Soybean Protein Hydrolysate Enhances Growth and Freeze-Drying Survival of Bifidobacterium breve and Bifidobacterium longum Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Soy Protein Isolate
2.2. Enzymatic Hydrolysis of Soy Protein Isolate
2.3. Characterization of SPH
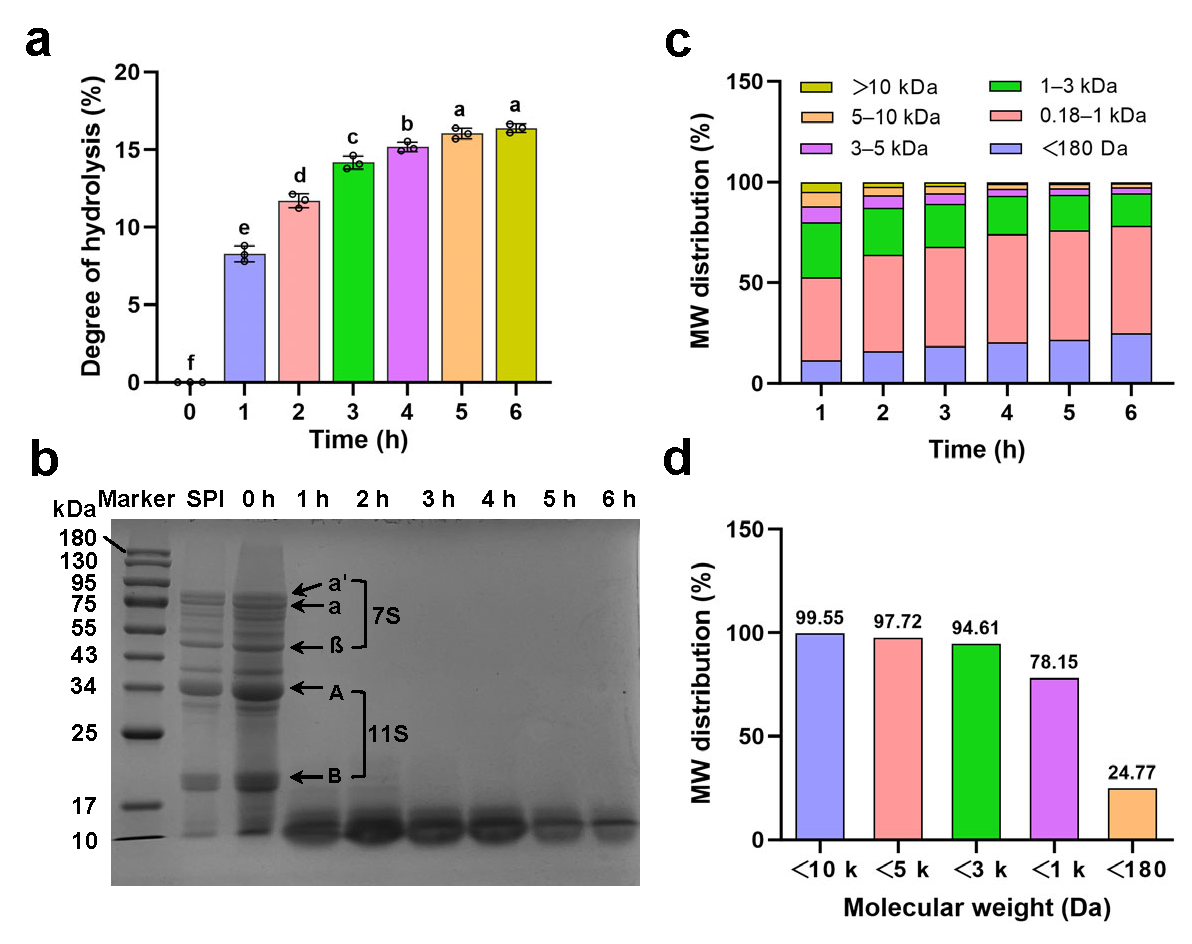
2.3.1. Degree of Hydrolysis
