Evaluation of Quality and Storage Characteristics of Freeze-Dried Powdered Mycelium Sausages According to Packaging Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Shiitake Mushroom Mycelium Culture
2.2. Freeze-Drying Shiitake Mushroom Mycelium and Sausage Production
2.3. Proximate Composition
2.4. pH
2.5. Storage Loss
- A = Weight on Day 0
- B = Weight by storage period (7, 15, 30, 50 days).
2.6. Color
2.7. Water Activity (Aw)
2.8. Aerobic Plate Count (APC)
2.9. Thiobarbituric Acid Reactive Substances (TBARS)
2.10. Volatile Basic Nitrogen (VBN)
- V1: Sample titration volume (mL);
- V2: Titrant volume (mL);
- m: Sample mass (g);
- a: 0.02 N H2SO4 titration strength;
- b: Dilution factor.
2.11. Electronic Nose (E-Nose)
2.12. Electronic Tongue (E-Tongue)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition
3.2. pH and Storage Loss
3.3. Color
3.4. Aw and APC
3.5. TBARS and VBN
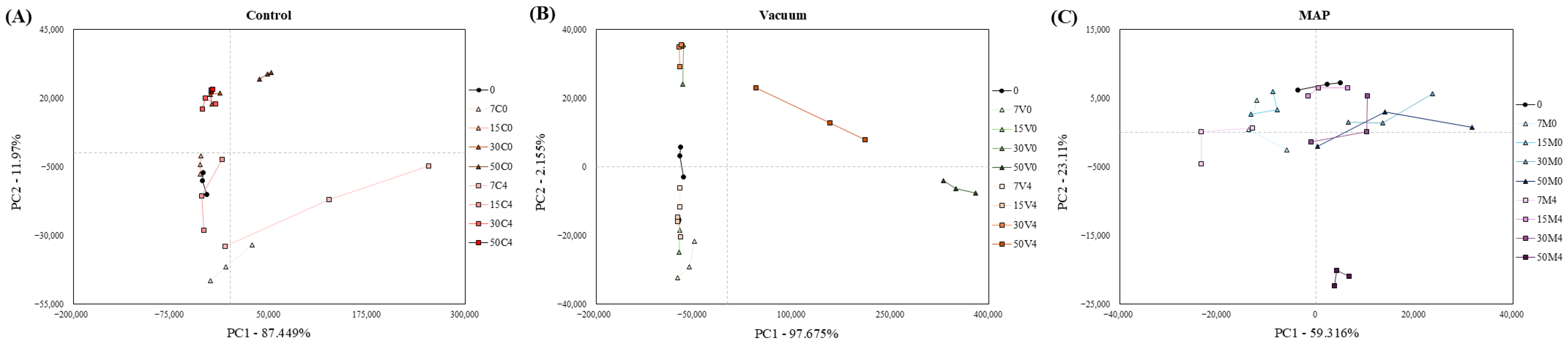
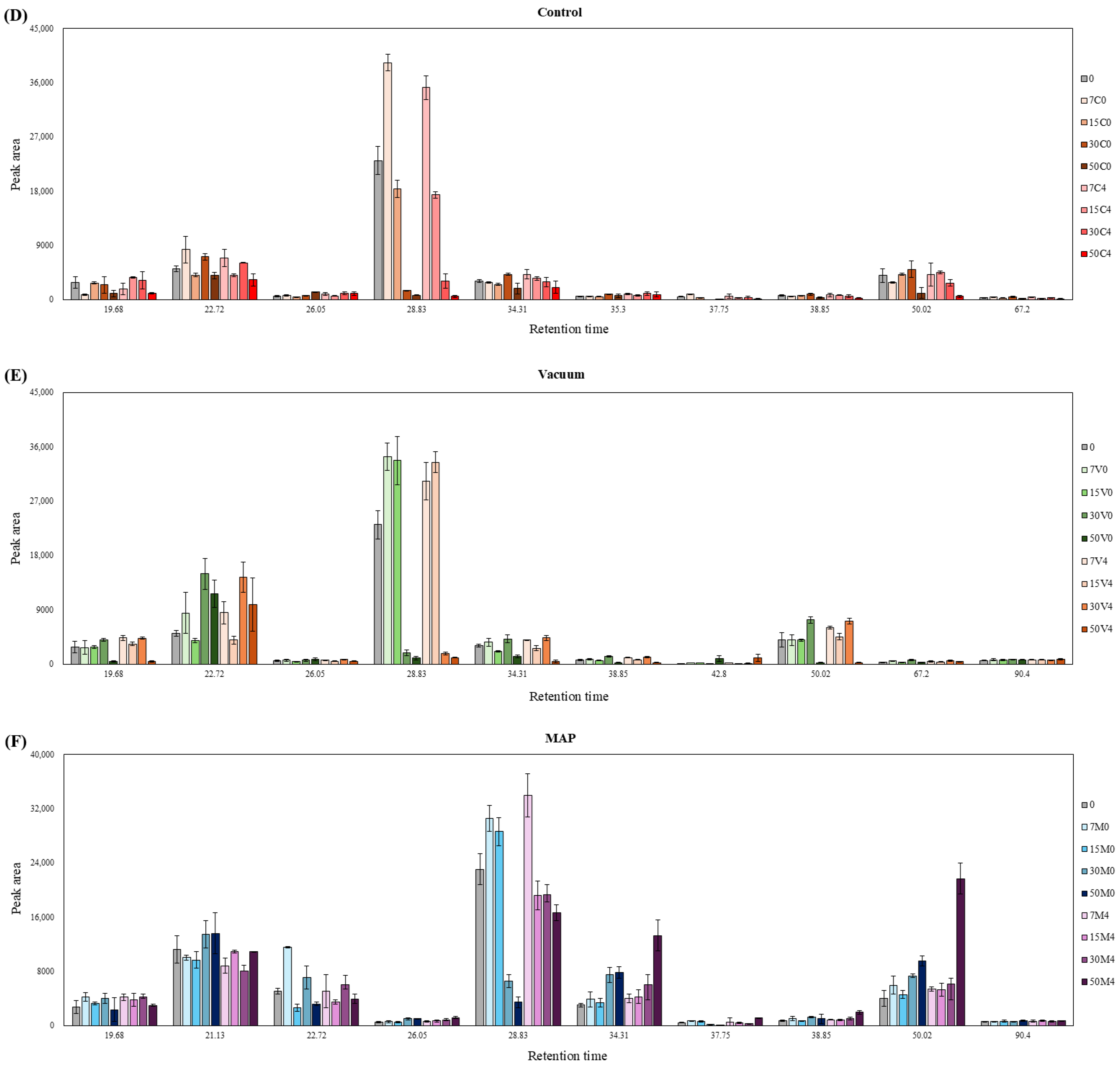
3.6. E-Nose
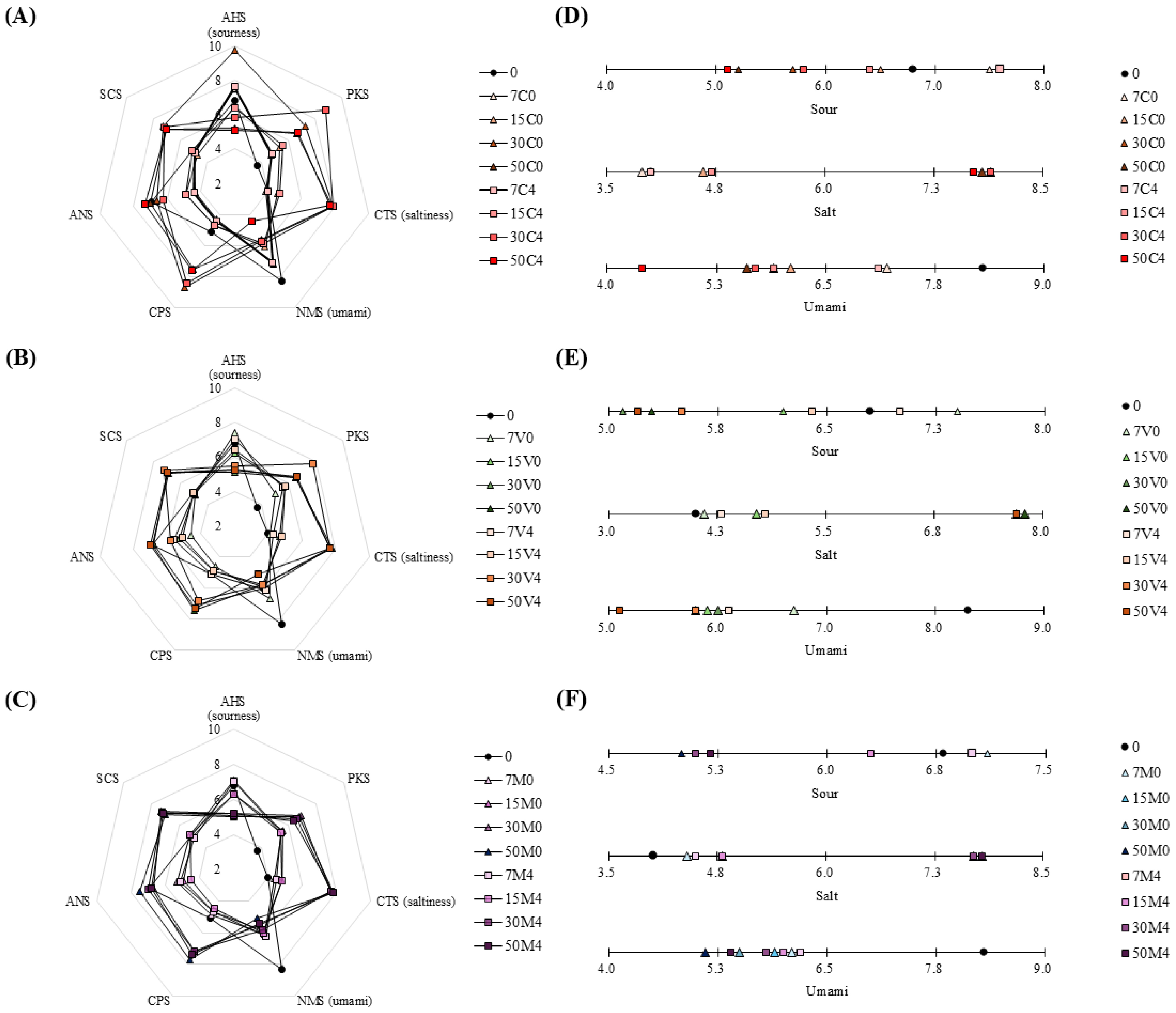
3.7. E-Tongue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMMS | Shiitake mushroom mycelium sausage |
| MAP | Modified atmosphere packaging |
| RT | Retention time |
| Aw | Water activity |
| APC | Aerobic plate count |
| TBARS | Thiobarbituric acid reactive substances |
| VBN | Volatile basic nitrogen |
| e-nose | Electronic nose |
| e-tongue | Electronic tongue |
| 0 | Day 0 |
| 7C0 | Control 0 °C, day 7 |
| 15C0 | Control 0 °C, day 15 |
| 30C0 | Control 0 °C, day 30 |
| 50C0 | Control 0 °C, day 50 |
| 7C4 | Control 4 °C, day 7 |
| 15C4 | Control 4 °C, day 15 |
| 30C4 | Control 4 °C, day 30 |
| 50C4 | Control 4 °C, day 50 |
| 7V0 | Vacuum 0 °C, day 7 |
| 15V0 | Vacuum 0 °C, day 15 |
| 30V0 | Vacuum 0 °C, day 30 |
| 50V0 | Vacuum 0 °C, day 50 |
| 7V4 | Vacuum 4 °C, day 7 |
| 15V4 | Vacuum 4 °C, day 15 |
| 30V4 | Vacuum 4 °C, day 30 |
| 50V4 | Vacuum 4 °C, day 50 |
| 7M0 | MAP 0 °C, day 7 |
| 15M0 | MAP 0 °C, day 15 |
| 30M0 | MAP 0 °C, day 30 |
| 50M0 | MAP 0 °C, day 50 |
| 7M4 | MAP 4 °C, day 7 |
| 15M4 | MAP 4 °C, day 15 |
| 30M4 | MAP 4 °C, day 30 |
| 50M4 | MAP 4 °C, day 50 |
References
- Flint, M.; Bowles, S.; Lynn, A. Paxman JR. Novel plant-based meat alternatives: Future opportunities and health considerations. Proc. Nutr. Soc. 2023, 82, 370–385. [Google Scholar] [CrossRef]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-based meat alternatives: Technological, nutritional, environmental, market, and social challenges and opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Kim, S.H.; Kim, C.J.; Lee, E.Y.; Kumari, S.; Hossain, M.J.; Alam, A.N.; Muazzam, A.; Hwang, Y.H.; Joo, S.T. From farms to labs: The new trend of sustainable meat alternatives. Food Sci. Anim. Resour. 2025, 45, 13–30. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, W.; Li, L.; Gao, Y.; Lai, K.H. Recent advances in the processing and manufacturing of plant-based meat. J. Agric. Food Chem. 2023, 71, 1276–1290. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.; Chen, J.; Zhou, J. Novel fungal alternative proteins from Penicillium limosum for enhancing structural and functional properties of plant-based meat analogues. Food Chem. 2024, 444, 138627. [Google Scholar] [CrossRef]
- Maseko, K.H.; Regnier, T.; Bartels, P.; Meiring, B. Mushroom mycelia as sustainable alternative proteins for the production of hybrid cell-cultured meat: A review. J. Food Sci. 2025, 90, e70060. [Google Scholar] [CrossRef]
- Perez-Montes, A.; Rangel-Vargas, E.; Lorenzo, J.M.; Romero, L.; Santos, E.M. Edible mushrooms as a novel trend in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 37, 118–124. [Google Scholar] [CrossRef]
- Gat, Y.; Gawande, P. Freeze-Drying Effect on Nutrients and Their Stability. In Freeze Drying of Food Products: Fundamentals, Processes and Applications; Wiley Online Library: Hoboken, NJ, USA, 2024; pp. 179–201. [Google Scholar]
- Nethra, P.V.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Akhila, P.P.; Sudheesh, C.; Mir, S.A.; Shijin, A.; George, J. Critical factors affecting the shelf life of packaged fresh red meat–A review. Meas. Food. 2023, 10, 100086. [Google Scholar] [CrossRef]
