Extraction Techniques for Brewer’s Spent Grain Protein: A Comparative Review of Efficiency, Purity, and Functionality
Abstract
1. Introduction
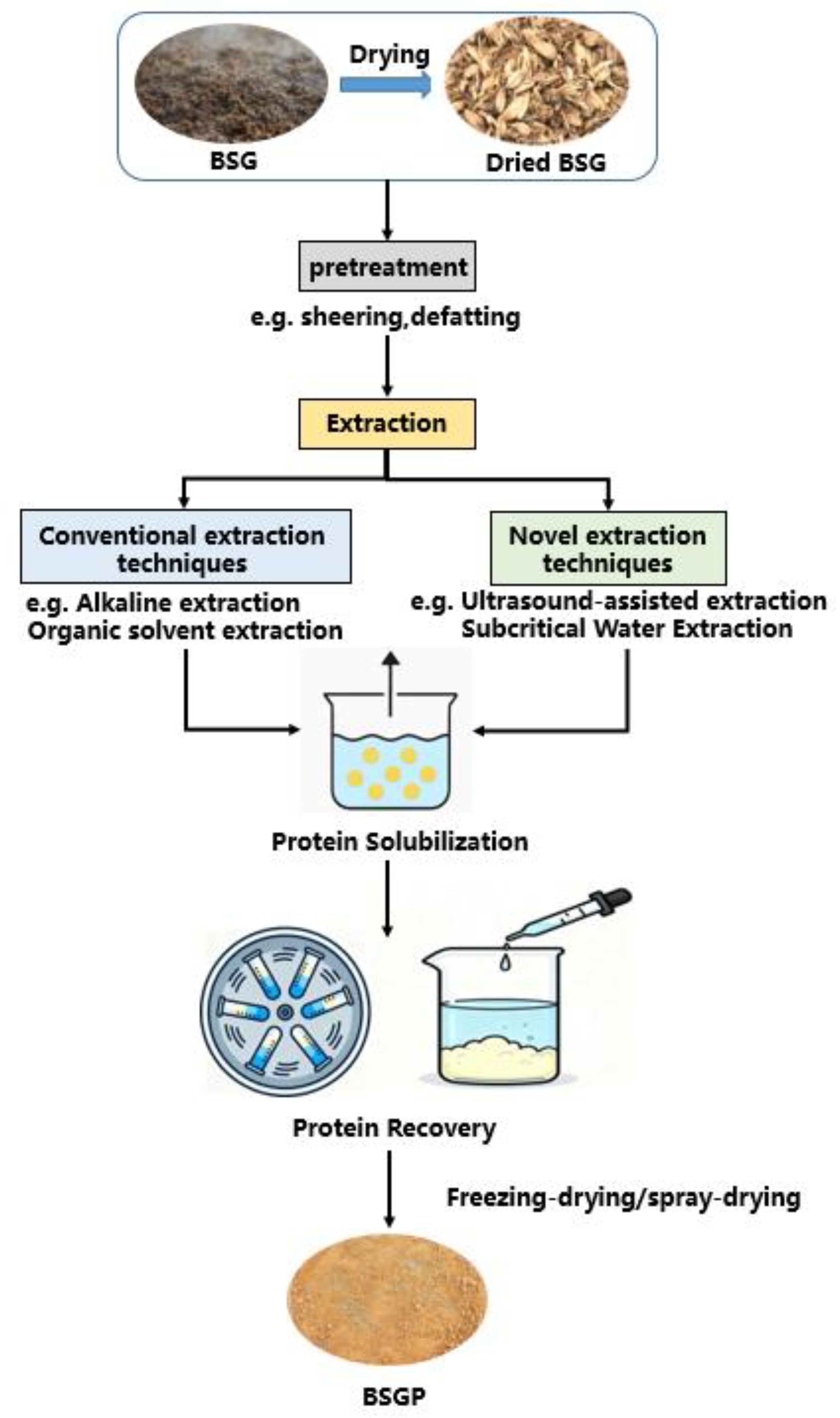
2. Extraction Methods for BSGP
2.1. Pretreatment
2.2. Conventional Extraction Technologies
2.2.1. Alkaline Extraction
2.2.2. Hydrothermal Extraction and Ethanol Extraction
2.2.3. Enzymatic Extraction
2.3. Novel Extraction Technologies
2.3.1. Ultrasound-Assisted Extraction
2.3.2. Microwave-Assisted Extraction
2.3.3. Subcritical Water Extraction
2.3.4. Pressurized Solvent Extraction
2.3.5. Deep Eutectic Solvent Extraction
3. Property Modification by Extraction Techniques
3.1. Structural Properties Modification by Extraction Techniques
3.1.1. Molecular Weight Distribution
3.1.2. Secondary Structure
3.1.3. Surface Hydrophobicity
3.2. Techno-Functional Properties Modification by Extraction Techniques
3.2.1. Protein Solubility
3.2.2. Water-Holding and Oil-Holding Capacities
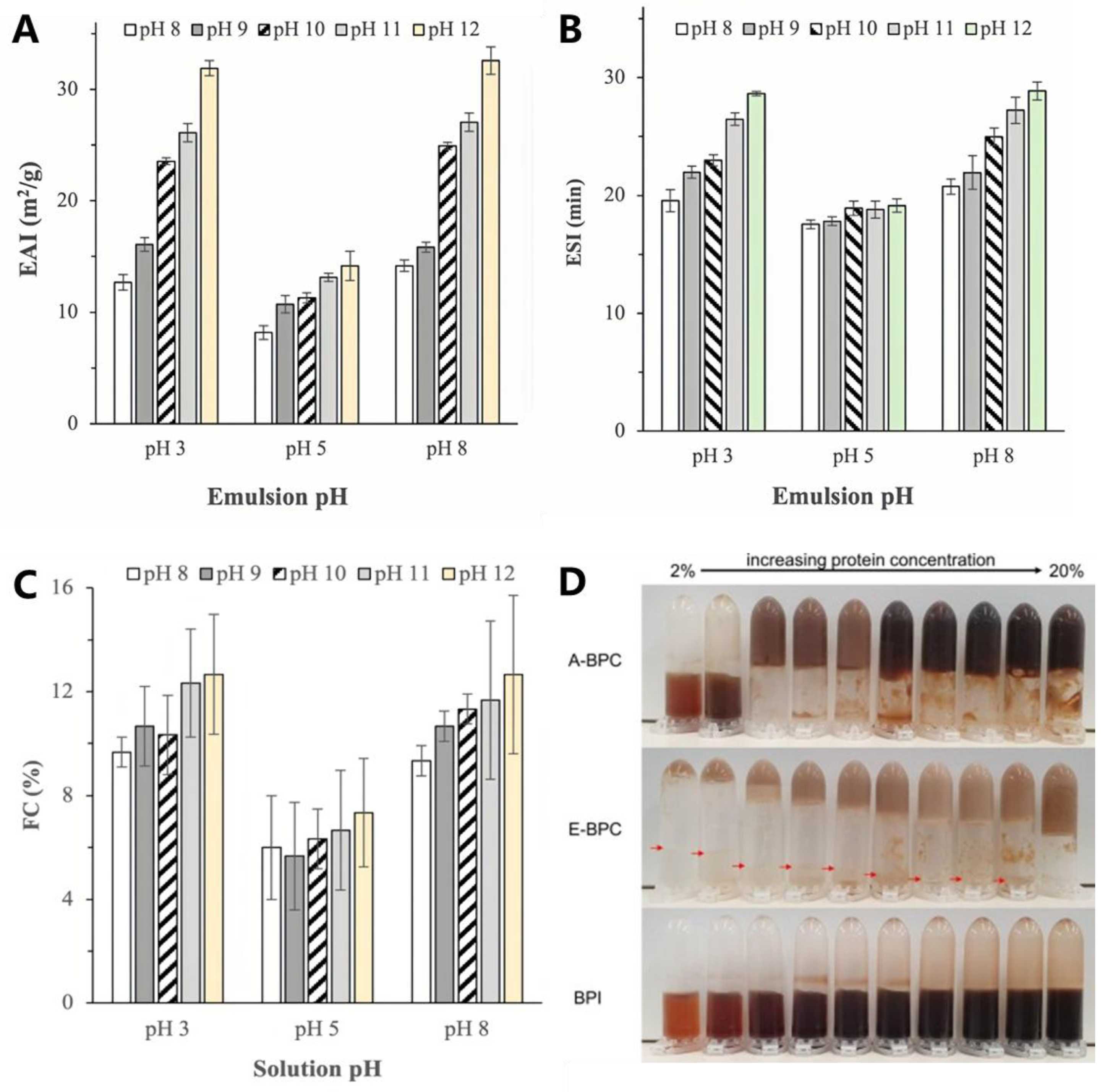
3.2.3. Emulsifying Properties
3.2.4. Foaming Properties
3.2.5. Gelation Properties
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSG | brewer’s spent grain |
| BSGP | brewer’s spent grain protein |
| UAE | ultrasound-assisted extraction |
| MAE | microwave-assisted extraction |
| DES | deep eutectic solvent |
| HBA | hydrogen bond acceptors |
| HCA | hydrogen bond donors |
| WHC | water-holding capacities |
| OHC | oil-holding capacities |
| EC | emulsion capacity |
| ES | emulsion stability |
| EAI | emulsion activity index |
| ESI | emulsion stability index |
| ECI | emulsion capacity index |
| EVI | emulsion volume index |
| FC | foaming capacity |
| FS | foaming stability |
References
- Jaeger, A.; Zannini, E.; Sahin, A.W.; Arendt, E.K. Barley Protein Properties, Extraction and Applications, with a Focus on Brewers’ Spent Grain Protein. Foods 2021, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio-Lopes, T.; Teixeira, J.A.; Pintado, M. Current extraction techniques towards bioactive compounds from brewer’s spent grain—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical composition of lipids in brewer’s spent grain: A promising source of valuable phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Devnani, B.; Moran, G.C.; Grossmann, L. Extraction, Composition, Functionality, and Utilization of Brewer’s Spent Grain Protein in Food Formulations. Foods 2023, 12, 1543. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef]
- Petit, G.; Korbel, E.; Jury, V.; Aider, M.; Rousselière, S.; Audebrand, L.K.; Turgeon, S.L.; Mikhaylin, S. Environmental Evaluation of New Brewer’s Spent Grain Preservation Pathways for Further Valorization in Human Nutrition. ACS Sustain. Chem. Eng. 2020, 8, 17335–17344. [Google Scholar] [CrossRef]
