Simple and Robust Approach for Determination of Total Protein Content in Plant Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Reagents and Working Solutions
2.2. Sample Preparation
2.3. Protein Electrophoresis in Polyacrylamide Gel
2.4. Nitrogen-Based and Biochemical Methods for Protein Assay
2.5. Statistical Analysis
3. Results and Discussion
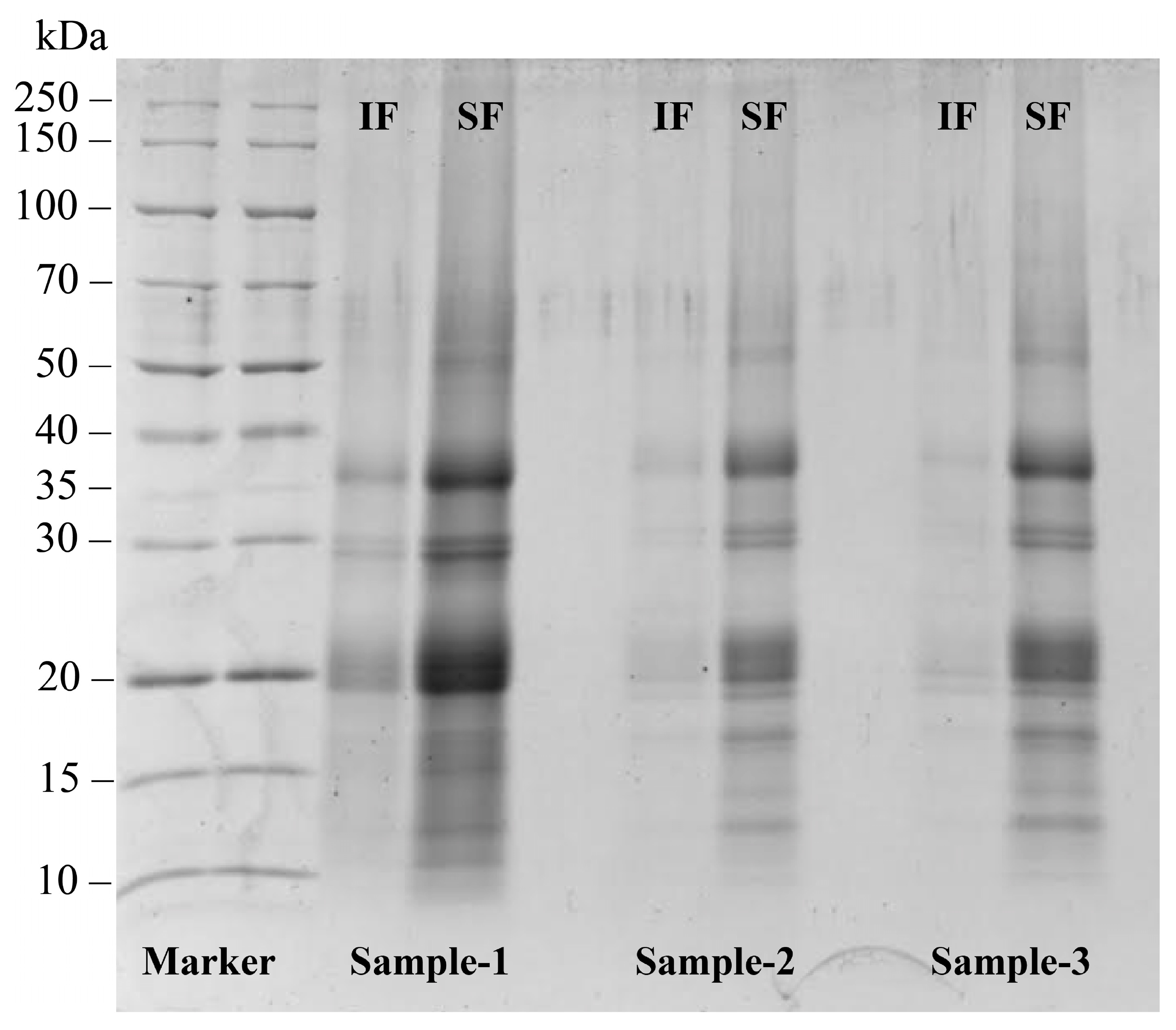
3.1. Electrophoresis
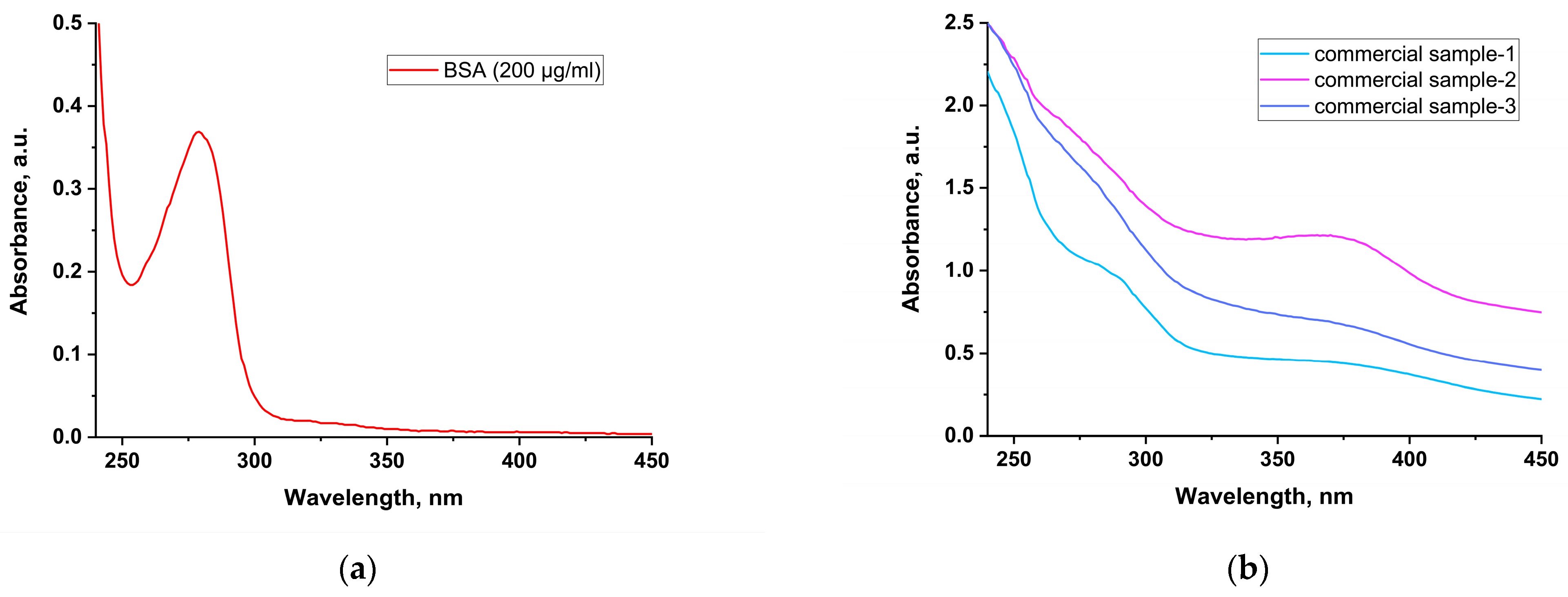
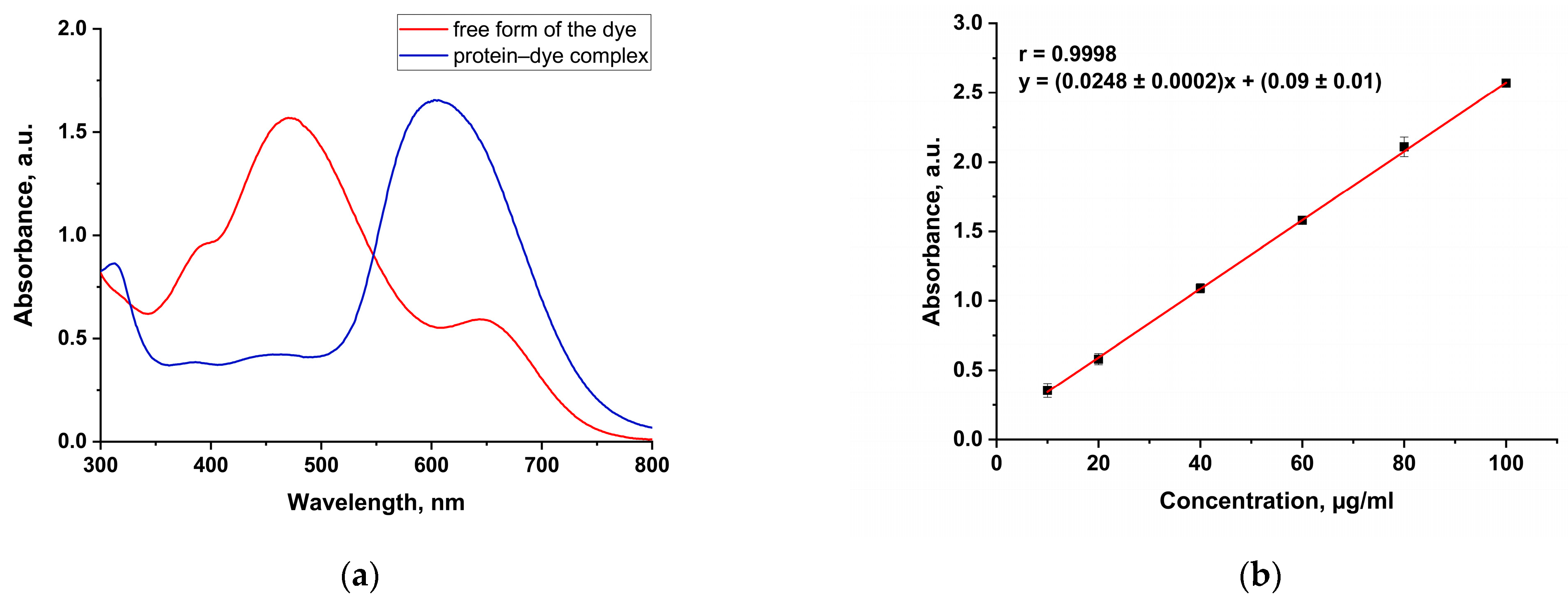
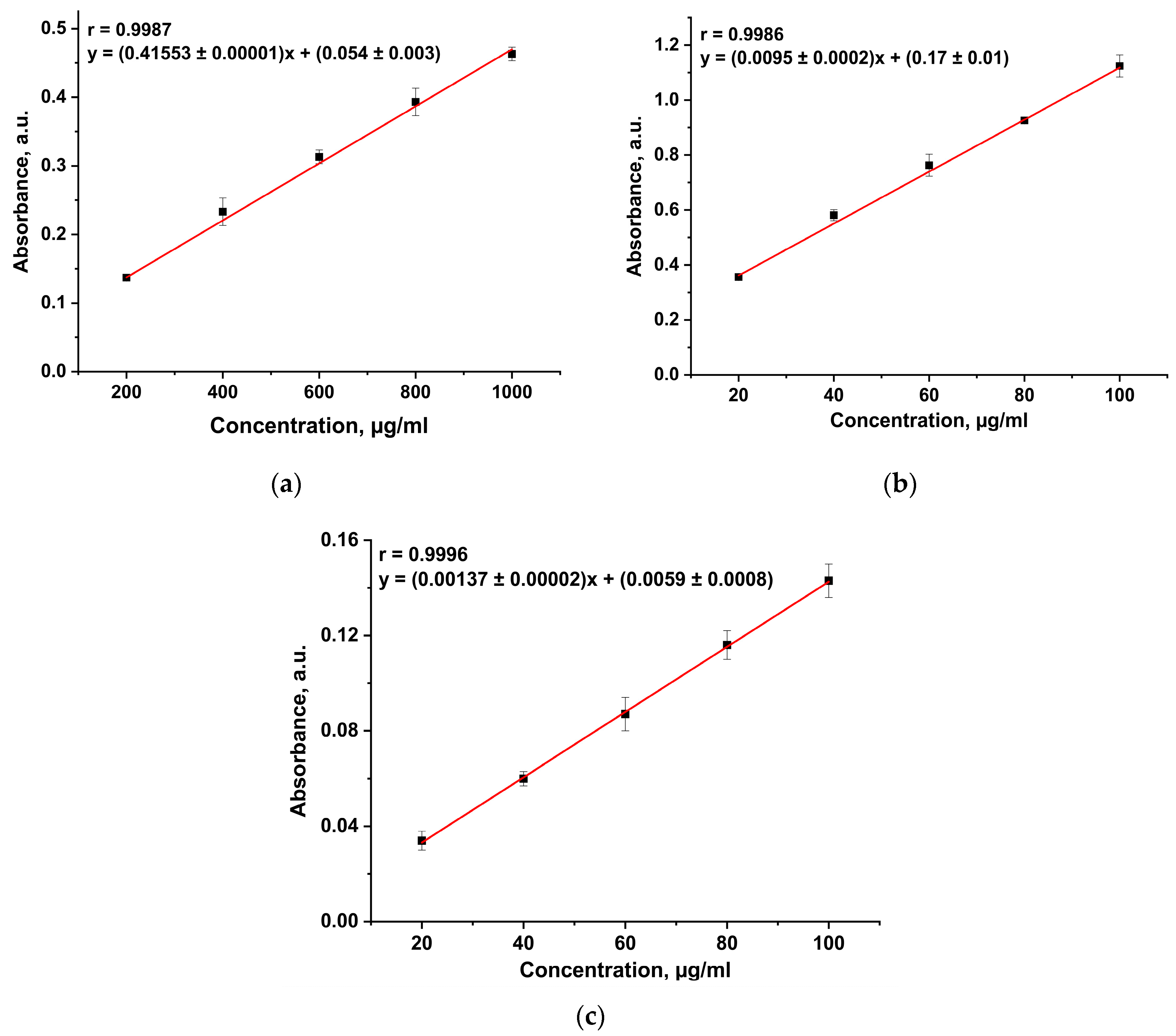
3.2. Protein Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adenekan, M.K.; Fadimu, G.J.; Odunmbaku, L.A.; Oke, E.K. Effect of isolation techniques on the characteristics of pigeon pea (Cajanus cajan) protein isolates. Food Sci. Nutr. 2018, 6, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino Acid Nutrition in Animals: Protein Synthesis and Beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Courtney-Martin, G.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein Requirements during Aging. Nutrients 2016, 8, 492. [Google Scholar] [CrossRef] [PubMed]
- Nevara, G.A.; Giwa Ibrahim, S.a.; Syed Muhammad, S.K.; Zawawi, N.; Mustapha, N.A.; Karim, R. Oilseed meals into foods: An approach for the valorization of oilseed by-products. Crit. Rev. Food Sci. Nutr. 2023, 63, 6330–6343. [Google Scholar] [CrossRef] [PubMed]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- González-Pérez, S.; Vereijken, J.M. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- de Boer, J.; Aiking, H. On the merits of plant-based proteins for global food security: Marrying macro and micro perspectives. Ecol. Econ. 2011, 70, 1259–1265. [Google Scholar] [CrossRef]