- Cenci-Goga, B.T.; Iulietto, M.F.; Sechi, P.; Borgogni, E.; Karama, M.; Grispoldi, L. New trends in meat packaging. Microbiol. Res. 2020, 11, 56–67. [Google Scholar] [CrossRef]
- Hua, Q.; Li, D. Effects of probiotic coating and vacuum packaging on the microbial safety and quality of fresh-cut cantaloupe stored at different temperatures. J. Food Sci. 2025, 90, e17665. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Dinakarkumar, Y.; Shin, H. A comprehensive overview on the preservation techniques and packaging of processed meat products: Emphasis on natural derivatives. J. King Saud Univ.-Sci. 2024, 36, 103032. [Google Scholar] [CrossRef]
- Abdullah, F.A.A.; Dordevic, D.; Kabourkova, E. Oxidation status and antioxidant activity of analogue meat products in modified atmosphere packaging. Appl. Sci. 2024, 14, 6713. [Google Scholar] [CrossRef]
- Gamarra-Castillo, O.; Echeverry-Montaña, N.; Marbello-Santrich, A.; Hernández-Carrión, M.; Restrepo, S. Meat substitute development from fungal protein (Aspergillus oryzae). Foods 2022, 11, 2940. [Google Scholar] [CrossRef]
- Nian, L.; Zhou, K.; Li, Q.; Hu, X.; Su, X.; Yan, X.; Tian, L.; Xu, B. Incorporation of yeast protein improves the textural properties of a fungal meat analogue from Pleurotus eryngii mycelium. Innov. Food Sci. Emerg. Technol. 2025, 107, 104322. [Google Scholar] [CrossRef]
- Piyaboon, O.; Chawasirikhunthol, C.; Phetprom, P.; Srimai, J. The Development of ground pork patty substitutes from mycelium of Pleurotus pulmonarius. Rev. Inf. Sci. Technol. 2025, 8, 32–38. [Google Scholar]
- Choi, H.; Gwon, H.G.; Kim, D.; Kim, M.; You, S.; Jo, Y.J. Structural characterization and rheological analysis of Lentinus edodes mycelium (mycoprotein)–hydrocolloid composites for food formulation. Future Foods 2025, 11, 100568. [Google Scholar] [CrossRef]
- Choi, D.M.; Lee, S.H.; KIm, H.Y. Antioxidant Activity of Different Fractions from Freeze-Dried Broccoli and Its Effect on Storage Properties of Emulsion-Type Pork Sausage. J. Korean Soc. Food Sci. Nutr. 2022, 51, 580–587. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, W.; Mao, Y.; Zhang, Y.; Li, K.; Luo, X.; Yang, X. Effects of CO2 on the physicochemical, microbial, and sensory properties of pork patties packaged under optimized O2 levels. Meat Sci. 2024, 209, 109422. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F. Plant protein heat-induced gels: Formation mechanisms and regulatory strategies. Coatings 2023, 13, 1899. [Google Scholar] [CrossRef]
- Cook, D.; Northcutt, J.; Dawson, P. Storage Effects on the Quality of Animal-and Plant-Based Sausage Patties. Eur. J. Agric. Food Sci. 2024, 6, 8–16. [Google Scholar] [CrossRef]
- Liu, J.; Luo, D.; Li, X.; Xu, B.; Zhang, X.; Liu, J. Effects of inulin on the structure and emulsifying properties of protein components in dough. Food Chem. 2016, 210, 235–241. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, T.S.; Kim, G.W.; Kim, H.Y. Quality properties of various dietary fibers as isolated soy protein (ISP) replacements in pork emulsion systems. J. Anim. Sci. Technol. 2020, 62, 94. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Hanus, P.; Ormian, M.; Kačániová, M.; Sokołowicz, Z.; Topczewska, J. The effect of temperature and storage duration on the quality and attributes of the breast meat of hens after their laying periods. Foods 2023, 12, 4340. [Google Scholar] [CrossRef]
- Jung, D.Y.; Lee, H.J.; Shin, D.J.; Kim, C.H.; Jo, C. Mechanism of improving emulsion stability of emulsion-type sausage with oyster mushroom (Pleurotus ostreatus) powder as a phosphate replacement. Meat Sci. 2022, 194, 108993. [Google Scholar] [CrossRef] [PubMed]
- Korus, A. Effect of pre-treatment and drying methods on the content of minerals, B-group vitamins and tocopherols in kale (Brassica oleracea L. var. acephala) leaves. J. Food Sci. Technol. 2022, 59, 279–287. [Google Scholar] [CrossRef]
