Effects of Grass Carp Antifreeze Peptide on Freeze-Thaw Characteristics and Structure of Wet Gluten Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals Instruments
2.2. Preparation of Wet Gluten Protein
2.3. Apparent Specific Heat of Wet Gluten Protein
2.4. Freeze–Thaw Characteristics of Wet Gluten Protein
2.5. Melting Characteristics and Fw of Wet Gluten Protein
2.6. Water Fluidity and Distribution of Wet Gluten Protein
2.7. Dynamic Rheological Characteristics of Wet Gluten Protein
2.8. Structural Characterization of Wet Gluten Protein
2.8.1. Content of Free S-H in Wet Gluten Protein
2.8.2. Secondary Structure of Wet Gluten Protein
2.8.3. SDS-PAGE of Wet Gluten Protein
2.8.4. Distribution of Relative Molecular Weight in Wet Gluten Protein
2.8.5. Microstructure of Wet Gluten Protein
2.9. Statistical Analysis
3. Results and Discussion
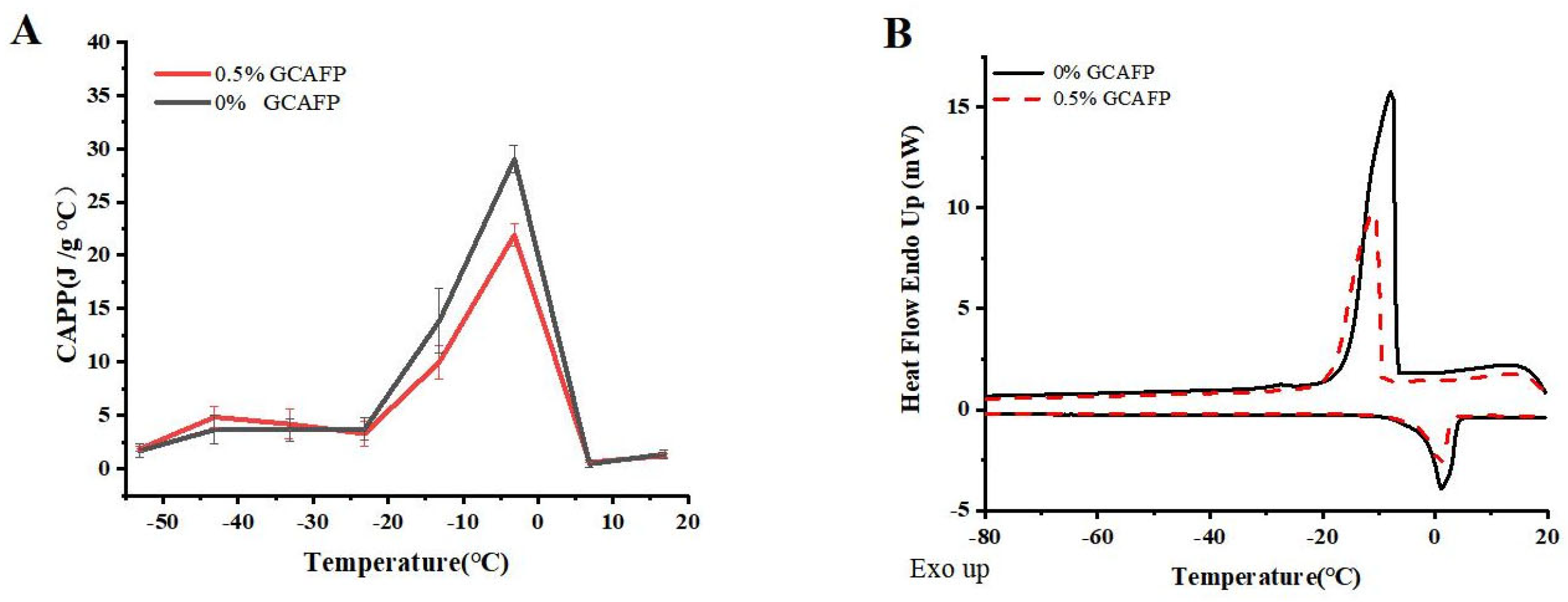
3.1. Effects of GCAFP on Thermodynamic Properties of Wet Gluten Protein
3.1.1. Effects of GCAFP on the Apparent Specific Heat of Wet Gluten Protein
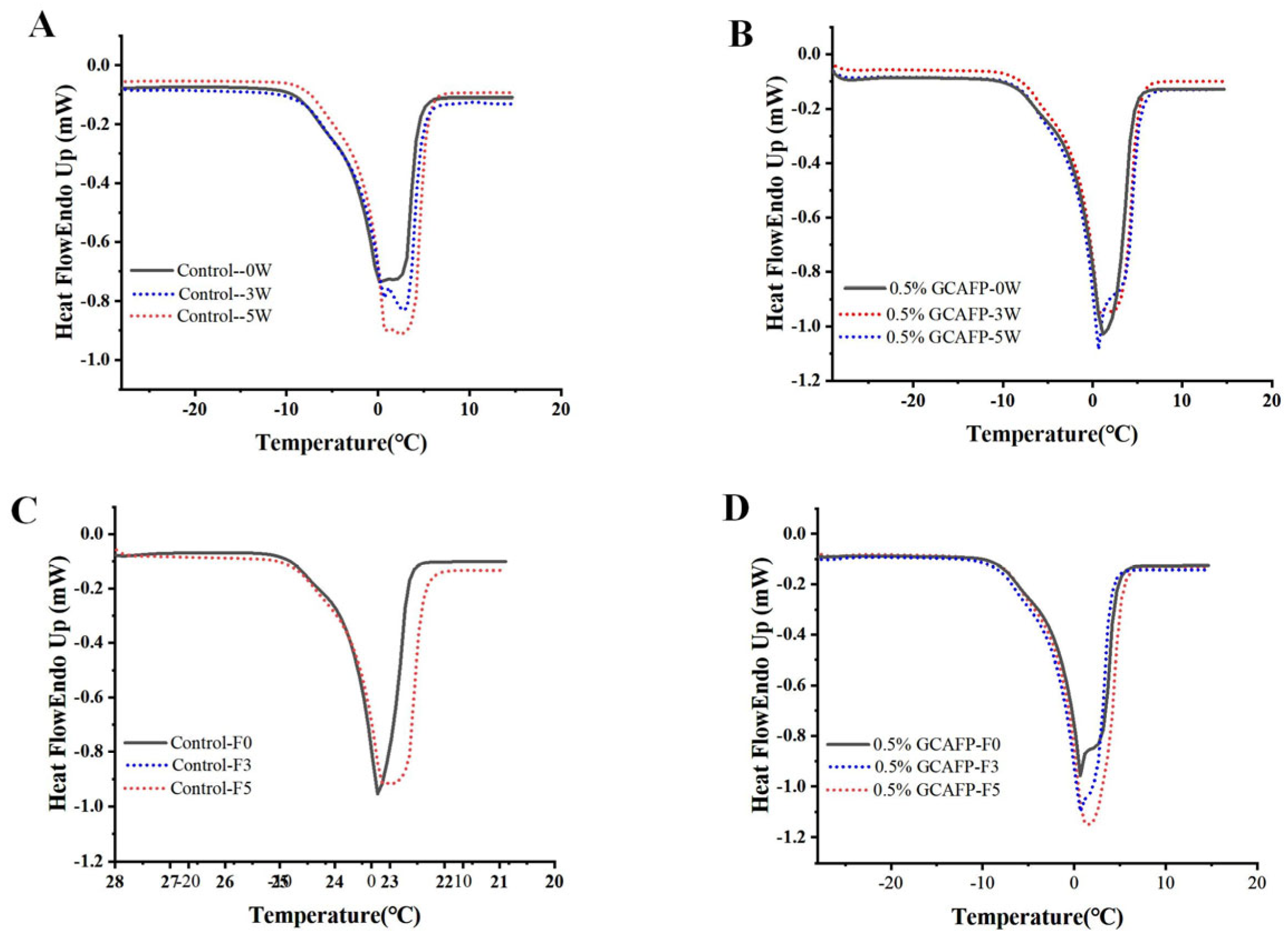
3.1.2. Effects of GCAFP on the Freeze–Thaw Characteristics of Wet Gluten Protein
3.1.3. Effects of GCAFP on the Melting Characteristics and Fw of Wet Gluten Protein
3.2. Effects of GCAFP on Water Fluidity of Wet Gluten Protein
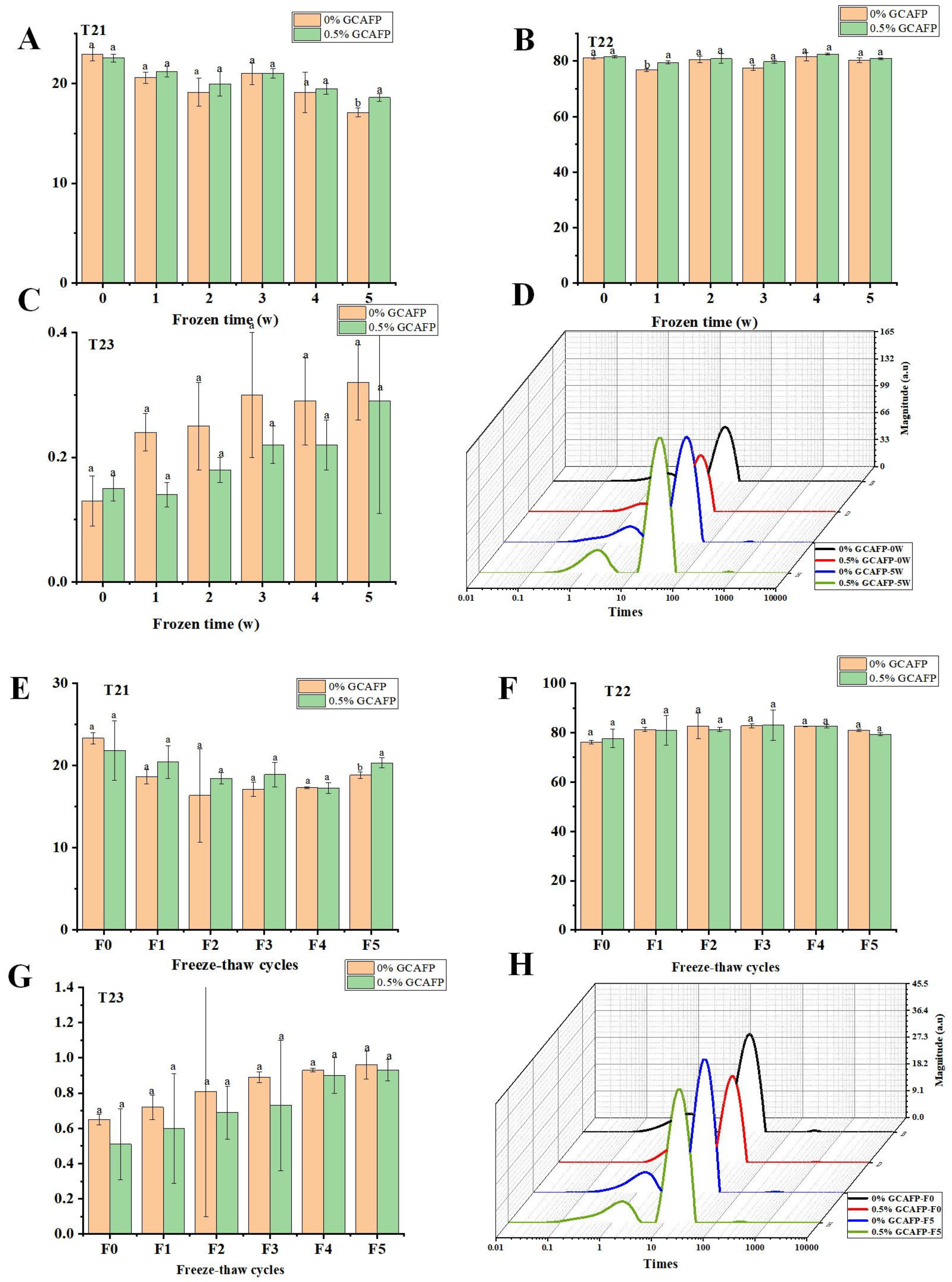
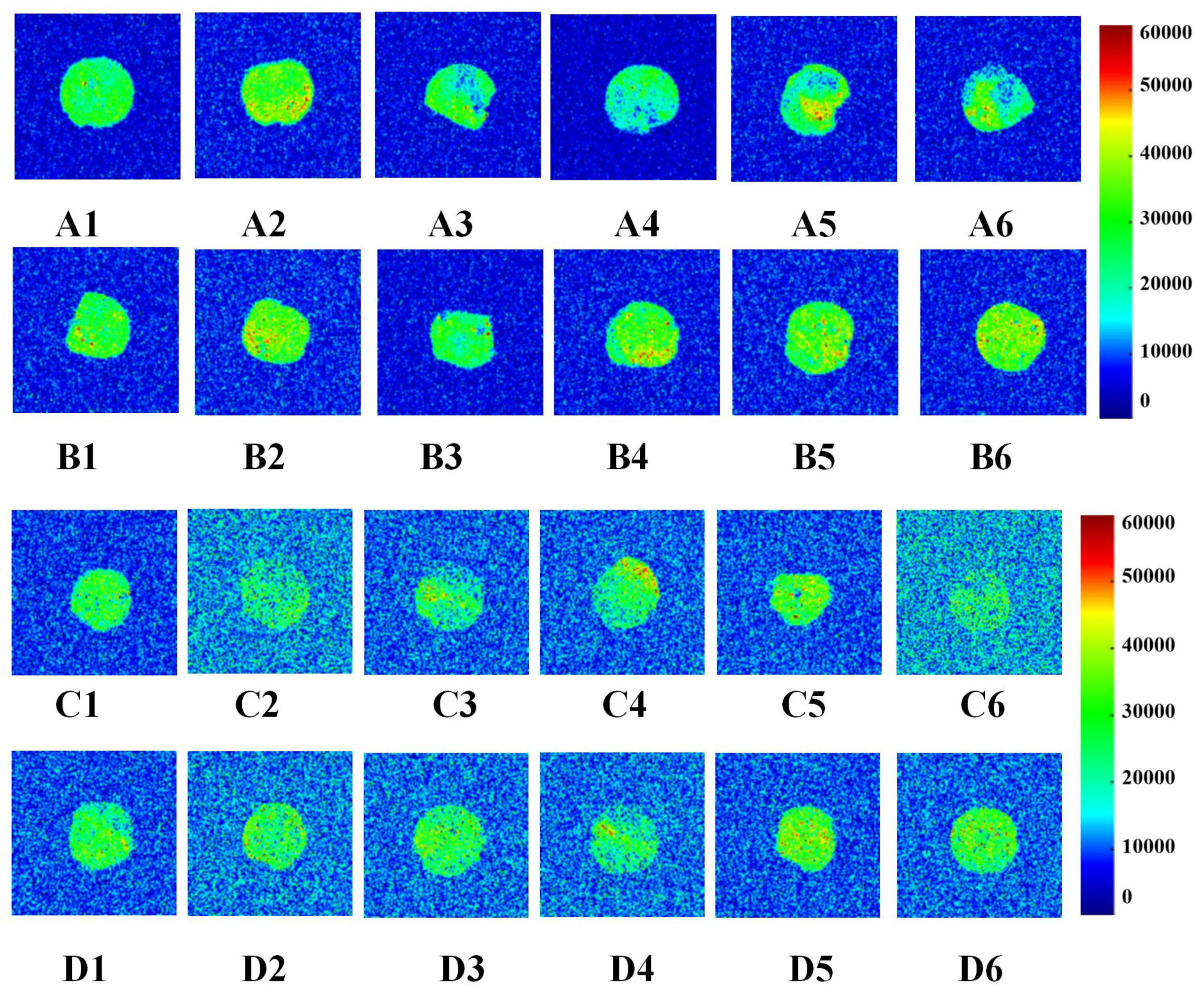
3.3. Effects of GCAFP on Water Distribution of Wet Gluten Protein
3.3.1. Water Distribution During Frozen Storage
3.3.2. Water Distribution During Freeze–Thaw Cycles
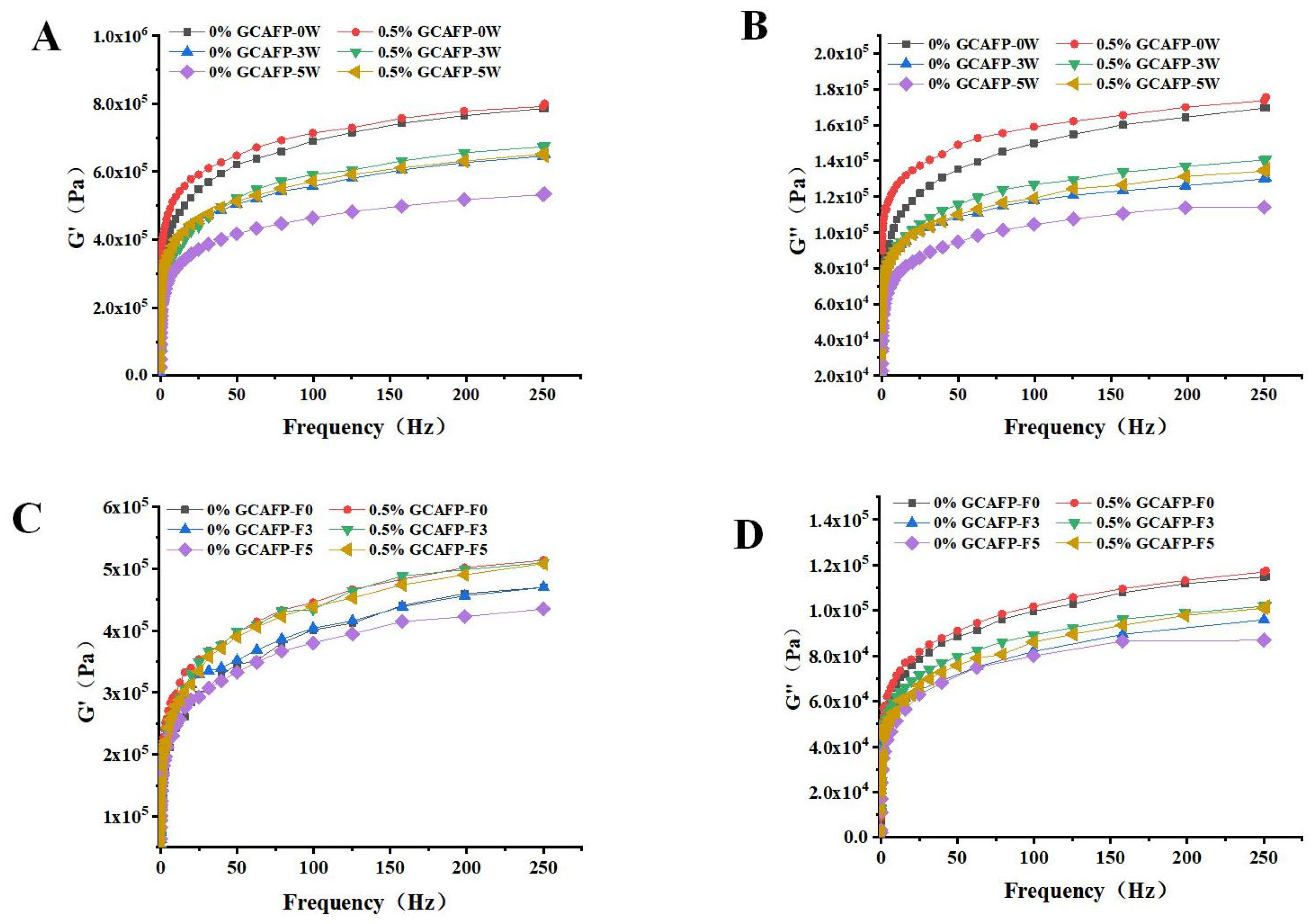
3.4. Effects of GCAFP on Rheological Properties of Wet Gluten Protein
3.5. Effects of GCAFP on the Structure of Wet Gluten Protein
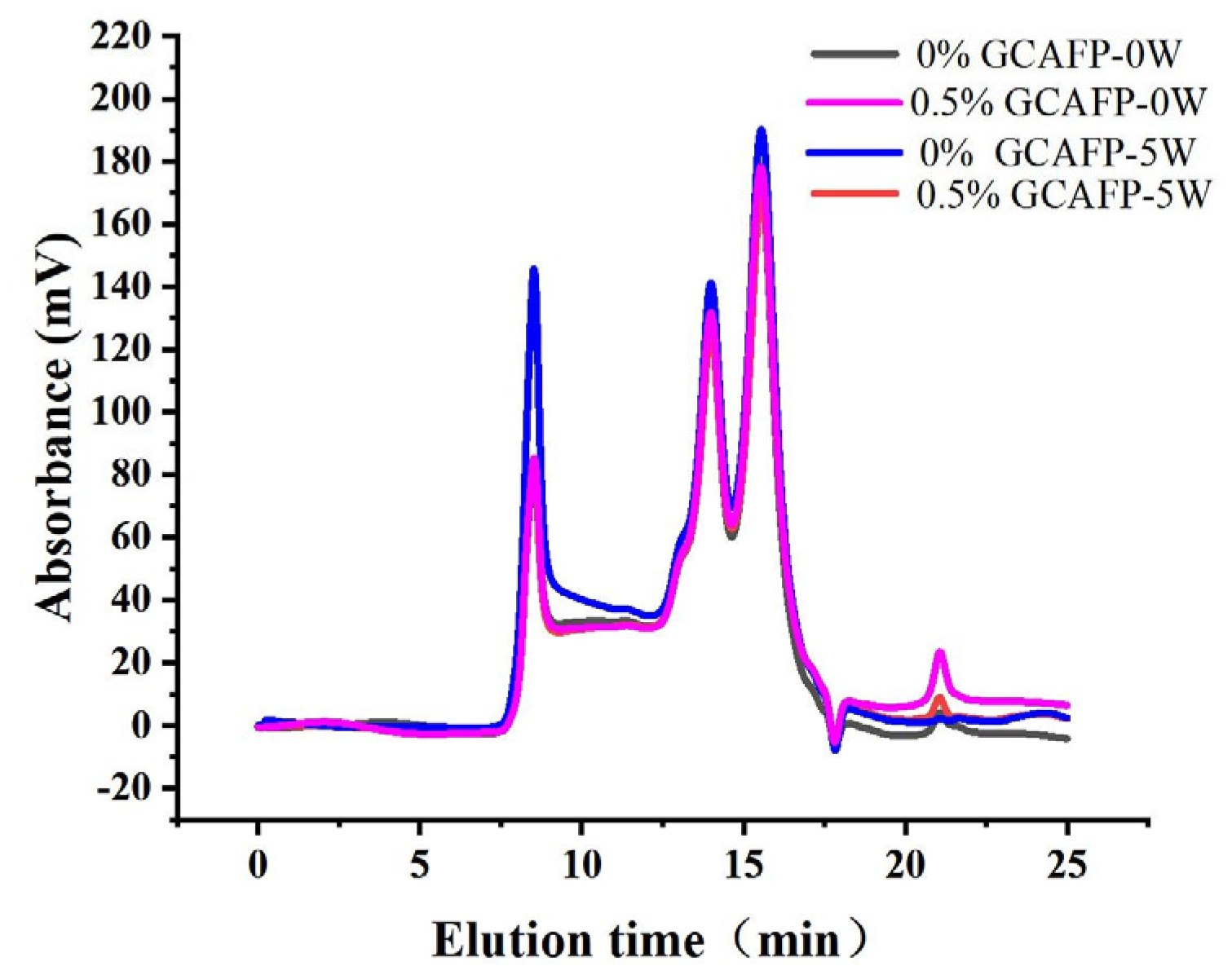
3.5.1. Effects of GCAFP on the Distribution of Relative Molecular Weight of Gluten Protein
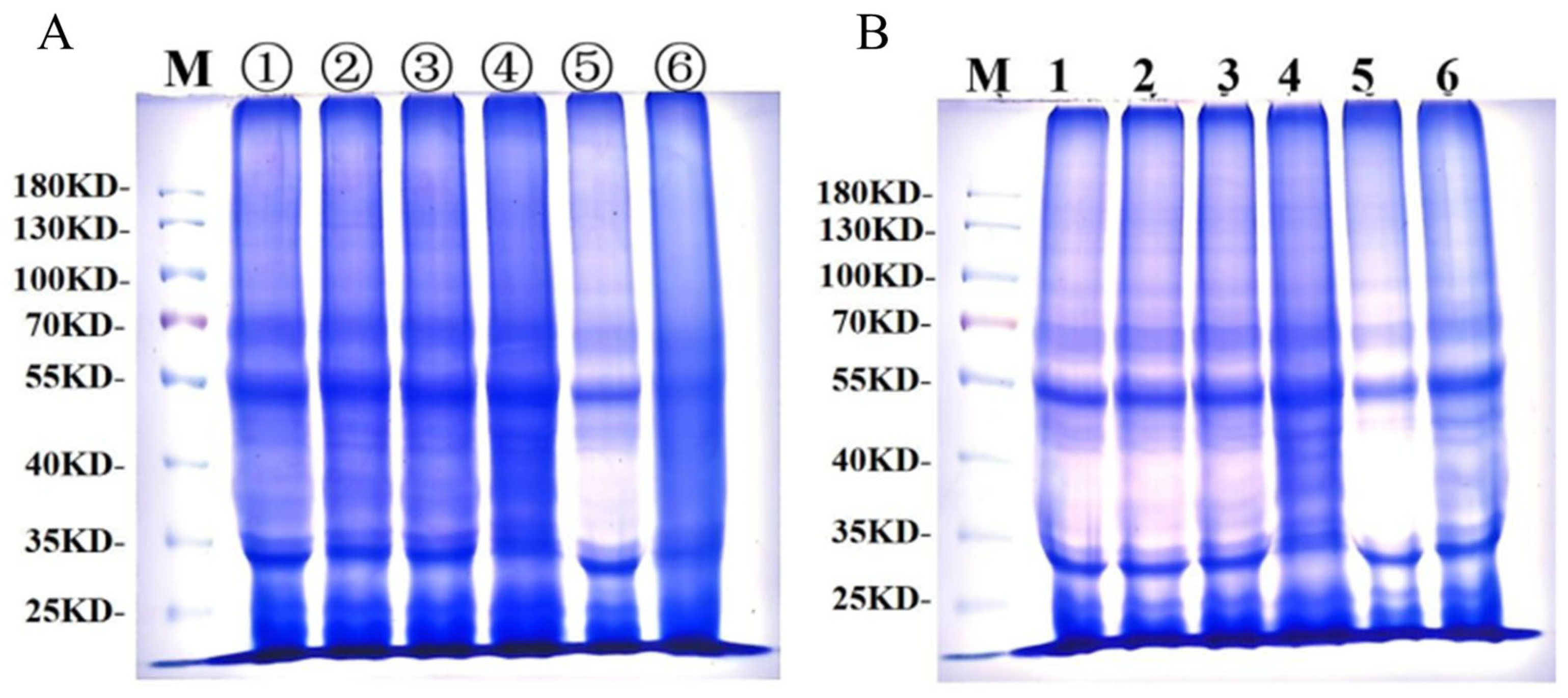
3.5.2. Effects of GCAFP on Wet Gluten Protein Electrophoretic Band
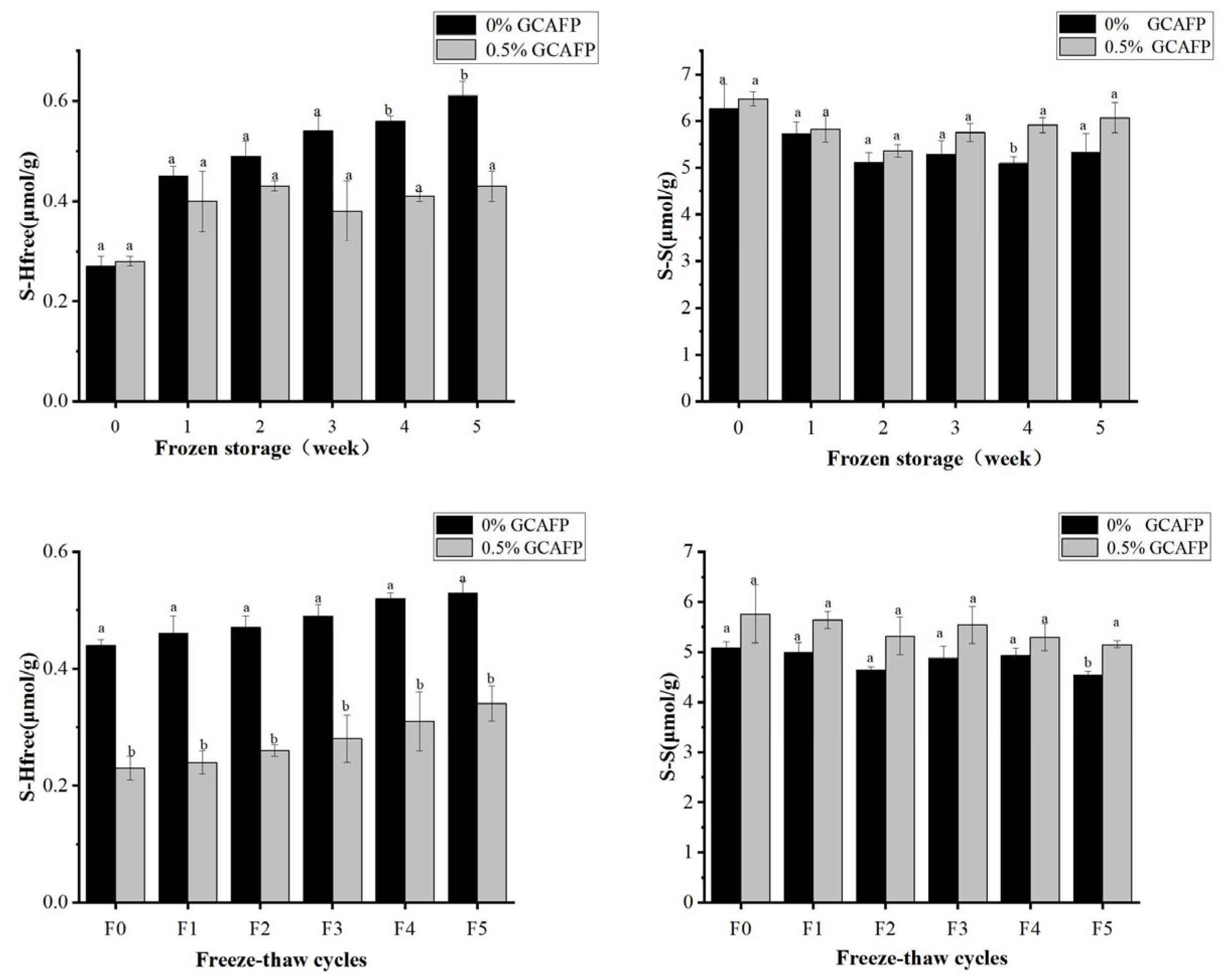
3.5.3. Effects of GCAFP on the Content of Free Thiol Group in Wet Gluten Protein
3.5.4. Effects of GCAFP on the Secondary Structure of Wet Gluten Protein
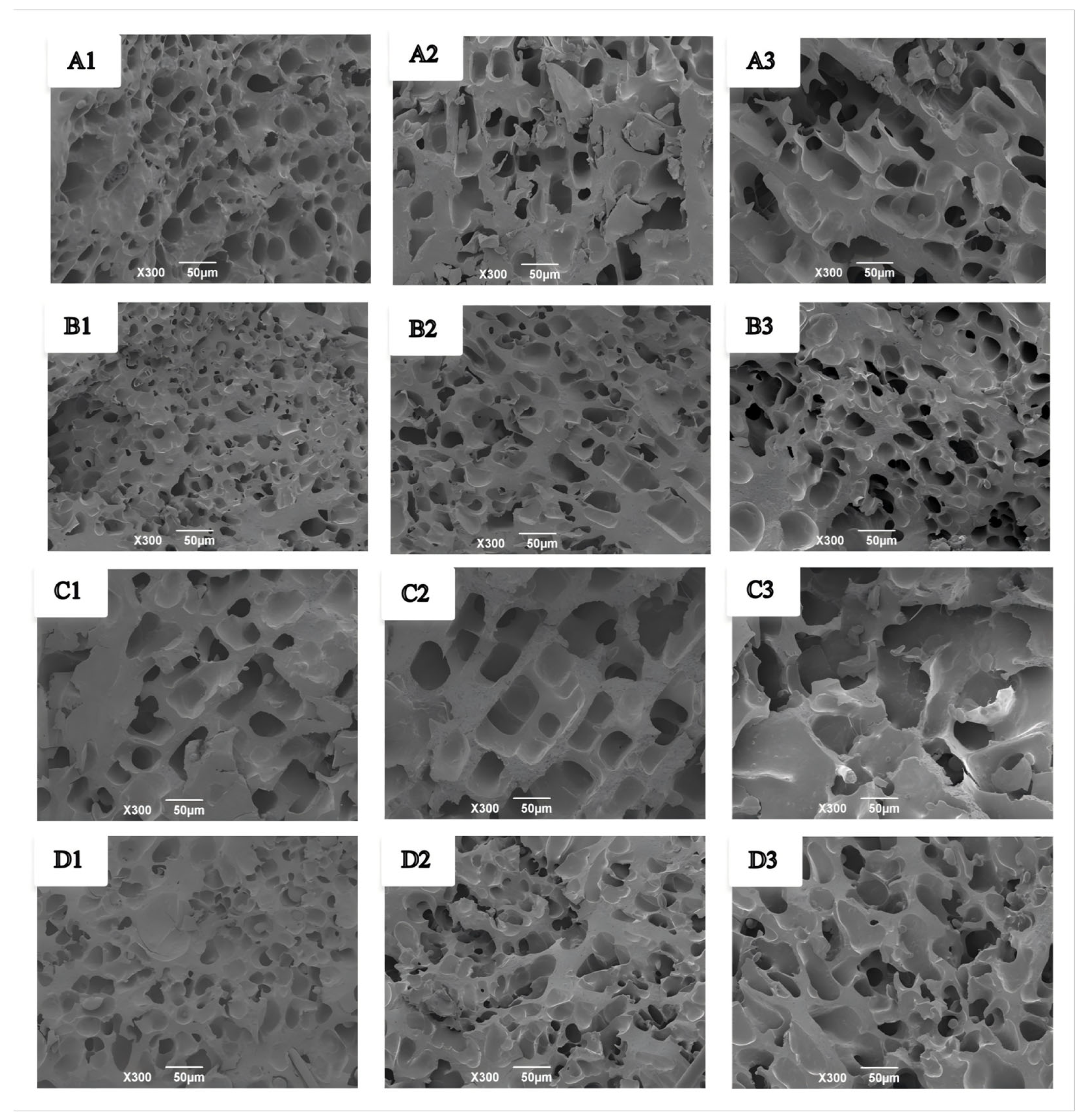
3.5.5. Effects of GCAFP on Protein Microstructure of Wet Gluten
