Bioaccessibility and Gut Microbiota Modulation of Phenolics in Prunus mume vs. Fructus mume
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. In Vitro Simulated Gastrointestinal Digestion
2.4. In Vitro Fecal Fermentation
2.5. Determination of Total Phenol Content
2.6. Analysis of Phenolic Composition and Content by UPLC-Q-Exactive Orbitrap/MS
2.7. Determination of Antioxidant Activity in Vitro
2.8. Gut Microbiota Composition Analysis
2.9. Statistical Analysis
3. Results and Discussion
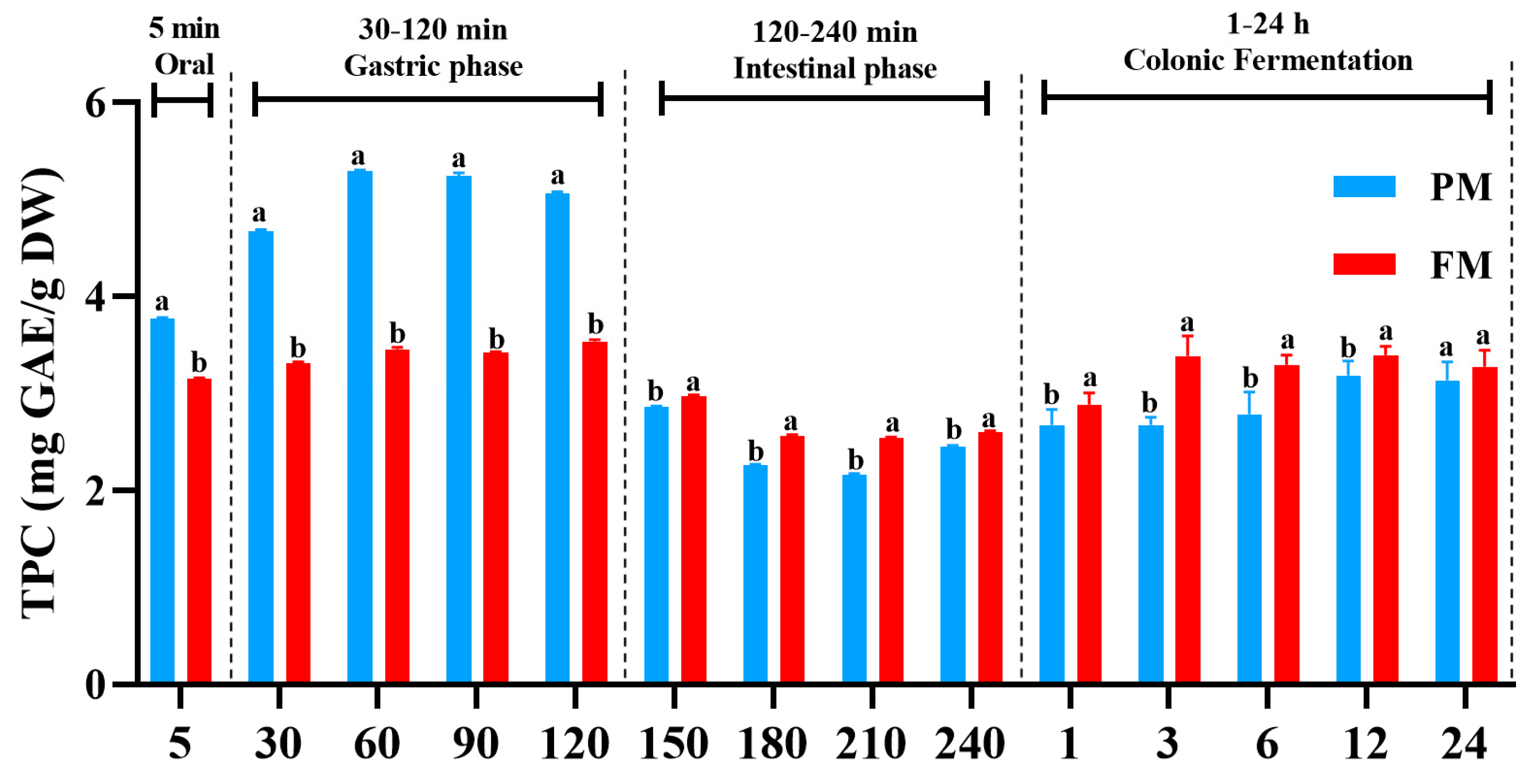
3.1. Impact of Processing on the Release of Total Polyphenols During Different Digestion Stages
3.2. Impact of Processing on Phenolic Bioavailability
3.2.1. Chemical Characterization of Released Phenolics and Metabolites
3.2.2. Quantitation of Released Phenolics and Metabolites
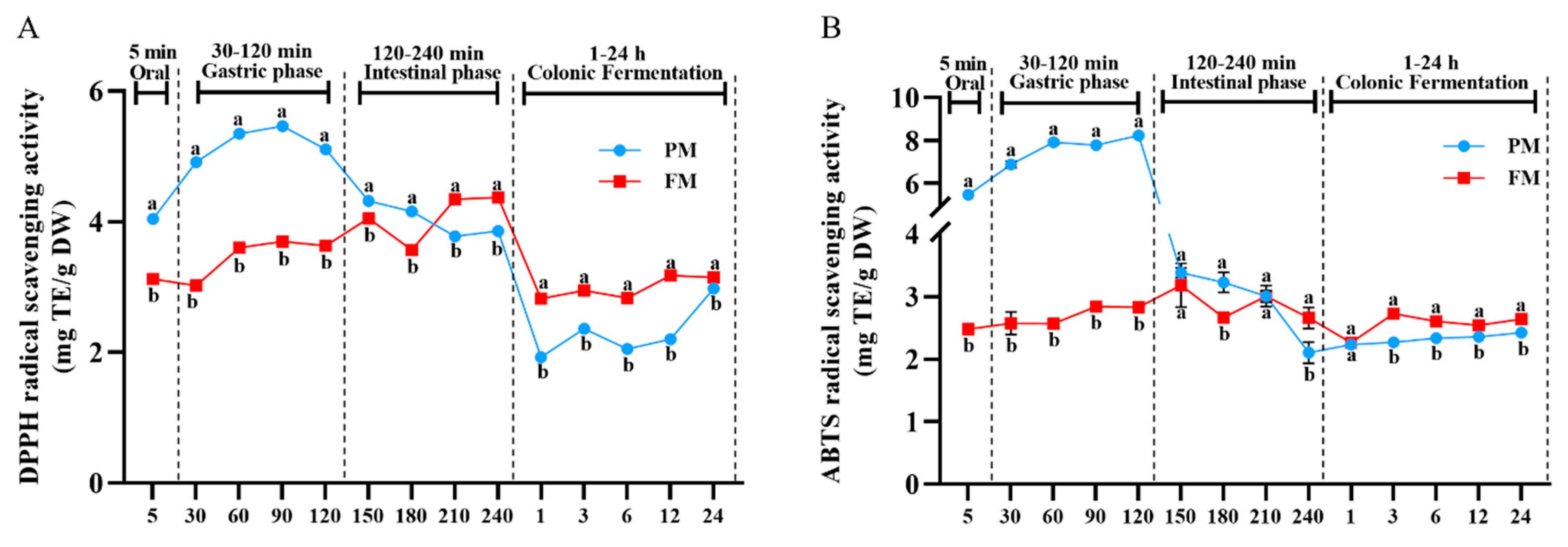
3.3. Impact of Processing on Antioxidant Activity Change During Different Digestion Stages
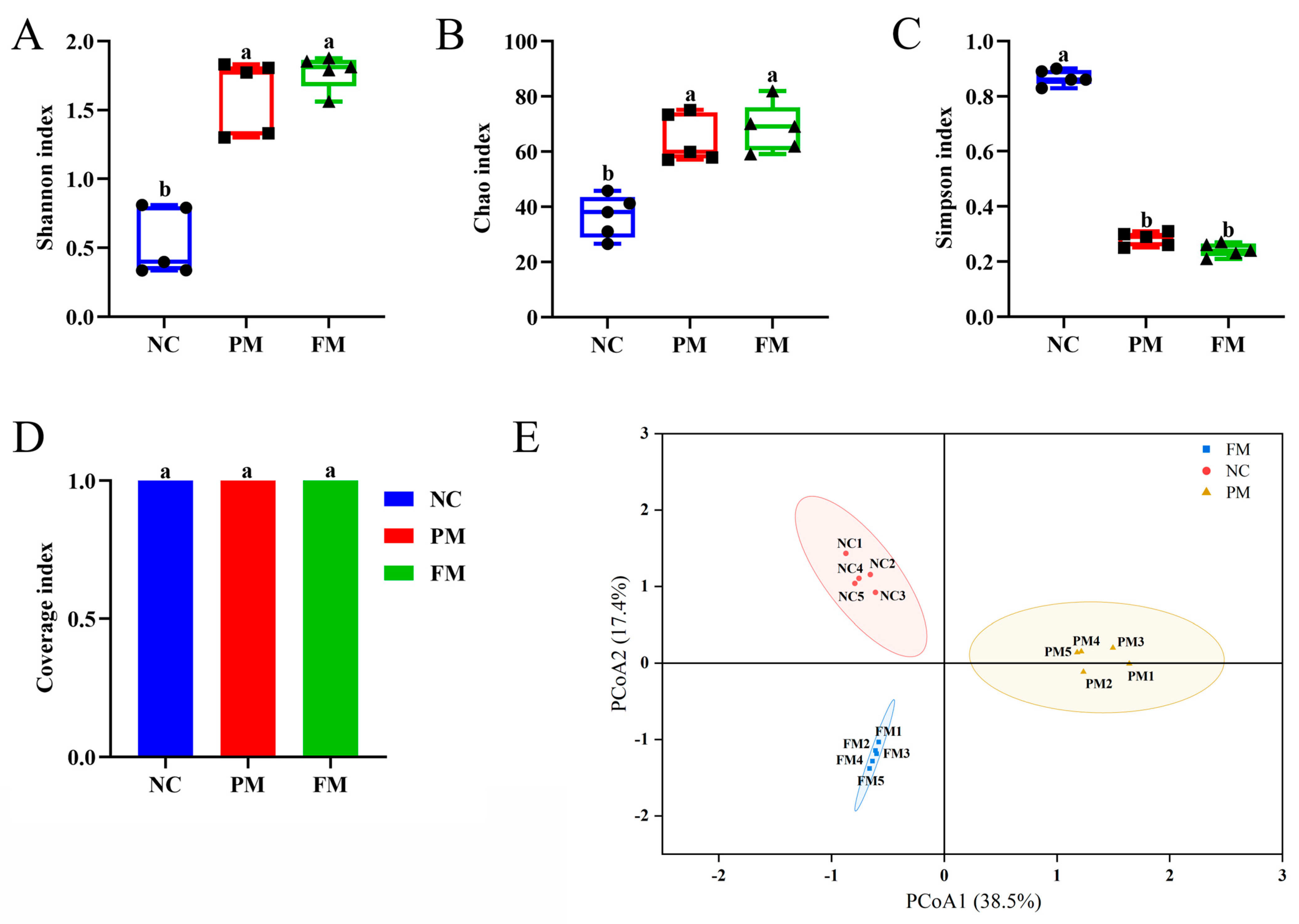
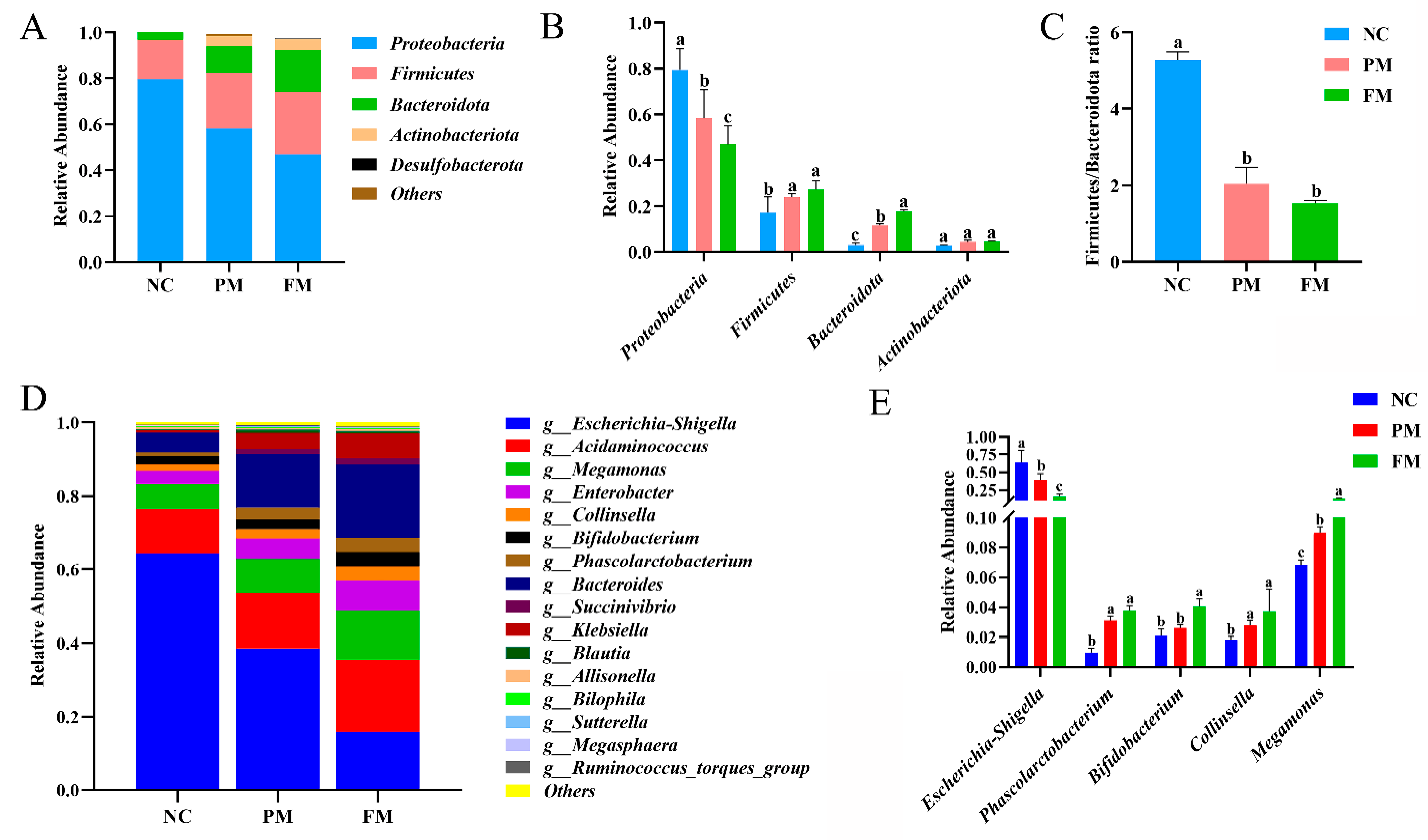
3.4. Impact of Processing on Gut Microbiota Regulation Activity
| Peak. | RT (min) | Phenolics | Formula | Ion Peak (m/z) | Fragment (m/z) [Relative Abundance, %] | Source | References |
|---|---|---|---|---|---|---|---|
| 1 | 4.94 | Protocatechuic acid | C7H6O4 | 153.01839 | 109.02888 [100] | PM: IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [50] |
| 2 | 5.22 | Neochlorogenic acid | C16H18O9 | 353.08844 | 191.05551 [91], 179.03433 [68], 135.04401 [12] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [51] |
| 3 | 5.53 | Procyanidin B1 | C30H26O12 | 577.13623 | 289.07211 [100], 407.07773 [64], 125.02317 [75], 425.08859 [40], 451.10410 [10] | PM: OP, GP, IP FM: IP | [52] |
| 4 | 6.14 | Chlorogenic acid | C16H18O9 | 353.08823 | 191.05548 [100] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [53] |
| 5 | 6.21 | Catechin | C15H14O6 | 289.07220 | 245.08144 [30] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: F1, F3, F6, F12, F24 | [39] |
| 6 | 6.38 | 3-(3,4-Dihydroxyphenyl) propionic acid | C9H10O4 | 181.04991 | 137.05969 [100] | PM: F3, F6, F12, F24 FM: F12, F24 | [54] |
| 7 | 6.49 | Cryptochlorogenic acid | C16H18O9 | 353.0833 | 173.04472 [100], 179.03421 [67], 191.05536 [31], 135.04398 [15] | PM: GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [51] |
| 8 | 6.45 | p-Hydroxybenzoic acid | C7H5O3 | 137.02339 | 93.03323 [100] | PM: GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [55] |
| 9 | 6.57 | 3,4-Dihydroxyphenylacetic acid | C8H8O4 | 167.03420 | 123.04397 [54] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [56] |
| 10 | 7.08 | Epicatechin | C15H14O6 | 289.07224 | 245.08212 [30], 125.02299 [9] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [15] |
| 11 | 7.12 | Caffeic acid | C9H8O4 | 179.03423 | 135.04404 [100] | PM: IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12 | [15] |
| 12 | 7.22 | Syringic acid | C9H10O5 | 197.04510 | 182.02152 [100], 153.05495 [14] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [39] |
| 13 | 7.27 | Vanillic acid | C8H8O4 | 167.03427 | 152.01057 [100] | PM: GP, F1, F3, F6, F12, F24 FM: OP, F1, F3, F6, F12, F24 | [39] |
| 14 | 8.05 | Benzoic acid | C7H6O2 | 121.02822 | 77.03805 [11] | PM: IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [39] |
| 15 | 8.73 | 2-O-Rhamnosylvitexin | C27H30O14 | 577.15686 | 413.09126 [48]; 293.04826 [69] | PM: F1, F3, F6, F12, F24 FM: F1, F3, F6, F12, F24 | [57] |
| 16 | 8.84 | Rutin | C27H30O16 | 609.14771 | 300.02783 [100] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [53] |
| 17 | 8.93 | p-Coumaric acid | C9H8O3 | 163.03915 | 119.04906 [100] | PM: IP, F1, F3, F6, F12 FM: OP, GP, IP, F1, F3, F6, F12 | [53] |