2.3.2. SDS-PAGE
2.3.3. Molecular Weight Distribution
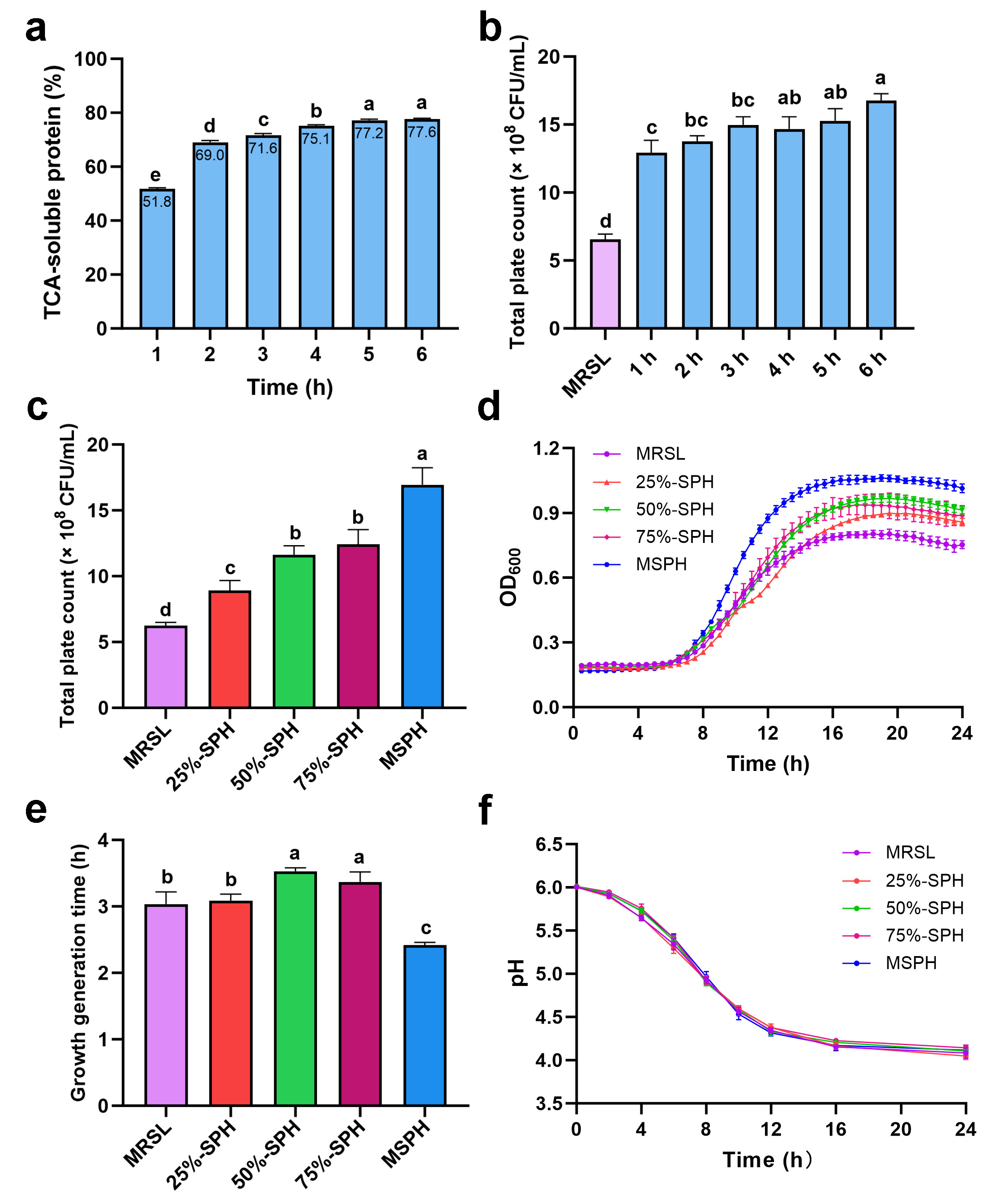
2.3.4. Trichloroacetic Acid–Soluble Protein Content
2.4. Amino Acid Composition Analysis
2.5. Microorganisms and Culture Conditions
2.6. Determination of Growth of Bifidobacteria Strains In Vitro
2.7. Effects of SPH as Sole Nitrogen on the Freeze-Drying Survival Rate