- Coronado, M.A.; Montero, G.; Montes, D.G.; Valdez-Salas, B.; Ayala, J.R.; García, C.; Carrillo, M.; León, J.A.; Moreno, A. Physicochemical Characterization and SEM-EDX Analysis of Brewer’s Spent Grain from the Craft Brewery Industry. Sustainability 2020, 12, 7744. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. Enzymatic Hydrolysis of Brewers’ Spent Grain Proteins and Technofunctional Properties of the Resulting Hydrolysates. J. Agric. Food Chem. 2007, 55, 8703–8710. [Google Scholar] [CrossRef]
- Connolly, A.; Piggott, C.O.; FitzGerald, R.J. Characterisation of protein-rich isolates and antioxidative phenolic extracts from pale and black brewers’ spent grain. Int. J. Food Sci. Technol. 2013, 48, 1670–1681. [Google Scholar] [CrossRef]
- Niemi, P.; Martins, D.; Buchert, J.; Faulds, C.B. Pre-hydrolysis with carbohydrases facilitates the release of protein from brewer’s spent grain. Bioresour. Technol. 2013, 136, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.L.; Keppler, J.K.; Dinani, S.T.; Chen, W.N.; Boom, R. Brewers’ spent grain proteins: The extraction method determines the functional properties. Innov. Food Sci. Emerg. Technol. 2024, 94, 103666. [Google Scholar] [CrossRef]
- Kriisa, M.; Taivosalo, A.; Föste, M.; Kütt, M.-L.; Viirma, M.; Priidik, R.; Korzeniowska, M.; Tian, Y.; Laaksonen, O.; Yang, B.; et al. Effect of enzyme-assisted hydrolysis on brewer’s spent grain protein solubilization—Peptide composition and sensory properties. Appl. Food Res. 2022, 2, 100108. [Google Scholar] [CrossRef]
- Qin, F.; Johansen, A.Z.; Mussatto, S.I. Evaluation of different pretreatment strategies for protein extraction from brewer’s spent grains. Ind. Crops Prod. 2018, 125, 443–453. [Google Scholar] [CrossRef]
- Silva, A.M.M.d.; Almeida, F.S.; Silva, M.F.d.; Goldbeck, R.; Sato, A.C.K. How do pH and temperature influence extraction yield, physicochemical, functional, and rheological characteristics of brewer spent grain protein concentrates? Food Bioprod. Process. 2023, 139, 34–45. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.A.; Bhat, H.F. Technological, Regulatory, and Ethical Aspects of In Vitro Meat: A Future Slaughter-Free Harvest. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1192–1208. [Google Scholar] [CrossRef]
- Chai, K.F.; Chen, W.N. Recovery of antioxidative protein hydrolysates with functional properties from fermented brewer’s spent grain via microwave-assisted three phase partitioning. Innov. Food Sci. Emerg. Technol. 2024, 91, 103551. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Duan, Y.; Zhang, H.; Ma, H. A Mini-Review on Brewer’s Spent Grain Protein: Isolation, Physicochemical Properties, Application of Protein, and Functional Properties of Hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Sanz, M.T.; Benito-Román, O.; Beltrán, S.; Trigueros, E. Subcritical water as hydrolytic medium to recover and fractionate the protein fraction and phenolic compounds from craft brewer’s spent grain. Food Chem. 2021, 351, 129264. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59 (Suppl. 5), 1203S–1212S. [Google Scholar] [CrossRef] [PubMed]