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AsAFP | Oat antifreeze protein |
| GCAFP | Grass carp antifreeze peptide |
| EDTA | Ethylene Diamine Tetraacetic Acid |
| Fw | Freezable water content |
| Capp | Apparent specific heat |
| Tm | Melting temperature |
| Tm,δ | Melting temperature range |
| Tf | The peak temperature of the freezing curve |
| Tm,o | The onset temperature of the heat absorption curve |
| Tm,p | The peak temperature of the melting curve |
| Tm,e | The end temperature of the melting curve |
| Fw | The ratio of frozen water to total water |
| NMR | Nuclear magnetic resonance |
| Mw | Molecular weight |
| SDS | Sodium dodecyl sulfonate |
| SDS-P | SDS-soluble polymer |
| SDS-I | SDS-insoluble protein |
| ISP | Ice structuring protein |
| SaAFP | Salmon antifreeze peptide |
| Tris-HCl | Tris (hydroxymethyl) aminomethane hydrochloride |
| LD | Freezing temperature |
| ∆Hm | Melting enthalpy |
| DSC | Differential scanning calorimetry |
| SEM | Scanning electron microscopy |
| DTT | Dithiothreitol |
| SDS-M | SDS-soluble monomeric protein |
| GMP | Glutenin macropolymer |
References
- Chen, X.; Wu, J.-H.; Li, L.; Wang, S.-Y. The cryoprotective effects of antifreeze peptides from pigskin collagen on texture properties and water mobility of frozen dough subjected to freeze–thaw cycles. Eur. Food Res. Technol. 2017, 243, 1149–1156. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Zhang, H.; Guo, L.; Hong, T.; Zhang, W.; Jin, Y.; Xu, X. Effect of pigskin gelatin on baking, structural and thermal properties of frozen dough: Comprehensive studies on alteration of gluten network. Food Hydrocoll. 2020, 102, 105591. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Y.Y.; Wen, R.X.; Qin, L.; Chen, Q. A review of the mechanism of action of plant antifre-eze proteins and their application in food. Food Sci. 2019, 40, 305–312. [Google Scholar]
- Chen, X.; Wu, J.; Li, L.; Wang, S. Cryoprotective activity and action mechanism of antifreeze peptides obtained from tilapia scales on Streptococcus thermophilus during cold stress. J. Agric. Food Chem. 2019, 67, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Wang, J.H.; Al, Z.L.; Fan, H. Effects of plant-derived antifreeze proteins on properties of gluten: A review. Food Ferment. Ind. 2022, 48, 267–273. [Google Scholar]
