An Exploratory Study on the Influence of Frying on Chemical Constituent Transformation and Antioxidant Activity in Ziziphi Spinosae Semen: A Multimodal Analytical Strategy Based on UPLC–Q–TOF–MS and GC–IMS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stir-Fried ZSS
2.3. Preparation of Ziziphi Spinosae Semen Oil
2.4. Non-Targeted Metabolomics Analysis Based on UPLC-Q-TOF-MS
2.4.1. Preparation of Sample Solutions
2.4.2. Preparation of Reference Standard Solutions
2.4.3. Chromatographic Conditions
2.4.4. Mass Spectrometric Conditions
2.5. Analysis of Volatile Components Using GC-IMS
2.6. Evaluation of In Vitro Antioxidant Activity of Ziziphi Spinosae Semen Oil
2.7. Statistical Analysis
3. Results
3.1. Analysis of UPLC-Q-TOF-MS Detection Results
3.1.1. UPLC-Q-TOF-MS Component Identification Results
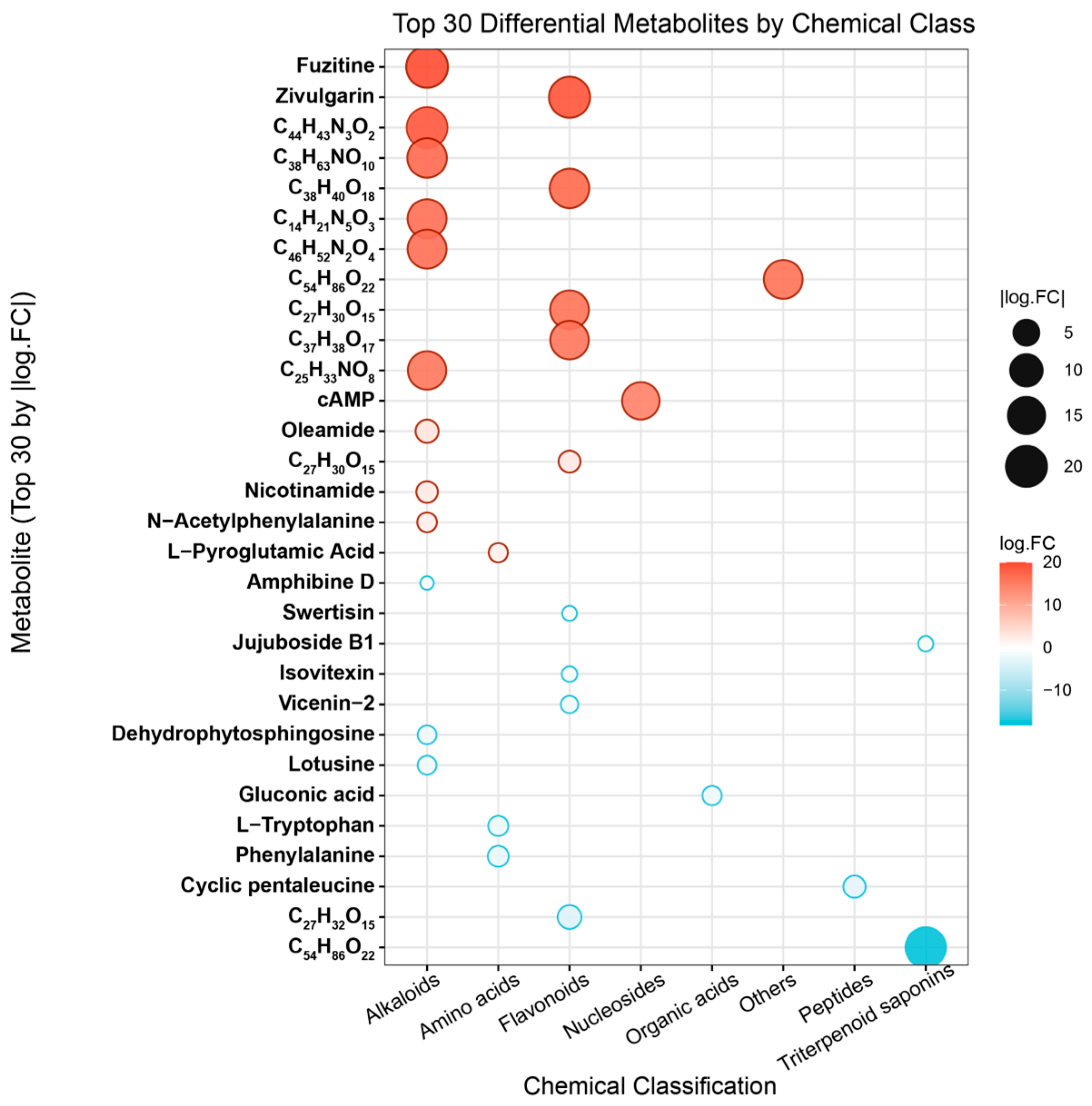
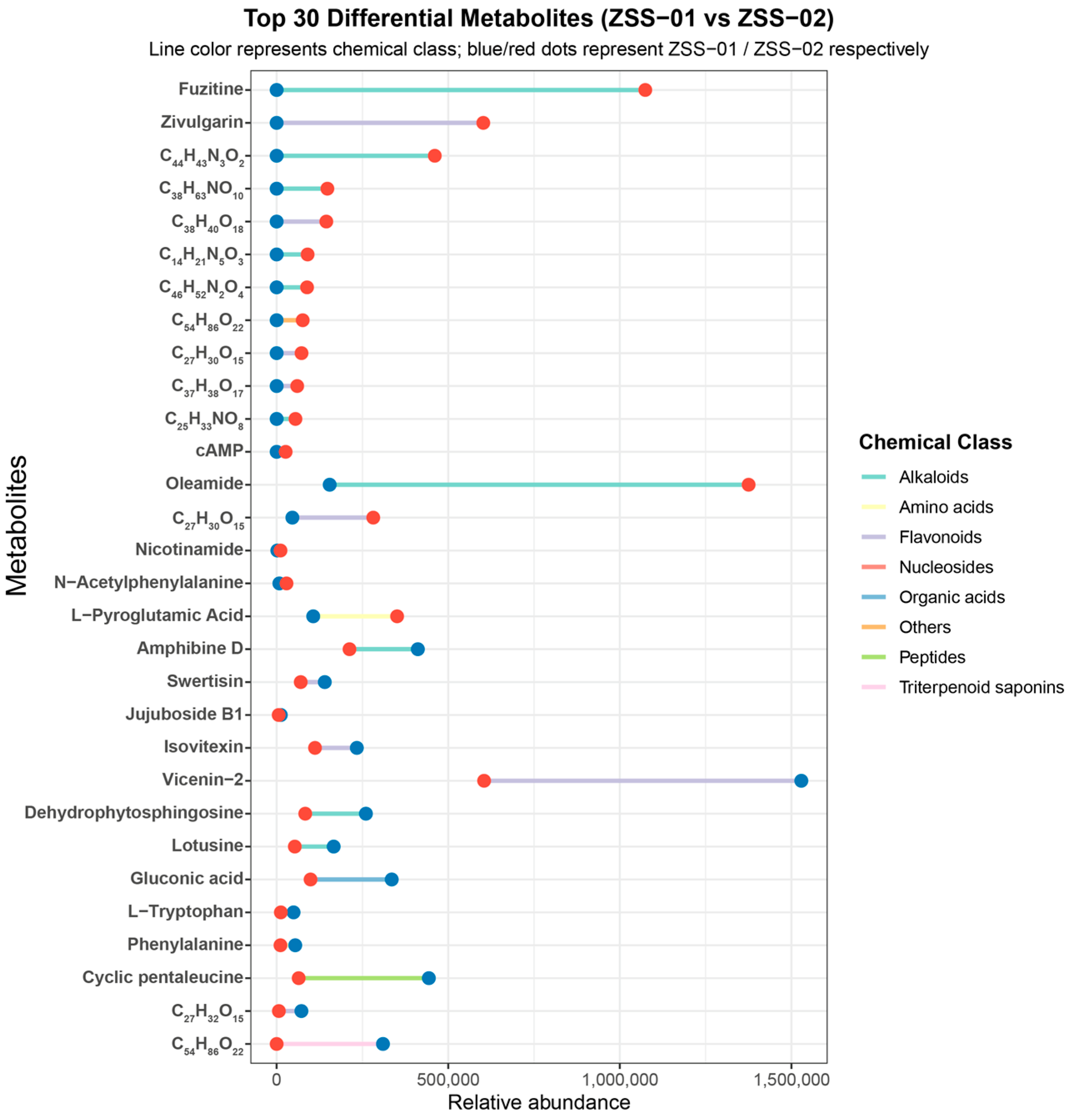
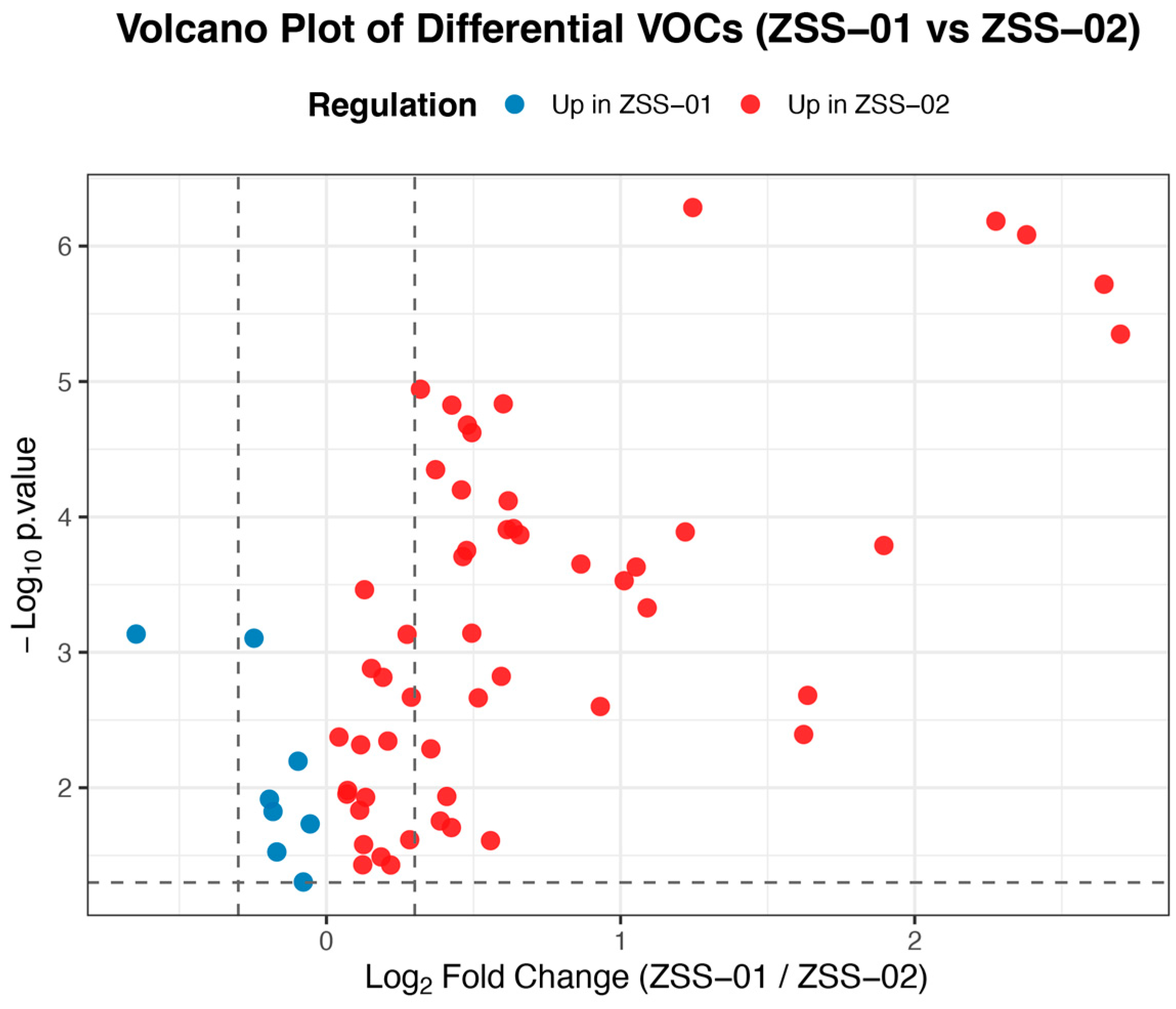
3.1.2. Qualitative Comparison of Component Distribution and Chemical Category Differences
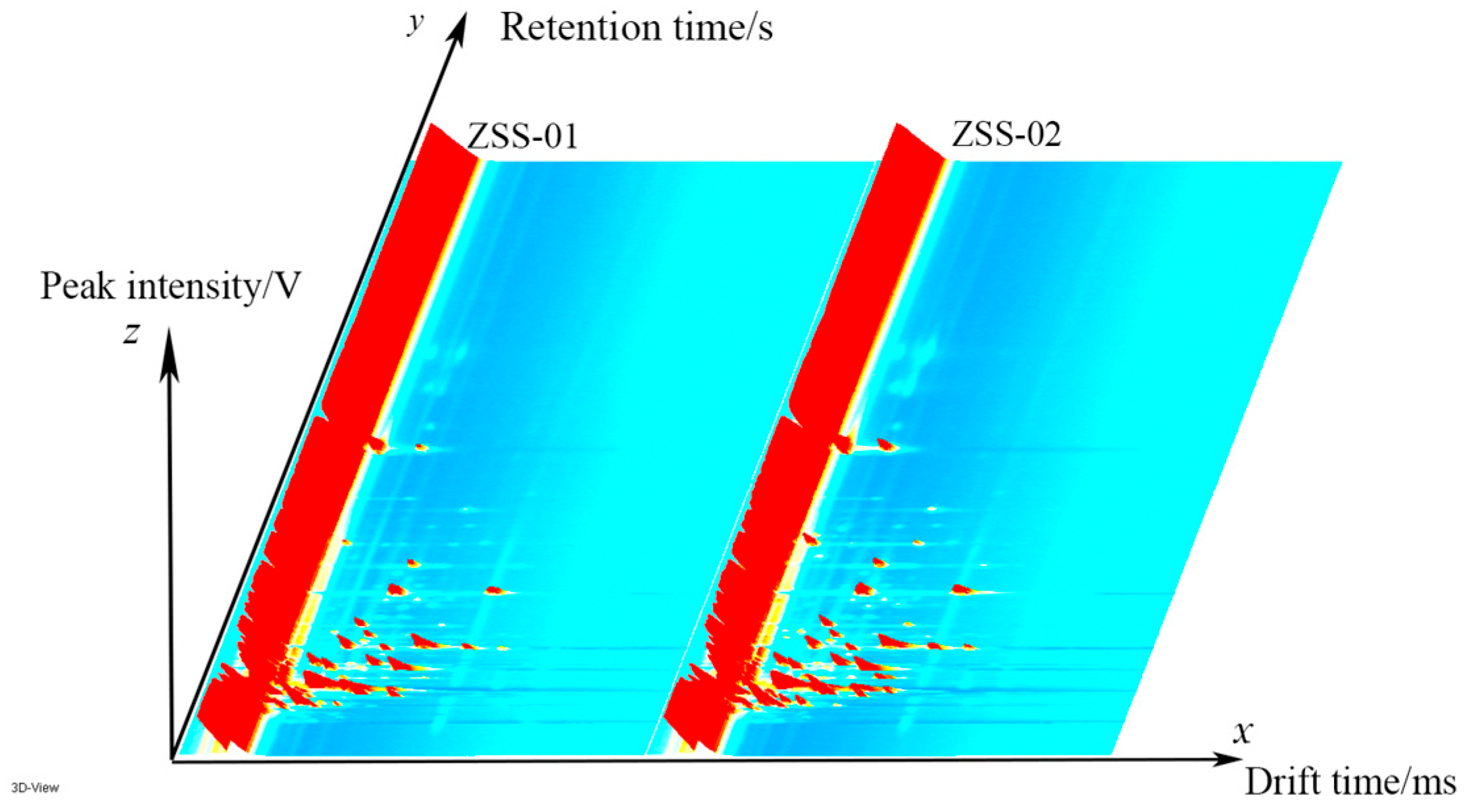
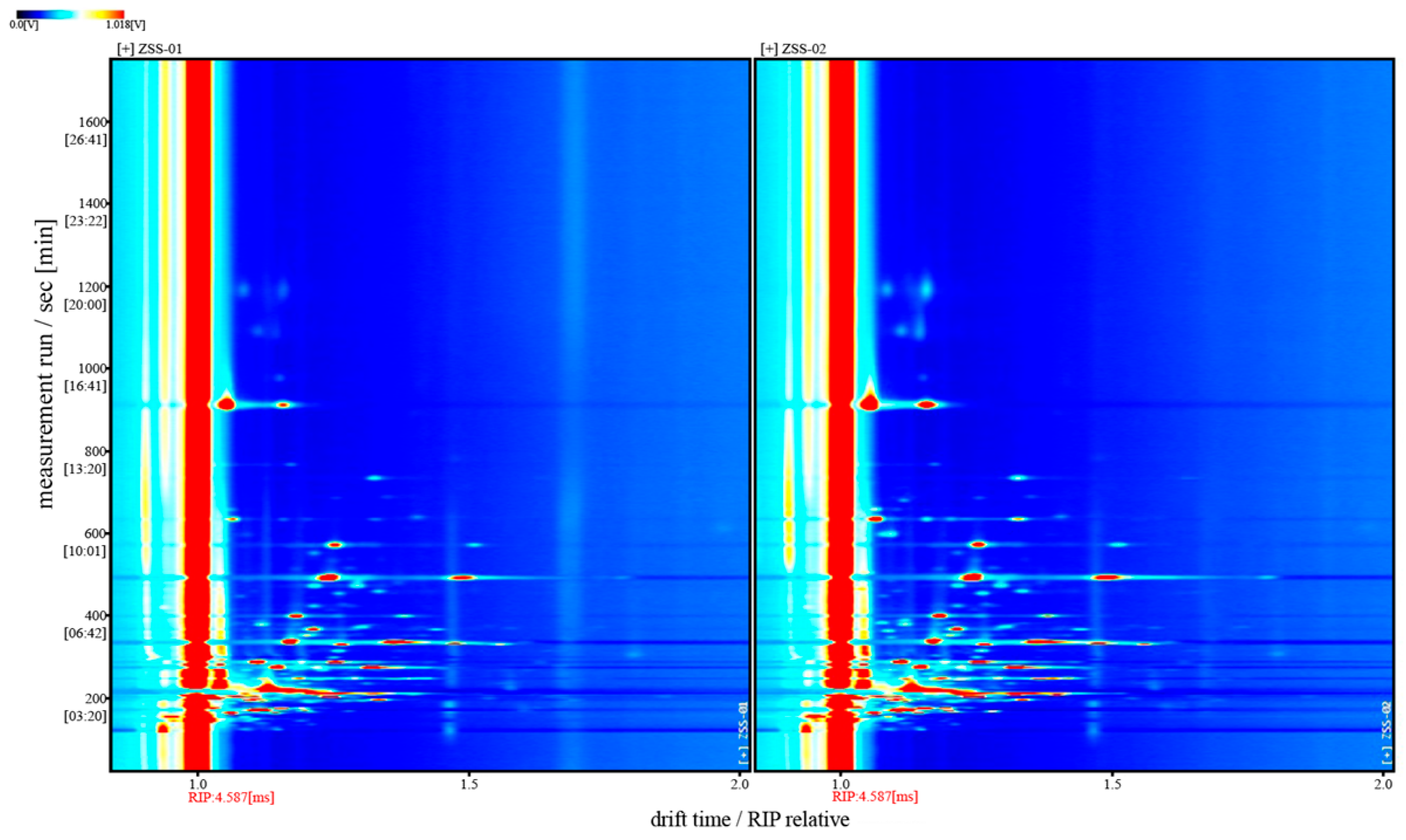
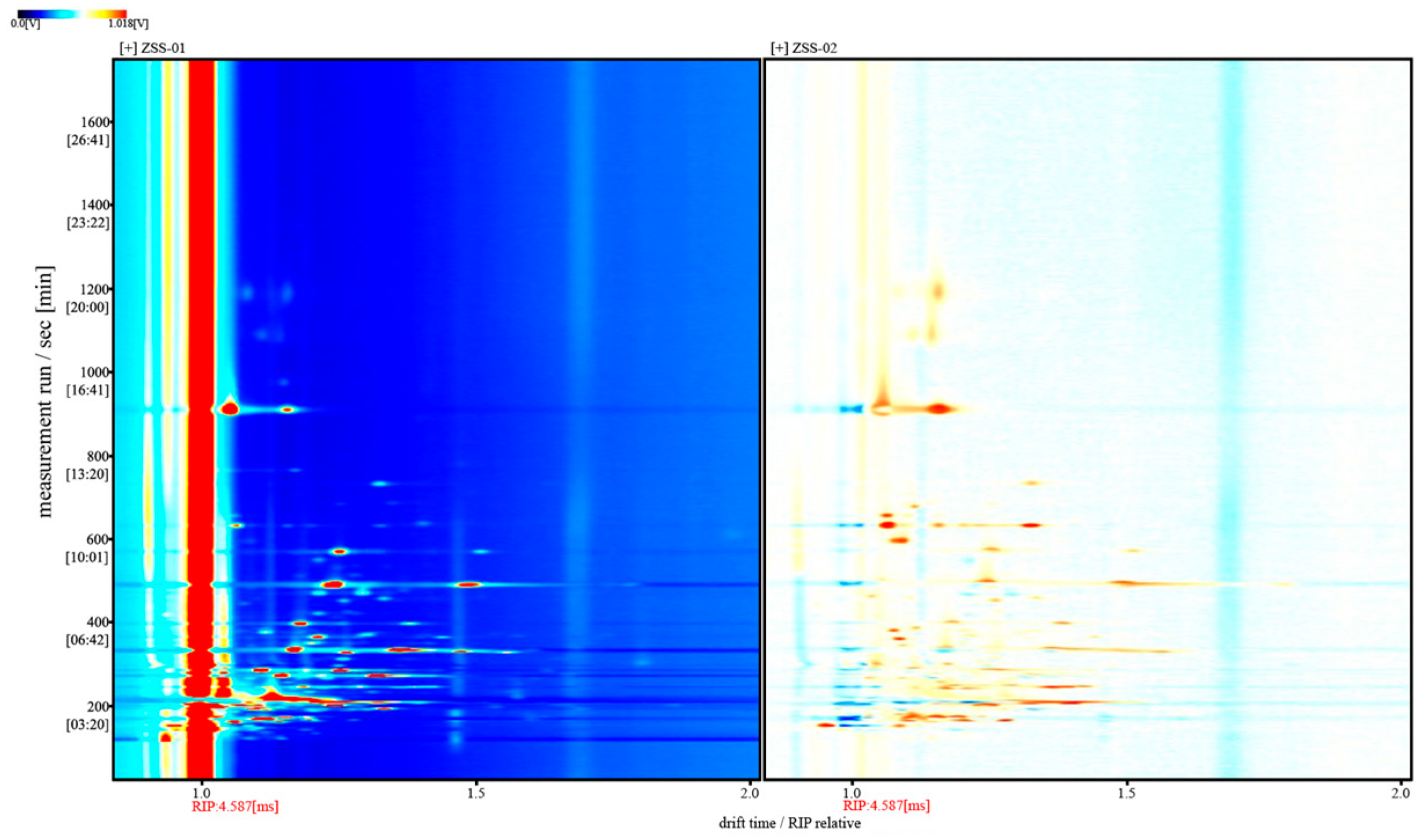
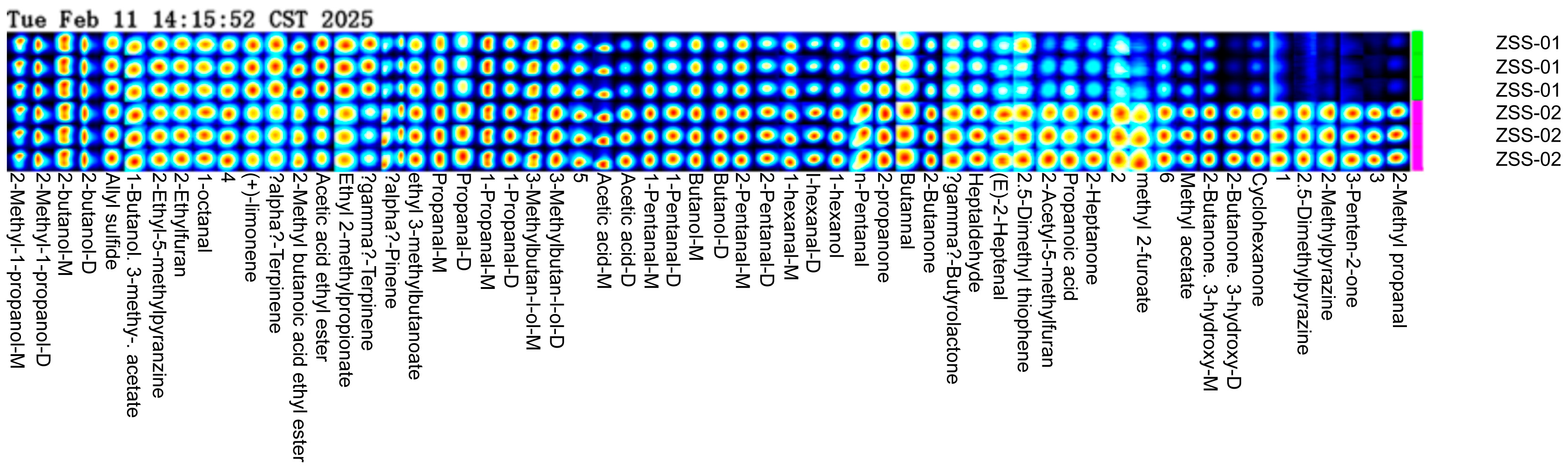
3.2. Analysis of GC-IMS Detection Results
3.2.1. Analysis of Volatile Component Differences in Ziziphi Spinosae Semen Oil with Different Processing Methods
3.2.2. Analysis of GC–IMS Volatile Organic Components in Ziziphi Spinosae Semen Oil with Different Processing Methods
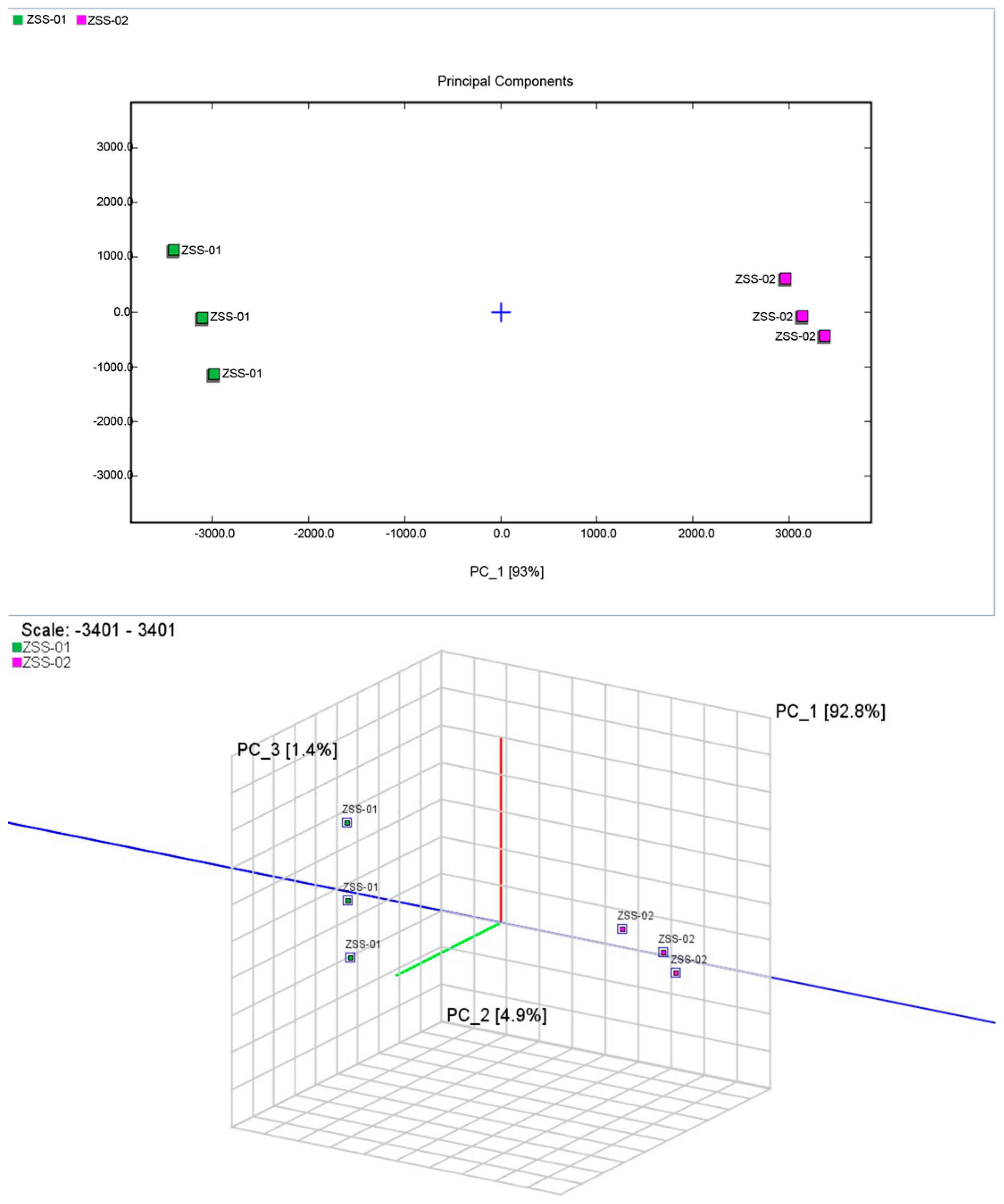
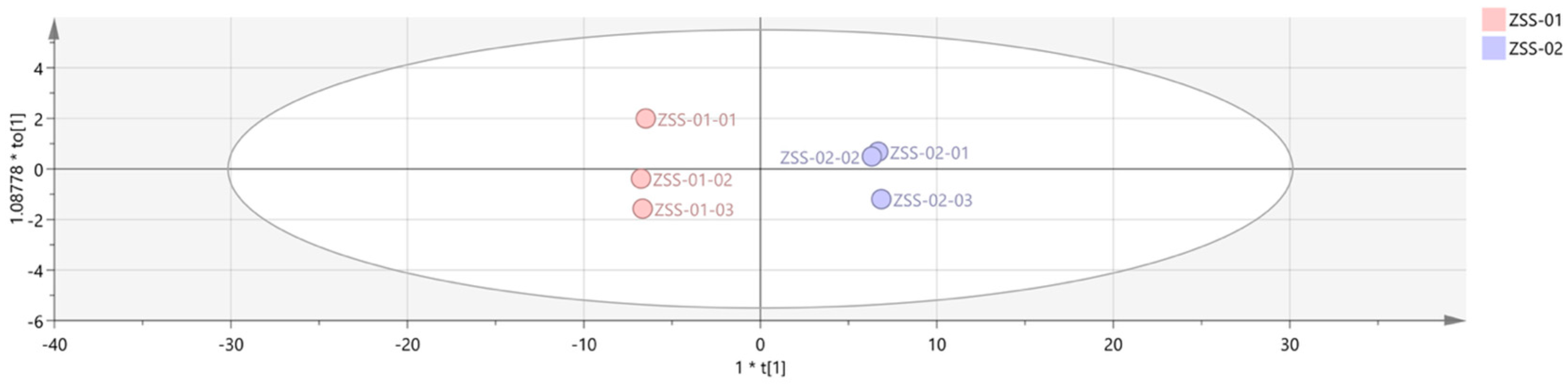
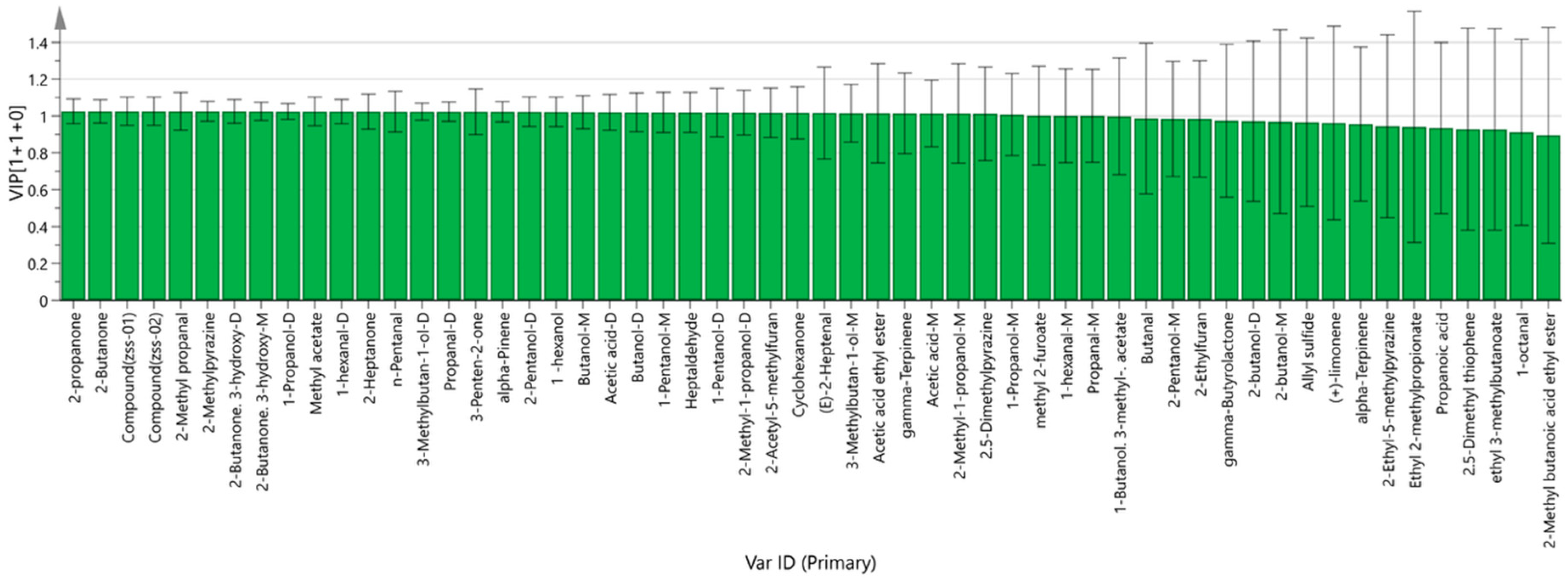
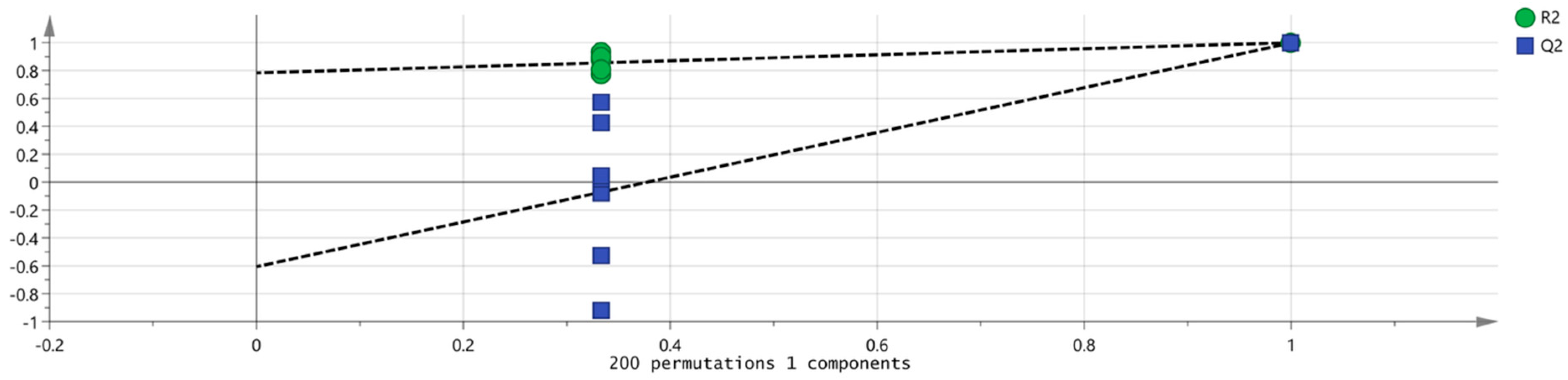
3.2.3. Multivariate Statistical Analysis
3.3. Analysis of Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Xiao, F.; Li, J.; Han, R.; Li, G.; Wan, Z.; Shao, S.; Zhao, D.; Yan, M. Immunomodulatory activity of semen Ziziphi Spinosae protein: A potential plant protein functional food raw material. NPJ Sci. Food 2023, 7, 32. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.; Zhang, Y.; Xie, J. Ziziphi Spinosae Semen: A Natural Herb Resource for Treating Neurological Disorders. Curr. Top. Med. Chem. 2022, 22, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ho, C.T.; Bai, N. Ziziphi Spinosae Semen: An updated review on pharmacological activity, quality control, and application. J. Food Biochem. 2022, 46, e14153. [Google Scholar] [CrossRef]
- Li, H.; Ni, M.; Zhao, R.; Wang, H. Optimization of Fermentation Processing of Spine Date Seed & Tartary Buck-wheat Yoghurt Using Response Surface Methodology. China Dairy Ind. 2020, 48, 59–64. [Google Scholar] [CrossRef]
- Bi, A.; Liu, R.; Xie, M.; He, B.; Yan, T.; Du, Y.; Jia, Y. Semen Ziziphi Spinosae alleviates cardiomyocyte apoptosis in rats with coronary heart disease via the AMPK/SIRT1/PGC-1α signaling pathway activation. Phytomedicine 2025, 142, 156743. [Google Scholar] [CrossRef]
- Du, C.; Han, R.; Wu, J.; Zhao, N.; Pei, X.; Qin, X.; Yan, Y. Study on the antidepressive effects and mechanism of raw and fried Ziziphi Spinosae Semen via metabolomics and gut microbiota analysis. Biomed. Chromatogr. 2024, 38, e5873. [Google Scholar] [CrossRef]