2.8. Scanning Electron Microscopy (SEM) Analysis
2.9. Confocal Laser Scanning Microscopy Imaging
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effects of Enzymatic Hydrolysis on Protein Degradation
3.1.1. Degree of Hydrolysis
3.1.2. Molecular Weight Distribution
3.2. Effects of Hydrolysis Time and Nitrogen Substitution Ratio on the Growth of B. breve 1206
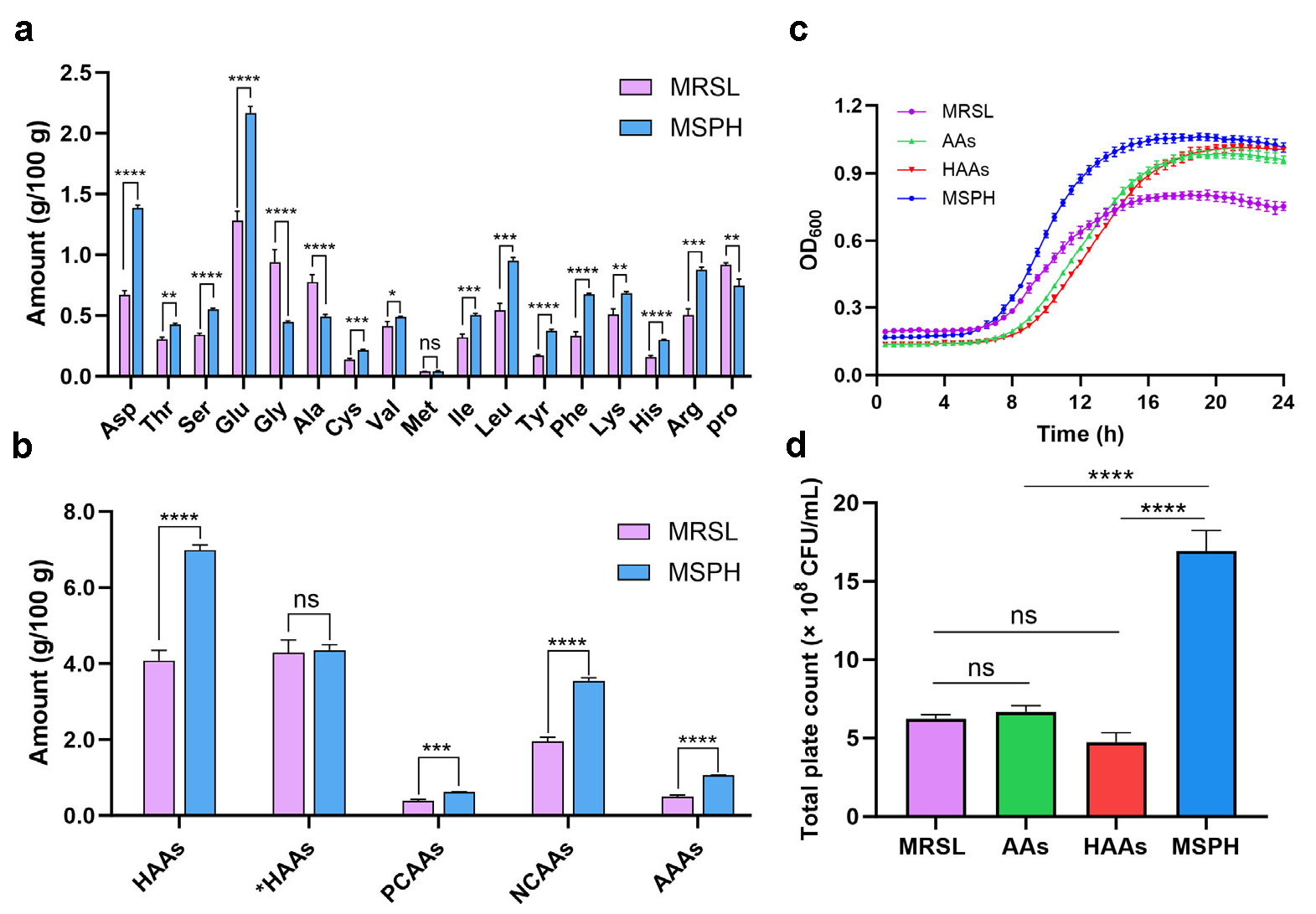
3.3. Amino Acid Compositions of MRSL and MSPH
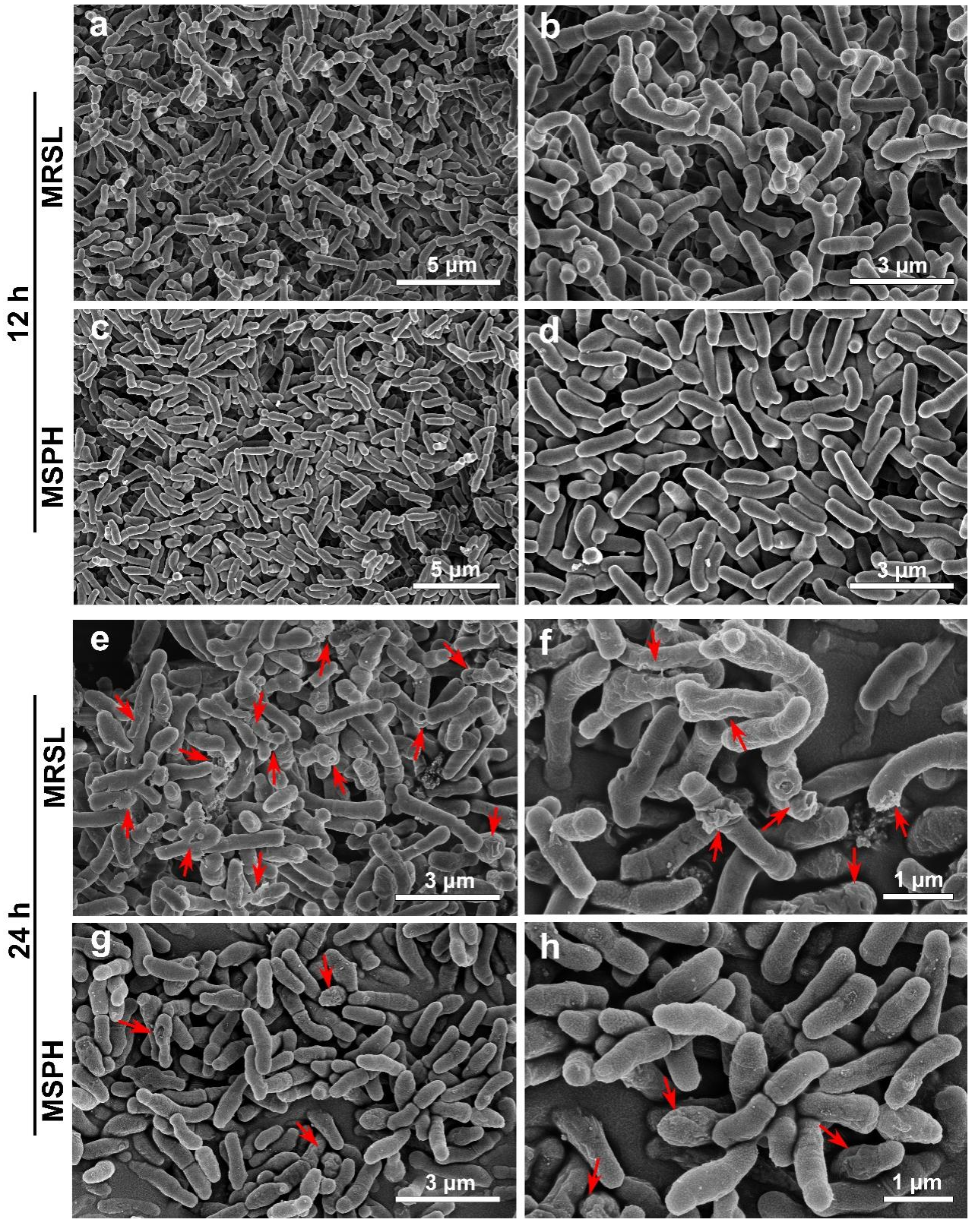
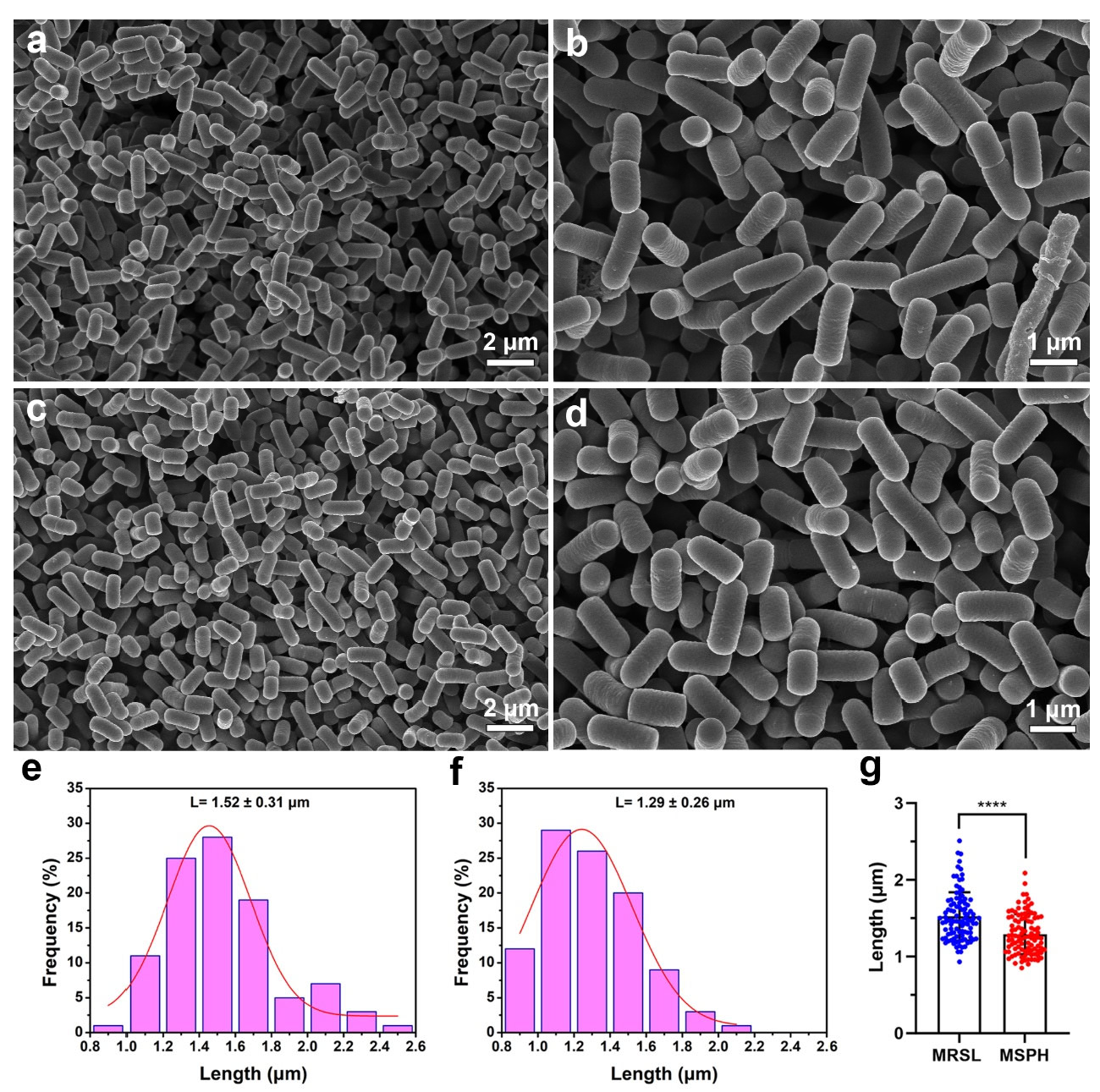
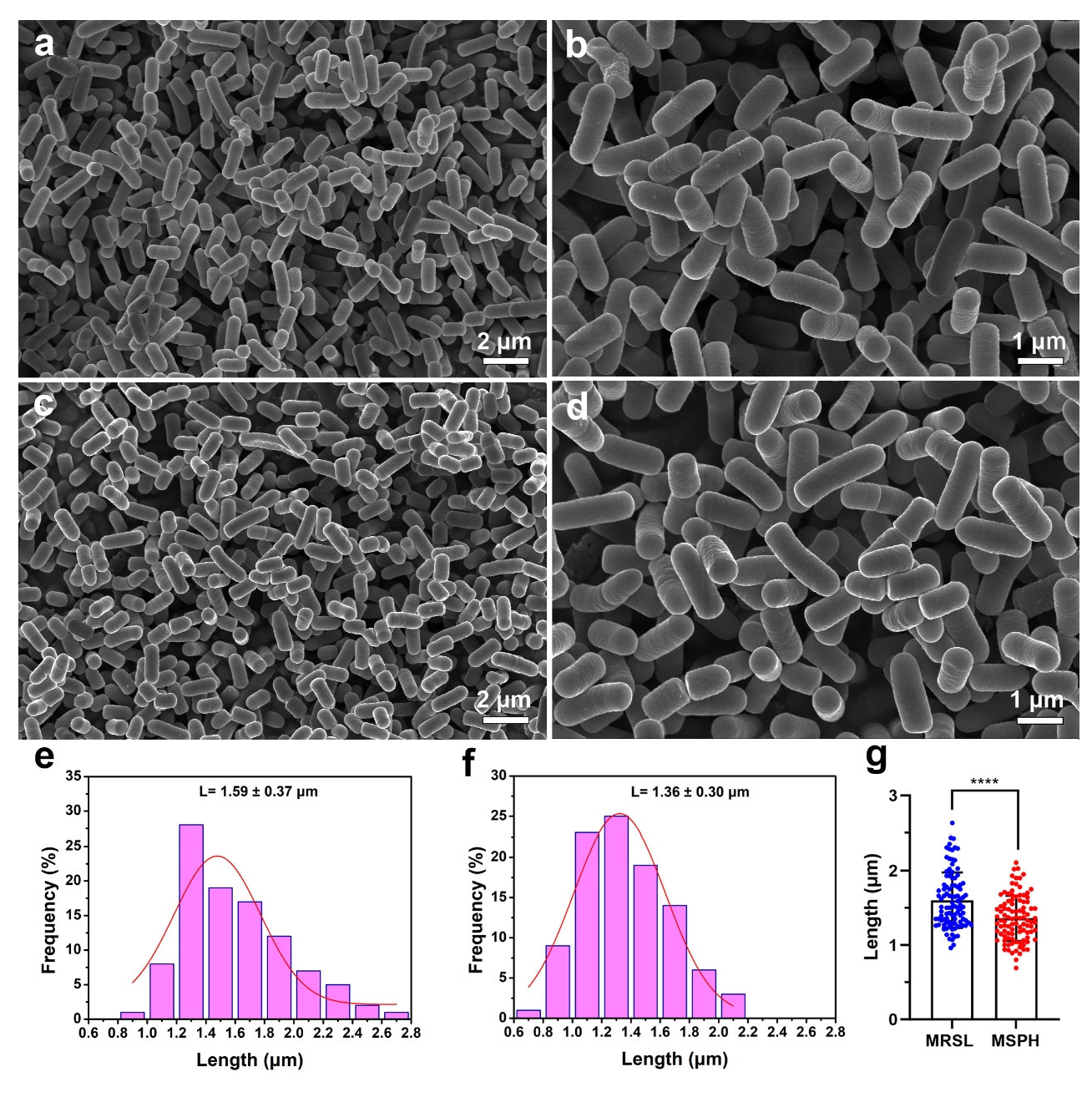
3.4. Morphology Analysis of MRSL and MSPH
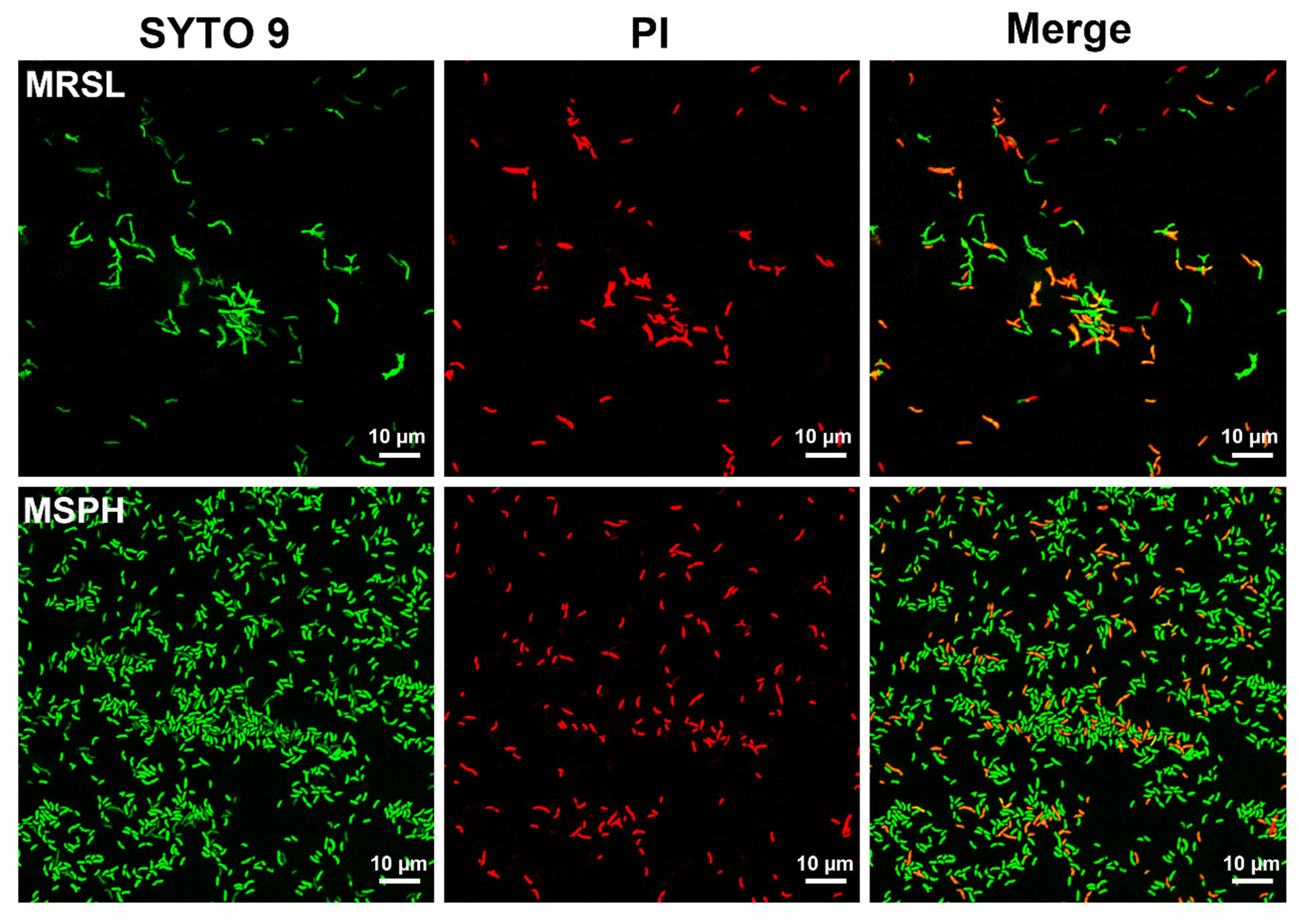