| 18 | 9.19 | Hyperoside | C21H20O12 | 463.08923 | 300.02783 [58], 301.03546 [34] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [58] |
| 19 | 9.36 | Isoquercetin | C21H20O12 | 463.08932 | 300.02779 [100] | PM: OP, GP, IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [59] |
| 20 | 9.57 | Ferulic acid | C10H10O4 | 193.04988 | 178.02635 [90], 134.03616 [100], 149.05968 [35] | PM: IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [15] |
| 21 | 9.66 | Isoferulic acid | C10H10O4 | 193.04984 | 178.02621 [100], 134.03612 [65] | PM: F1, F3, F6, F12, F24 FM: F1, F3, F6, F12, F24 | [60] |
| 22 | 9.87 | Kaempferol-3-O-rutinoside | C27H30O15 | 593.15247 | 285.04080 [62] | PM: F1, F3, F6, F12, F24 FM: F1, F3, F6, F12, F24 | [61] |
| 23 | 14.63 | Quercetin | C15H10O7 | 301.03583 | 151.00272 [21], 178.99788 [15] | PM: GP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [15] |
| 24 | 16.17 | Naringenin | C15H12O5 | 271.06155 | 151.00255 [43], 119.04892 [14], 177.01808 [6] | PM: IP, F1, F3, F6, F12, F24 FM: OP, GP, IP, F1, F3, F6, F12, F24 | [15] |
| NO. | Phenolics | Sample | OP | GP | IP (PCafter) | PCbefore | Intestinal (Bioaccessibility) |
|---|---|---|---|---|---|---|---|
| 1 | Protocatechuic acid | PM | Nd | Nd | 0.04 ± 0.03 a | 0.67 ± 0.04 a | 5.97% a |
| FM | 0.08 ± 0.02 B | 0.08 ± 0.01 B | 0.24 ± 0.04 bA | 2.84 ± 0.07 b | 8.45% b | ||
| 2 | Neochlorogenic acid | PM | 300.23 ± 24.17 aA | 325.68 ± 13.74 aA | 22.35 ± 0.42 aB | 200.91 ± 42.38 a | 11.12% a |
| FM | 231.63 ± 25.03 bA | 220.22 ± 1.40 bA | 12.57 ± 1.17 bB | 195.34 ± 6.25 a | 6.43% b | ||
| 3 | Procyanidin B1 | PM | 2.79 ± 0.05 B | 3.88 ± 0.12 A | 0.03 ± 0.00 aC | 11.44 ± 1.38 a | 0.26% a |
| FM | Nd | Nd | 0.06 ± 0.00 b | 2.92 ± 0.15 b | 2.05% b | ||
| 4 | Chlorogenic acid | PM | 17.94 ± 0.34 aA | 2.48 ± 0.39 aB | 2.80 ± 0.10 aB | 18.25 ± 1.67 a | 15.34% a |
| FM | 14.66 ± 0.67 bB | 23.23 ± 0.78 bA | 2.22 ± 0.09 aC | 14.57 ± 0.55 b | 15.24% a | ||
| 5 | Catechin | PM | 9.18 ± 0.16 B | 12.58 ± 0.38 A | 0.24 ± 0.05 C | 34.60 ± 2.12 a | 0.69% |
| FM | Nd | Nd | Nd | 12.99 ± 1.00 b | Nd | ||
| 6 | 3-(3,4-Dihydroxyphenyl) propionic acid | PM | Nd | Nd | Nd | Nd | Nd |
| FM | Nd | Nd | Nd | Nd | Nd | ||
| 7 | Cryptochlorogenic acid | PM | Nd | 0.23 ± 0.09 aA | 0.41 ± 0.00 aA | 2.92 ± 0.39 a | 14.04% a |
| FM | 5.12 ± 0.20 A | 5.01 ± 0.18 bA | 0.17 ± 0.02 bB | 22.60 ± 0.46 b | 0.75% b | ||
| 8 | p-Hydroxybenzoic acid | PM | Nd | 0.02 ± 0.00 aB | 1.39 ± 0.01 aA | 6.79 ± 0.23 a | 20.47% a |
| FM | 1.31 ± 0.06 A | 1.22 ± 0.02 bA | 2.50 ± 0.04 bA | 13.69 ± 0.74 b | 18.26% b | ||
| 9 | 3,4-Dihydroxyphenylacetic acid | PM | 323.95 ± 20.39 aB | 535.14 ± 17.56 aA | 250.13 ± 48.81 aB | Nd | Nd |
| FM | 205.53 ± 0.46 bB | 257.18 ± 8.29 bA | 111.18 ± 3.92 bAB | Nd | Nd | ||
| 10 | Epicatechin | PM | 45.76 ± 1.60 aB | 66.86 ± 1.92 aA | 0.98 ± 0.12 aC | 45.09 ± 2.12 a | 2.17% a |
| FM | 0.63 ± 0.00 bB | 0.88 ± 0.02 bA | 0.45 ± 0.03 bC | 4.90 ± 0.38 b | 9.18% b | ||
| 11 | Caffeic acid | PM | Nd | Nd | 0.61 ± 0.10 a | 67.40 ± 5.26 a | 0.90% a |
| FM | 0.68 ± 0.04 B | 0.72 ± 0.03 B | 3.25 ± 0.08 bA | 50.26 ± 4.59 b | 6.47% b | ||
| 12 | Syringic acid | PM | 0.07 ± 0.02 aAB | 0.10 ± 0.01 aB | 0.36 ± 0.02 aA | Nd | Nd |
| FM | 0.35 ± 0.06 bB | 0.38 ± 0.01 bB | 0.47 ± 0.01 bA | Nd | Nd | ||
| 13 | Vanillic acid | PM | Nd | 0.38 ± 0.05 | Nd | Nd | Nd |
| FM | 0.21 ± 0.00 | Nd | Nd | Nd | Nd | ||
| 14 | Benzoic acid | PM | Nd | Nd | 53.74 ± 54.05 a | Nd | Nd |
| FM | 147.53 ± 6.19 bB | 179.20 ± 3.02 bA | 98.10 ± 1.54 bC | Nd | Nd | ||
| 15 | 2-O-Rhamnosylvitexin | PM | Nd | Nd | Nd | Nd | Nd |
| FM | Nd | Nd | Nd | Nd | Nd | ||
