Allergy to Thaumatin-like Proteins—What Do We Know?
Abstract
1. Introduction
2. Thaumatin-like Proteins
3. Allergy to TLPs
4. TLP Sources
4.1. Food Allergens
4.1.1. Mal d 2
4.1.2. Pru p 2
4.1.3. Pru av 2
4.1.4. Cap a 1
4.1.5. Act d 2
4.1.6. Mus a 4
4.2. Inhalant Allergens
4.2.1. Ole e 13
4.2.2. Jun a 3
4.2.3. Cry j 3
4.2.4. Cup s 3
4.2.5. Jun v 3
4.2.6. Can s 7
4.3. Other Allergens
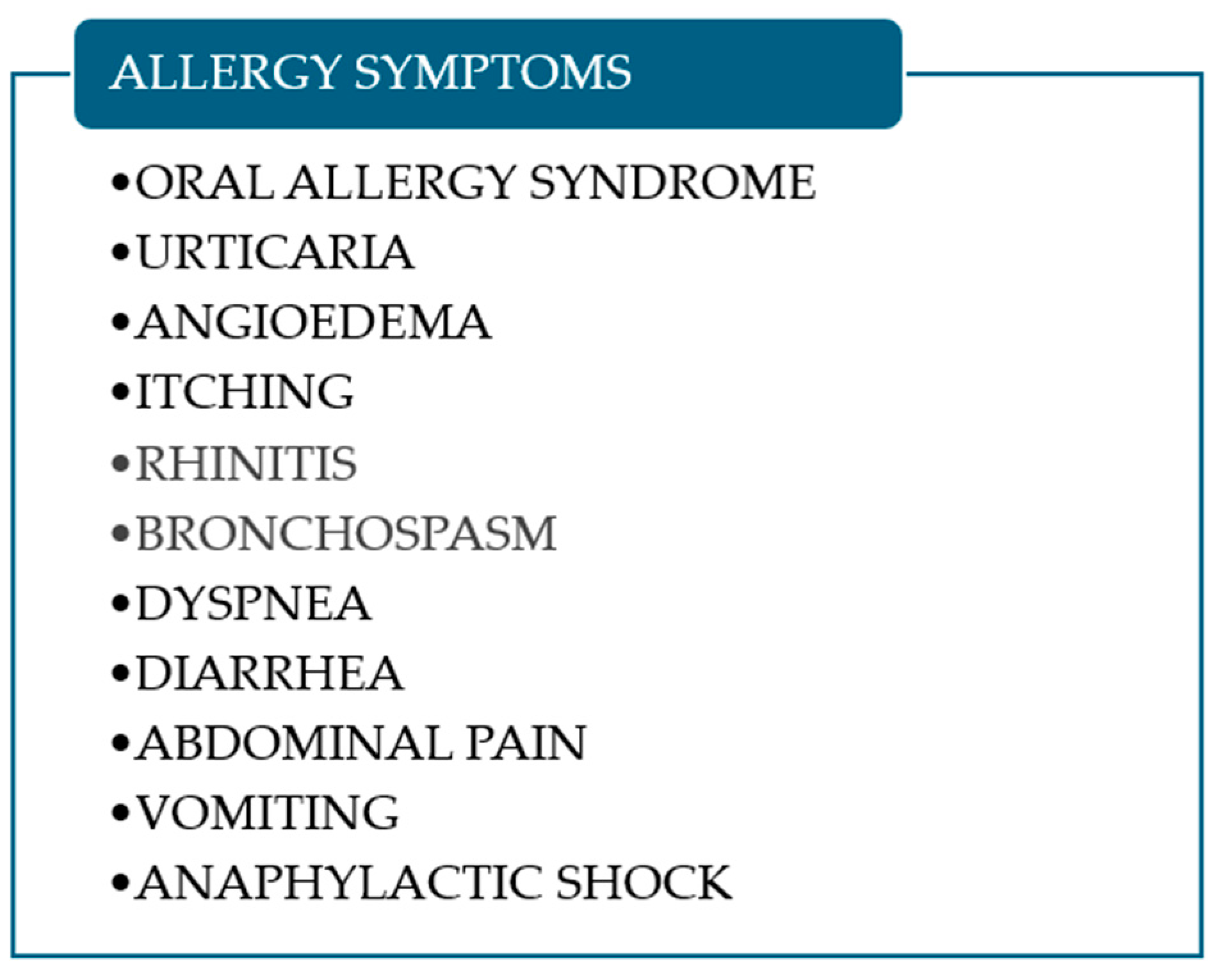
5. Clinical Presentation
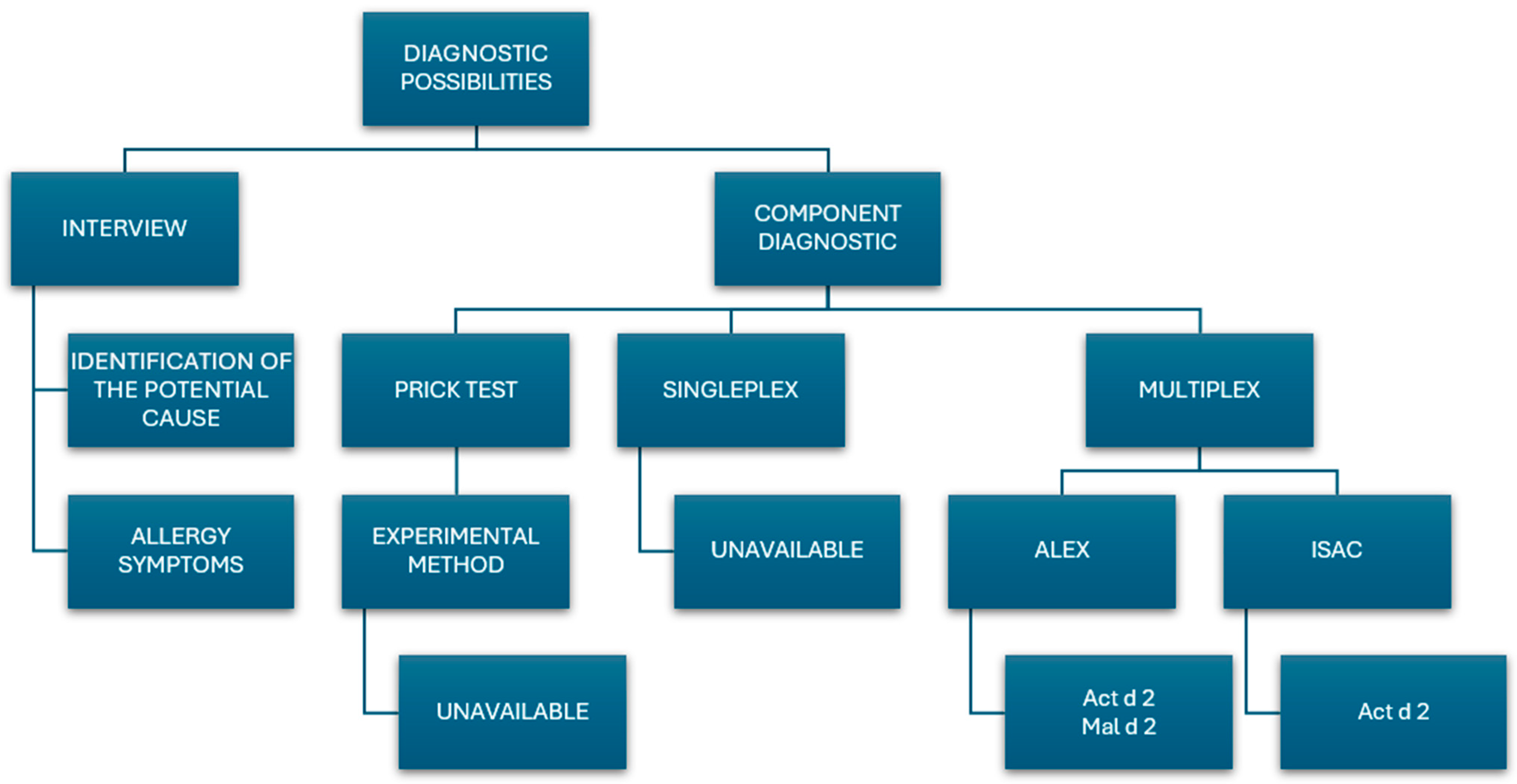
6. Diagnostic Possibilities
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ukleja-Sokołowska, N.; Zacniewski, R.; Lis, K.; Żbikowska-Gotz, M.; Kuźmiński, A.; Bartuzi, Z. Exercise induced anaphylaxis in kiwi allergic patient: Case report. Allergy Asthma Clin. Immunol. 2021, 17, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olivieri, B.; Skypala, I.J. New arrivals in anaphylaxis to foods. Curr. Opin. Allergy Clin. Immunol. 