- Naveed, A.; Zubair, M.; Baig, A.; Farid, M.; Ahmed, W.; Rehman, R.; Ayub, M.A.; Hassoun, A.; Cropotova, J. Effect of storage on the nutritional and antioxidant properties of brown Basmati rice. Food Sci. Nutr. 2023, 11, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Weiss, J.; Kinchla, A.J.; Nolden, A.A.; Grossmann, L. Methods for testing the quality attributes of plant-based foods: Meat- and processed-meat analogs. Foods 2021, 10, 260. [Google Scholar] [CrossRef]
- Oh, Y.N.; Kim, H.Y. Characterization of plant-based sausage quality using shiitake mushroom mycelium and soybean and wheat proteins. Food Sci. Anim. Resour. 2025, 45, 1514–1531. [Google Scholar] [CrossRef]
- Ameer, A.; Seleshe, S.; Kang, S.N. Effect of modified atmosphere packaging varying in CO2 and N2 composition on quality characteristics of dry fermented sausage during refrigeration storage. Food Sci. Anim. Resour. 2022, 42, 639–654. [Google Scholar] [CrossRef]
- Faisal, M.; Jacobson, T.; Meineret, L.; Vorup, P.; Bordallo, H.N.; Kirkensgaard, J.J.K.; Ulvskov, P.; Blennow, A. Development of pH indicator composite films based on anthocyanins and neutral red for monitoring minced meat and fish in modified gas atmosphere (MAP). Coatings 2024, 14, 725. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. Supercritical carbon dioxide treatment improves the functional properties of pea protein: Application in eggless cakes. Food Chem. 2025, 475, 143224. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Andreescu, S. Chemical and biological sensors for food-quality monitoring and smart packaging. Foods 2018, 7, 168. [Google Scholar] [CrossRef]
- Go, H.Y.; Park, S.Y.; Kim, H.Y. Analysis of cured pork loin ham quality using wet-aging and a pulsed electric field system. Food Sci. Biotechnol. 2023, 32, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food biosci. 2022, 46, 101615. [Google Scholar]
- Uysal, C.; Enişte, İ.; Çifçi, M.; Şimşek, A.; Kılıç, B. Effects of different packaging methods and storage temperatures on physicochemical, microbiological, textural and sensorial properties of emulsion-type sausage chips. J. Stored Prod. Res. 2022, 98, 102002. [Google Scholar] [CrossRef]
- Stasiewicz, M.; Lipiński, K.; Cierach, M. Quality of meat products packaged and stored under vacuum and modified atmosphere conditions. J. Food Sci. Technol. 2014, 51, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.H.; Kaur, M.; Farahnaky, A.; Torley, P.J.; Osborn, A.M. Microbial and Quality Attributes of Beef Steaks under High-CO2 Packaging: Emitter Pads versus Gas Flushing. Foods 2024, 13, 2913. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, C.; Chen, S.; Xue, Y.; Wang, Y.; Wu, Y. Effects of modified atmosphere packaging with different gas ratios on the quality changes of golden pompano (Trachinotus ovatus) fillets during superchilling storage. Foods 2022, 11, 1943. [Google Scholar] [CrossRef]
- Reyes, T.M.; Wagoner, M.P.; Zorn, V.E.; Coursen, M.M.; Wilborn, B.S.; Bonner, T.; Brandebourg, T.D.; Rodning, S.P.; Sawyer, J.T. Vacuum packaging can extend fresh color characteristics of beef steaks during simulated display conditions. Foods 2022, 11, 520. [Google Scholar] [CrossRef]
- Holck, A.L.; Pettersen, M.K.; Moen, M.H.; Sørheim, O. Prolonged shelf life and reduced drip loss of chicken filets by the use of carbon dioxide emitters and modified atmosphere packaging. J. Food Prot. 2014, 77, 1133–1141. [Google Scholar] [CrossRef]
- Wang, B.; Xu, C.C.; Liu, C.; Qu, Y.H.; Zhang, H.; Luo, H.L. The effect of dietary lycopene supplementation on drip loss during storage of lamb meat by iTRAQ analysis. Antioxidants 2021, 10, 198. [Google Scholar] [CrossRef]
- You, S.; Yang, S.; Li, L.; Zheng, B.; Zhang, Y.; Zeng, H. Processing technology and quality change during storage of fish sausages with textured soy protein. Foods 2022, 11, 3546. [Google Scholar] [CrossRef]
- Miller, O.; Scarlett, C.J.; Akanbi, T.O. Lipid stability profiles of uncooked plant-based meat analogue burger patties and comparison of their textural and sensory properties with traditional beef burger patties. J. Food Compos. Anal. 2025, 147, 108002. [Google Scholar] [CrossRef]