| 16 | Rutin | PM | 1.91 ± 0.13 aA | 2.33 ± 0.04 aA | 2.56 ± 0.34 aA | 4.34 ± 0.97 a | 58.99% a |
| FM | 2.95 ± 0.06 bA | 3.28 ± 0.00 bA | 2.64 ± 0.23 aA | 4.18 ± 0.39 a | 63.16% a | ||
| 17 | p-Coumaric acid | PM | Nd | Nd | 3.88 ± 0.66 a | 33.93 ± 3.95 a | 11.44% a |
| FM | 0.92 ± 0.08 B | 0.99 ± 0.08 B | 10.89 ± 0.61 bA | 28.40 ± 3.88 b | 38.34% b | ||
| 18 | Hyperoside | PM | 1.10 ± 0.09 aA | 1.59 ± 0.12 aA | 1.33 ± 0.13 aA | 0.84 ± 0.11 a | 158.33% a |
| FM | 1.42 ± 0.00 bA | 1.45 ± 0.05 aA | 1.28 ± 0.02 aB | 0.91 ± 0.44 a | 140.66% a | ||
| 19 | Isoquercetin | PM | 0.84 ± 0.07 aA | 1.21 ± 0.08 aA | 1.02 ± 0.08 aA | 1.90 ± 0.32 a | 53.68% a |
| FM | 1.08 ± 0.00 bA | 1.10 ± 0.04 aA | 0.98 ± 0.00 aB | 1.14 ± 0.37 b | 85.96% a | ||
| 20 | Ferulic acid | PM | Nd | Nd | 0.20 ± 0.01 a | 6.97 ± 0.80 a | 2.87% a |
| FM | 0.12 ± 0.02 A | 0.11 ± 0.01 A | 0.67 ± 0.04 bA | 6.47 ± 0.74 a | 10.36% b | ||
| 21 | Isoferulic acid | PM | Nd | Nd | Nd | 40.72 ± 4.77 a | Nd |
| FM | Nd | Nd | Nd | 26.86 ± 2.34 b | Nd | ||
| 22 | Kaempferol-3-O-rutinoside | PM | Nd | Nd | Nd | Nd | Nd |
| FM | Nd | Nd | Nd | Nd | Nd | ||
| 23 | Quercetin | PM | Nd | 0.04 ± 0.01 a | Nd | 0.18 ± 0.06 a | Nd |
| FM | 0.18 ± 0.02 B | 0.36 ± 0.03 bA | 0.12 ± 0.00 C | 0.48 ± 0.06 b | 25.00% | ||
| 24 | Naringenin | PM | Nd | Nd | 7.71 ± 0.33 a | 2.31 ± 0.20 a | 333.77% a |
| FM | 4.41 ± 1.00 B | 5.60 ± 0.27 B | 9.41 ± 0.20 bA | 10.39 ± 0.39 b | 90.57% b |
| NO. | Phenolics | Sample | F1 | F3 | F6 | F12 | F24 |
|---|---|---|---|---|---|---|---|
| 1 | Protocatechuic acid | PM | 0.13 ± 0.01 aC | 0.15 ± 0.00 aC | 0.18 ± 0.02 aB | 0.24 ± 0.01 aA | 0.01 ± 0.00 aD |
| FM | 0.49 ± 0.04 bB | 0.65 ± 0.06 bA | 0.70 ± 0.07 bA | 0.46 ± 0.05 bB | 0.04 ± 0.01 bC | ||
| 2 | Neochlorogenic acid | PM | 215.69 ± 22.41 aA | 186.65 ± 17.14 aA | 202.25 ± 16.86 aA | 210.26 ± 6.87 aA | 180.12 ± 11.52 aA |
| FM | 209.95 ± 19.78 aB | 270.42 ± 17.58 bA | 188.76 ± 20.15 aC | 244.86 ± 24.17 aAB | 254.70 ± 15.23 bAB | ||
| 3 | Procyanidin B1 | PM | Nd | Nd | Nd | Nd | Nd |
| FM | Nd | Nd | Nd | Nd | Nd | ||
| 4 | Chlorogenic acid | PM | 44.31 ± 4.26 aB | 39.88 ± 3.70 aC | 53.33 ± 5.89 aAB | 62.17 ± 6.17 aA | 54.14 ± 5.37 aAB |
| FM | 68.15 ± 5.05 bB | 94.00 ± 9.27 bA | 67.94 ± 5.29 bB | 90.07 ± 8.34 bA | 95.48 ± 8.90 bA | ||
| 5 | Catechin | PM | 0.58 ± 0.07 aB | 0.16 ± 0.01 aD | 0.30 ± 0.02 aC | 0.21 ± 0.01 aCD | 0.78 ± 0.05 aA |
| FM | 0.09 ± 0.01 bB | 0.08 ± 0.01 bB | 0.15 ± 0.03 bA | 0.04 ± 0.00 bC | 0.02 ± 0.00 bC | ||
| 6 | 3-(3,4-Dihydroxyphenyl) propionic acid | PM | Nd | 19.93 ± 1.29 D | 32.35 ± 1.55 C | 75.20 ± 0.81 aB | 191.25 ± 11.83 aA |
| FM | Nd | Nd | Nd | 17.50 ± 6.61 bB | 105.15 ± 8.25 bA | ||
| 7 | Cryptochlorogenic acid | PM | 38.97 ± 3.37 aB | 38.76 ± 3.62 aB | 48.12 ± 4.41 aAB | 59.02 ± 5.47 aA | 58.66 ± 5.98 aA |
| FM | 77.42 ± 6.99 bB | 101.06 ± 8.83 bA | 76.16 ± 6.18 bB | 103.66 ± 1.18 bA | 103.97 ± 2.99 bA | ||
| 8 | p-Hydroxybenzoic acid | PM | 2.93 ± 0.21 aB | 3.91 ± 0.22 aA | 3.70 ± 0.34 aA | 1.68 ± 0.11 aC | 0.26 ± 0.02 aD |
| FM | 4.08 ± 0.49 bB | 4.78 ± 0.43 aAB | 5.42 ± 0.53 bA | 0.35 ± 0.02 bC | 0.25 ± 0.05 aC | ||
| 9 | 3,4-Dihydroxyphenylacetic acid | PM | 522.38 ± 19.13 aA | 462.38 ± 92.82 aA | 529.90 ± 9.62 aA | 494.60 ± 17.64 aA | 477.02 ± 6.48 aA |
| FM | 312.20 ± 4.91 bB | 380.69 ± 8.76 bA | 354.27 ± 17.77 bA | 318.83 ± 11.23 bB | 177.24 ± 4.55 bC | ||
| 10 | Epicatechin | PM | 0.96 ± 0.02 aB | 0.62 ± 0.04 aC | 0.62 ± 0.06 aC | 0.51 ± 0.04 aC | 1.54 ± 0.17 aA |
| FM | 0.52 ± 0.08 bBC | 0.42 ± 0.06 bD | 0.65 ± 0.07 aA | 0.57 ± 0.05 aAB | 0.45 ± 0.07 bCD | ||
| 11 | Caffeic acid | PM | 0.41 ± 0.03 aC | 0.94 ± 0.07 aB | 1.10 ± 0.12 aB | 1.40 ± 0.16 aA | 0.03 ± 0.00 aD |