3.5. CLSM Analysis of Live/Dead Bacteria in MRSL and MSPH
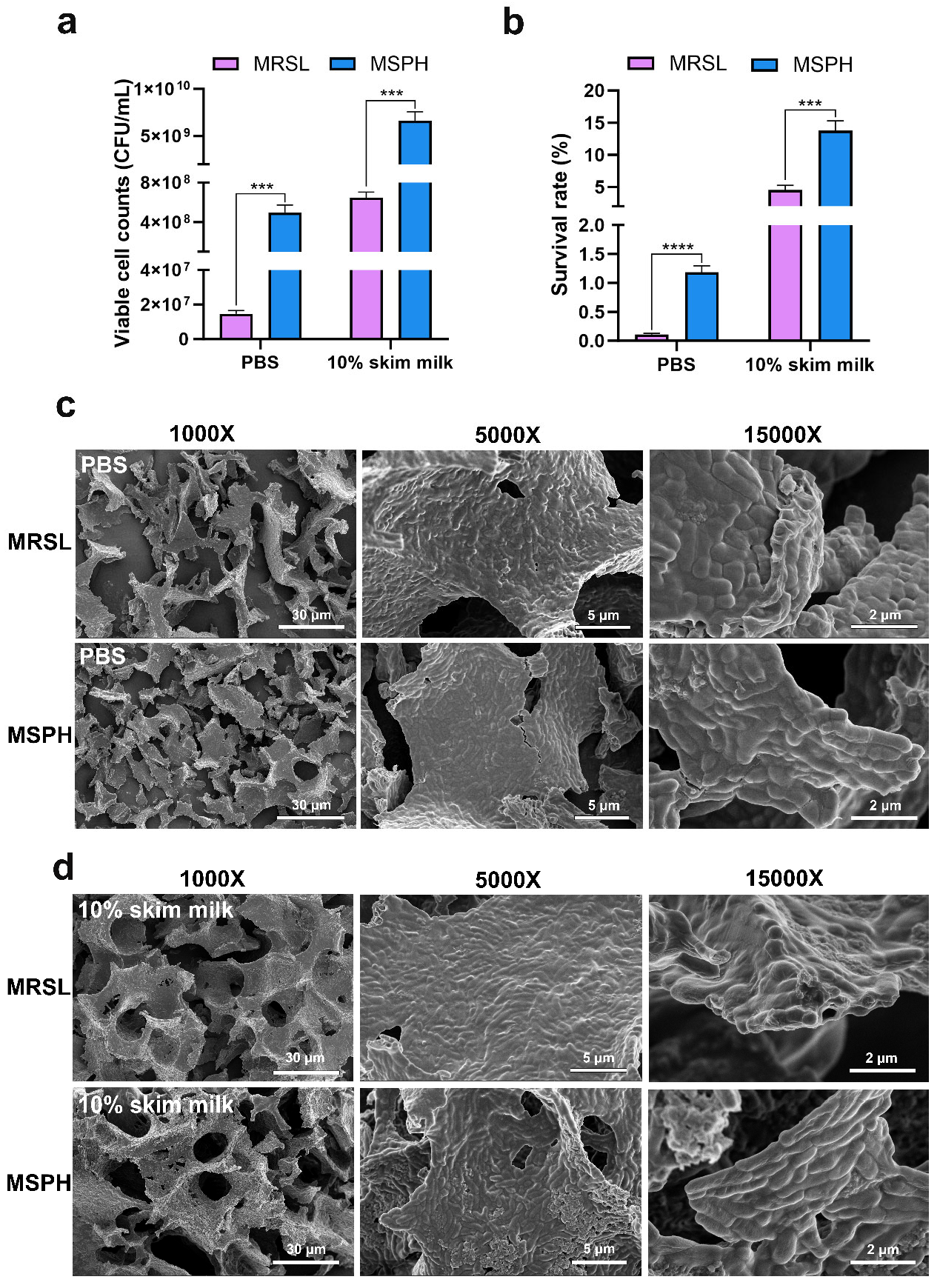
3.6. Morphological Changes and Survival Rate After Freeze-Drying
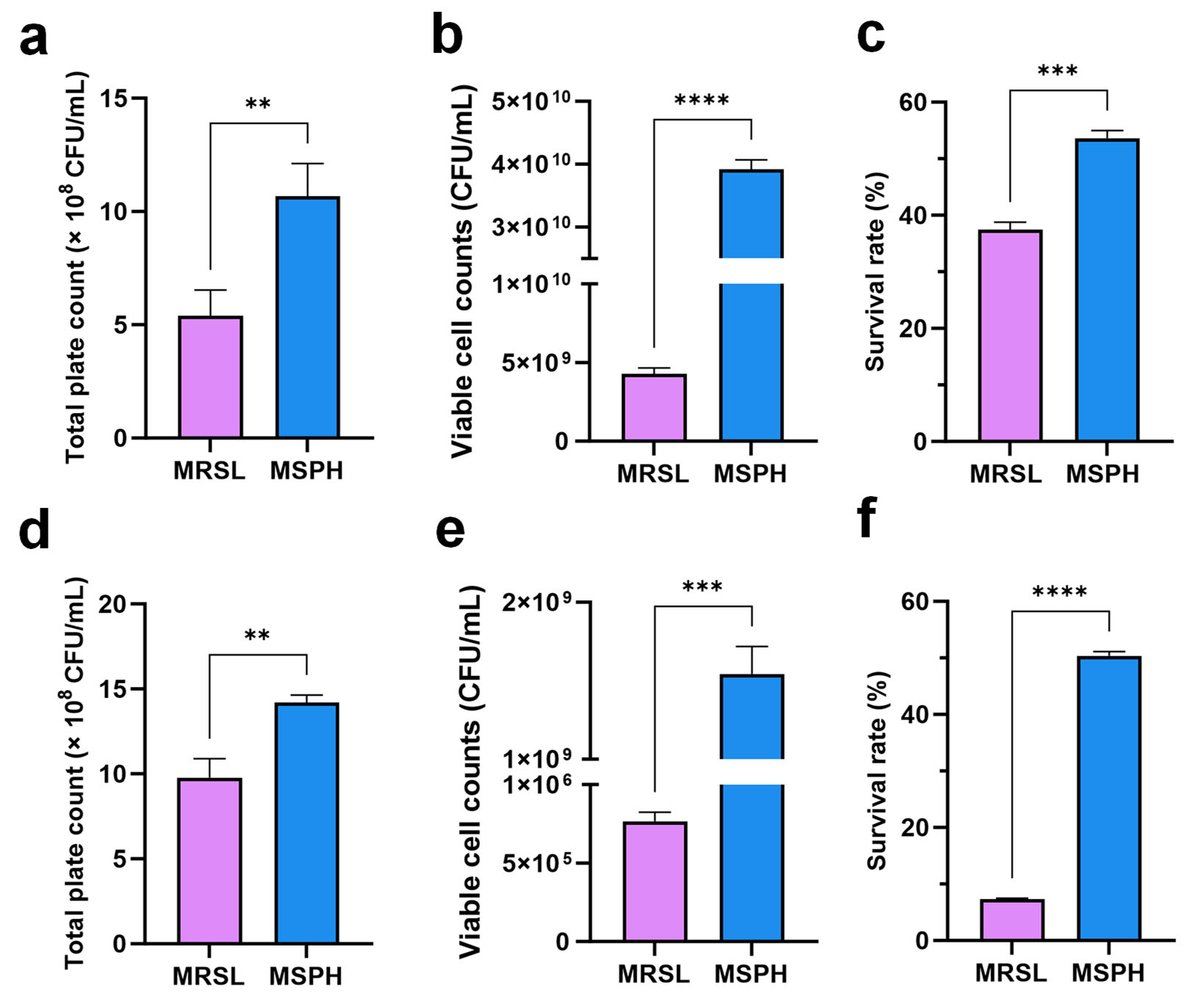
3.7. Effects of MSPH on Bacterial Viability, Freeze-Drying Survival, and Morphology Changes in B. longum 070103 and 050101
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Probiotics market size, share & trends analysis report by product, by ingredient (bacteria, yeast), by distribution channel, by end use (human probiotics, animal probiotics), by region, and segment forecasts, 2024–2030. In Grand View Research; Research and Markets: Dublin, Ireland, 2024.