- Baskaran, A.; Kaari, M.; Venugopal, G.; Manikkam, R.; Jerrine, J.; Bhaskar, P.V. Anti freeze proteins (Afp): Properties, sources and applications—A review. Int. J. Biol. Macromol. 2021, 189, 292–305. [Google Scholar] [CrossRef]
- Kontogiorgos, V.; Goff, H.D.; Kasapis, S. Effect of aging and ice-structuring proteins on the physical properties of frozen flour–water mixtures. Food Hydrocoll. 2007, 22, 1135–1147. [Google Scholar] [CrossRef]
- Vassilis, K.; Douglas, G.H.; Stefan, K. Effect of aging and ice structuring proteins on the morphology of frozen hydrated gluten networks. Biomacromolecules 2007, 8, 1293–1299. [Google Scholar] [CrossRef]
- Li, L.L.; Jia, C.L.; Huang, W.N.; Jin, L. Effect of Ice-structuring Protein on the Stability of Frozen Hydrated Gluten. Food Sci. 2019, 31, 25–28. [Google Scholar]
- Sun, L.J. Study on the Preparation of Antifreeze Peptide from Fish Skin and Its Cryopro-Tective Effects on Frozen Doughs. Master’s Thesis, Jiangnan University, Wuxi, China, 2017. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Ai, Z.; Zhang, H. Thermal, rheological properties and microstructure of hydrated gluten as influenced by antifreeze protein from oat (Avena sativa L.). J. Cereal Sci. 2020, 93, 102934. [Google Scholar] [CrossRef]
- Mohammed, O.; Bin, X. Characteristics and applications of plant-derived antifreeze proteins in frozen dough: A review. Int. J. Biol. Macromol. 2023, 255, 128202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J. Isolation and Purification of Oat (Avena sativa L.) Antifreeze Proteins and Their Effects on Frozen Dough. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2017. [Google Scholar]
- Ding, X.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Xiao, J. Effect of barley antifreeze protein on thermal properties and water state of dough during freezing and freeze-thaw cycles. Food Hydrocoll. 2015, 47, 32–40. [Google Scholar] [CrossRef]
- Gan, C.-L.; Guo, S.-S.; Rong, J.-H.; Xiong, S.-B. Thermo-physical Properties of Crisped Grass Crap Meat during Low Temperature Phase Transition. Food Sci. 2009, 30, 224–228. [Google Scholar]
- Zhang, Z.H. Manual of Chemical Analysis-Thermal Analysis; Chemical Industry Press: Beijing, China, 2000. [Google Scholar]