| FM | 1.70 ± 0.27 bB | 2.67 ± 0.18 bA | 2.92 ± 0.24 bA | 2.45 ± 0.28 bA | Nd | ||
| 12 | Syringic acid | PM | 0.22 ± 0.01 aC | 5.46 ± 0.57 aA | 2.57 ± 0.22 aB | 0.42 ± 0.01 aC | 0.60 ± 0.08 aC |
| FM | 5.00 ± 0.59 bB | 2.90 ± 0.21 bC | 8.06 ± 0.77 bA | 5.63 ± 0.51 bB | 0.60 ± 0.01 aD | ||
| 13 | Vanillic acid | PM | 0.84 ± 0.09 aBC | 0.78 ± 0.05 aBC | 1.15 ± 0.17 aA | 0.75 ± 0.07 aC | 1.02 ± 0.04 aAB |
| FM | 1.10 ± 0.15 aAB | 0.94 ± 0.05 bBC | 1.06 ± 0.06 aBC | 0.80 ± 0.17 aC | 1.35 ± 0.03 bA | ||
| 14 | Benzoic acid | PM | 205.28 ± 23.70 aD | 1874.22± 115.46 aB | 2826.56 ± 1.73 aA | 586.49 ± 22.32 aC | 94.96 ± 18.70 aE |
| FM | 895.92 ± 70.05 bB | 2920.44 ± 50.39 bA | 3118.91 ± 53.83 bA | 471.66 ± 18.92 bC | 271.28 ± 17.98 bD | ||
| 15 | 2-O-Rhamnosylvitexin | PM | 0.26 ± 0.06 aB | 0.45 ± 0.00 aA | 0.34 ± 0.05 aB | 0.28 ± 0.02 aB | 0.32 ± 0.05 aB |
| FM | 0.29 ± 0.02 aAB | 0.27 ± 0.02 bAB | 0.33 ± 0.01 aA | 0.25 ± 0.04 aB | 0.26 ± 0.02 aB | ||
| 16 | Rutin | PM | 4.75 ± 0.41 aAB | 5.32 ± 0.53 aA | 5.82 ± 0.47 aA | 5.14 ± 0.43 aA | 4.43 ± 0.57 aB |
| FM | 5.34 ± 0.53 aA | 6.67 ± 0.52 bA | 5.98 ± 0.62 aA | 6.16 ± 0.61 aA | 6.06 ± 0.65 bA | ||
| 17 | p-Coumaric acid | PM | 2.27 ± 0.28 aB | 5.28 ± 0.56 aA | 5.68 ± 0.17 aA | 1.92 ± 0.18 aB | Nd |
| FM | 5.01 ± 0.57 bC | 6.89 ± 0.56 bB | 8.65 ± 0.88 bA | 1.35 ± 0.12 bD | Nd | ||
| 18 | Hyperoside | PM | 3.19 ± 0.25 aA | 3.21 ± 0.33 aA | 3.62 ± 0.36 aA | 2.89 ± 0.26 aA | 1.82 ± 0.19 aB |
| FM | 2.84 ± 0.22 aB | 3.64 ± 0.36 aA | 3.40 ± 0.29 aA | 2.96 ± 0.25 aAB | 2.41 ± 0.14 bB | ||
| 19 | Isoquercetin | PM | 2.52 ± 0.09 aAB | 2.63 ± 0.12 aA | 2.86 ± 0.11 aA | 2.37 ± 0.26 aB | 1.42 ± 0.10 aC |
| FM | 2.31 ± 0.27 aAB | 2.90 ± 0.21 aA | 2.67 ± 0.29 aA | 2.37 ± 0.22 aAB | 1.90 ± 0.13 bB | ||
| 20 | Ferulic acid | PM | 0.71 ± 0.01 aB | 0.80 ± 0.07 aAB | 0.87 ± 0.03 aA | 0.87 ± 0.06 aA | 0.88 ± 0.05 aA |
| FM | 0.34 ± 0.03 bC | 0.46 ± 0.01 bBC | 0.55 ± 0.06 bB | 0.66 ± 0.06 bA | 0.35 ± 0.02 bC | ||
| 21 | Isoferulic acid | PM | 11.7 ± 1.12 aA | 12.63 ± 0.72 aA | 14.13 ± 1.38 aA | 8.53 ± 0.76 aB | 6.99 ± 0.72 aB |
| FM | 2.08 ± 0.19 bC | 2.88 ± 0.26 bB | 3.71 ± 0.35 bA | 3.99 ± 0.31 bA | 3.99 ± 0.32 bA | ||
| 22 | Kaempferol-3-O-rutinoside | PM | 0.17 ± 0.03 aA | 0.16 ± 0.01 aA | 0.20 ± 0.03 aA | 0.18 ± 0.02 aA | 0.12 ± 0.01 aB |
| FM | 0.16 ± 0.02 aB | 0.24 ± 0.02 bA | 0.21 ± 0.01 aA | 0.22 ± 0.03 aA | 0.18 ± 0.02 bB | ||
| 23 | Quercetin | PM | 0.66 ± 0.05 aC | 0.76 ± 0.02 aBC | 0.80 ± 0.06 aAB | 0.92 ± 0.09 aA | 0.84 ± 0.05 aAB |
| FM | 1.55 ± 0.19 bB | 1.62 ± 0.13 bAB | 1.79 ± 0.14 bA | 1.95 ± 0.13 bA | 1.91 ± 0.17 bA | ||
| 24 | Naringenin | PM | 30.21 ± 3.52 aB | 33.81 ± 2.86 aB | 37.76 ± 3.52 aAB | 44.01 ± 4.34 aA | 44.20 ± 4.01 aA |
| FM | 49.82 ± 4.81 bA | 47.00 ± 4.77 bAB | 41.40 ± 4.80 bB | 45.47 ± 5.59 aAB | 53.23 ± 4.08 bA |
3.5. Correlation Between Phenolics Compounds and Gut Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; Luo, W.; Li, H.; Huang, X.; Ni, Z.; Gao, H.; Iqbal, S.; Gao, Z. Association between Blooming Time and Climatic Adaptation in Prunus mume. Ecol. Evol. 2020, 10, 292–306. [Google Scholar] [CrossRef]
- Ye, J. Study on Preparation Technology and Quality Standard of Dark Plum Standard Slices. Master’s Thesis, Hubei University of Chinese Medicine, Wuhan, China, 2018. [Google Scholar]
- Wang, R.; Cheng, H.; Yang, Y.; Ou, J.; Song, Q.; Zhou, H.; Peng, H.; Wang, J.; Fang, C.W. Ultra High Performance Liquid Chromatography with Quadrupole Time-of-flight Tandem Mass Spectrometry and Liquid Chromatography with Tandem Mass Spectrometry Combined with Chemometric Analysis as an Approach for the Quality Evaluation of Mume fructus. J. Sep. Sci. 2022, 45, 1884–1893. [Google Scholar] [CrossRef]