- Xia, X.; Zhou, T.; Yu, J.; Cui, H.; Zhang, F.; Hayat, K.; Zhang, X.; Ho, C.T. Formation of fluorescent Maillard reaction intermediates of peptide and glucose during thermal reaction and its mechanism. J. Agric. Food Chem. 2023, 71, 8569–8579. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Cichy, K.; Swada, J. Evaluation of Cooking Time and Impact of Retort Processing, Packaging Material, and Calcium Chloride Additive on Pulse (Phaseolus vulgaris, Phaseolus coccineus, and Cicer arietinum) Quality Attributes. J. Food Process. Preserv. 2025, 2025, 1164668. [Google Scholar] [CrossRef]
- Ding, C.; Khir, R.; Pan, Z.; Wood, D.F.; Venkitasamy, C.; Tu, K.; El-Mashad, H.; Berrios, J. Influence of infrared drying on storage characteristics of brown rice. Food Chem. 2018, 264, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kała, K.; Krakowska, A.; Szewczyk, A.; Ostachowicz, B.; Szczurek, K.; Fijałkowska, A.; Muszyńska, B. Determining the amount of potentially bioavailable phenolic compounds and bioelements in edible mushroom mycelia of Agaricus bisporus, Cantharellus cibarius, and Lentinula edodes. Food Chem. 2021, 352, 129456. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Łopacka, J.; Półtorak, A.; Wierzbicka, A. Effect of MAP, vacuum skin-pack and combined packaging methods on physicochemical properties of beef steaks stored up to 12 days. Meat Sci. 2016, 119, 147–153. [Google Scholar] [CrossRef]
- Neo, C.W.X.; How, Y.H.; Kong, I.; Talib, R.A.; Pui, L.P. Development of plant-based burger patties with pea protein isolate and barnyard millet flour and its storage stability in aerobic and vacuum packaging. J. Food Saf. 2024, 44, e13134. [Google Scholar] [CrossRef]
- Zduńczyk, W.; Modzelewska-Kapituła, M.; Tkacz, K. Influence of Oxygen and Carbon Dioxide Content in Modified Atmosphere Packaging on the Colour and Water-Holding Capacity of Pork Loin. Appl. Sci. 2024, 14, 3420. [Google Scholar] [CrossRef]
- Liu, R. Discussion on the Control of Water Activity on Microorganism in Traditional Chinese Medicine Preparation. World Sci. Res. J. 2022, 8, 723–727. [Google Scholar]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking microbial contamination to food spoilage and food waste: The role of smart packaging, spoilage risk assessments, and date labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef]
- Pangastuti, H.A.; Permana, L.; Fitriani, V.; Mareta, D.T.; Wahyuningtyas, A. The effect of citric acid on chemical and physical characteristics of andaliman (Zanthoxylum acanthopodium) chili paste in retort packaging. IOP Conf. Ser. Earth Environ. Sci. 2020, 537, 012031. [Google Scholar] [CrossRef]
- Zardetto, S.; Pasini, G.; Romani, S.; Rocculi, P.; Dalla Rosa, M. Evaluation of physico-chemical changes and FT-NIR spectra in fresh egg pasta packed in modified atmosphere during storage at different temperatures. Food Packag. Shelf Life 2021, 28, 100648. [Google Scholar] [CrossRef]
- Qian, Y.F.; Shi, C.J.; Liu, C.C.; Zhang, J.J.; Yang, S.P. Effects of CO2 and O2 in Modified Atmosphere Packaging on Water Retention, Protein Stability, and Microbial Growth in Atlantic Salmon Fillets. Fishes 2025, 10, 141. [Google Scholar] [CrossRef]
- Corrêa, P.F.; da Silva, C.F.; Ferreira, J.P.; Guerra, J.M.C. Vegetable-based frankfurter sausage production by different emulsion gels and assessment of physical-chemical, microbiological and nutritional properties. Food Chem. Adv. 2023, 3, 100354. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, D.; Li, X.; Ding, T.; Liang, C.; Zheng, X.; Yang, W.; Hou, C. Dynamic changes of bacteria and screening of potential spoilage markers of lamb in aerobic and vacuum packaging. Food Microbiol. 2022, 104, 103996. [Google Scholar] [CrossRef]
- Park, J.H.; Hwang, D.; Kang, M.; Kim, H.J. Predictive modeling and scenario-based risk estimation of Escherichia coli growth in meatball products: A comparison of aerobic and vacuum packaging conditions. LWT 2025, 216, 117271. [Google Scholar] [CrossRef]