- Li, G.; Yuan, S.; Tang, Z.; Song, Z.; Shi, X.; Liu, H. Effect of different extraction methods on the lipid composition and antioxidant activity of Ziziphi spinosae semens oil. Food Res. Int. 2024, 192, 114745. [Google Scholar] [CrossRef]
- Shan, S.; Xie, Y.; Zhang, C.; Jia, B.; Li, H.; Li, Z. Identification of polyphenol from Ziziphi spinosae semen against human colon cancer cells and colitis-associated colorectal cancer in mice. Food Funct. 2020, 11, 8259–8272. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Liu, J.; Zhang, S.; Huang, Y.; Zhang, Y.; Wang, Z. Sour Jujube (Ziziphus jujuba var. spinosa): A Bibliometric Review of Its Bioactive Profile, Health Benefits and Trends in Food and Medicine Applications. Foods 2024, 13, 636. [Google Scholar] [CrossRef]
- Du, C.H.; Heng, Y.R.; Li, Z.; Pei, X.P.; Yan, Y. Processing of Ziziphi Spinosae Semen: Evolution and research progress. China J. Chin. Mater. Medica 2022, 47, 2572–2583. [Google Scholar] [CrossRef]
- Fan, L.; Gu, C.; Jiang, Y.; Cao, G.; Sun, L.; Ho, R.J.Y.; Wu, D.; Han, Y.; Hong, Y. Screening of different chemical components of sedative and hypnotic effects of Ziziphi Spinosae Semen before and after frying and determination of the Q-Marker. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1207, 123349. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; Mamadalieva, N.Z.; Böhmdorfer, S.; Rosenau, T.; Zengin, G.; Mamadalieva, R.Z.; Musayeib, N.M.A.; Ashour, M.L. GC-MS Based Identification of the Volatile Components of Six Astragalus Species from Uzbekistan and Their Biological Activity. Plants 2021, 10, 124. [Google Scholar] [CrossRef]
- Katherinatama, A.; Asikin, Y.; Shimoda, K.; Shimomura, M.; Mitsube, F.; Takara, K.; Wada, K. Characterization of Free and Glycosidically Bound Volatile and Non-Volatile Components of Shiikuwasha (Citrus depressa Hayata) Fruit. Foods 2024, 13, 3428. [Google Scholar] [CrossRef]
- McGorrin, R.J. Key Aroma Compounds in Oats and Oat Cereals. J. Agric. Food Chem. 2019, 67, 13778–13789. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, C.; Ren, D.; Bai, R.; Li, W.; Wang, J.; Shan, Z.; Dong, W.; Yi, L. Headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry (HS-SPME-GC-MS) and odor activity value (OAV) to reveal the flavor characteristics of ripened Pu-erh tea by co-fermentation. Front. Nutr. 2023, 10, 1138783. [Google Scholar] [CrossRef]
- Tufariello, M.; Pati, S.; Palombi, L.; Grieco, F.; Losito, I. Use of Multivariate Statistics in the Processing of Data on Wine Volatile Compounds Obtained by HS-SPME-GC-MS. Foods 2022, 11, 910. [Google Scholar] [CrossRef]
- Parastar, H.; Weller, P. Feature selection and extraction strategies for non-targeted analysis using GC-MS and GC-IMS: A tutorial. Anal. Chim. Acta 2025, 1343, 343635. [Google Scholar] [CrossRef]
- Jia, Q.Q.; Li, J.X.; Yang, S.; Su, D.D. Gas chromatography-ion mobility spectrometry-based fingerprint analysis of volatile flavor compounds in ginger cultivated under different conditions. Curr. Res. Food Sci. 2025, 10, 101041. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, Q.; Liu, R.; Xu, H.; Yin, Y.; Wang, Y.; Wang, H.; Bi, K. Quality control of Semen Ziziphi Spinosae standard decoction based on determination of multi-components using TOF-MS/MS and UPLC-PDA technology. J. Pharm. Anal. 2019, 9, 406–413. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Chen, X.; Hou, X.; Yu, X.; Han, M.; Qiu, S.; Li, Y.; Qin, S.; Wang, F. UPLC-Q-TOF/MS-based metabolomic analysis reveals the effects of asomate on the citrus fruit. Curr. Res. Food Sci. 2023, 6, 100523. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-F.; Liang, Z.-S.; Shan, C.-J.; Viernstein, H.; Unger, F. Comprehensive evaluation of natural antioxidants and antioxidant potentials in Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou fruits based on geographical origin by TOPSIS method. Food Chem. 2011, 124, 1612–1619. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Sławińska, N.; Olas, B. The current state of knowledge about thermal processing of edible seeds; a special emphasis on their bioactive constituents and antioxidant activity. Food Chem. 2024, 458, 140526. [Google Scholar] [CrossRef]
- Liu, R.X.; Hao, X.J.; Zhang, H.J.; Zhang, L.; Gui, X.J.; Lin, Z.Z.; Luo, C.N.; Tian, L.Y.; Wang, Y.L.; Feng, W.H.; et al. A rapid identification of authenticity and specifications of Chinese medicine Fritillariae Cirrhosae Bulbus based on E-eye technology. China J. Chin. Mater. Medica 2020, 45, 3441–3451. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhang, M.; Qi, J.; Zhao, W.; Gu, J.; Guo, W.; Li, Y. Screening of specific quantitative peptides of beef by LC-MS/MS coupled with OPLS-DA. Food Chem. 2022, 387, 132932. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, F.; Abbasi, A.M.; Chang, X.; Guo, X. Effect of steaming processing on phenolic profiles and cellular antioxidant activities of Castanea mollissima. Molecules 2019, 24, 703. [Google Scholar] [CrossRef] [PubMed]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Karav, S.; Witkowska, A.M. Dietary Polyphenols, Food Processing and Gut Microbiome: Recent Findings on Bioavailability, Bioactivity, and Gut Microbiome Interplay. Antioxidants 2024, 13, 1220. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Zhai, E.; Zhang, J.; Zhu, J.; Zhou, R.; Niu, Y.; Xiao, Z. Formation Mechanism of Lipid and Flavor of Lard Under the Intervention of Heating Temperature via UPLC-TOF-MS/MS with OPLS-DA and HS-GC-IMS Analysis. Foods 2025, 14, 2441. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yoon, N.; Lee, Y.J.; Woo, K.S.; Kim, H.Y.; Lee, J.; Jeong, H.S. Influence of Thermal Processing on Free and Bound Forms of Phenolics and Antioxidant Capacity of Rice Hull (Oryza sativa L.). Prev. Nutr. Food Sci. 2020, 25, 310–318. [Google Scholar] [CrossRef] [PubMed]
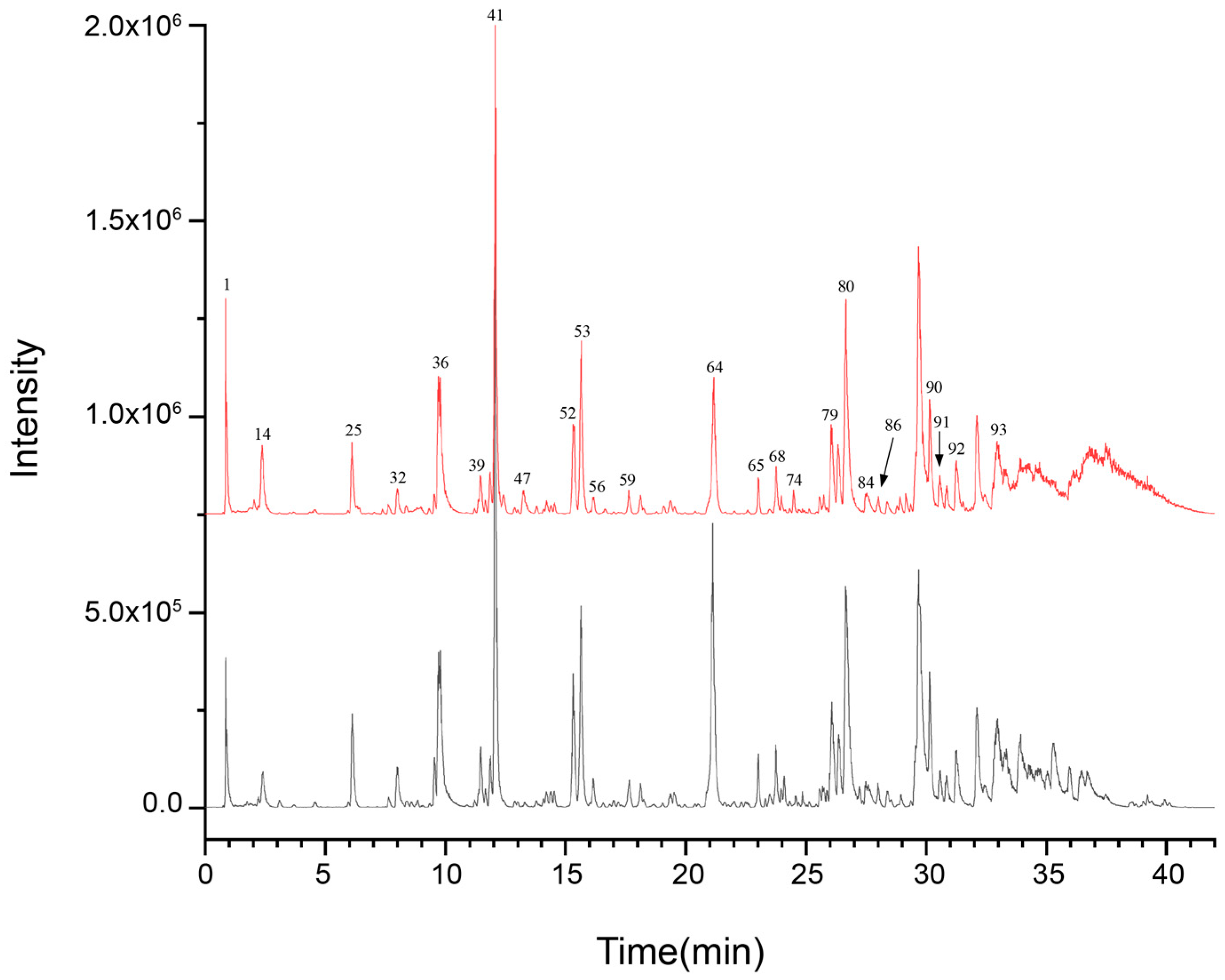
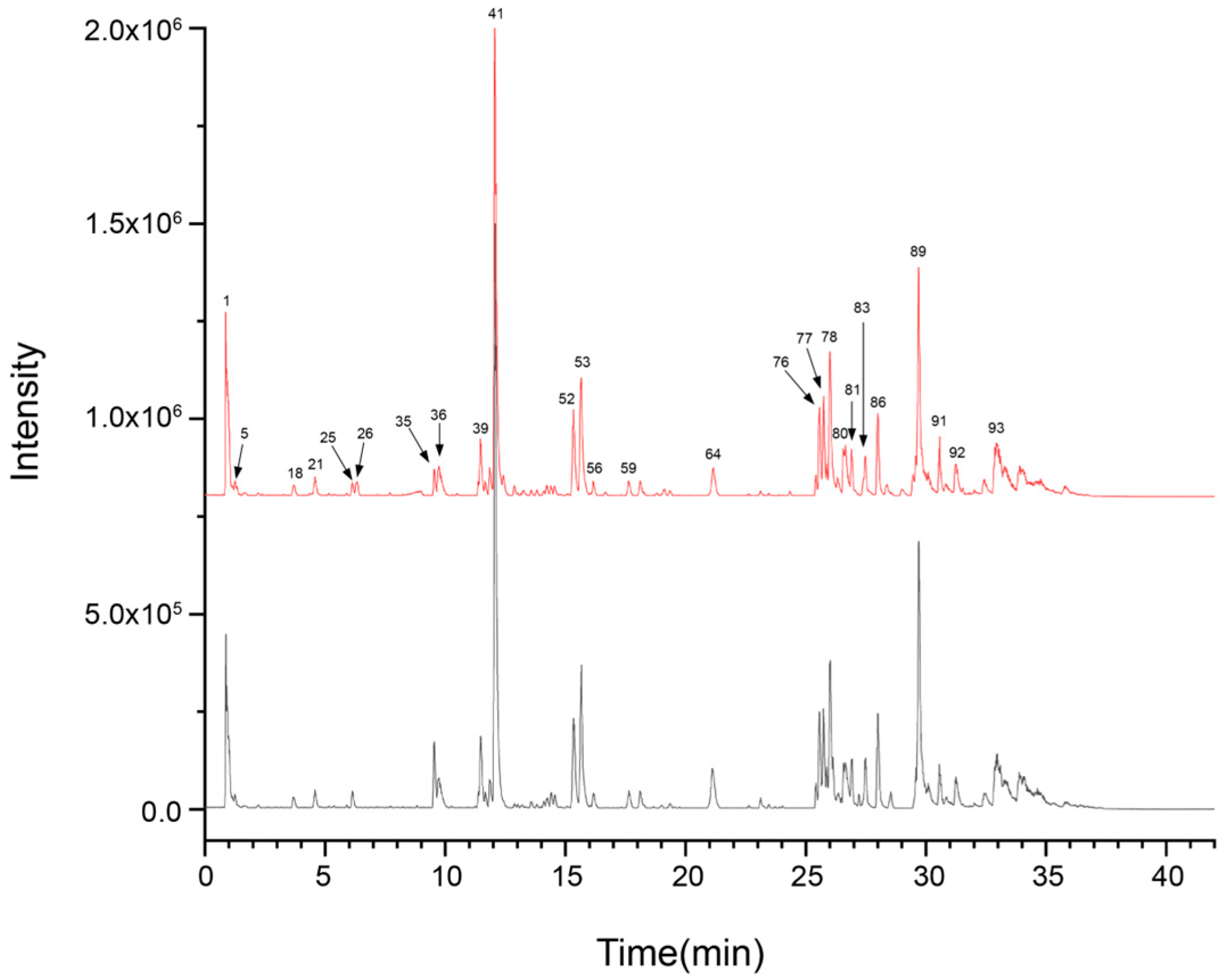
| No. | t/min | Compound Identification | Molecular Formula | Ion Form | Sample Presence | Measured m/z | Characteristic Fragment Ions (m/z) |
|---|---|---|---|---|---|---|---|
| 1 | 0.902 | Maltotetraose | C24H42O21 | [M+FA-H]- | S>R | 711 | 711; 665; 503; 341; 179 |
| 2 | 0.92 | Gluconic acid | C6H12O7 | [M-H]- | R>S | 195 | 195; 129; 75 |
| 3 | 0.938 | D-(+)-Raffinose (1) | C18H32O16 | [M+FA-H]- | S>R | 549 | 549; 503 341; 221; 179 |
| 4 | 1.036 | Trigonelline (1) | C7H7NO2 | [M+H]+ | R>S | 138 | 138; 92; 78; 65 |
| 5 | 1.201 | Citric acid (1) | C6H8O7 | [M-H]- | S>R | 192 | 191; 111; 87 |
| 6 | 1.328 | L-Pyroglutamic Acid (1) | C5H7NO3 | [M-H]- | S>R | 128 | 128; 85 |