- Zhang, L.; Jin, Q.; Luo, J.; Wu, J.; Wang, S.; Wang, Z.; Gong, S.; Zhang, W.; Lan, X. Intracellular Expression of Antifreeze Peptides in Food Grade Lactococcus lactis and Evaluation of Their Cryoprotective Activity. J. Food Sci. 2018, 83, 1311–1320. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, Y.; Wang, L.; Ban, J.; Wei, Y.; Fan, F.; Guo, B. Dynamic Study on Water State and Water Migration during Gluten–Starch Model Dough Development under Different Gluten Protein Contents. Foods 2024, 13, 996. [Google Scholar] [CrossRef]
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef]
- Huang, W.; Kim, Y.; Li, X.; Rayas-Duarte, P. Rheofermentometer parameters and bread specific volume of frozen sweet dough influenced by ingredients and dough mixing temperature. J. Cereal Sci. 2008, 48, 639–646. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, J.H.; Zhang, S.H.; Ai, Z.L.; Pan, Z.L.; Li, Z.; Fan, H.P. Effects of carrot antifreeze protein on the quality of freeze-thaw dough under subfreezing. Trans. Chin. Soc. Agric. Eng. 2022, 38, 258–265. [Google Scholar]
- Ying, L.; Zhuoting, Q.; Mei, L.; Mengfei, Z.; Xia, Z.; Le, W.; Feng, J.; Xiaobei, Z.; Jinshui, W. Further interpretation of the strengthening effect of curdlan on frozen cooked noodles quality during frozen storage: Studies on water state and properties. Food Chem. 2021, 346, 128908. [Google Scholar] [CrossRef]
- Jia, C.L. Studies on Cryoprotective Characteristics of Frozen Doughs Using Thermostable Ice Structuring Protein. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2013. [Google Scholar]
- Bhattacharya, M.; Langstaff, T.M.; Berzonsky, W.A. Effect of frozen storage and freeze–thaw cycles on the rheological and baking properties of frozen doughs. Food Res. Int. 2003, 36, 365–372. [Google Scholar] [CrossRef]
- Jia, C.; Huang, W.; Rayas-Duarte, P.; Zou, Q.; Zhang, L.; Li, Y. Hydration, polymerization and rheological properties of frozen gluten-water dough as influenced by thermostable ice structuring protein extract from Chinese privet (Ligustrum vulgare) leaves. J. Cereal Sci. 2014, 59, 132–136. [Google Scholar] [CrossRef]
- Omkar, V.; David, N.; Ling, H.S. Analysis of structural rearrangements of poly(lactic acid) in the presence of water. J. Phys. Chem. B 2014, 118, 4185–4193. [Google Scholar]