- Chen, Y.; Wong, R.W.K.; Seneviratne, C.J.; Hägg, U.; Mcgrath, C.; Samaranayake, L.P.; Kao, R. The Antimicrobial Efficacy of Fructus mume Extract on Orthodontic Bracket: A Monospecies-Biofilm Model Study in Vitro. Arch. Oral Biol. 2011, 56, 16–21. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Wang, W.; Zhang, D.; Ma, Y.; Zhang, D.; Chen, M. Fructus mume (Wu Mei) Attenuates Acetic Acid-Induced Ulcerative Colitis by Regulating Inflammatory Cytokine, Reactive Oxygen Species, and Neuropeptide Levels in Model Rats. J. Med. Food 2022, 25, 389–401. [Google Scholar] [CrossRef]
- Yan, S.; Wang, P.; Wei, H.; Jia, R.; Zhen, M.; Li, Q.; Xue, C.; Li, J. Treatment of Ulcerative Colitis with Wu-Mei-Wan by Inhibiting Intestinal Inflammatory Response and Repairing Damaged Intestinal Mucosa. Phytomedicine 2022, 105, 154362. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, H.; Gao, X.; Gao, H.; He, Q.; Li, G.; Yao, J.; Liu, Z. Natural Herbal Remedy Wumei Decoction Ameliorates Intestinal Mucosal Inflammation by Inhibiting Th1/Th17 Cell Differentiation and Maintaining Microbial Homeostasis. Inflamm. Bowel Dis. 2022, 28, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Chen, S.-G.; Zhao, Y.-Y.; Luo, C.-X.; Li, J.; Gao, Y.-Q. Total Phenolic Contents of 33 Fruits and Their Antioxidant Capacities before and after in Vitro Digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Kaulmann, A.; André, C.M.; Schneider, Y.-J.; Hoffmann, L.; Bohn, T. Carotenoid and Polyphenol Bioaccessibility and Cellular Uptake from Plum and Cabbage Varieties. Food Chem. 2016, 197, 325–332. [Google Scholar] [CrossRef]
- Mubarak, A.; Swinny, E.E.; Ching, S.Y.L.; Jacob, S.R.; Lacey, K.; Hodgson, J.M.; Croft, K.D.; Considine, M.J. Polyphenol Composition of Plum Selections in Relation to Total Antioxidant Capacity. J. Agric. Food Chem. 2012, 60, 10256–10262. [Google Scholar] [CrossRef]
- You, B.; Yang, S.; Yu, J.; Xian, W.; Deng, Y.; Huang, W.; Li, W.; Yang, R. Effect of Thermal and Dry Salt-Curing Processing on Free and Bound Phenolics and Antioxidant Activity in Prunus mume Fruits Together with the Phenolic Bioaccessibility. LWT 2021, 145, 111355. [Google Scholar] [CrossRef]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic Insight into the Profile, in Vitro Bioaccessibility and Bioactive Properties of Polyphenols and Glucosinolates from Four Brassicaceae Microgreens. Food Res. Int. 2021, 140, 110039. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, J.; Cui, J.; Fan, Y.; Li, N.; Wang, C.; Liu, Y.; Dong, Y. Stability and Mechanism of Phenolic Compounds from Raspberry Extract under in Vitro Gastrointestinal Digestion. LWT 2021, 139, 110552. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, 1900952. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; Wang, X.; Yan, X.; Guo, Q.; Yue, Y.; Yue, T.; Yuan, Y. The Bioaccessibility, Bioavailability, Bioactivity, and Prebiotic Effects of Phenolic Compounds from Raw and Solid-Fermented Mulberry Leaves during in Vitro Digestion and Colonic Fermentation. Food Res. Int. 2023, 165, 112493. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, Q.; Huang, H.; Hou, K.; Dong, R.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. The Effect of Bound Polyphenols on the Fermentation and Antioxidant Properties of Carrot Dietary Fiber in Vivo and in Vitro. Food Funct. 2020, 11, 748–758. [Google Scholar] [CrossRef]
