave-assisted extraction. Food Bioproc. Tech. 2024, 17, 2009–2022. [Google Scholar] [CrossRef]
- Mohammadzadeh, N.; Ghiasian, M.; Faradmal, J.; Dastan, D. Quantitative and qualitative analyses of the constituents of the hydroalcoholic extract of Quercus infectoria gall from Kermanshah and evaluation of its antioxidant and antibacterial activities. J. Rep. Pharm. Sci. 2021, 10, 287. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Fan, C.; Feng, T.; Wang, X.; Xia, S.; Swing, C.J. Liposomes for encapsulation of liposoluble vitamins (A, D, E and K): Comparation of loading ability, storage stability and bilayer dynamics. Food Res. Int. 2023, 163, 112264. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.; Srivastav, P.P. Encapsulation of Centella asiatica leaf extract in liposome: Study on structural stability, degradation kinetics and fate of bioactive compounds during storage. Food Chem. Adv. 2023, 2, 100202. [Google Scholar] [CrossRef]
- Amiri, H.; Shabanpour, B.; Pourashouri, P. Encapsulation of marine bioactive compounds using liposome technique: Evaluation of physicochemical properties and oxidative stability during storage. Food Struct. 2023, 35, 100308. [Google Scholar] [CrossRef]
- Yoshida, P.A.; Yokota, D.; Foglio, M.A.; Rodrigues, R.F.; Pinho, S.C.D. Liposomes incorporating essential oil of Brazilian cherry (Eugenia uniflora L.): Characterization of aqueous dispersions and lyophilized formulations. J. Microencapsul. 2010, 27, 416–425. [Google Scholar] [CrossRef]
- Ciont, C.; Difonzo, G.; Pasqualone, A.; Chis, M.S.; Ranga, F.; Szabo, K.; Simon, E.; Naghiu, A.; Barbu-Tudoran, L.; Caponio, F.; et al. Phenolic profile of micro-and nano-encapsulated olive leaf extract in biscuits during in vitro gastrointestinal digestion. Food Chem. 2023, 428, 136778. [Google Scholar] [CrossRef] [PubMed]
- Petelska, A.D.; Figaszewski, Z.A. Effect of pH on the interfacial tension of bilayer lipid membrane formed from phosphatidylcholine or phosphatidylserine. Biochim. Biophys. Acta Biomembr. 2002, 1561, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, H.C.; Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Van de Ven, M.; Kattenberg, M.; Van Ginkel, G.; Levine, Y.K. Study of the orientational ordering of carotenoids in lipid bilayers by resonance-Raman spectroscopy. Biophys. J. 1984, 45, 1203–1209. [Google Scholar] [CrossRef]
- Cui, H.; Zang, Z.; Jiang, Q.; Bao, Y.; Wu, Y.; Li, J.; Chen, Y.; Liu, X.; Yang, S.; Si, X.; et al. Utilization of ultrasound and glycation to improve functional properties and encapsulated efficiency of proteins in anthocyanins. Food Chem. 2023, 419, 135899. [Google Scholar] [CrossRef] [PubMed]
- Vongsvivut, J.; Heraud, P.; Zhang, W.; Kralovec, J.A.; McNaughton, D.; Barrow, C.J. Quantitative determination of fatty acid compositions in micro-encapsulated fish-oil supplements using Fourier transform infrared (FTIR) spectroscopy. Food Chem. 2012, 135, 603–609. [Google Scholar] [CrossRef]
- Kim, W.; Wang, Y.; Vongsvivut, J.; Ye, Q.; Selomulya, C. On surface composition and stability of β-carotene microcapsules comprising pea/whey protein complexes by synchrotron-FTIR microspectroscopy. Food Chem. 2023, 426, 136565. [Google Scholar] [CrossRef]
- Garofulić, I.E.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Retention of polyphenols in encapsulated sour cherry juice in dependence of drying temperature and wall material. LWT 2017, 83, 110–117. [Google Scholar] [CrossRef]
- Chen, W.T.; Kuo, Y.L.; Chen, C.H.; Wu, H.T.; Chen, H.W.; Fang, W.P. Improving the stability and bioactivity of curcumin using chitosan-coated liposomes through a combination mode of high-pressure processing. LWT 2022, 168, 113946. [Google Scholar] [CrossRef]
- Zabodalova, L.; Ishchenko, T.; Skvortcova, N.; Baranenko, D.; Chernjavskij, V. Liposomal beta-carotene as a functional additive in dairy products. Agron. Res. 2014, 12, 825–834. [Google Scholar]