- Aiking, H. Future protein supply. Trends Food Sci Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Adascălului, M.; Multescu, M.; Mihai, A.L.; Bobea, S.A.; Florea, C.; Belc, N. Cytotoxicity Assessment and Nutritional Profiling of Bio-Active Compounds Obtained from Food Waste. Processes 2023, 11, 89. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Wilson, K. Basic principles. In Principles and Techniques of Biochemistry and Molecular Biology, 7th ed.; Wilson, K., Walker, J., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 1–37. [Google Scholar]
- Hayes, M. Measuring Protein Content in Food: An Overview of Methods. Foods 2020, 9, 1340. [Google Scholar] [CrossRef]
- Finete, V.d.L.M.; Gouvêa, M.M.; Marques, F.F.d.C.; Netto, A.D.P. Is it possible to screen for milk or whey protein adulteration with melamine, urea and ammonium sulphate, combining Kjeldahl and classical spectrophotometric methods? Food Chem. 2013, 141, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Goldring, J.P.D. Measuring Protein Concentration with Absorbance, Lowry, Bradford Coomassie Blue, or the Smith Bicinchoninic Acid Assay Before Electrophoresis. In Electrophoretic Separation of Proteins: Methods and Protocols; Kurien, B.T., Scofield, R.H., Eds.; Springer: New York, NY, USA, 2019; pp. 31–39. [Google Scholar]
- Settaluri, V.S.; Al-Mamari, K.M.K.; Al-Balushi, S.I.M.; Al-Risi, M.K.Z.; Ali, M.B. Review of Biochemical and Nutritional Constituents in Different Green Leafy Vegetables in Oman. Food Nutr. Sci. 2015, 6, 765–769. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, J. A practical method for extending the biuret assay to protein determination of corn-based products. Food Chem. 2017, 224, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Mondal, M.; Ghosh, P.; Saha, M.; Chatterjee, S. Quantification of total protein content from some traditionally used edible plant leaves: A comparative study. J. Med. Plants Stud. 2020, 8, 1164. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Gill, D.M. A micro-biuret method for estimating proteins. Anal. Biochem. 1964, 9, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Boctor, F.N. Hematin interference with lowry protein determination. Anal. Biochem. 1972, 50, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Rickert, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of kjeldahl and dumas methods for determining protein contents of soybean products. J. Am. Oil Chem. Soc. 2003, 80, 1169. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Grimsley, G.R.; Pace, C.N. Spectrophotometric Determination of Protein Concentration. Curr. Protoc. Protein Sci. 2003, 33, 3.1.1–3.1.9. [Google Scholar] [CrossRef] [PubMed]
| Essential Amino Acids | Content, g/100 g Protein | |
|---|---|---|
| «Ideal» Protein | Sunflower Meal Protein | |
| Histidine | 1.5 | 2.6 |
| Isoleucine | 3.0 | 5.0 |
| Leucine | 5.9 | 6.8 |
| Lysine | 4.5 | 4.4 |
| Threonine | 2.3 | 4.0 |
| Tryptophan | 0.6 | - |
| Valine | 3.9 | 5.4 |
| Methionine | 2.2 | 5.1 |
| Phenylalanine + tyrosine | 3.8 | 8.9 |
| Component | Content, % |
|---|---|
| Proteins | 20–40 |
| Carbohydrates | 4–18 |
| Lipids | 47–65 |
| Fatty acids: | |
| -palmitic acid | 5–7 |
| -stearic acid | 2–6 |
| -arachidonic acid | 0–0.3 |
| -oleic acid | 15–37 |
| -linoleic acid | 51–73 |
| -linolenic acid | <0.3 |
| Tocopherol | 0.07 |
| Carotenoids | 0.01–0.02 |
| Vitamin B1 | 0.002 |
| Chlorogenic acid | 0.5–2.8 |
| Quinic acid | 0.12–0.25 |
| Caffeic acid | 0.05–0.29 |
| Minerals | 3–4 |