- Jankovic, I.; Sybesma, W.; Phothirath, P.; Ananta, E.; Mercenier, A. Application of probiotics in food products—Challenges and new approaches. Curr. Opin. Biotechnol. 2010, 21, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Food. 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Harmstorf, I.; Phillips, M. Survival of Lactobacillus acidophilus and Bifidobacterium animalis ssp. lactis in stirred fruit yogurts. LWT—Food Sci. Technol. 2008, 41, 1317–1322. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Shao, W.; Xiao, C.; Yong, T.; Zhang, Y.; Hu, H.; Xie, T.; Liu, R.; Huang, L.; Li, X.; Xie, Y.; et al. A polysaccharide isolated from Ganoderma lucidum ameliorates hyperglycemia through modulating gut microbiota in type 2 diabetic mice. Int. J. Biol. Macromol. 2022, 197, 23–38. [Google Scholar] [CrossRef]
- Huang, L.; Wu, Y.; Fan, Y.; Su, Y.; Liu, Z.; Bai, J.; Zhao, X.; Li, Y.; Xie, X.; Zhang, J.; et al. The growth-promoting effects of protein hydrolysates and their derived peptides on probiotics: Structure-activity relationships, mechanisms and future perspectives. Crit. Rev. Food Sci. Nutr. 2024, 65, 4401–4420. [Google Scholar] [CrossRef]
- Stgelais, D.; Roy, D.; Hache, S.; Desjardins, M.L.; Gauthier, S.F. Growth of nonproteolytic Lactococcus lactis in culture medium supplemented with different casein hydrolyzates. J. Dairy Sci. 1993, 76, 3327–3337. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, J.; Zhao, M.; Zhao, H.; Regenstein, J.M.; Li, Y.; Wu, J. Isolation and characterization of three novel peptides from casein hydrolysates that stimulate the growth of mixed cultures of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. J. Agric. Food Chem. 2011, 59, 7045–7053. [Google Scholar] [CrossRef]
- Jameel, S. Climate change, food systems and the Islamic perspective on alternative proteins. Trends Food Sci. Technol. 2023, 138, 480–490. [Google Scholar] [CrossRef]
- Messina, M.; Messina, V. The role of soy in vegetarian diets. Nutrients 2010, 2, 855–888. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, H.; Zhang, C.; Zhang, J.; Uddin, J.; Liu, X. Effect of soybean oligopeptide on the growth and metabolism of Lactobacillus acidophilus JCM 1132. RSC Adv. 2020, 10, 16737–16748. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zhang, Y.; He, L.; Zhang, C.; Liu, X. Different effects of soybean protein and its derived peptides on the growth and metabolism of Bifidobacterium animalis subsp. animalis JCM 1190. Food Funct. 2021, 12, 5731–5744. [Google Scholar] [CrossRef]
- Han, K.; Luo, D.; Zou, Y.; Dong, S.; Wan, Z.; Yang, X. Modulation of gut microbiota by soybean 7S globulin peptide that involved lipopolysaccharide–peptide interaction. J. Agric. Food Chem. 2019, 67, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xia, S.; Zhang, Y.; Zhu, S.; Li, H.; Liu, X. Identification of soybean peptides and their effect on the growth and metabolism of Limosilactobacillus reuteri LR08. Food Chem. 2022, 369, 130923. [Google Scholar] [CrossRef] [PubMed]
- Niamah, A.K.; Sahi, A.A.; Al-Sharifi, A.S.N. Effect of feeding soy milk fermented by probiotic bacteria on some blood criteria and weight of experimental animals. Probiotics Antimicrob. Proteins 2017, 9, 284–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.; Lu, W.; Zhang, C.; Chen, D.; Shah, N.P.; Xiao, C. Optimizing Levilactobacillus brevis NPS-QW 145 fermentation for gamma-aminobutyric acid (GABA) production in soybean sprout yogurt-like product. Foods 2023, 12, 977. [Google Scholar] [CrossRef]
- Acker, J.P.; McGann, L.E. Cell-cell contact affects membrane integrity after intracellular freezing. Cryobiology 2000, 40, 54–63. [Google Scholar] [CrossRef]
- Pehkonen, K.S.; Roos, Y.H.; Miao, S.; Ross, R.P.; Stanton, C. State transitions and physicochemical aspects of cryoprotection and stabilization in freeze-drying of Lactobacillus rhamnosus GG (LGG). J. Appl. Microbiol. 2008, 104, 1732–1743. [Google Scholar] [CrossRef]
- Peiren, J.; Buyse, J.; De Vos, P.; Lang, E.; Clermont, D.; Hamon, S.; Begaud, E.; Bizet, C.; Pascual, J.; Ruvira, M.A.; et al. Improving survival and storage stability of bacteria recalcitrant to freeze-drying: A coordinated study by European culture collections. Appl. Microbiol. Biotechnol. 2015, 99, 3559–3571. [Google Scholar] [CrossRef]
- Chen, Z.; E, J.; Ma, R.; Zhang, J.; Yao, C.; Wang, R.; Zhang, Q.; Yang, Y.; Li, J.; Wang, J. The effect of aspartic acid on the freeze-drying survival rate of Lactobacillus plantarum LIP-1 and its inherent mechanism. LWT 2022, 155, 112929. [Google Scholar] [CrossRef]
- Senz, M.; van Lengerich, B.; Bader, J.; Stahl, U. Control of cell morphology of probiotic Lactobacillus acidophilus for enhanced cell stability during industrial processing. Int. J. Food Microbiol. 2015, 192, 34–42. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, A.; van Sinderen, D. Bifidobacteria and their role as members of the human gut microbiota. Front. Microbiol. 2016, 7, 925. [Google Scholar] [CrossRef] [PubMed]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef]
- Zhong, Z.; Hu, R.; Zhao, J.; Liu, W.; Kwok, L.Y.; Sun, Z.; Zhang, H.; Chen, Y. Acetate kinase and peptidases are associated with the proteolytic activity of Lactobacillus helveticus isolated from fermented food. Food Microbiol. 2021, 94, 103651. [Google Scholar] [CrossRef]
- Buruleanu, C.L.; Nicolescu, C.L.; Avram, D.; Manea, I.; Bratu, M.G. Effects of yeast extract and different amino acids on the dynamics of some components in cabbage juice during fermentation with Bifidobacterium lactis BB-12. Food Sci. Biotechnol. 2012, 21, 691–699. [Google Scholar] [CrossRef]
- Janer, C.; Arigoni, F.; Lee, B.H.; Pelaez, C.; Requena, T. Enzymatic ability of Bifidobacterium animalis subsp. lactis to hydrolyze milk proteins: Identification and characterization of endopeptidase O. Appl. Environ. Microbiol. 2005, 71, 8460–8465. [Google Scholar] [CrossRef]