- Skrabanja, V.; Laerke, H.N.; Kreft, I. Protein-polyphenol interactions and in vivo digestibility of buckwheat groat proteins. Pflugers Arch. 2000, 440 (Suppl. 1), R129–R131. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries—Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Junttila, M.H. Extraction of brewers’ spent grain in near subcritical conditions: A method to obtain high protein contents extracts. J. Agric. Food Res. 2022, 10, 100378. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Coldea, T.E.; Zhao, H. Modification of structural and functional characteristics of brewer’s spent grain protein by ultrasound assisted extraction. LWT 2021, 139, 110582. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Du, L.; Arauzo, P.J.; Meza Zavala, M.F.; Cao, Z.; Olszewski, M.P.; Kruse, A. Towards the Properties of Different Biomass-Derived Proteins via Various Extraction Methods. Molecules 2020, 25, 488. [Google Scholar] [CrossRef]
- Karlsen, F.; Lund, I.; Skov, P.V. Optimisation of alkaline extraction of protein from brewer’s spent grain. J. Inst. Brew. 2022, 128, 150–161. [Google Scholar] [CrossRef]
- Zhang, J.; Perez-Gavilan, A.; Neves, A.C. Evaluation of the In Vitro Bioactivities’ Profiles of Brewers’ Spent Grain Protein and Hydrolysates with and without Cellulase Pretreatment. Nutraceuticals 2022, 2, 218–233. [Google Scholar] [CrossRef]
- Chin, Y.L.; Chai, K.F.; Chen, W.N. Upcycling of brewers’ spent grains via solid-state fermentation for the production of protein hydrolysates with antioxidant and techno-functional properties. Food Chem. X 2022, 13, 100184. [Google Scholar] [CrossRef]
- Connolly, A.; Cermeño, M.; Crowley, D.; O’Callaghan, Y.; O’Brien, N.M.; FitzGerald, R.J. Characterisation of the in vitro bioactive properties of alkaline and enzyme extracted brewers’ spent grain protein hydrolysates. Food Res. Int. 2019, 121, 524–532. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kuhn, D.D.; Ogejo, J.A.; O’Keefe, S.F.; Fraguas, C.F.; Wiersema, B.D.; Jin, Q.; Yu, D.; Huang, H. Wet fractionation process to produce high protein and high fiber products from brewer’s spent grain. Food Bioprod. Process. 2019, 117, 266–274. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Impact of the use of pressurized liquids on the extraction and functionality of proteins and bioactives from brewer’s spent grain. Food Chem. 2021, 359, 129874. [Google Scholar] [CrossRef]