- Nauman, K.; Jaspal, M.H.; Asghar, B.; Manzoor, A.; Akhtar, K.H.; Ali, U.; Ali, S.; Nasir, J.; Sohaib, M.; Badar, I.H. Effect of different packaging atmosphere on microbiological shelf life, physicochemical attributes, and sensory characteristics of chilled poultry fillets. Food Sci. Anim. Resour. 2022, 42, 153–174. [Google Scholar] [CrossRef]
- Yim, D.G.; Jin, S.K.; Hur, S.J. Microbial changes under packaging conditions during transport and comparison between sampling methods of beef. J. Anim. Sci. Technol. 2019, 61, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Mei, J.; Xie, J. Effects of packaging methods and temperature variations on the quality and microbial diversity of grouper (Epinephelus lanceolatus) during cold storage. Food Biosci. 2024, 60, 104315. [Google Scholar] [CrossRef]
- Zhao, T.; Echegaray, N.; Lorenzo, J.M.; Köroğlu, D.G.; Capanoglu, E.; Ziora, Z.M.; Esatbeyoglu, T. Exploring Meat Analogs: A Review of Alternative Proteins with a Focus on Food Safety and Regulatory Challenges. Food Control 2025, 182, 111777. [Google Scholar] [CrossRef]
- Kabisch, J.; Joswig, G.; Böhnlein, C.; Fiedler, G.; Franz, C.M. Microbiological status of vegan ground meat products from German retail. J. Consum. Prot. Food Saf. 2024, 19, 33–40. [Google Scholar] [CrossRef]
- Geeraerts, W.; De Vuyst, L.; Leroy, F. Ready-to-eat meat alternatives, a study of their associated bacterial communities. Food Biosci. 2020, 37, 100681. [Google Scholar] [CrossRef]
- Piranavatharsan, U.; Jinadasa, B.K.K.K.; Jayasinghe, C.V.L. Validation of thiobarbituric acid reactive substances (TBARS) method for measuring secondary lipid oxidation products in fresh Indian mackerel (Rastrelliger kanagurta). Food Humanit. 2023, 1, 1194–1199. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Muzolf-Panek, M. Predictive modelling of TBARS changes in the intramuscular lipid fraction of raw ground pork enriched with plant extracts. J. Food Sci. Technol. 2022, 59, 1756–1768. [Google Scholar] [CrossRef]
- Liu, M.; Lin, C.; Wu, R.; Cao, S.; Liang, Z.; Zhang, R. Heat Stress Response and Comprehensive Evaluation of Heat Resistance of Lentinula edodes Mycelia. J. Agric. Sci. Technol. 2024, 26, 90. [Google Scholar]
- Münch, K.; Stoyanov, S.; Schroën, K.; Berton-Carabin, C. Effect of Nonprotein Components for Lipid Oxidation in Emulsions Stabilized by Plant Protein Extracts. ACS Food Sci. Technol. 2024, 4, 926–934. [Google Scholar] [CrossRef]
- Bukowski, M.R.; Goslee, S. Climate-based variability in the essential fatty acid composition of soybean oil. Am. J. Clin. Nutr. 2024, 119, 58–68. [Google Scholar] [CrossRef]
- Moretto, L.; Tonolo, F.; Folda, A.; Scalcon, V.; Bindoli, A.; Bellamio, M.; Feller, E.; Rigobello, M.P. Comparative analysis of the antioxidant capacity and lipid and protein oxidation of soy and oats beverages. Food Prod. Process. Nutr. 2021, 3, 1. [Google Scholar] [CrossRef]
- Solinho, J.; Santos, J.; Vázquez, M.; Pinheiro, R. Comparative study of preservation techniques for refrigerated Atlantic bonito Fillets: Effects of modified atmosphere packaging, vacuum packaging, and alginate coating on shelf life and quality. Food Packag. Shelf Life 2025, 50, 101556. [Google Scholar] [CrossRef]
- Li, S.; Guo, X.; Shen, Y.; Pan, J.; Dong, X. Effects of oxygen concentrations in modified atmosphere packaging on pork quality and protein oxidation. Meat Sci. 2022, 189, 108826. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Kang, S.M. Effect of Oxygen Concentration in Modified Atmosphere Packaging on the Superoxide Dismutase Activity and Oxidation Stability of Ground Beef. Resour. Sci. Res. 2022, 4, 48–55. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Holman, B.W.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Weng, P.; Zhang, Y.; Peng, J.; Ma, F.; Zhou, H. Analysis of Potential Markers of Pork Freshness Based on Volatile Organic Compounds. Foods 2025, 14, 832. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Song, Y.R.; Lee, S.J.; Lee, J.K.; Lee, S.K. Effects of gas composition in the modified atmosphere packaging on the shelf-life of Longissimus dorsi of Korean native black pigs-duroc crossbred during refrigerated storage. Anim. Biosci. 2014, 27, 1157. [Google Scholar]