| 7 | 1.454 | Uridine (1) | C9H12N2O6 | [M-H]- | R>S | 243 | 243; 200; 152; 110; 82 |
| 8 | 1.498 | Nicotinamide (1) | C6H6N2O | [M+H]+ | S>R | 123 | 123; 80; 53 |
| 9 | 1.641 | 1,4-Butanediyl bis[α-D-mannopyranoside] | C16H30O12 | [M+FA-H]- | S>R | 459 | 459; 413; 251; 179; 161 |
| 10 | 1.652 | cAMP (1) | C10H12N5O6P | [M+H]+ | S-only | 330 | 330; 136 |
| 11 | 1.759 | Phenylalanine (1) | C9H11NO2 | [M+H]+ | R>S | 166 | 166; 120; 103; 91 |
| 12 | 2.043 | Guanosine (1) | C10H13N5O5 | [M-H]- | S>R | 282 | 282; 150; 133 |
| 13 | 2.232 | Adenosine (1) | C10H13N5O4 | [M+H]+ | S-only | 268 | 268; 136; 119; 94 |
| 14 | 2.408 | Verpacamide A | C11H19N5O2 | [M+H]+ | S>R | 254 | 254; 195; 167; 125; 98; 70 |
| 15 | 2.618 | Xanthosine (1) | C10H12N4O6 | [M-H]- | R>S | 283 | 283; 151; 108; 80 |
| 16 | 3.111 | L-Tryptophan (1) | C11H12N2O2 | [M-H]- | R>S | 203 | 203; 116 |
| 17 | 3.521 | / | C14H21N5O3 | [M+H]+ | S-only | 308 | 308; 195; 167; 125; 98; 70 |
| 18 | 3.704 | 1-β-D-glucopyranosyl-2,3-dihydro-2-oxo-1H-Indole-3-acetic Acid (1) | C16H19NO8 | [M-H]- | S>R | 352 | 352; 308; 190; 188; 146 |
| 19 | 4.413 | / | C46H52N2O4 | [M+NH4]+ | S-only | 714 | 714; 498; 313; 200; 158 |
| 20 | 4.589 | Vanillic acid 4-O-β-D-glucoside | C14H18O9 | [M-H]- | R>S | 329 | 329; 167; 152; 123; 108 |
| 21 | 4.591 | 2-((R)-2-oxo-1-((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro- 2H-pyran-2-yl)indolin-3-yl)acetic acid | C16H19NO8 | [M-H]- | S>R | 352 | 352; 308; 188; 160; 146 |
| 22 | 4.795 | 2,6-Dihydroxybenzoic acid 2-O-β-D-apiofuranosyl(1→2)-β-D- glucopyranoside | C18H24O13 | [M-H]- | R>S | 447 | 447; 315; 271; 152; 108 |
| 23 | 5.908 | Dihydrophaseic acid 4′-O-β-glucopyranoside (1) | C21H32O10 | [M-H]- | R>S | 443 | 443; 237; 189; 119 |
| 24 | 6.132 | 2,6-Dihydroxybenzoic acid 2-O-β-D-apiofuranosyl(1→2)-β-D- xylopyranoside | C17H22O12 | [M-H]- | R>S | 417 | 417; 285; 241; 152; 108 |
| 25 | 6.136 | Coclaurine-6-glucoside | C23H29NO8 | [M+H]+ | R>S | 448 | 448; 286; 269; 237; 175 |
| 26 | 6.321 | / | C44H43N3O2 | [M-H]- | S-only | 644 | 644; 600; 470; 297; 279; 209; 128 |
| 27 | 6.749 | Catechin (1) | C15H14O6 | [M-H]- | R>S | 289 | 289; 245; 203; 123; 109 |
| 28 | 7.041 | 4-[(1,2,3,4-Tetrahydro-6,7-dimethoxy-2-methyl- 1-isoquinolinyl)methyl] phenyl β-D-glucopyranoside | C25H33NO8 | [M+H]+ | S-only | 476 | 476; 314; 271; 269 |
| 29 | 7.65 | Magnocurarine | C19H24NO3+ | M+ | S>R | 314 | 314; 269; 175 |
| 30 | 7.735 | N-Acetylphenylalanine | C11H13NO3 | [M-H]- | S>R | 206 | 206; 164; 147.; 103; 91; 72 |
| 31 | 7.74 | (R)-6,8-di-β-D-glucopyranosyl-2,3-dihydro- 5,7-dihydroxy-2-(4-hydroxyphenyl)- 4H-1-Benzopyran-4-one | C27H32O15 | [M-H]- | R>S | 595 | 595; 475; 415; 385; 355 |
| 32 | 8.007 | Coclaurine (1) | C17H19NO3 | [M+H]+ | R>S | 286 | 286.1445; 269.1186; 237.0917; 209.0963; 107.0498 |
| 33 | 8.122 | (-)-Epicatechin (1) | C15H14O6 | [M-H]- | S>R | 289 | 289.0733; 203.0742 |
| 34 | 8.786 | Tembetarine | C20H26NO4+ | M+ | S>R | 344 | 344; 301; 299; 267; 235; 175 |
| 35 | 9.551 | Vicenin-2 (1) | C27H30O15 | [M-H]- | R>S | 593 | 593; 503; 473; 383; 353 |
| 36 | 9.761 | Magnoflorine (1) | C20H24NO4+ | M+ | R>S | 342 | 342; 297; 282; 265; 237 |
| 37 | 10.008 | Lotusine | C19H24NO3+ | M+ | R>S | 314 | 314; 269; 175 |