- Tang, D.-S.; Tian, Y.-J.; He, Y.-Z.; Li, L.; Hu, S.-Q.; Li, B. Optimisation of ultrasonic-assisted protein extraction from brewer’s spent grain. Czech J. Food Sci. 2010, 28, 9–17. [Google Scholar] [CrossRef]
- Tang, D.-S.; Yin, G.-M.; He, Y.-Z.; Hu, S.-Q.; Li, B.; Li, L.; Liang, H.-L.; Borthakur, D. Recovery of protein from brewer’s spent grain by ultrafiltration. Biochem. Eng. J. 2009, 48, 1–5. [Google Scholar] [CrossRef]
- Barrios, C.; Fernández-Delgado, M.; López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A techno-economic perspective on a microwave extraction process for efficient protein recovery from agri-food wastes. Ind. Crops Prod. 2022, 186, 115166. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Ramos, C.; Trigueros, E.; Beltrán, S.; Sanz, M.T. Study of subcritical water scale-up from laboratory to pilot system for brewer’s spent grain valorization. Ind. Crops Prod. 2023, 191, 115927. [Google Scholar] [CrossRef]
- Wahlström, R.; Rommi, K.; Willberg-Keyriläinen, P.; Ercili-Cura, D.; Holopainen-Mantila, U.; Hiltunen, J.; Mäkinen, O.; Nygren, H.; Mikkelson, A.; Kuutti, L. High Yield Protein Extraction from Brewer’s Spent Grain with Novel Carboxylate Salt—Urea Aqueous Deep Eutectic Solvents. ChemistrySelect 2017, 2, 9355–9363. [Google Scholar] [CrossRef]
- Casillo, A.; D’Angelo, C.; Imbimbo, P.; Monti, D.M.; Parrilli, E.; Lanzetta, R.; Gomez d’Ayala, G.; Mallardo, S.; Corsaro, M.M.; Duraccio, D. Aqueous Extracts from Hemp Seeds as a New Weapon against Staphylococcus epidermidis Biofilms. Int. J. Mol. Sci. 2023, 24, 16026. [Google Scholar] [CrossRef]
- Rommi, K.; Niemi, P.; Kemppainen, K.; Kruus, K. Impact of thermochemical pre-treatment and carbohydrate and protein hydrolyzing enzyme treatment on fractionation of protein and lignin from brewer’s spent grain. J. Cereal Sci. 2018, 79, 168–173. [Google Scholar] [CrossRef]
- Ibarruri, J.; Cebrián, M.; Hernández, I. Solid State Fermentation of Brewer’s Spent Grain Using Rhizopus sp. to Enhance Nutritional Value. Waste Biomass Valorization 2019, 10, 3687–3700. [Google Scholar] [CrossRef]
- Zeng, J.; Huang, W.; Tian, X.; Hu, X.; Wu, Z. Brewer’s spent grain fermentation improves its soluble sugar and protein as well as enzymatic activities using Bacillus velezensis. Process Biochem. 2021, 111, 12–20. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. pH-shift processing of Nannochloropsis oculata microalgal biomass to obtain a protein-enriched food or feed ingredient. Algal Res. 2015, 11, 95–102. [Google Scholar] [CrossRef]
- Vilg, J.V.; Undeland, I. pH-driven solubilization and isoelectric precipitation of proteins from the brown seaweed Saccharina latissima-effects of osmotic shock, water volume and temperature. J. Appl. Phycol. 2017, 29, 585–593. [Google Scholar] [CrossRef]