- Gomes, A.M.P.; Malcata, F.X.; Klaver, F.A.M. Growth enhancement of Bifidobacterium lactis Bo and Lactobacillus acidophilus Ki by milk hydrolyzates. J. Dairy Sci. 1998, 81, 2817–2825. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl method for organic nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, J.; Ma, D.; Li, Z.; Zhang, S.; Li, Y. Alcalase-hydrolyzed insoluble soybean meal hydrolysate aggregates: Structure, bioactivity, function properties, and influences on the stability of oil-in-water emulsions. Int. J. Biol. Macromol. 2024, 265, 131014. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Cui, Q.; Sun, Y.; Cheng, J.; Guo, M. Effect of two-step enzymatic hydrolysis on the antioxidant properties and proteomics of hydrolysates of milk protein concentrate. Food Chem. 2022, 366, 130711. [Google Scholar] [CrossRef] [PubMed]
- Coscueta, E.R.; Cunha Fernandes, N.; Brassesco, M.E.; Rosa, A.; Almeida, A.; Pintado, M.M. Turning discarded blue shark (Prionace glauca) skin into a valuable nutraceutical resource: An enzymatic collagen hydrolysate. Food Biosci. 2024, 60, 104472. [Google Scholar] [CrossRef]
- Henn, R.L.; Netto, F.M. Biochemical characterization and enzymatic hydrolysis of different commercial soybean protein isolates. J. Agric. Food Chem. 1998, 46, 3009–3015. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, Y.; Yu, J.; Wang, F.; Li, X.; Liu, Y.; Ma, X. Changes of proteins and amino acids in soymilk during lactic acid fermentation and subsequent storage. J. Food Meas. Charact. 2022, 16, 4728–4737. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, P.; Ismail, B.B.; Yang, Z.; Zou, Z.; Suo, Y.; Ye, X.; Liu, D.; Guo, M. Chickpea-derived modified antimicrobial peptides KTA and KTR inactivate Staphylococcus aureus via disrupting cell membrane and interfering with peptidoglycan synthesis. J. Agric. Food Chem. 2024, 72, 2727–2740. [Google Scholar] [CrossRef]
- Zhao, H.; Bai, F.; Zhou, F.; Piotr, W.; Jiang, X.; Zhang, B. Characterization of soybean protein hydrolysates able to promote the proliferation of Streptococcus thermophilus ST. J. Food Sci. 2013, 78, M575–M581. [Google Scholar] [CrossRef]
- Proulx, M.; Ward, P.; Gauthier, S.F.; Roy, D. Comparison of bifidobacterial growth-promoting activity of ultrafiltered casein hydrolyzate fractions. Lait 1994, 74, 139–152. [Google Scholar] [CrossRef]
- Etoh, S.; Asamura, K.; Obu, A.; Sonomoto, K.; Ishizaki, A. Purification and identification of a growth-stimulating peptide for Bifidobacterium bifidum from natural rubber serum powder. Biosci. Biotechnol. Biochem. 2000, 64, 2083–2088. [Google Scholar] [CrossRef]
- Zuo, W.; Chen, W.; Zou, S. Separation of growth-stimulating peptides for Bifidobacterium from soybean conglycinin. World J. Gastroenterol. 2005, 11, 5801–5806. [Google Scholar] [CrossRef] [PubMed]
- Poch, M.; Bezkorovainy, A. Bovine milk κ-casein trypsin digest is a growth enhancer for the genus Bifidobacterium. J. Agric. Food Chem. 1991, 39, 73–77. [Google Scholar] [CrossRef]
- Tang, X.; Tian, Q.; Cheng, X.; Li, N.; Mao, X.-Y. Bifidobacterial growth-promoting effect of yak milk κ-casein hydrolysates produced with different proteases. Int. J. Food Sci. Tech. 2013, 48, 1682–1687. [Google Scholar] [CrossRef]
- Poupard, J.A.; Husain, I.; Norris, R.F. Biology of the bifidobacteria. Bacteriol. Rev. 1973, 37, 136–165. [Google Scholar] [CrossRef]
- Husain, I.; Poupard, J.A.; Norris, R.F. Influence of nutrition on the morphology of a strain of Bifidobacterium bifidum. J. Bacteriol. 1972, 111, 841–844. [Google Scholar] [CrossRef]
- Wright, C.T.; Klaenhammer, T.R. Calcium-induced alteration of cellular morphology affecting the resistance of Lactobacillus acidophilus to freezing. Appl. Environ. Microbiol. 1981, 41, 807–815. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, X.; Zhang, Y.; Zou, X.; Tian, L.; Hong, H.; Luo, Y.; Tan, Y. Silver carp (Hypophthalmichthys molitrix) by-product hydrolysates: A new nitrogen source for Bifidobacterium animalis ssp. lactis BB-12. Food Chem. 2023, 404, 134630. [Google Scholar] [CrossRef]
- Cui, S.; Gu, Z.; Wang, W.; Tang, X.; Zhang, Q.; Mao, B.; Zhang, H.; Zhao, J. Characterization of peptides available to different bifidobacteria. LWT 2022, 169, 113958. [Google Scholar] [CrossRef]
- Rajab, S.; Tabandeh, F.; Shahraky, M.K.; Alahyaribeik, S. The effect of Lactobacillus cell size on its probiotic characteristics. Anaerobe 2020, 62, 102103. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, Y.K.; Kim, M.G.; Seo, H.S.; Park, S.; Lee, S.H. Key factors for the survival of Lactiplantibacillus plantarum IDCC 3501 in manufacturing and storage. Appl. Microbiol. Biotechnol. 2024, 108, 12. [Google Scholar] [CrossRef]
- Parlindungan, E.; Dekiwadia, C.; Tran, K.T.M.; Jones, O.A.H.; May, B.K. Morphological and ultrastructural changes in Lactobacillus plantarum B21 as an indicator of nutrient stress. LWT 2018, 92, 556–563. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Y.; Li, L.; Liang, T.; Du, M.; Yang, L.; Yang, J.; Yang, R.; Zhao, H.; Chen, M.; et al. Bifidobacterium longum 070103 fermented milk improve glucose and lipid metabolism disorders by regulating gut microbiota in mice. Nutrients 2022, 14, 4050. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zhao, X.; Wu, Q.; Guo, W.; Yang, N.; Fan, Y.; Zhang, Y.; Li, Y.; Xie, X.; Chen, M. Soybean Protein Hydrolysate Enhances Growth and Freeze-Drying Survival of Bifidobacterium breve and Bifidobacterium longum Strains. Foods 2025, 14, 4071. https://doi.org/10.3390/foods14234071
Huang L, Zhao X, Wu Q, Guo W, Yang N, Fan Y, Zhang Y, Li Y, Xie X, Chen M. Soybean Protein Hydrolysate Enhances Growth and Freeze-Drying Survival of Bifidobacterium breve and Bifidobacterium longum Strains. Foods. 2025; 14(23):4071. https://doi.org/10.3390/foods14234071
Chicago/Turabian StyleHuang, Lanyan, Xinyu Zhao, Qingping Wu, Weipeng Guo, Ning Yang, Yue Fan, Ying Zhang, Ying Li, Xinqiang Xie, and Moutong Chen. 2025. "Soybean Protein Hydrolysate Enhances Growth and Freeze-Drying Survival of Bifidobacterium breve and Bifidobacterium longum Strains" Foods 14, no. 23: 4071. https://doi.org/10.3390/foods14234071
APA StyleHuang, L., Zhao, X., Wu, Q., Guo, W., Yang, N., Fan, Y., Zhang, Y., Li, Y., Xie, X., & Chen, M. (2025). Soybean Protein Hydrolysate Enhances Growth and Freeze-Drying Survival of Bifidobacterium breve and Bifidobacterium longum Strains. Foods, 14(23), 4071. https://doi.org/10.3390/foods14234071