| 38 | 10.487 | Apigenin 6,8-C-di-β-galactopyranoside | C27H30O15 | [M-H]- | S-only | 593 | 593; 503; 473; 413; 383 |
| 39 | 11.488 | Isovitexin-2″-O-glucopyranoside | C27H30O15 | [M-H]- | R>S | 593 | 593; 413; 341; 293 |
| 40 | 11.872 | Isospinosin | C28H32O15 | [M-H]- | R>S | 607 | 607; 487; 427 |
| 41 | 12.084 | Spinosin (1) | C28H32O15 | [M-H]- | R>S | 607 | 607; 427; 307 |
| 42 | 12.22 | Azelaic acid (1) | C9H16O4 | [M-H]- | R>S | 187 | 187; 169; 125; 123; 97 |
| 43 | 12.416 | Zivulgarin | C28H32O15 | [M-H]- | S-only | 607 | 607; 427; 307 |
| 44 | 12.44 | Isovitexin | C21H20O10 | [M-H]- | R>S | 431 | 431; 341; 311; 283 |
| 45 | 12.888 | Apigenin-6-C-glucoside-7-O-glucoside | C27H30O15 | [M-H]- | S>R | 593 | 593; 413; 283; 269 |
| 46 | 12.9 | Swertisin (1) | C22H22O10 | [M-H]- | R>S | 445 | 445; 325; 297; 282 |
| 47 | 13.253 | Fuzitine | C20H24NO4+ | M+ | S-only | 342 | 342; 297; 282; 265; 237; 222; 191 |
| 48 | 13.585 | Kaemperol-3-O-rutinoside (1) | C27H30O15 | [M-H]- | R>S | 593 | 593; 285; 255 |
| 49 | 13.818 | 6″′-Vanilloylspinosin | C36H38O18 | [M-H]- | S>R | 757 | 757; 427; 209 |
| 50 | 14.242 | 6″′-p-Hydroxylbenzoylspinosin | C35H36O17 | [M-H]- | S>R | 727 | 727; 427; 179; 137 |
| 51 | 14.42 | 5,7-Dihydroxy-6-[2-O-[6-O-[(2E)-3-(4-hydroxy-3-methoxyphenyl)- 1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]- β-D-glucopyranosyl]-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | C37H38O18 | [M-H]- | R>S | 769 | 769; 593; 413; 293; 235 |
| 52 | 15.358 | 6″′-Sinapoylspinosin | C39H42O19 | [M-H]- | R>S | 813 | 813; 693; 607; 427; 325 |
| 53 | 15.669 | 6″′-Feruloylspinosin | C38H40O18 | [M-H]- | R>S | 783 | 783; 607; 427; 235 |
| 54 | 15.717 | 6″′-p-Coumaroylspinosin | C37H38O17 | [M-H]- | R>S | 753 | 753; 607; 427 |
| 55 | 15.756 | 6-[2-O-β-D-Glucopyranosyl-6-O-[2-[(3R)-1-β-D-glucopyranosyl- 2,3-dihydro-2-oxo-1H-indol-3-yl]acetyl]-β-D-glucopyranosyl]- 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one | C44H49NO22 | [M-H]- | R>S | 942 | 942; 762; 589; 293 |
| 56 | 16.175 | 6-[2-O-β-D-Glucopyranosyl-6-O-[2-[(3S)-1-β-D-glucopyranosyl- 2,3-dihydro-2-oxo-1H-indol-3-yl]acetyl]-β-D-glucopyranosyl]- 5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one | C44H49NO22 | [M-H]- | R>S | 942 | 942; 762; 607; 589; 427; 352 |
| 57 | 16.669 | 5-hydroxy-8-[2-O-[6-O-[(2E)-3-(4-hydroxy-3-methoxyphenyl)-1-oxo- 2-propen-1-yl]-β-D-glucopyranosyl]-β-D-glucopyranosyl]- 2-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one | C38H40O18 | [M-H]- | S-only | 783 | 783; 427; 235 |
| 58 | 16.846 | 5-Hydroxy-2-(4-hydroxyphenyl)-6-[2-O-[6-O-[3-(4-hydroxyphenyl)- 1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]-β-D-glucopyranosyl]- 7-methoxy-4H-1-benzopyran-4-one | C37H38O17 | [M-H]- | S-only | 753 | 753; 607; 427 |
| 59 | 17.656 | 6″′-(-)-Phaseolspinosin | C43H50O19 | [M-H]- | R>S | 869 | 869; 839; 607; 427 |
| 60 | 18.261 | 6’’-Feruloyspinosin (1) | C38H40O18 | [M-H]- | R>S | 783 | 783; 607; 427; 235 |
| 61 | 18.668 | 6-[6-O-[2-[(3S)-1-β-D-Glucopyranosyl-2,3-dihydro-2-oxo-1H-indol-3-yl]acetyl]- 2-O-[6-O-[3-(4-hydroxy-3-methoxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]- β-D-glucopyranosyl]-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one | C54H57NO25 | [M+H]+ | R>S | 1120 | 1120; 782; 764; 665; 393; 327; 177 |