- Szwed, M.; Torgersen, M.L.; Kumari, R.V.; Yadava, S.K.; Pust, S.; Iversen, T.G.; Skotland, T.; Giri, J.; Sandvig, K. Biological response and cytotoxicity induced by lipid nanocapsules. J. Nanobiotechnol. 2020, 18, 1–19. [Google Scholar] [CrossRef]
- Obiedallah, M.M.; Mironov, M.A.; Belyaev, D.V.; Ene, A.; Vakhrusheva, D.V.; Krasnoborova, S.Y.; Bershitsky, S.Y.; Shchepkin, D.V.; Minin, A.S.; Ishmetova, R.I.; et al. Optimization, characterization, and cytotoxicity studies of novel anti-tubercular agent-loaded liposomal vesicles. Sci. Rep. 2024, 14, 524. [Google Scholar]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Dcunha, R.; Mutalik, S.P.; Reji, R.A.; Mutalik, S.; Kalthur, S.G.; Hegde, P.; Murari, M.S.; Raghu, S.V.; Banerjee, S.; Kumar, A.; et al. Liposome-based freezing medium improves the outcome of mouse prepubertal testicular tissue cryopreservation. Reprod. Sci. 2024, 31, 3532–3548. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, R.; Seguin, C.; Fournel, S.; Frisch, B.; Heurtault, B.; Hadjsadok, A. Anti-inflammatory effects of free and liposome-encapsulated Algerian thermal waters in RAW 264.7 macrophages. Int. J. Pharm. 2022, 614, 121452. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.Y.; Rod-In, W.; Monmai, C.; Choi, G.S.; Park, W.J. Anti-inflammatory effects of neutral lipids, glycolipids, phospholipids from Halocynthia aurantium tunic by suppressing the activation of NF-κB and MAPKs in LPS-stimulated RAW264. 7 macrophages. PLoS ONE 2022, 17, e0270794. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, L.; Hong, P.; Lang, W.; Hui, J.; Yang, Y.; Zheng, X. β-Carotene prevents weaning-induced intestinal inflammation by modulating gut microbiota in piglets. Anim. Biosci. 2021, 34, 1221. [Google Scholar] [CrossRef] [PubMed]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, β-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef]
- Cui, B.; Liu, S.; Wang, Q.; Lin, X. Effect of β-carotene on immunity function and tumour growth in hepatocellular carcinoma rats. Molecules 2012, 17, 8595–8603. [Google Scholar] [CrossRef] [PubMed]

| EYPC/RPO (w/w) | Average Vesicle Size (nm) | PDI | Zeta Potential (mV) | pH |
|---|---|---|---|---|
| Empty nanoliposome | 196.20 ± 2.16 a | 0.396 ± 0.003 e | −39.57 ± 0.06 a | 4.60 ± 0.006 a |
| 1:1 | 239.3 ± 7.22 d | 0.385 ± 0.004 d | −38.73 ± 0.65 b | 4.65 ± 0.000 b |
| 3:1 | 231.6 ± 5.65 d | 0.378 ± 0.004 c | −38.63 ± 0.21 b | 4.66 ± 0.000 c |
| 5:1 | 217.2 ± 3.84 c | 0.366 ± 0.002 b | −36.60 ± 0.10 c | 4.68 ± 0.006 d |
| 7:1 | 209.0 ± 2.60 c | 0.359 ± 0.003 a | −35.17 ± 0.50 d | 4.67 ± 0.006 c |
| 10:1 | 199.3 ± 4.18 b | 0.354 ± 0.006 a | −34.50 ± 0.56 d | 4.72 ± 0.000 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodsamai, T.; Chaijan, M.; Rodjan, P.; Tamman, A.; Supaweera, N.; Yin, M.; Kim, S.R.; Panpipat, W. Design and Bioanalysis of Nanoliposome Loaded with Premium Red Palm Oil for Improved Nutritional Delivery and Stability. Foods 2025, 14, 566. https://doi.org/10.3390/foods14040566
Rodsamai T, Chaijan M, Rodjan P, Tamman A, Supaweera N, Yin M, Kim SR, Panpipat W. Design and Bioanalysis of Nanoliposome Loaded with Premium Red Palm Oil for Improved Nutritional Delivery and Stability. Foods. 2025; 14(4):566. https://doi.org/10.3390/foods14040566
Chicago/Turabian StyleRodsamai, Tanatchapond, Manat Chaijan, Prawit Rodjan, Arlee Tamman, Nassareen Supaweera, Mingyu Yin, Siriporn Riebroy Kim, and Worawan Panpipat. 2025. "Design and Bioanalysis of Nanoliposome Loaded with Premium Red Palm Oil for Improved Nutritional Delivery and Stability" Foods 14, no. 4: 566. https://doi.org/10.3390/foods14040566
APA StyleRodsamai, T., Chaijan, M., Rodjan, P., Tamman, A., Supaweera, N., Yin, M., Kim, S. R., & Panpipat, W. (2025). Design and Bioanalysis of Nanoliposome Loaded with Premium Red Palm Oil for Improved Nutritional Delivery and Stability. Foods, 14(4), 566. https://doi.org/10.3390/foods14040566