2023, 23, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Azofra García, J.; Cuesta-Herranz, J.; Perea Lam, N.; Díaz-Perales, A. Anaphylaxis mediated by thaumatin-like proteins. J. Investig. Allergol. Clin. Immunol. 2014, 24, 448–449. [Google Scholar] [PubMed]
- van der Wel, H.; Loeve, K. Isolation and characterization of thaumatin I and II, the sweet-tasting proteins from Thaumatococcus daniellii Benth. Eur. J. Biochem. 1972, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Breiteneder, H. Thaumatin-like proteins—A new family of pollen and fruit allergens. Allergy 2004, 59, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Potvin, C.; Trudel, J.; Asselin, A. Some thaumatin-like proteins hydrolyse polymeric beta-1,3-glucans. Plant J. 1999, 19, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Arandia, M.; Murua-García, A.; Palacin, A.; Tordesillas, L.; Gómez-Casado, C.; Blanca-Lopez, N.; Ramos, T.; Canto, G.; Blanco, C.; Cuesta-Herranz, J.; et al. The role of N-glycosylation in kiwi allergy. Food Sci. Nutr. 2014, 2, 260–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jimenez-Lopez, J.C.; Robles-Bolivar, P.; Lopez-Valverde, F.J.; Lima-Cabello, E.; Kotchoni, S.O.; Alché, J.D. Ole e 13 is the unique food allergen in olive: Structure-functional, substrates docking, and molecular allergenicity comparative analysis. J. Mol. Graph. Model. 2016, 66, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Kawamoto, S. Spectrum of allergens for Japanese cedar pollinosis and impact of component-resolved diagnosis on allergen-specific immunotherapy. Allergol. Int. 2015, 64, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Palacín, A.; Rivas, L.A.; Gómez-Casado, C.; Aguirre, J.; Tordesillas, L.; Bartra, J.; Blanco, C.; Carrillo, T.; Cuesta-Herranz, J.; Bonny, J.A.; et al. The involvement of thaumatin-like proteins in plant food cross-reactivity: A multicenter study using a specific protein microarray. PLoS ONE 2012, 7, e44088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO/IUIS Allergen Nomenclature Home Page. Available online: https://allergen.org/ (accessed on 20 December 2024).