| 62 | 18.995 | 6-[6-O-[2-[(3R)-1-β-D-Glucopyranosyl-2,3-dihydro-2-oxo-1H-indol-3-yl]acetyl]- 2-O-[6-O-[3-(4-hydroxy-3-methoxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]- β-D-glucopyranosyl]-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one | C54H57NO25 | [M+H]+ | R>S | 1120 | 1120; 782; 764; 665; 393; 327; 177 |
| 63 | 20.925 | Amphibine D | C36H49N5O5 | [M+H]+ | R>S | 632 | 632; 289; 261; 148 |
| 64 | 21.123 | Frangufoline | C36H42N2O2 | [M+H]+ | R>S | 535 | 535; 148; 133 |
| 65 | 23.013 | Jujuboside A (1) | C58H94O26 | [M+Cl]- | R>S | 1242 | 1241; 1205; 1073; 749 |
| 66 | 23.13 | Pinellic acid | C18H34O5 | [M-H]- | R>S | 329 | 329; 229; 211; 171 |
| 67 | 23.422 | Jujuboside A1 | C58H94O26 | [M+Cl]- | S>R | 1242 | 1241; 1206; 1074; 749 |
| 68 | 23.761 | Jujuboside B (1) | C52H84O21 | [M+FA-H]- | R>S | 1090 | 1090; 1044; 912; 749 |
| 69 | 23.912 | 9,10,11-Trihydroxy-12-octadecenoic acid | C18H34O5 | [M-H]- | R>S | 329 | 329; 211; 171 |
| 70 | 24.034 | 9,12,13-Trihydroxy-10-octadecenoic acid | C18H34O5 | [M-H]- | R>S | 329 | 329; 211; 171 |
| 71 | 24.045 | Jujuboside B1 | C52H84O21 | [M+FA-H]- | R>S | 1090 | 1090; 1044; 912; 749 |
| 72 | 24.099 | (3β,16β,23R)-16,23:16,30-Diepoxy-20-hydroxydammar-24-en-3-yl O-6-deoxy-α-L-mannopyranosyl-(1→2)- O-[O-β-D-xylopyranosyl-(1→2)-6-O-acetyl-β-D-glucopyranosyl-(1→3)]- α-L-arabinopyranoside | C54H86O22 | [M+FA-H]- | R-only | 1131 | 1132; 1086; 1026; 954; 912; 893; 749 |
| 73 | 24.331 | / | C38H63NO10 | [M+Cl]- | S-only | 728 | 728; 692; 522; 283; 265; 199 |
| 74 | 24.491 | / | C54H86O22 | [M+FA-H]- | S-only | 1132 | 1132; 1086; 1026; 954; 893; 749 |
| 75 | 24.57 | Dehydrophytosphingosine | C18H37NO3 | [M+H]+ | R>S | 316 | 316; 298; 280 |
| 76 | 25.571 | 1-[(2S)-3-hydroxy-2-[[(9Z,12Z)-1-oxo-9,12-octadecadienyl]oxy]propyl hydrogen phosphate]-D-myo-Inositol | C27H49O12P | [M-H]- | R>S | 595 | 595; 315; 279; 241; 153 |
| 77 | 25.742 | D-myo-Inositol, 1-[(2R)-2-hydroxy-3-[(1-oxohexadecyl)oxy]propyl hydrogen phosphate] | C25H49O12P | [M-H]- | S>R | 571 | 571; 315; 255; 241; 153; 79 |
| 78 | 26.017 | 1-[2-Hydroxy-3-[[(9Z)-1-oxo-9-octadecen-1-yl]oxy]propyl hydrogen phosphate]-myo-inositol | C27H51O12P | [M-H]- | R>S | 597 | 597; 315; 281; 241; 153 |
| 79 | 26.094 | 1-Linoleoyl-sn-glycero-3-phosphorylcholine | C26H50NO7P | [M+H]+ | R>S | 520 | 520; 502; 184; 104 |
| 80 | 26.675 | 1-Oleoyl-sn-glycero-3-phosphocholine | C26H52NO7P | [M+FA-H]- | R>S | 566 | 566; 506; 281; 224 |
| 81 | 26.915 | Epiceanothic acid | C30H46O5 | [M-H]- | R>S | 485 | 485; 439; 423 |
| 82 | 27.23 | Cyclic pentaleucine | C30H55N5O5 | [M+H]+ | R>S | 566 | 566; 340; 295; 227; 199 |
| 83 | 27.461 | 1-Oleoyl-sn-glycerol 3-phosphate | C21H41O7P | [M-H]- | S>R | 435 | 435; 281; 152; 78 |
| 84 | 27.491 | Gamma-Linolenoyl carnitine | C25H43NO4 | [M+H]+ | R>S | 422 | 422; 376; 160; 114 |
| 85 | 27.992 | O-Palmitoleoylcarnitine | C23H43NO4 | [M+H]+ | R>S | 398 | 398; 352; 160; 114 |
| 86 | 28.009 | Ceanothic acid | C30H46O5 | [M-H]- | R>S | 485 | 485; 423 |
| 87 | 28.403 | O-linoleyl-L-carnitine | C25H45NO4 | [M+H]+ | R>S | 424 | 424; 378; 160; 114 |
| 88 | 29.59 | 3β-O-(trans-p-Coumaroyl)maslinic acid | C39H54O6 | [M-H]- | R>S | 617 | 617; 453; 145 |