- Ribotta, P.D.; León, A.E.; Añón, M.C. Effect of freezing and frozen storage of doughs on bread quality. J. Agric. Food Chem. 2001, 49, 913–918. [Google Scholar] [CrossRef]
- Ding, X.L. Study on the Preparation and the Antifreeze Mechanism of Antifreeze Protein from Barley (Hordeum vulgare). Ph.D. Thesis, Jiangnan University, Wuxi, China, 2015. [Google Scholar]
- Bekiroglu, H.; Ozulku, G.; Sagdic, O. Effects of Casein Hydrolysate Prepared with Savinase on the Quality of Bread Made by Frozen Dough. Foods 2023, 12, 3854. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Bell, A.E.; Schofield, J.D. The dynamic rheological properties of glutens and gluten sub-fractions from wheats of good and poor bread making quality. J. Cereal Sci. 1995, 22, 29–44. [Google Scholar] [CrossRef]
- Hayta, M.; Schofield, J.D. Dynamic rheological behavior of wheat glutens during heating. J. Sci. Food Agric. 2005, 85, 1992–1998. [Google Scholar] [CrossRef]
- Kokini, J.L.; Cocero, A.M.; Madeka, H.; De Graaf, E. The development of state diagrams for cereal proteins. Trends Food Sci. Technol. 1994, 5, 281–288. [Google Scholar] [CrossRef]
- Kline, L. Factors affecting the stability of frozen bread doughs. I. Prepared by the straight dough method. Bak. Dig. 1968, 42, 44. [Google Scholar]
- Cornec, M.; Popineau, Y.; Lefebvre, J. Characterisation of gluten subfractions by SE-HPLC and dynamic rheological analysis in shear. J. Cereal Sci. 1994, 19, 131–139. [Google Scholar] [CrossRef]
- Hayta, M.; Schofield, J.D. Heat and additive induced biochemical transitions in gluten from good and poor breadmaking quality wheats. J. Cereal Sci. 2004, 40, 245–256. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, J.; Chen, L.; Zhou, Y.; Wu, J. Preparation, isolation and hypothermia protection activity of antifreeze peptides from shark skin collagen. LWT Food Sci. Technol. 2014, 55, 210–217. [Google Scholar] [CrossRef]
- Sharadanant, R.; Khan, K. Effect of Hydrophilic Gums on the Quality of Frozen Dough: II. Bread Characteristics. Cereal Chem. 2003, 80, 773–780. [Google Scholar] [CrossRef]
- Singh, H.; MacRitchie, F. Application of Polymer Science to Properties of Gluten. J. Cereal Sci. 2001, 33, 231–243. [Google Scholar] [CrossRef]
- Ahui, Z.; Peiwen, S.; Runqiang, Y.; Zhenxin, G.; Dong, J.; Pei, W. Isolation of novel wheat bran antifreeze polysaccharides and the cryoprotective effect on frozen dough quality. Food Hydrocoll. 2022, 125, 107446. [Google Scholar] [CrossRef]