- Liang, C.; Zhang, D.; Zheng, X.; Wen, X.; Yan, T.; Zhang, Z.; Hou, C. Effects of different storage temperatures on the physicochemical properties and bacterial community structure of fresh lamb meat. Food Sci. Anim. Resour. 2021, 41, 509–526. [Google Scholar] [CrossRef]
- Lee, H.; Cho, H.; Lim, Y.; Park, S.; Lee, J. Safety, quality, and microbial community analysis of salt-fermented shrimp (Saeu-jeot) in South Korea. Heliyon 2024, 10, e26006. [Google Scholar] [CrossRef]
- Baek, U.B.; Kim, H.Y. Physicochemical properties of restructured black goat jerky with various types of ultra-ground seaweed powders. Food Sci. Anim. Resour. 2024, 44, 483–497. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Yu, Y.; Zhang, X.; Chen, J.; Zhang, H. Quality change in camellia oil during intermittent frying. Foods 2022, 11, 4047. [Google Scholar] [CrossRef]
- Martín-Tornero, E.; Barea-Ramos, J.D.; Lozano, J.; Durán-Merás, I.; Martín-Vertedor, D. E-nose quality evaluation of extra virgin olive oil stored in different containers. Chemosensors 2023, 11, 85. [Google Scholar] [CrossRef]
- Ritter, S.W.; Thiel, Q.P.; Gastl, M.I.; Becker, T.M. Optimizing the fermentation parameters in the Lactic Acid Fermentation of Legume-based Beverages– a statistically based fermentation. Microb. Cell Fact. 2024, 23, 253. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Zhang, L. Optimization of lactic acid fermentation conditions for fermented tofu whey beverage with high-isoflavone aglycones. LWT 2019, 111, 211–217. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef]
- Wang, W.K.; Zhu, Y.; Tang, Y.; Lu, N.; Song, J.L.; Yuan, W.D.; Jia, Y. Non-volatile taste components of different cultivated mushrooms at mycelia, primordium, and fruit body cultivation stages. Int. J. Food Prop. 2016, 19, 1938–1948. [Google Scholar] [CrossRef]
- Shu, C.; Liu, B.; Zhao, H.; Cui, K.; Jiang, W. Effect of near-freezing temperature storage on the quality and organic acid metabolism of apple fruit. Agriculture 2024, 14, 1057. [Google Scholar] [CrossRef]
- You, Z.; Bai, Y.; Bo, D.; Feng, Y.; Shen, J.; Wang, Y.; Li, J.; Bai, Y. A review of taste-active compounds in meat: Identification, influencing factors, and taste transduction mechanism. J. Food Sci. 2024, 89, 8128–8155. [Google Scholar] [CrossRef]
- Rysová, J.; Šmídová, Z. Effect of salt content reduction on food processing technology. Foods 2021, 10, 2237. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Baldwin, E.A.; Plotto, A.; Rosskopf, E.; Hong, J.C.; Bai, J. Electronic tongue discrimination of four tomato cultivars harvested at six maturities and exposed to blanching and refrigeration treatments. Postharvest Biol. Technol. 2019, 136, 42–49. [Google Scholar] [CrossRef]
- Wang, X.; Cui, B.; Lin, H.; Pan, R.; Zeng, J.; Fang, X.; Liu, Y.; Chen, Z.Y.; Chen, Y.; Zhu, H. Research progress in saltiness perception and salty substitutes. J. Agric. Food Chem. 2025, 73, 2745–2759. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lee, H.; Choi, J.; Kim, K.; Kim, A.Y. Potential reduction of salt consumption by preparing noodles with entrapped NaCl in mycelial cell wall cavities of Lentinus edodes. Food Bioprocess Technol. 2019, 12, 704–713. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.; Cittadini, A.; Domínguez, R.; Lorenzo, J.M. Metallic-based salt substitutes to reduce sodium content in meat products. Curr. Opin. Food Sci. 2021, 38, 21–31. [Google Scholar] [CrossRef]
- Yaşar, K.; Şahingil, D.; Hayaloğlu, A.A. Reducing sodium in Kashar cheese by partial substitution of NaCl with KCl: Proteolysis, texture, meltability and sensory characteristics. Int. Dairy J. 2025, 166, 106239. [Google Scholar] [CrossRef]
| Properties (%) | Storage Day | Treatment | |||||
|---|---|---|---|---|---|---|---|
| Control | Vacuum | MAP | |||||
| 0 °C | 4 °C | 0 °C | 4 °C | 0 °C | 4 °C | ||
| Moisture | 0 | 73.36 ± 0.08 A | 73.36 ± 0.08 A | 73.36 ± 0.08 A | 73.36 ± 0.08 A | 73.36 ± 0.08 A | 73.36 ± 0.08 A |