| 89 | 29.721 | Betulinic acid (1) | C30H48O3 | [M+H-H2O]+ | R>S | 439 | 439; 393; 259; 241; 137 |
| 90 | 30.258 | Oleamide | C18H35NO | [M+H]+ | S>R | 282 | 282.; 69 |
| 91 | 30.588 | Oleic Acid | C18H34O2 | [M+H]+ | S-only | 283 | 283; 135; 121; 107; 93 |
| 92 | 31.292 | Soyacerebroside I | C40H75NO9 | [M+H]+ | R>S | 714 | 715; 697; 534; 516; 262 |
| 93 | 32.997 | D-myo-Inositol, 1-[(2R)-2-[(1-oxohexadecyl)oxy]- 3-[[(9Z,12Z)-1-oxo-9,12-octadecadienyl]oxy]propyl hydrogen phosphate] | C43H79O13P | [M-H]- | S>R | 834 | 834; 553; 391; 279; 255; 241 |
| NO | Compound | CAS | Formula | MW | RI | Rt/s | Dt/ms |
|---|---|---|---|---|---|---|---|
| 1 | γ-Butyrolactone | C96480 | C4H6O2 | 86.1 | 1603 | 1184.01 | 1.08377 |
| 2 | 2-Acetyl-5-methylfuran | C1193799 | C7H8O2 | 124.1 | 1601 | 1177.167 | 1.16054 |
| 3 | Propanoic acid | C79094 | C3H6O2 | 74.1 | 1561 | 1086.512 | 1.11613 |
| 4 | methyl 2-furoate | C611132 | C6H6O3 | 126.1 | 1558 | 1081.145 | 1.14754 |
| 5 | Acetic acid-M | C64197 | C2H4O2 | 60.1 | 1467 | 901.108 | 1.05442 |
| 6 | Acetic acid-D | C64197 | C2H4O2 | 60.1 | 1471 | 908.609 | 1.1589 |
| 7 | 2-Ethyl-5-methylpyrazine | C13360640 | C7H10N2 | 122.2 | 1387 | 767.807 | 1.17362 |
| 8 | 1 -hexanol | C111273 | C6H14O | 102.2 | 1364 | 733.551 | 1.32865 |
| 9 | 2,5-Dimethylpyrazine | C123320 | C6H8N2 | 108.1 | 1326 | 679.134 | 1.11963 |
| 10 | (E)-2-Heptenal | C18829555 | C7H12O | 112.2 | 1331 | 684.874 | 1.25487 |
| 11 | 2-Butanone, 3-hydroxy-M | C513860 | C4H8O2 | 88.1 | 1291 | 630.85 | 1.06623 |
| 12 | 2-Butanone, 3-hydroxy-D | C513860 | C4H8O2 | 88.1 | 1292 | 633.923 | 1.3317 |
| 13 | Cyclohexanone | C108941 | C6H10O | 98.1 | 1292 | 632.899 | 1.16192 |
| 14 | 1-octanal | C124130 | C8H16O | 128.2 | 1295 | 637.633 | 1.40796 |
| 15 | 2-Methylpyrazine | C109080 | C5H6N2 | 94.1 | 1271 | 595.45 | 1.09174 |
| 16 | 1-Pentanol-M | C71410 | C5H12O | 88.1 | 1256 | 569.178 | 1.25342 |
| 17 | 1-Pentanol-D | C71410 | C5H12O | 88.1 | 1257 | 571.552 | 1.51276 |
| 18 | 3-Methylbutan-1-ol-M | C123513 | C5H12O | 88.1 | 1203 | 487.447 | 1.2419 |
| 19 | 3-Methylbutan-1-ol-D | C123513 | C5H12O | 88.1 | 1205 | 490.438 | 1.49425 |
| 20 | (+)-limonene | C138863 | C10H16 | 136.2 | 1192 | 472.182 | 1.21612 |
| 21 | 2-Heptanone | C110430 | C7H14O | 114.2 | 1179 | 454.127 | 1.26294 |
| 22 | Heptaldehyde | C111717 | C7H14O | 114.2 | 1184 | 460.198 | 1.33668 |
| 23 | 2,5-Dimethyl thiophene | C638028 | C6H8S | 112.2 | 1175 | 447.978 | 1.0794 |
| 24 | α-Terpinene | C99865 | C10H16 | 136.2 | 1159 | 423.141 | 1.21508 |
| 25 | Butanol-M | C71363 | C4H10O | 74.1 | 1139 | 396.254 | 1.18384 |
| 26 | Butanol-D | C71363 | C4H10O | 74.1 | 1141 | 398.882 | 1.38147 |
| 27 | 3-Penten-2-one | C625332 | C5H8O | 84.1 | 1129 | 382.432 | 1.07735 |
| 28 | Allyl sulfide | C592881 | C6H10S | 114.2 | 1126 | 379.317 | 1.11942 |
| 29 | 2-Pentanol-M | C6032297 | C5H12O | 88.1 | 1116 | 365.725 | 1.21353 |
| 30 | 2-Pentanol-D | C6032297 | C5H12O | 88.1 | 1117 | 367.424 | 1.44382 |
| 31 | 1-Butanol, 3-methyl-, acetate | C123922 | C7H14O2 | 130.2 | 1121 | 372.521 | 1.30432 |
| 32 | 2-Methyl-1-propanol-M | C78831 | C4H10O | 74.1 | 1088 | 333.153 | 1.17268 |
| 33 | 2-Methyl-1-propanol-D | C78831 | C4H10O | 74.1 | 1089 | 333.742 | 1.36549 |
| 34 | 1-hexanal-M | C66251 | C6H12O | 100.2 | 1084 | 329.618 | 1.26829 |