- Di Naso, F.C.; Simões Dias, A.; Porawski, M.; Marroni, N.A.P. Exogenous Superoxide Dismutase: Action on Liver Oxidative Stress in Animals with Streptozotocin-Induced Diabetes. Exp. Diabetes Res. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of Polyphenols in Apple Pomace: A Comparative Study of Different Extraction and Hydrolysis Procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- He, Z.; Tao, Y.; Zeng, M.; Zhang, S.; Tao, G.; Qin, F.; Chen, J. High Pressure Homogenization Processing, Thermal Treatment and Milk Matrix Affect in Vitro Bioaccessibility of Phenolics in Apple, Grape and Orange Juice to Different Extents. Food Chem. 2016, 200, 107–116. [Google Scholar] [CrossRef]
- Velázquez, E.; Tournier, H.A.; Mordujovich De Buschiazzo, P.; Saavedra, G.; Schinella, G.R. Antioxidant Activity of Paraguayan Plant Extracts. Fitoterapia 2003, 74, 91–97. [Google Scholar] [CrossRef]
- Xian, W.; Yang, S.; Deng, Y.; Yang, Y.; Tan, Z.; Li, W.; Yang, R. Potential of Establishing the Corresponding Human Microbial Community in Pseudo Germ-Free Mice through Fecal Microbe Transfer from Three Urolithin Metabotypes. J. Agric. Food Chem. 2022, 70, 9388–9398. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In Vitro Bio-Accessibility and Antioxidant Activity of Grape Polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Bazzocco, S.; Mattila, I.; Guyot, S.; Renard, C.M.G.C.; Aura, A.-M. Factors Affecting the Conversion of Apple Polyphenols to Phenolic Acids and Fruit Matrix to Short-Chain Fatty Acids by Human Faecal Microbiota in Vitro. Eur. J. Nutr. 2008, 47, 442–452. [Google Scholar] [CrossRef]
- Aura, A.-M.; Mattila, I.; Seppänen-Laakso, T.; Miettinen, J.; Oksman-Caldentey, K.-M.; Orešič, M. Microbial Metabolism of Catechin Stereoisomers by Human Faecal Microbiota: Comparison of Targeted Analysis and a Non-Targeted Metabolomics Method. Phytochem. Lett. 2008, 1, 18–22. [Google Scholar] [CrossRef]
- Parada, J.; Aguilera, J.M. Food Microstructure Affects the Bioavailability of Several Nutrients. J. Food Sci. 2007, 72, R21–R32. [Google Scholar] [CrossRef] [PubMed]
- Morzel, M.; Canon, F.; Guyot, S. Interactions between Salivary Proteins and Dietary Polyphenols: Potential Consequences on Gastrointestinal Digestive Events. J. Agric. Food Chem. 2022, 70, 6317–6327. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Victoria Moreno-Arribas, M.; Bartolomé, B. Gut Microbial Catabolism of Grape Seed Flavan-3-Ols by Human Faecal Microbiota. Targetted Analysis of Precursor Compounds, Intermediate Metabolites and End-Products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. Digestive Fate of Polyphenols: Updated View of the Influence of Chemical Structure and the Presence of Cell Wall Material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the Free and Bound Phenolic Profiles and Cellular Antioxidant Activities of Litchi Pulp Extracts from Different Solvents. BMC Complement. Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Verny, M.-A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic Acid Bioavailability Largely Depends on Its Metabolism by the Gut Microflora in Rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of Dietary Flavonoid Glycosides by Strains of Intestinal Bacteroides from Humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef]
- Shim, S.-M.; Yoo, S.-H.; Ra, C.-S.; Kim, Y.-K.; Chung, J.-O.; Lee, S.-J. Digestive Stability and Absorption of Green Tea Polyphenols: Influence of Acid and Xylitol Addition. Food Res. Int. 2012, 45, 204–210. [Google Scholar] [CrossRef]