- Hsieh, L.S.; Moos, M., Jr.; Lin, Y. Characterization of apple 18 and 31 kd allergens by microsequencing and evaluation of their content during storage and ripening. J. Allergy Clin. Immunol. 1995, 96, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Marzban, G.; Puehringer, H.; Dey, R.; Brynda, S.; Ma, Y.; Martinelli, A.; Zaccarini, M.; Van Der Weg, E.; Housley, Z.; Kolarich, D.; et al. Localisation and distribution of the major allergens in apple fruits. Plant Sci. 2005, 169, 387–394. [Google Scholar] [CrossRef]
- Smole, U.; Bublin, M.; Radauer, C.; Ebner, C.; Breiteneder, H. Mal d 2, the thaumatin-like allergen from apple, is highly resistant to gastrointestinal digestion and thermal processing. Int. Arch. Allergy Immunol. 2008, 147, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Krebitz, M.; Wagner, B.; Ferreira, F.; Peterbauer, C.; Campillo, N.; Witty, M.; Kolarich, D.; Steinkellner, H.; Scheiner, O.; Breiteneder, H. Plant-based heterologous expression of Mal d 2, a thaumatin-like protein and allergen of apple (Malus domestica), and its characterization as an antifungal protein. J. Mol. Biol. 2003, 329, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rivas, M.; Bolhaar, S.; González-Mancebo, E.; Asero, R.; van Leeuwen, A.; Bohle, B.; Ma, Y.; Ebner, C.; Rigby, N.; Sancho, A.I.; et al. Apple allergy across Europe: How allergen sensitization profiles determine the clinical expression of allergies to plant foods. J. Allergy Clin. Immunol. 2006, 118, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, C.; Ma, Y.; Marsh, J.; Rigby, N.; Smole, U.; Radauer, C.; Alessandri, S.; Briza, P.; Zuidmeer, L.; Maderegger, B.; et al. Purification and characterisation of relevant natural and recombinant apple allergens. Mol. Nutr. Food Res. 2008, 52 (Suppl. 2), S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Palacín, A.; Tordesillas, L.; Gamboa, P.; Sanchez-Monge, R.; Cuesta-Herranz, J.; Sanz, M.L.; Barber, D.; Salcedo, G.; Díaz-Perales, A. Characterization of peach thaumatin-like proteins and their identification as major peach allergens. Clin. Exp. Allergy 2010, 40, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Inschlag, C.; Hoffmann-Sommergruber, K.; O’Riordain, G.; Ahorn, H.; Ebner, C.; Scheiner, O.; Breiteneder, H. Biochemical characterization of Pru a 2, a 23-kD thaumatin-like protein representing a potential major allergen in cherry (Prunus avium). Int. Arch. Allergy Immunol. 1998, 116, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Izumi, E.; Hidaka, S.; Hiroi, A.; Kinugasa, S.; Yano, E.; Zaima, N.; Moriyama, T. Thaumatin-Like Protein (Pru av 2) Is a Cherry Allergen That Triggers Percutaneous Sensitization in Mice. Foods 2021, 10, 134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leitner, A.; Jensen-Jarolim, E.; Grimm, R.; Wüthrich, B.; Ebner, H.; Scheiner, O.; Kraft, D.; Ebner, C. Allergens in pepper and paprika. Immunologic investigation of the celery-birch-mugwort-spice syndrome. Allergy 1998, 53, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gavrović-Jankulović, M.; Ćirković, T.; Vucković, O.; Atanasković-Marković, M.; Petersen, A.; Gojgić, G.; Burazer, L.; Jankov, R.M. Isolation and biochemical characterization of a thaumatin-like kiwi allergen. J. Allergy Clin. Immunol. 2002, 110, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Gavrovic-Jankulovic, M.; Polovic, N.; Prisic, S.; Jankov, R.M.; Atanaskovic-Markovic, M.; Vuckovic, O.; Velickovic, T.C. Allergenic potency of kiwi fruit during fruit development. Food Agric. Immunol. 2005, 16, 117–128. [Google Scholar] [CrossRef]