| 7 | 72.59 ± 0.20 Ba | 72.51 ± 0.32 Ba | 72.52 ± 0.24 Ba | 72.52 ± 0.17 Ba | 72.66 ± 0.10 Ba | 72.42 ± 0.28 Ba | |
| 15 | 72.36 ± 0.16 Bab | 72.28 ± 0.26 Bb | 72.34 ± 0.41 Bab | 72.78 ± 0.38 ABa | 72.52 ± 0.19 Bab | 72.31 ± 0.29 Bab | |
| 30 | 72.57 ± 0.40 Ba | 72.21 ± 0.47 Ba | 72.47 ± 0.50 Ba | 72.61 ± 0.41 Ba | 72.42 ± 0.20 Ba | 72.40 ± 0.17 Ba | |
| 50 | 71.80 ± 0.58 Cab | 70.99 ± 0.42 Cb | 72.11 ± 0.61 Ba | 71.78 ± 0.64 Cab | 72.37 ± 0.50 Ba | 71.72 ± 0.36 Cab | |
| Protein | 0 | 25.04 ± 0.09 B | 25.04 ± 0.09 B | 25.04 ± 0.09 C | 25.04 ± 0.09 C | 25.04 ± 0.09 B | 25.04 ± 0.09 B |
| 7 | 25.76 ± 0.31 Aab | 25.47 ± 0.24 Aab | 25.32 ± 0.25 Bb | 25.42 ± 0.37 BCab | 25.85 ± 0.24 Aa | 25.51 ± 0.28 ABab | |
| 15 | 25.82 ± 0.24 Aa | 25.74 ± 0.26 Aa | 25.77 ± 0.12 Aa | 25.62 ± 0.19 ABa | 25.86 ± 0.24 Aa | 25.58 ± 0.66 Aa | |
| 30 | 25.54 ± 0.36 Aa | 25.77 ± 0.25 Aa | 25.74 ± 0.14 Aa | 25.91 ± 0.31 Aa | 25.90 ± 0.59 Aa | 26.03 ± 0.08 Aa | |
| 50 | 25.65 ± 0.25 Aa | 25.78 ± 0.29 Aa | 25.67 ± 0.09 Aa | 25.52 ± 0.38 ABa | 25.64 ± 0.19 Aa | 25.92 ± 0.25 Aa | |
| Ash | 0 | 1.14 ± 0.02 A | 1.14 ± 0.02 A | 1.14 ± 0.02 A | 1.14 ± 0.02 A | 1.14 ± 0.02 A | 1.14 ± 0.02 A |
| 7 | 1.14 ± 0.04 Aa | 1.14 ± 0.02 Aa | 1.15 ± 0.03 Aa | 1.14 ± 0.04 Aa | 1.12 ± 0.01 Aa | 1.13 ± 0.02 Aa | |
| 15 | 1.11 ± 0.01 Aa | 1.13 ± 0.08 Aa | 1.11 ± 0.08 Aa | 1.10 ± 0.05 Aa | 1.12 ± 0.06 Aa | 1.14 ± 0.05 Aa | |
| 30 | 1.13 ± 0.02 Aab | 1.13 ± 0.02 Aab | 1.14 ± 0.01 Aa | 1.13 ± 0.02 Aab | 1.13 ± 0.01 Aab | 1.11 ± 0.01 Ab | |
| 50 | 1.14 ± 0.06 Aa | 1.14 ± 0.03 Aa | 1.13 ± 0.02 Aa | 1.14 ± 0.02 Aa | 1.14 ± 0.02 Aa | 1.13 ± 0.03 Aa | |
| Traits | Storage Day | Treatment | |||||
|---|---|---|---|---|---|---|---|
| Control | Vacuum | MAP | |||||
| 0 °C | 4 °C | 0 °C | 4 °C | 0 °C | 4 °C | ||
| pH | 0 | 6.09 ± 0.05 A | 6.09 ± 0.05 A | 6.09 ± 0.05 A | 6.09 ± 0.05 A | 6.09 ± 0.05 A | 6.09 ± 0.05 A |
| 7 | 6.05 ± 0.01 Aa | 6.03 ± 0.01 ABa | 6.04 ± 0.01 Aa | 6.03 ± 0.01 ABa | 6.06 ± 0.05 Aa | 6.05 ± 0.01 Aa | |
| 15 | 6.03 ± 0.03 Aa | 6.02 ± 0.01 Bbc | 6.03 ± 0.01 Aab | 6.01 ± 0.01 Bbc | 6.01 ± 0.01 ABbc | 6.02 ± 0.00 Bc | |
| 30 | 6.03 ± 0.00 Aa | 6.03 ± 0.00 ABbc | 6.03 ± 0.00 Aa | 6.02 ± 0.00 ABc | 6.00 ± 0.00 Bd | 6.00 ± 0.00 Bd | |
| 50 | 5.95 ± 0.10 Bab | 5.87 ± 0.01 Cbc | 5.94 ± 0.03 Ba | 5.90 ± 0.04 Cab | 5.87 ± 0.02 Cb | 5.86 ± 0.01 Cb | |
| Storage loss (%) | 0 | 0.00 B | 0.00 C | 0.00 C | 0.00 D | 0.00 C | 0.00 D |
| 7 | 0.80 ± 0.53 ABd | 2.00 ± 0.40 Bc | 4.07 ± 0.76 Bb | 4.67 ± 0.50 Cb | 3.87 ± 0.90 Bb | 8.80 ± 0.53 Ca | |
| 15 | 1.40 ± 0.87 Ad | 3.13 ± 0.42 Ac | 6.53 ± 0.64 Ab | 6.73 ± 0.42 Bb | 4.13 ± 0.61 Bc | 12.33 ± 0.50 Ba | |
| 30 | 1.53 ± 0.42 Ae | 3.27 ± 0.50 Ad | 6.60 ± 0.92 Abc | 7.40 ± 0.92 Bb | 5.93 ± 0.70 Ac | 13.67 ± 0.95 Aa | |
| 50 | 1.67 ± 0.31 Af | 3.80 ± 0.20 Ae | 7.87 ± 0.90 Ac | 9.73 ± 0.61 Ab | 6.40 ± 0.72 Ad | 14.47 ± 0.76 Aa | |
| Characteristics | Storage Day | Treatment | |||||
|---|---|---|---|---|---|---|---|
| Control | Vacuum | MAP | |||||
| 0 °C | 4 °C | 0 °C | 4 °C | 0 °C | 4 °C | ||
| CIE L* | 0 | 65.87 ± 0.15 A | 65.87 ± 0.15 A | 65.87 ± 0.15 A | 65.87 ± 0.15 A | 65.87 ± 0.15 A | 65.87 ± 0.15 A |
| 7 | 64.10 ± 0.35 Ba | 64.70 ± 0.35 Ba | 64.47 ± 0.93 Ba | 64.33 ± 0.60 Ba | 63.97 ± 0.15 Ba | 63.90 ± 0.53 Ba | |
| 15 | 63.40 ± 0.26 Cc | 63.53 ± 0.06 Cc | 64.57 ± 0.15 Ba | 64.10 ± 0.10 Bb | 63.87 ± 0.15 Bb | 63.83 ± 0.06 Bb | |
| 30 | 61.47 ± 0.47 Dc | 61.43 ± 0.12 Dc | 64.53 ± 0.72 Ba | 64.43 ± 0.55 Ba | 63.27 ± 0.61 Cb | 63.07 ± 0.76 BCb | |
| 50 | 61.20 ± 0.20 Dc | 61.13 ± 0.55 Dc | 64.53 ± 0.15 Ba | 64.07 ± 0.32 Ba | 63.10 ± 0.17 Cb | 62.67 ± 0.32 Cb | |
| CIE a* | 0 | 3.40 ± 0.10 C | 3.40 ± 0.10 C | 3.40 ± 0.10 A | 3.40 ± 0.10 A | 3.40 ± 0.10 D | 3.40 ± 0.10 C |
| 7 | 3.40 ± 0.17 Ca | 3.63 ± 0.23 Ba | 3.40 ± 0.17 Aa | 3.43 ± 0.06 Aa | 3.60 ± 0.10 CDa | 3.53 ± 0.15 Ca | |
| 15 | 3.50 ± 0.10 BCb | 3.73 ± 0.06 Ba | 3.33 ± 0.06 Ab | 3.43 ± 0.21 Ab | 3.80 ± 0.10 BCa | 3.93 ± 0.06 Ba | |
| 30 | 3.70 ± 0.10 Bab | 3.87 ± 0.29 Ba | 3.30 ± 0.10 Ac | 3.47 ± 0.12 Abc | 3.93 ± 0.12 Ba | 4.03 ± 0.32 Ba | |