- Lu, K.; Zhao, X.; Ho, S.H.; Ma, R.; Xie, Y.; Chen, J. Biorefining and the Functional Properties of Proteins from Lipid and Pigment Extract Residue of Chlorella pyrenoidosa. Mar. Drugs 2019, 17, 454. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Khan, A.; Hashem, A.; Abd Allah, E.F.; Al-Harrasi, A. The molecular mass and isoelectric point of plant proteomes. BMC Genom. 2019, 20, 631. [Google Scholar] [CrossRef]
- Kozlowski, L.P. Proteome-pI: Proteome isoelectric point database. Nucleic Acids Res. 2017, 45, D1112–D1116. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and protein recovery from brewer’s spent grain using hydrothermal pretreatment and their conversion to edible filamentous fungi—A brewery biorefinery concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Ethanolic extraction of flavonoids, phenolics and antioxidants from Vernonia amygdalina leaf using two-level factorial design. J. King Saud Univ.—Sci. 2020, 32, 7–16. [Google Scholar] [CrossRef]
- Gouseti, O.; Larsen, M.E.; Amin, A.; Bakalis, S.; Petersen, I.L.; Lametsch, R.; Jensen, P.E. Applications of Enzyme Technology to Enhance Transition to Plant Proteins: A Review. Foods 2023, 12, 2518. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.J.; Jadhav, S.B.; Bankar, S.B.; Kumari Dubey, K.; Singhal, R.S. Improvements in the extraction of bioactive compounds by enzymes. Curr. Opin. Food Sci. 2019, 25, 62–72. [Google Scholar] [CrossRef]
- Hanmoungjai, P.; Pyle, D.L.; Niranjan, K. Enzyme-assisted water-extraction of oil and protein from rice bran. J. Chem. Technol. Biotechnol. 2002, 77, 771–776. [Google Scholar] [CrossRef]
- Tang, J.; Yao, D.; Xia, S.; Cheong, L.; Tu, M. Recent progress in plant-based proteins: From extraction and modification methods to applications in the food industry. Food Chem. X 2024, 23, 101540. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Recovery of high value-added protein from enzyme-assisted aqueous extraction (EAE) of soybeans by dead-end ultrafiltration. Food Sci. Nutr. 2019, 7, 858–868. [Google Scholar] [CrossRef]
- Daniel, R.M.; Danson, M.J.; Eisenthal, R.; Lee, C.K.; Peterson, M.E. The effect of temperature on enzyme activity: New insights and their implications. Extremophiles 2008, 12, 51–59. [Google Scholar] [CrossRef]
- Karki, B.; Lamsal, B.P.; Jung, S.; van Leeuwen, J.; Pometto, A.L.; Grewell, D.; Khanal, S.K. Enhancing protein and sugar release from defatted soy flakes using ultrasound technology. J. Food Eng. 2010, 96, 270–278. [Google Scholar] [CrossRef]
- Yu, D.; Sun, Y.; Wang, W.; O’Keefe, S.F.; Neilson, A.P.; Feng, H.; Wang, Z.; Huang, H. Recovery of protein hydrolysates from brewer’s spent grain using enzyme and ultrasonication. Int. J. Food Sci. Technol. 2019, 55, 357–368. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.M.; Kentish, S.; Ashokkumar, M. Use of Power Ultrasound to Improve Extraction and Modify Phase Transitions in Food Processing. Food Rev. Int. 2013, 29, 67–91. [Google Scholar] [CrossRef]