- Gavrović-Jankulović, M.; Cirković, T.; Burazer, L.; Vucković, O.; Jankov, R.M. IgE cross-reactivity between meadow fescue pollen and kiwi fruit in patients’ sera with sensitivity to both extracts. J. Investig. Allergol. Clin. Immunol. 2002, 12, 279–286. [Google Scholar] [PubMed]
- Uberti, F.; Peñas, E.; Manzoni, Y.; di Lorenzo, C.; Ballabio, C.; Fiocchi, A.; Terracciano, L.; Restani, P. Molecular characterization of allergens in raw and processed kiwifruit. Pediatr. Allergy Immunol. 2015, 26, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Palacin, A.; Quirce, S.; Sanchez-Monge, R.; Bobolea, I.; Diaz-Perales, A.; Martin-Muñoz, F.; Pascual, C.; Salcedo, G. Sensitization profiles to purified plant food allergens among pediatric patients with allergy to banana. Pediatr. Allergy Immunol. 2011, 22, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Alcántara, M.; Quiralte, J.; Villalba, M.; Garzón, F.; Rodríguez, R. Airway disease and thaumatin-like protein in an olive-oil mill worker. N. Engl. J. Med. 2008, 358, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Alvarez-García, E.; Bartra, J.; Alcántara, M.; Palomares, O.; Villalba, M.; Rodríguez, R. The allergenic structure of the thaumatin-like protein Ole e 13 is degraded by processing of raw olive fruits. J. Investig. Allergol. Clin. Immunol. 2014, 24, 162–168. [Google Scholar] [PubMed]
- Midoro-Horiuti, T.; Goldblum, R.M.; Kurosky, A.; Wood, T.G.; Brooks, E.G. Variable expression of pathogenesis-related protein allergen in mountain cedar (Juniperus ashei) pollen. J. Immunol. 2000, 164, 2188–2192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujimura, T.; Futamura, N.; Midoro-Horiuti, T.; Togawa, A.; Goldblum, R.M.; Yasueda, H.; Saito, A.; Shinohara, K.; Masuda, K.; Kurata, K.; et al. Isolation and characterization of native Cry j 3 from Japanese cedar (Cryptomeria japonica) pollen. Allergy 2007, 62, 547–553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kondo, Y.; Tokuda, R.; Urisu, A.; Matsuda, T. Assessment of cross-reactivity between Japanese cedar (Cryptomeria japonica) pollen and tomato fruit extracts by RAST inhibition and immunoblot inhibition. Clin. Exp. Allergy 2002, 32, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Togawa, A.; Panzani, R.C.; Garza, M.A.; Kishikawa, R.; Goldblum, R.M.; Midoro-Horiuti, T. Identification of italian cypress (Cupressus sempervirens) pollen allergen Cup s 3 using homology and cross-reactivity. Ann. Allergy Asthma Immunol. 2006, 97, 336–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebo, D.G.; Rihs, H.P.; Mertens, C.H.; Van Gasse, A.L.; van der Poorten, M.L.; Hagendorens, M.M.; Sabato, V.; Elst, J. Exploring the thaumatin-like protein (TLP) as a candidate cannabis allergen in North-Western Europe. Allergy 2024, 79, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Larramendi, C.H.; López-Matas, M.Á.; Ferrer, A.; Huertas, A.J.; Pagán, J.A.; Navarro, L.Á.; García-Abujeta, J.L.; Andreu, C.; Carnés, J. Prevalence of sensitization to Cannabis sativa. Lipid-transfer and thaumatin-like proteins are relevant allergens. Int. Arch. Allergy Immunol. 2013, 162, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ashok Kumar, H.G.; Hegde, V.L.; Shetty, S.M.; Venkatesh, Y.P. Characterization and gene cloning of an acidic thaumatin-like protein (TLP 1), an allergen from sapodilla fruit (Manilkara zapota). Allergol. Int. 2013, 62, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Lehto, M.; Airaksinen, L.; Puustinen, A.; Tillander, S.; Hannula, S.; Nyman, T.; Toskala, E.; Alenius, H.; Lauerma, A. Thaumatin-like protein and baker’s respiratory allergy. Ann. Allergy Asthma Immunol. 