- Rechner, A.R.; Spencer, J.P.E.; Kuhnle, G.; Hahn, U.; Rice-Evans, C.A. Novel Biomarkers of the Metabolism of Caffeic Acid Derivatives in Vivo. Free Radic. Biol. Med. 2001, 30, 1213–1222. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Structural Features of Procyanidin Interactions with Salivary Proteins. J. Agric. Food Chem. 2001, 49, 940–945. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Huang, Q.; Fu, X. In Vitro Digestion of the Whole Blackberry Fruit: Bioaccessibility, Bioactive Variation of Active Ingredients and Impacts on Human Gut Microbiota. Food Chem. 2022, 370, 131001. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green Tea Polyphenols Decrease Weight Gain, Ameliorate Alteration of Gut Microbiota, and Mitigate Intestinal Inflammation in Canines with High-Fat-Diet-Induced Obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Sun, N.; Huang, H.; Xie, J.; Chen, Y.; Hu, X.; Hu, X.; Dong, R.; Yu, Q. Catabolism of Polyphenols Released from Mung Bean Coat and Its Effects on Gut Microbiota during in Vitro Simulated Digestion and Colonic Fermentation. Food Chem. 2022, 396, 133719. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Li, D.; Wang, R.; Wang, A.; Strappe, P.; Wu, Q.; Shang, W.; Wang, X.; Zhuang, M.; Blanchard, C.; et al. Gut Microbiota Derived Structural Changes of Phenolic Compounds from Colored Rice and Its Corresponding Fermentation Property. Food Funct. 2022, 13, 10759–10768. [Google Scholar] [CrossRef]
- Ren, X.; Xu, J.; Zhang, Y.; Chen, G.; Zhang, Y.; Huang, Q.; Liu, Y. Bacterial Alterations in Post-Cholecystectomy Patients Are Associated With Colorectal Cancer. Front. Oncol. 2020, 10, 1418. [Google Scholar] [CrossRef]
- Mottawea, W.; Sultan, S.; Landau, K.; Bordenave, N.; Hammami, R. Evaluation of the Prebiotic Potential of a Commercial Synbiotic Food Ingredient on Gut Microbiota in an Ex Vivo Model of the Human Colon. Nutrients 2020, 12, 2669. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium Faecium Abundant Colonization in Human Gastrointestinal Tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? eBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Wang, R.; Sun, J.; Li, G.; Zhang, M.; Niu, T.; Kang, X.; Zhao, H.; Chen, J.; Sun, E.; Li, Y. Effect of Bifidobacterium animalis Subsp. Lactis MN-Gup on Constipation and the Composition of Gut Microbiota. BM 2021, 12, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, J.H.; Ahn, S.H.; Lee, S.I.; Han, Y.S.; Choi, Y.O.; Lee, S.Y.; Ahn, K.M.; Ji, G.E. Effect of Probiotic Mix (Bifidobacterium Bifidum, Bifidobacterium Lactis, Lactobacillus Acidophilus) in the Primary Prevention of Eczema: A Double-blind, Randomized, Placebo-controlled Trial. Pediatr. Allergy Immunol. 2010, 21, e386–e393. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, F.A.; Nascimento, B.B.; Andrade, M.E.R.; De Barros, P.A.V.; Cartelle, C.T.; Martins, F.S.; Nicoli, J.R.; Arantes, R.M.E.; Generoso, S.V.; Fernandes, S.O.A.; et al. Treatment with Bifidobacterium Longum 51A Attenuates Intestinal Damage and Inflammatory Response in Experimental Colitis. Benef. Microbes 2020, 11, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Won, Y.-S.; Cho, H.-D.; Hong, S.-M.; Moon, K.-D.; Seo, K.-I. Protective Effect of Prunus mume Fermented with Mixed Lactic Acid Bacteria in Dextran Sodium Sulfate-Induced Colitis. Foods 2020, 10, 58. [Google Scholar] [CrossRef]
- Zhao, Y.; Leng, F.; Fan, S.; Ding, Y.; Chen, T.; Zhou, H.; Xiao, X. Changes of Barley Bound Phenolics and Their Characteristics During Simulated Gastrointestinal Digestion and Colonic Fermentation In Vitro. Foods 2025, 14, 1114. [Google Scholar] [CrossRef]
- Jakobek, L.; Pöc, K.; Valenteković, M.; Matić, P. The Behavior of Phenolic Compounds from Apples during Simulated Gastrointestinal Digestion with Focus on Chlorogenic Acid. Foods 2024, 13, 693. [Google Scholar] [CrossRef]