- Xie, X.; Jin, X.; Huang, J.; Yi, J.; Li, X.; Huang, Z.; Lin, Q.; Guo, B. High resveratrol-loaded microcapsules with trehalose and OSA starch as the wall materials: Fabrication, characterization, and evaluation. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124825. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and Ultrasound to Enhance Protein Extraction from Peanut Flour under Alkaline Conditions: Effects in Yield and Functional Properties of Protein Isolates. Food Bioprocess Technol. 2016, 10, 543–555. [Google Scholar] [CrossRef]
- Jahan, K.; Fatima, S.; Osama, K.; Younis, K.; Yousuf, O. Boosting protein yield from mustard (Brassica juncea) meal via microwave-assisted extraction and advanced optimization methods. Biomass Convers. Biorefinery 2023, 13, 16241–16251. [Google Scholar] [CrossRef]
- Prandi, B.; Di Massimo, M.; Tedeschi, T.; Rodríguez-Turienzo, L.; Rodríguez, Ó. Ultrasound and Microwave-assisted Extraction of Proteins from Coffee Green Beans: Effects of Process Variables on the Protein Integrity. Food Bioprocess Technol. 2022, 15, 2712–2722. [Google Scholar] [CrossRef]
- Martins-Vieira, J.C.; Torres-Mayanga, P.C.; Lachos-Perez, D. Hydrothermal Processing of Lignocellulosic Biomass: An Overview of Subcritical and Supercritical Water Hydrolysis. BioEnergy Res. 2022, 16, 1296–1317. [Google Scholar] [CrossRef]
- Rodríguez-Seoane, P.; del Pozo, C.; Puy, N.; Bartrolí, J.; Domínguez, H. Hydrothermal Extraction of Valuable Components from Leaves and Petioles from Paulownia elongata x fortunei. Waste Biomass Valorization 2020, 12, 4525–4535. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Herrero, M.; Simo, C.; Ibanez, E.; Cifuentes, A. Capillary electrophoresis-mass spectrometry of Spirulina platensis proteins obtained by pressurized liquid extraction. Electrophoresis 2005, 26, 4215–4224. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Oyoun, F.; Toncheva, A.; Henriquez, L.C.; Grougnet, R.; Laoutid, F.; Mignet, N.; Alhareth, K.; Corvis, Y. Deep Eutectic Solvents: An Eco-friendly Design for Drug Engineering. ChemSusChem 2023, 16, e202300669. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Wang, S.; Miao, S.; Sun, D.-W. Modifying structural and techno-functional properties of quinoa proteins through extraction techniques and modification methods. Trends Food Sci. Technol. 2024, 143, 104285. [Google Scholar] [CrossRef]
- Hadinoto, K.; Ling, J.K.; Pu, S.; Tran, T.T. Effects of Alkaline Extraction pH on Amino Acid Compositions, Protein Secondary Structures, Thermal Stability, and Functionalities of Brewer’s Spent Grain Proteins. Int. J. Mol. Sci. 2024, 25, 6369. [Google Scholar] [CrossRef]