- Zhao, L.; Li, L.; Li, B.; Liu, G.-Q.; Liu, X.-X. Effect of Frozen Storage on Molecular Weight, Size Distribution and Conformation of Gluten by SAXS and SEC-MALLS. Molecules 2012, 17, 7169–7182. [Google Scholar] [CrossRef]
- Jood, S.; Schofield, J.D.; Tsiami, A.A.; Bollecker, S. Effect of glutenin subfractions on bread-making quality of wheat. Int. J. Food Sci. Technol. 2001, 36, 573–584. [Google Scholar] [CrossRef]
- Liu, G.Q.; Yan, N.J.; Zhao, L.; Li, B.; Li, L.; Yang, X.Q. Effect of Frozen Storage on Secondary Structure of Gluten Proteins. J. South China Univ. Technol. (Nat. Sci. Ed.) 2012, 40, 115–120. [Google Scholar]
- Ding, X.L.; Li, T.T.; Zhang, H.; Guan, C.R.; Qian, J.Y.; Zhou, X.Y. Effect of Barley Antifreeze Protein on Dough and Bread during Freezing and Freeze-Thaw Cycles. Foods 2020, 9, 1698. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Q.F.; Li, X.X. Effect of carrot antifreeze protein on quality of frozen dough and steamed bread. Cereals Oils 2021, 34, 51–54. [Google Scholar]
| Gluten Protein | 0% GCAFP | 0.5% GCAFP |
|---|---|---|
| Tf (°C) | −8.50 ± 1.31 a | −10.75 ± 2.49 a |
| Tm,o (°C) | −1.70 ± 0.41 a | −2.05 ± 0.37 a |
| Tm,p (°C) | −0.65 ± 0.28 a | −1.15 ± 0.04 a |
| Tm,δ (°C) | 3.60 ± 1.4 a | 5.65 ± 0.12 b |
| ∆Hm (J/g) | 145.7 ± 0.54 a | 113.9 ± 1.38 b |
| Fw (%) | 81.04 ± 2.65 a | 61.28 ± 1.02 b |
| Gluten Protein | Frozen Storage Time (W) | Tm,o (°C) | Tm,δ (°C) | ∆Hm (J/g) | WA (%) | Fw (%) |
|---|---|---|---|---|---|---|
| 0% GCAFP | 0 | −3.35 ± 1.34 a | 6.85 ± 1.34 b | 96.91 ± 2.47 b | 53.90 ± 0.92 a | 57.75 ± 0.09 b |
| 1 | −2.60 ± 1.84 a | 4.80 ± 4.36 a | 102.07 ± 3.64 a | 51.94 ± 0.42 a | 58.83 ± 1.43 b | |
| 2 | −1.75 ± 0.07 a | 6.83 ± 0.72 b | 123.95 ± 13.20 a | 51.37 ± 11.38 a | 69.52 ± 17.56 ab | |
| 3 | −1.23 ± 0.46 a | 4.70 ± 3.39 a | 117.14 ± 0.37 a | 47.89 ± 0.84 a | 73.30 ± 1.06 ab | |
| 5 | −1.15 ± 0.64 a | 4.15 ± 3.32 a | 124.14 ± 4.76 a | 43.65 ± 0.84 a | 81.89 ± 7.37 a | |
| 0.5% GCAFP | 0 | −3.25 ± 1.34 b | 7.30 ± 1.98 a | 96.08 ± 1.30 b | 51.64 ± 0.63 a | 54.91 ± 0.15 b |
| 1 | −2.60 ± 1.59 b | 6.23 ± 1.25 a | 105.35 ± 5.21 a | 48.36 ± 0.23 a | 56.97 ± 17.52 b | |