2010, 104, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Campo, P.; Palacin, A.; Doña, I.; Gomez-Casado, C.; Galindo, L.; Díaz-Perales, A.; Blanca, M. Antigenic proteins involved in occupational rhinitis and asthma caused by obeche wood (Triplochiton scleroxylon). PLoS ONE 2013, 8, e53926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jamakhani, M.; Lele, S.S.; Rekadwad, B. In silico assessment data of allergenicity and cross-reactivity of NP24 epitopes from Solanum lycopersicum (Tomato) fruit. Data Brief 2018, 21, 660–674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muñoz-García, E.; Luengo-Sánchez, O.; Haroun-Díaz, E.; Maroto, A.S.; Palacín, A.; Díaz-Perales, A.; de las Heras Gozalo, M.; Labrador-Horrillo, M.; Vivanco, F.; Cuesta-Herranz, J.; et al. Identification of thaumatin-like protein and aspartyl protease as new major allergens in lettuce (Lactuca sativa). Mol. Nutr. Food Res. 2013, 57, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Betancor, D.; Gomez-Lopez, A.; Nuñez-Borque, E.; Fernandez-Bravo, S.; Barroso, B.; Esteban, V.; Pastor-Vargas, C.; Cuesta-Herranz, J. Allergy to Persimmon (Diospyros kaki), A Chitinase and Thaumatin-Like Protein: 2 Newly Identified Allergens. J. Investig. Allergol. Clin. Immunol. 2023, 33, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.; Watts, T.J. Food-dependent exercise-induced anaphylaxis to orange, with possible underlying thaumatin-like protein allergy. Ann. Allergy Asthma Immunol. 2023, 130, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Poncet, P.; Sénéchal, H.; Charpin, D. Update on pollen-food allergy syndrome. Expert. Rev. Clin. Immunol. 2020, 16, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Lis, K.; Bartuzi, Z. Multi-parameter tests for molecular diagnosis of allergies—Current possibilities. Alerg. Astma Immunol. 2020, 25, 122–140. [Google Scholar]

| Allergen | Source | Molecular Mass | Route of Exposure |
|---|---|---|---|
| Mal d 2 | Apple | 23 kDa | Oral |
| Pru p 2 | Peach | 25–28 kDa | Oral |
| Pru av 2 | Sweet cherry | 23 kDa | Oral |
| Cap a 1 | Bell pepper | 23 kDa | Oral |
| Act d 2 | Green kiwi | 24 kDa | Oral |
| Mus a 4 | Banana | 20 kDa | Oral |
| Ole e 13 | Olive | 23 kDa | Inhalation |
| Jun v 1 | Eastern red cedar | 10 kDa | Inhalation |
| Jun a 3 | Mountain cedar | 30 kDa | Inhalation |
| Cup s 3 | Common cypress | 34 kDa | Inhalation |
| Cry j 3 | Japanese cedar | 27 kDa | Inhalation |
| Can s 7 | Indian hemp | Inhalation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydzyńska, M.; Bartuzi, Z.; Rosada, T.; Grześk-Kaczyńska, M.; Ukleja-Sokołowska, N. Allergy to Thaumatin-like Proteins—What Do We Know? Foods 2025, 14, 543. https://doi.org/10.3390/foods14040543
Rydzyńska M, Bartuzi Z, Rosada T, Grześk-Kaczyńska M, Ukleja-Sokołowska N. Allergy to Thaumatin-like Proteins—What Do We Know? Foods. 2025; 14(4):543. https://doi.org/10.3390/foods14040543
Chicago/Turabian StyleRydzyńska, Magdalena, Zbigniew Bartuzi, Tomasz Rosada, Magdalena Grześk-Kaczyńska, and Natalia Ukleja-Sokołowska. 2025. "Allergy to Thaumatin-like Proteins—What Do We Know?" Foods 14, no. 4: 543. https://doi.org/10.3390/foods14040543
APA StyleRydzyńska, M., Bartuzi, Z., Rosada, T., Grześk-Kaczyńska, M., & Ukleja-Sokołowska, N. (2025). Allergy to Thaumatin-like Proteins—What Do We Know? Foods, 14(4), 543. https://doi.org/10.3390/foods14040543