- Meng, G.T.; Ma, C.Y. Fourier-transform infrared spectroscopic study of globulin from Phaseolus angularis (red bean). Int. J. Biol. Macromol. 2001, 29, 287–294. [Google Scholar] [CrossRef]
- Hou, F.; Ding, W.; Qu, W.; Oladejo, A.O.; Xiong, F.; Zhang, W.; He, R.; Ma, H. Alkali solution extraction of rice residue protein isolates: Influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef]
- Makinen, O.E.; Zannini, E.; Arendt, E.K. Modifying the Cold Gelation Properties of Quinoa Protein Isolate: Influence of Heat-Denaturation pH in the Alkaline Range. Plant Foods Hum. Nutr. 2015, 70, 250–256. [Google Scholar] [CrossRef]
- Hellebois, T.; Gaiani, C.; Planchon, S.; Renaut, J.; Soukoulis, C. Impact of heat treatment on the acid induced gelation of brewers’ spent grain protein isolate. Food Hydrocoll. 2021, 113, 106531. [Google Scholar] [CrossRef]
- Sathe, S.K.; Zaffran, V.D.; Gupta, S.; Li, T. Protein Solubilization. J. Am. Oil Chem. Soc. 2018, 95, 883–901. [Google Scholar] [CrossRef]
- Zhang, Y.; Sharan, S.; Rinnan, A.; Orlien, V. Survey on Methods for Investigating Protein Functionality and Related Molecular Characteristics. Foods 2021, 10, 2848. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.A.; Xiao, W.; van Boekel, M.; Minor, M.; Stieger, M. Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd). Food Chem. 2016, 209, 203–210. [Google Scholar] [CrossRef]
- Sathe, S.K.; Deshpande, S.S.; Salunkhe, D.K. Functional Properties of Winged Bean [Psophocarpus tetragonolobus (L.) DC] Proteins. J. Food Sci. 2006, 47, 503–509. [Google Scholar] [CrossRef]
- Wasswa, J.; Tang, J.; Gu, X.; Yuan, X. Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysate from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2007, 104, 1698–1704. [Google Scholar] [CrossRef]
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Park, M.; Beevers, J. The effect of ultrasound upon the physicochemical and emulsifying properties of wheat and soy protein isolates. J. Cereal Sci. 2016, 69, 77–84. [Google Scholar] [CrossRef]
- Ravindran, A.; Ramaswamy, H.S. Effect of sonication—Cooking on the immunoreactivity of soy slurry from germinated soybeans. Food Innov. Adv. 2023, 2, 60–68. [Google Scholar] [CrossRef]
- Moll, P.; Grossmann, L.; Kutzli, I.; Weiss, J. Influence of energy density and viscosity on foam stability—A study with pea protein (Pisum sativum L.). J. Dispers. Sci. Technol. 2019, 41, 1789–1796. [Google Scholar] [CrossRef]
- Das, D.; Mir, N.A.; Chandla, N.K.; Singh, S. Combined effect of pH treatment and the extraction pH on the physicochemical, functional and rheological characteristics of amaranth (Amaranthus hypochondriacus) seed protein isolates. Food Chem. 2021, 353, 129466. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, F. Plant Protein Heat-Induced Gels: Formation Mechanisms and Regulatory Strategies. Coatings 2023, 13, 1899. [Google Scholar] [CrossRef]