| Potassium | 0.67–0.75 |
| Phosphorus | 0.60–0.94 |
| Sulfur | 0.26–0.32 |
| Magnesium | 0.35–0.41 |
| Calcium | 0.08–0.10 |
| Sodium | 0.02 |
| Method | Composition and Methods of Reagent Preparation | References |
|---|---|---|
| Benedict’s method | Reagent A: A total of 17.3 g of sodium citrate and 10 g of sodium carbonate were dissolved in 40 mL of water. Reagent B: A total of 1.73 g of copper sulfate was dissolved in 10 mL of water. Working solution: Reagent A and reagent B were mixed and diluted with more water to make a final volume of 100 mL. | [19] |
| BCA method | Reagent A: A total of 0.1 g of sodium bicinchoninate, 2 g of sodium carbonate, 0.16 g of sodium tartrate, and 0.4 g of sodium hydroxide were mixed with 0.95 g of sodium bicarbonate and diluted in water to obtain a final volume of 100 mL. Reagent B: A total of 0.4 g of copper sulfate was dissolved in 10 mL of water. Working solution: A total of 1 mL of reagent B was mixed with 50 mL of reagent A. | [20] |
| Bradford’s method | A total of 10 mg of Coomassie Brilliant Blue G-250 dye was dissolved in 5 mL of 95% ethanol. A total of 10 mL of 85% phosphoric acid was added, and the solution was diluted with water to obtain a final volume of 100 mL. | [21] |
| Lowry’s method | Reagent A: A total of 2 g of sodium carbonate was dissolved in 100 mL of 0.1 M sodium hydroxide solution. Reagent B: A total of 0.025 g of copper sulfate was dissolved in 50 mL of 1% sodium citrate solution. Working solution: A total of 1 mL of reagent B was mixed with 50 mL of reagent A. | [22,23] |
| Method | Range of Concentrations | Method Principle | Disadvantages |
|---|---|---|---|