- Sirisena, S.; Ajlouni, S.; Ng, K. Simulated Gastrointestinal Digestion and in Vitro Colonic Fermentation of Date (Phoenix dactylifera L.) Seed Polyphenols. Int. J. Food Sci. Tech. 2018, 53, 412–422. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated Gastrointestinal Digestion and in Vitro Colonic Fermentation of Carob Polyphenols: Bioaccessibility and Bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Suleria, H.; Zhang, P.; Gu, C.; Ng, K. Sugarcane Polyphenol and Fiber to Affect Production of Short-Chain Fatty Acids and Microbiota Composition Using in Vitro Digestion and Pig Faecal Fermentation Model. Food Chem. 2022, 385, 132665. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Z.; Kong, K.W.; Xiang, P.; He, X.; Zhang, X. Probiotic Fermentation Improves the Bioactivities and Bioaccessibility of Polyphenols in Dendrobium officinale under in Vitro Simulated Gastrointestinal Digestion and Fecal Fermentation. Front. Nutr. 2022, 9, 1005912. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Bhumireddy, S.R.; Giuberti, G.; Mandal, R.; Lucini, L.; Wishart, D.S. Edible Nuts Deliver Polyphenols and Their Transformation Products to the Large Intestine: An in Vitro Fermentation Model Combining Targeted/Untargeted Metabolomics. Food Res. Int. 2019, 116, 786–794. [Google Scholar] [CrossRef]
- Chow, J.; Yang, X.; Hu, J.; Yu, Q.; Zhong, Y.; Hu, X.; Liang, J.; Zhu, C.; Yan, S.; Li, L.; et al. Gastrointestinal Absorption and Its Regulation of Hawthorn Leaves Flavonoids. Sci. Rep. 2025, 15, 658. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiang, T.; Bindawa Isah, M.; Chen, C.; Zhang, X. In Vitro Simulated Saliva, Gastric, and Intestinal Digestion Followed by Faecal Fermentation Reveals a Potential Modulatory Activity of Epimedium on Human Gut Microbiota. J. Pharm. Biomed. Anal. 2024, 245, 116151. [Google Scholar] [CrossRef]
- Cañas, S.; Rebollo-Hernanz, M.; Braojos, C.; Benítez, V.; Ferreras-Charro, R.; Dueñas, M.; Aguilera, Y.; Martín-Cabrejas, M.A. Understanding the Gastrointestinal Behavior of the Coffee Pulp Phenolic Compounds under Simulated Conditions. Antioxidants 2022, 11, 1818. [Google Scholar] [CrossRef]
- Obayiuwana, O.A.; Behrends, V.; Calle-Patino, Y.; Barone, M.; Turroni, S.; Brigidi, P.; Costabile, A.; Corona, G. Cooking, Digestion, and In Vitro Colonic Fermentation of Nigerian Wholegrains Affect Phenolic Acid Metabolism and Gut Microbiota Composition. Int. J. Mol. Sci. 2023, 24, 14111. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, F.; Peng, X.; Cheng, K.; Xiao, L.; Zhang, H.; Li, H.; Jiang, L.; Deng, Z. Metabolism of Phenolics of Tetrastigma Hemsleyanum Roots under In Vitro Digestion and Colonic Fermentation as Well as Their In Vivo Antioxidant Activity in Rats. Foods 2021, 10, 2123. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Tan, Z.; You, B.; Luo, J.; Huang, W.; Yang, R.; Li, W. Bioaccessibility and Gut Microbiota Modulation of Phenolics in Prunus mume vs. Fructus mume. Foods 2025, 14, 4067. https://doi.org/10.3390/foods14234067
Xie Q, Tan Z, You B, Luo J, Huang W, Yang R, Li W. Bioaccessibility and Gut Microbiota Modulation of Phenolics in Prunus mume vs. Fructus mume. Foods. 2025; 14(23):4067. https://doi.org/10.3390/foods14234067
Chicago/Turabian StyleXie, Qingzhuang, Zhaolun Tan, Bangyan You, Jinxin Luo, Wei Huang, Ruili Yang, and Wu Li. 2025. "Bioaccessibility and Gut Microbiota Modulation of Phenolics in Prunus mume vs. Fructus mume" Foods 14, no. 23: 4067. https://doi.org/10.3390/foods14234067
APA StyleXie, Q., Tan, Z., You, B., Luo, J., Huang, W., Yang, R., & Li, W. (2025). Bioaccessibility and Gut Microbiota Modulation of Phenolics in Prunus mume vs. Fructus mume. Foods, 14(23), 4067. https://doi.org/10.3390/foods14234067