| 2 | −2.95 ± 0.07 b | 7.75 ± 0.21 a | 108.7 ± 2.69 a | 50.26 ± 1.23 a | 63.96 ± 0.48 b | |
| 3 | −1.70 ± 0.20 a | 7.20 ± 0.14 a | 118.77 ± 7.18 a | 48.66 ± 0.88 a | 75.12 ± 5.26 a | |
| 5 | −1.55 ± 0.07 a | 6.56 ± 1.26 a | 121.80 ± 2.40 a | 48.89 ± 1.07 a | 78.41 ± 3.98 a |
| Gluten Protein | Freeze–Thaw | Tm,o (°C) | Tm,δ (°C) | ∆Hm (J/g) | WA (%) | Fw (%) |
|---|---|---|---|---|---|---|
| 0% GCAFP | 0 | −3.35 ± 1.34 b | 7.85 ± 1.34 a | 96.91 ± 2.47 a | 53.90 ± 0.92 a | 57.75 ± 0.09 b |
| F0 | −2.90 ± 0.71 ab | 7.55 ± 0.07 a | 106.22 ± 27.13 a | 48.33 ± 3.01 ab | 67.01 ± 21.16 ab | |
| F1 | −2.45 ± 0.21 ab | 7.00 ± 4.95 a | 119.50 ± 27.73 a | 47.93 ± 3.01 ab | 75.34 ± 22.05 ab | |
| F2 | −2.21 ± 0.28 ab | 7.05 ± 0.78 a | 117.80 ± 1.84 a | 49.36 ± 0.71 ab | 71.46 ± 3.40 ab | |
| F3 | −2.20 ± 0.14 ab | 7.15 ± 0.49 a | 120.40 ± 4.38 a | 51.84 ± 1.86 ab | 70.25 ± 4.16 ab | |
| F4 | −2.05 ± 1.06 ab | 6.35 ± 1.91 a | 123.41 ± 7.70 a | 48.97 ± 0.95 a | 71.25 ± 1.89 a | |
| F5 | −1.90 ± 0.14 a | 5.67 ± 1.27 a | 122.85 ± 5.30 a | 49.97 ± 1.37 a | 73.59 ± 1.16 a | |
| 0.5% GCAFP | 0 | −3.25 ± 0.72 a | 7.30 ± 1.98 a | 96.08 ± 1.30 a | 51.64 ± 0.63 ab | 54.91 ± 0.15 b |
| F0 | −3.05 ± 0.49 a | 7.70 ± 0.28 a | 103.72 ± 23.6 a | 54.93 ± 1.23 a | 56.40 ± 11.60 ab | |
| F1 | −2.90 ± 0.98 a | 7.65 ± 2.47 a | 104.09 ± 14.48 a | 49.01 ± 0.50 b | 58.54 ± 4.75 a | |
| F2 | −2.57 ± 0.72 a | 6.50 ± 1.65 a | 117.20 ± 9.90 a | 52.56 ± 0.18 a | 69.83 ± 9.34 a | |
| F3 | −2.35 ± 0.07 a | 5.15 ± 3.32 a | 118.74 ± 6.73 a | 51.84 ± 1.86 ab | 68.70 ± 6.35 a | |
| F4 | −2.40 ± 1.70 a | 7.05 ± 1.06 a | 111.3 ± 10.89 a | 50.84 ± 1.86 ab | 64.44 ± 8.60 a | |
| F5 | −2.50 ± 0.14 a | 6.20 ± 1.15 a | 116.25 ± 3.18 a | 51.39 ± 0.66 a | 68.40 ± 3.67 a |
| Gluten Protein | Frozen Storage Time (W) | G’ | G” |
|---|---|---|---|
| 0% GCAFP | 0 | 571,973 | 149,840 |
| 3 | 591,317 | 117,871 | |
| 5 | 464,525 | 101,221 | |
| 0.5% GCAFP | 0 | 689,859 | 159,039 |
| 3 | 713,542 | 126,809 | |
| 5 | 556,870 | 119,135 |
| Gluten Protein | Frozen Storage Time (W) | SDS-P | SDS-M | GMP Content | GMP Depolymerization Level |
|---|---|---|---|---|---|
| 0% GCAFP | 0 | 13.04 | 39.68 | 47.28 | - |
| 3 | 15.45 | 35.21 | 39.34 | 18.91 | |
| 5 | 17.60 | 46.87 | 35.53 | 24.85 | |
| 0.5% GCAFP | 0 | 12.79 | 38.81 | 48.40 | - |