| 35 | 1-hexanal-D | C66251 | C6H12O | 100.2 | 1085 | 330.502 | 1.5599 |
| 36 | ethyl 3-methylbutanoate | C108645 | C7H14O2 | 130.2 | 1068 | 314.592 | 1.26351 |
| 37 | 1-Propanol-M | C71238 | C3H8O | 60.1 | 1034 | 285.425 | 1.11053 |
| 38 | 1-Propanol-D | C71238 | C3H8O | 60.1 | 1034 | 285.719 | 1.24916 |
| 39 | 2-Methyl butanoic acid ethyl ester | C7452791 | C7H14O2 | 130.2 | 1048 | 296.915 | 1.25076 |
| 40 | 2-butanol-M | C78922 | C4H10O | 74.1 | 1018 | 272.65 | 1.14903 |
| 41 | 2-butanol-D | C78922 | C4H10O | 74.1 | 1020 | 274.538 | 1.32737 |
| 42 | n-Pentanal | C110623 | C5H10O | 86.1 | 981 | 245.833 | 1.18327 |
| 43 | α-Pinene | C80568 | C10H16 | 136.2 | 984 | 247.54 | 1.29202 |
| 44 | 2-Butanone | C78933 | C4H8O | 72.1 | 907 | 202.768 | 1.24549 |
| 45 | Acetic acid ethyl ester | C141786 | C4H8O2 | 88.1 | 891 | 194.507 | 1.33673 |
| 46 | Butanal | C123728 | C4H8O | 72.1 | 888 | 192.666 | 1.11855 |
| 47 | 2-propanone | C67641 | C3H6O | 58.1 | 838 | 169.356 | 1.11489 |
| 48 | Propanal-M | C123386 | C3H6O | 58.1 | 822 | 162.442 | 1.06094 |
| 49 | Propanal-D | C123386 | C3H6O | 58.1 | 824 | 163.232 | 1.14507 |
| 50 | Methyl acetate | C79209 | C3H6O2 | 74.1 | 852 | 175.71 | 1.19289 |
| 51 | 2-Methyl propanal | C78842 | C4H8O | 72.1 | 836 | 168.291 | 1.2809 |
| 52 | Ethyl 2-methylpropionate | C97621 | C6H12O2 | 116.2 | 965 | 235.545 | 1.19807 |
| 53 | 2-Ethylfuran | C3208160 | C6H8O | 96.1 | 963 | 234.725 | 1.31265 |
| 54 | γ-Terpinene | C99854 | C10H16 | 136.2 | 1244 | 549.933 | 1.21443 |
| Sample | DPPH IC50 | ABTS IC50 |
|---|---|---|
| ZSS-01 | 9.27 ± 0.84 a mg/mL | 9.33 ± 0.25 a mg/mL |
| ZSS-02 | 5.74 ± 0.20 b mg/mL | 8.12 ± 0.31 b mg/mL |
| Vc | 6.83 ± 0.60 c μg/mL | 2.80 ± 0.10 c μg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, X.; Shi, X.; Zhou, C.; Li, M.; Huang, R.; Liu, H.; Huang, D.; Zhang, G. An Exploratory Study on the Influence of Frying on Chemical Constituent Transformation and Antioxidant Activity in Ziziphi Spinosae Semen: A Multimodal Analytical Strategy Based on UPLC–Q–TOF–MS and GC–IMS. Foods 2025, 14, 4145. https://doi.org/10.3390/foods14234145
Ouyang X, Shi X, Zhou C, Li M, Huang R, Liu H, Huang D, Zhang G. An Exploratory Study on the Influence of Frying on Chemical Constituent Transformation and Antioxidant Activity in Ziziphi Spinosae Semen: A Multimodal Analytical Strategy Based on UPLC–Q–TOF–MS and GC–IMS. Foods. 2025; 14(23):4145. https://doi.org/10.3390/foods14234145
Chicago/Turabian StyleOuyang, Xinyi, Xiaonuo Shi, Chang Zhou, Mengyuan Li, Rujia Huang, Huiping Liu, Dan Huang, and Guomin Zhang. 2025. "An Exploratory Study on the Influence of Frying on Chemical Constituent Transformation and Antioxidant Activity in Ziziphi Spinosae Semen: A Multimodal Analytical Strategy Based on UPLC–Q–TOF–MS and GC–IMS" Foods 14, no. 23: 4145. https://doi.org/10.3390/foods14234145
APA StyleOuyang, X., Shi, X., Zhou, C., Li, M., Huang, R., Liu, H., Huang, D., & Zhang, G. (2025). An Exploratory Study on the Influence of Frying on Chemical Constituent Transformation and Antioxidant Activity in Ziziphi Spinosae Semen: A Multimodal Analytical Strategy Based on UPLC–Q–TOF–MS and GC–IMS. Foods, 14(23), 4145. https://doi.org/10.3390/foods14234145