| Direct spectrophotometric method | 20–2000 μg/mL | This method is based on the ability of aromatic amino acids (tryptophan, tyrosine, and, to a lesser extent, phenylalanine) to absorb ultraviolet (UV) light at 280 nm. | Since proteins differ in the content of aromatic amino acids, their absorption in the UV region of the spectrum varies greatly. When real samples are analyzed, an amino acid peak may be overlapped by an interfering matrix compound with an absorption maximum in the same region of the spectrum. |
| Benedict’s method | 200–2000 μg/mL | This method is based on the interaction of copper ions Cu2+ with peptide bonds of protein in an alkaline medium, forming a violet-colored complex. | This method is not recommended for reactions in solutions containing ammonium salts, due to the possibility of complex formation with copper, as well as in turbid or precipitated solutions. |
| BCA method | 200–2000 μg/mL | This method is based on reducing Cu2+ to Cu+ by interacting with the peptide bond of a protein and forming a colored Cu+ complex with bicinchoninic acid. | Determination is hindered by the presence of reducing substances, such as sugars, ascorbic acid, and thiol compounds. |
| Bradford’s method | 10–100 μg/mL | This method is based on the binding of Coomassie Blue G250 dye to amino acid residues in protein (primarily arginine and lysine), which shifts the absorption maximum from 470 nm (free dye) to 595 nm (bound dye), at which point the determination is carried out. | The dye most strongly binds to arginine and lysine residues, which can lead to errors in the determination of different types of proteins. BSA has an excessive dye-binding response and thus may underestimate the protein content of the analyzed sample. |