| 50 | 4.40 ± 0.10 Ab | 5.17 ± 0.15 Aa | 3.50 ± 0.10 Ac | 3.53 ± 0.06 Ac | 4.43 ± 0.25 Ab | 4.93 ± 0.15 Aa | |
| CIE b* | 0 | 16.57 ± 0.29 A | 16.57 ± 0.29 A | 16.57 ± 0.29 A | 16.57 ± 0.29 A | 16.57 ± 0.29 A | 16.57 ± 0.29 A |
| 7 | 14.57 ± 0.92 Bb | 14.63 ± 0.45 Bb | 14.43 ± 0.40 Bb | 14.47 ± 0.25 Bb | 16.20 ± 0.10 Aa | 16.23 ± 0.15 Aa | |
| 15 | 14.60 ± 0.17 Bb | 14.53 ± 0.32 Bb | 14.67 ± 0.15 Bb | 14.60 ± 0.46 Bb | 16.43 ± 0.74 Aa | 16.33 ± 0.29 Aa | |
| 30 | 14.83 ± 0.38 Bb | 14.80 ± 0.36 Bb | 14.73 ± 0.15 Bb | 14.77 ± 0.83 Bb | 16.33 ± 0.70 Aa | 16.13 ± 0.76 Aa | |
| 50 | 14.77 ± 0.51 Bb | 14.87 ± 0.32 Bb | 14.83 ± 0.31 Bb | 14.87 ± 0.25 Bb | 16.80 ± 0.10 Aa | 16.80 ± 0.10 Aa | |
| Traits | Storage Day | Treatment | |||||
|---|---|---|---|---|---|---|---|
| Control | Vacuum | MAP | |||||
| 0 °C | 4 °C | 0 °C | 4 °C | 0 °C | 4 °C | ||
| Aw | 0 | 0.81 ± 0.00 C | 0.81 ± 0.00 C | 0.81 ± 0.00 C | 0.81 ± 0.00 D | 0.81 ± 0.00 D | 0.81 ± 0.00 D |
| 7 | 0.84 ± 0.00 Bab | 0.84 ± 0.00 Ba | 0.83 ± 0.00 Bc | 0.84 ± 0.00 Ca | 0.83 ± 0.00 Cbc | 0.84 ± 0.00 Ca | |
| 15 | 0.84 ± 0.00 Aa | 0.84 ± 0.00 Aa | 0.84 ± 0.00 Bc | 0.84 ± 0.00 Bb | 0.84 ± 0.00 Bb | 0.84 ± 0.00 Bb | |
| 30 | 0.84 ± 0.00 Aa | 0.84 ± 0.00 Aa | 0.84 ± 0.00 Ad | 0.84 ± 0.00 ABc | 0.84 ± 0.00 Abc | 0.84 ± 0.00 Aab | |
| 50 | 0.84 ± 0.00 Aa | 0.84 ± 0.00 Aab | 0.84 ± 0.00 Ac | 0.84 ± 0.00 Aabc | 0.84 ± 0.00 Abc | 0.84 ± 0.00 Aabc | |
| APC | 0 | 0.18 ± 0.52 B | 0.18 ± 0.52 B | 0.18 ± 0.52 B | 0.18 ± 0.52 B | 0.18 ± 0.52 B | 0.18 ± 0.52 B |
| 7 | 0.37 ± 0.68 Ba | 0.55 ± 0.76 Ba | 0.18 ± 0.52 Ba | 0.37 ± 0.68 Ba | 0.18 ± 0.52 Ba | 0.18 ± 0.52 Ba | |
| 15 | 0.51 ± 0.72 Bab | 1.00 ± 0.85 Ba | 0.33 ± 0.62 Bab | 0.63 ± 0.70 Bab | 0.16 ± 0.46 Bb | 0.51 ± 0.65 Bab | |
| 30 | 2.10 ± 0.17 Ab | 3.68 ± 0.52 Aa | 1.50 ± 1.00 Ab | 2.00 ± 0.00 Ab | 0.25 ± 0.71 Bc | 0.54 ± 1.00 Bc | |
| 50 | 2.59 ± 0.36 Ab | 3.90 ± 0.82 Aa | 2.50 ± 0.71 Ab | 2.65 ± 0.49 Ab | 2.00 ± 0.00 Ab | 2.00 ± 0.00 Ab | |
| TBARS | 0 | 0.41 ± 0.01 C | 0.41 ± 0.01 C | 0.41 ± 0.01 B | 0.41 ± 0.01 C | 0.41 ± 0.01 C | 0.41 ± 0.01 D |
| 7 | 0.46 ± 0.01 Bb | 0.46 ± 0.01 Bb | 0.46 ± 0.01 Ab | 0.46 ± 0.01 Bb | 0.53 ± 0.01 Ba | 0.54 ± 0.01 Ca | |
| 15 | 0.47 ± 0.01 Bb | 0.49 ± 0.03 Bb | 0.46 ± 0.02 Ab | 0.48 ± 0.01 Bb | 0.56 ± 0.03 Ba | 0.59 ± 0.02 BCa | |
| 30 | 0.50 ± 0.04 ABbc | 0.54 ± 0.02 Ab | 0.46 ± 0.01 Ac | 0.47 ± 0.02 Bc | 0.60 ± 0.02 Aa | 0.64 ± 0.03 Ba | |
| 50 | 0.53 ± 0.03 Ac | 0.56 ± 0.01 Abc | 0.50 ± 0.04 Ac | 0.52 ± 0.02 Ac | 0.63 ± 0.01 Ab | 0.75 ± 0.10 Aa | |
| VBN | 0 | 0.37 ± 0.17 D | 0.37 ± 0.17 D | 0.37 ± 0.17 C | 0.37 ± 0.17 D | 0.37 ± 0.17 D | 0.37 ± 0.17 D |
| 7 | 2.17 ± 0.13 Ca | 2.24 ± 0.22 Ca | 2.09 ± 0.13 Ba | 2.09 ± 0.13 Ca | 2.17 ± 0.13 Ca | 2.31 ± 0.13 Ca | |
| 15 | 2.24 ± 0.22 Cc | 2.61 ± 0.13 Ca | 2.09 ± 0.13 Bc | 2.31 ± 0.13 Cbc | 2.54 ± 0.13 Cab | 2.65 ± 0.06 Ca | |
| 30 | 3.88 ± 0.13 Bd | 7.47 ± 0.34 Bc | 2.39 ± 0.13 Be | 3.73 ± 0.34 Bd | 8.29 ± 0.22 Bb | 30.02 ± 0.45 Ba | |
| 50 | 10.01 ± 0.47 Ae | 21.06 ± 0.22 Ac | 4.26 ± 0.22 Af | 18.07 ± 0.47 Ad | 22.33 ± 0.91 Ab | 36.89 ± 0.47 Aa | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, Y.-N.; Kim, H.-Y. Evaluation of Quality and Storage Characteristics of Freeze-Dried Powdered Mycelium Sausages According to Packaging Methods. Foods 2025, 14, 4080. https://doi.org/10.3390/foods14234080
Oh Y-N, Kim H-Y. Evaluation of Quality and Storage Characteristics of Freeze-Dried Powdered Mycelium Sausages According to Packaging Methods. Foods. 2025; 14(23):4080. https://doi.org/10.3390/foods14234080
Chicago/Turabian StyleOh, Yu-Na, and Hack-Youn Kim. 2025. "Evaluation of Quality and Storage Characteristics of Freeze-Dried Powdered Mycelium Sausages According to Packaging Methods" Foods 14, no. 23: 4080. https://doi.org/10.3390/foods14234080
APA StyleOh, Y.-N., & Kim, H.-Y. (2025). Evaluation of Quality and Storage Characteristics of Freeze-Dried Powdered Mycelium Sausages According to Packaging Methods. Foods, 14(23), 4080. https://doi.org/10.3390/foods14234080