| 3 | 14.25 | 41.90 | 43.85 | 9.40 | |
| 5 | 15.32 | 45.45 | 39.23 | 18.95 |
| Gluten Protein | Frozen Storage Time (W) | Secondary Structure (%) | |||
|---|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | ||
| 0% GCAFP | 0 | 19.32 ± 1.34 b | 50.56 ± 3.24 b | 20.21 ± 3.04 a | 9.91 ± 1.14 a |
| 3 | 17.44 ± 3.22 a | 55.54 ± 2.31 a | 19.75 ± 3.08 a | 7.27 ± 1.02 a | |
| 5 | 15.71 ± 2.68 a | 59.48 ± 2.56 a | 18.38 ± 2.59 b | 6.43 ± 0.81 a | |
| 0.5% GCAFP | 0 | 19.58 ± 3.17 a | 51.02 ± 4.30 a | 20.4 ± 4.35 a | 8.95 ± 0.96 b |
| 3 | 17.87 ± 2.65 a | 54.56 ± 2.39 a | 20.37 ± 2.36 a | 7.20 ± 0.63 ab | |
| 5 | 16.69 ± 1.98 a | 58.39 ± 1.02 a | 18.04 ± 3.37 a | 6.88 ± 0.34 a | |
| Gluten Protein | Freeze–Thaw | Secondary Structure (%) | |||
|---|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | ||
| 0% GCAFP | F0 | 20.08 ± 3.31 b | 49.76 ± 3.71 b | 19.361 ± 2.04 a | 10.80 ± 0.51 a |
| F3 | 18.51 ± 2.47 ab | 54.24 ± 2.96 ab | 18.47 ± 1.59 a | 8.78 ± 0.25 a | |
| F5 | 14.62 ± 1.82 a | 58.34 ± 3.85 a | 17.98 ± 1.15 a | 9.06 ± 0.48 a | |
| 0.5% GCAFP | F0 | 20.26 ± 4.26 a | 50.33 ± 2.82 a | 19.86 ± 2.06 a | 9.55 ± 0.74 a |
| F3 | 19.07 ± 2.84 a | 53.80 ± 3.56 a | 19.01 ± 1.52 a | 8.12 ± 0.62 b | |
| F5 | 15.39 ± 1.63 a | 57.09 ± 4.32 a | 18.04 ± 0.68 a | 9.48 ± 0.92 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, M.; Huang, B.; Jia, Y.; Shao, Y.; Mei, X.; Li, C. Effects of Grass Carp Antifreeze Peptide on Freeze-Thaw Characteristics and Structure of Wet Gluten Protein. Foods 2025, 14, 4336. https://doi.org/10.3390/foods14244336
Dang M, Huang B, Jia Y, Shao Y, Mei X, Li C. Effects of Grass Carp Antifreeze Peptide on Freeze-Thaw Characteristics and Structure of Wet Gluten Protein. Foods. 2025; 14(24):4336. https://doi.org/10.3390/foods14244336
Chicago/Turabian StyleDang, Meizhu, Bing Huang, Yangyang Jia, Yuanyuan Shao, Xingxing Mei, and Chunmei Li. 2025. "Effects of Grass Carp Antifreeze Peptide on Freeze-Thaw Characteristics and Structure of Wet Gluten Protein" Foods 14, no. 24: 4336. https://doi.org/10.3390/foods14244336
APA StyleDang, M., Huang, B., Jia, Y., Shao, Y., Mei, X., & Li, C. (2025). Effects of Grass Carp Antifreeze Peptide on Freeze-Thaw Characteristics and Structure of Wet Gluten Protein. Foods, 14(24), 4336. https://doi.org/10.3390/foods14244336