| Lowry’s method | 10–100 μg/mL | This method is based on the formation of colored products from aromatic amino acids using the Folin–Ciocalteu reagent in combination with the biuret reaction to detect peptide bonds. | Phenolic compounds, sugars, thiol compounds, and reducing agents interfere with analysis by enhancing background or leveling coloration with proteins. |
| Sample | Bradford’s Method | Benedict’s Method | BCA Method | Lowry’s Method | Dumas Method |
|---|---|---|---|---|---|
| Commercial sample-1 (90%) | 63.4 ± 1.7 | 91.0 ± 1.8 | 87.5 ± 3.3 | 89.7 ± 2.4 | 90.50 ± 0.07 |
| Commercial sample-2 (82.5%) | 44.2 ± 3.9 | 54.5 ± 5.3 | 75.3 ± 3.5 | 54.5 ± 3.3 | 51.56 ± 0.01 |
| Commercial sample-3 (78%) | 58.6 ± 1.1 | 62.2 ± 1.4 | 63.1 ± 1.3 | 76.1 ± 1.7 | 81.56 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vershinina, Y.S.; Mitin, I.V.; Garmay, A.V.; Sugakov, G.K.; Veselova, I.A. Simple and Robust Approach for Determination of Total Protein Content in Plant Samples. Foods 2025, 14, 358. https://doi.org/10.3390/foods14030358
Vershinina YS, Mitin IV, Garmay AV, Sugakov GK, Veselova IA. Simple and Robust Approach for Determination of Total Protein Content in Plant Samples. Foods. 2025; 14(3):358. https://doi.org/10.3390/foods14030358
Chicago/Turabian StyleVershinina, Yulia S., Ilya V. Mitin, Andrey V. Garmay, Gleb K. Sugakov, and Irina A. Veselova. 2025. "Simple and Robust Approach for Determination of Total Protein Content in Plant Samples" Foods 14, no. 3: 358. https://doi.org/10.3390/foods14030358
APA StyleVershinina, Y. S., Mitin, I. V., Garmay, A. V., Sugakov, G. K., & Veselova, I. A. (2025). Simple and Robust Approach for Determination of Total Protein Content in Plant Samples. Foods, 14(3), 358. https://doi.org/10.3390/foods14030358