- Carvajal-Millan, E.; Rascón-Chu, A.; Márquez-Escalante, J.A.; Micard, V.; de León, N.P.; Gardea, A. Maize bran gum: Extraction, characterization and functional properties. Carbohydr. Polym. 2007, 69, 280–285. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT—Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]


| Component (% Dry Basis) | [9] | [10] | [11] | [12] | [13] | [14] | [15] |
|---|---|---|---|---|---|---|---|
| Protein | 26.7 | 23.10 | 22.8 | 22.7 | 22.5 | 22.44 | 17.87 |
| Total fiber | n.d. | 34.0 | n.d. | 64.3 | 45.8 | n.d. | n.d. |
| Cellulose | n.d. | n.d. | 17.1 | n.d. | n.d. | 20.56 | 12.31 |
| Hemicellulose | 22.5 | n.d. | 13.1 | n.d. | n.d. | 25.97 | 26.28 |
| Lignin | n.d. | 23.39 | 19.4 | n.d. | n.d. | 19.57 | 3.48 |
| Lipids | n.d. | 13.51 | 11.0 | 9.0 | 7.2 | 5.30 | 6.72 |
| Ash | 3.3 | 3.29 | 4.7 | 3.9 | 4.4 | 3.54 | 2.33 |
| Starch | 1.0 | 1.48 | n.d. | n.d. | n.d. | 2.23 | n.d. |
| Phenolics | n.d. | 1.70 | n.d. | 0.09 | n.d. | n.d. | n.d. |
| Type of Technique | Extraction Technique | Pretreatment | Extraction Parameters | Extraction Yield (%) | Protein Purity (%) | References |
|---|---|---|---|---|---|---|
| Conventional Techniques | Hydrothermal extraction | - | Mixed with water (1:10 w/w), stirred at 40 °C, 2 h, centrifuged, supernatant collected | 6.8 | 40.7 | [28] |
| Alkaline extraction | Dried, ground at 22,000 rpm | Mixed with 110 mM NaOH (1:20 m/v), stirred, 50 °C/20 °C 1 h; Ph→3.8, centrifuged to collect precipitated protein | Pale BSG: 59.0 (50 °C) 28.55 (20 °C) Black BSG: 15.26 (50 °C) 11.0420 °C) | Pale BSG: 46 (50 °C) 45.74 (20 °C) Black BSG: 17.22 (50 °C) 19.42 (20 °C) | [10] | |
| Autoclaved (121 °C, 15 min), dried, ground (125–250 μm) | Mixed with 0.01 M NaOH (1:15 w/v, pH 12.4), extracted (60 °C, 30 min), centrifuged, repeated 3×, freeze-dried | 45 | 27 | [29] | ||
| Autoclaved. Dried, ground, defatted with methanol-chloroform (1:2 v/v, 15:1 w/v), stirred (1 h), filtered, dried (60 °C) | 38 | Approximately 27 | ||||
| Autoclaved. Dried, ground, delignin with 60% ethanol (1:4 w/v), heated (180 °C under reflux, 90 min), filtered, washed, and dried | Approximately 45 | 32 | ||||
| Dried at 60 °C for 6.5 h to ~5.9% moisture | 3 g BSG placed in a 33 mL extraction tank, 0.05 M NaOH. 40 °C, 60 min 103.4 bar | Approximately 19 | 69.7 | [25] | ||
| 3 g BSG placed in a 33 mL extraction tank, 0.1 M NaOH. 40 °C, 60 min 103.4 bar | Approximately 22 | Approximately 65 | ||||
| Dried,0.5% H2SO4,100 °C 20 min; pH adjusted to 3, left overnight; centrifuged to separate supernatant; dried | 3 g BSG placed in a 33 mL extraction tank, using 0.1 M NaOH as the extraction solvent. Extracted at 40 °C, 60 °C, and 80 °C for 60 min under a pressure of 103.4 bar | 40 °C: 37.6 60 °C: 57.5 80 °C: 65.3 | 40 °C: 60 60 °C: 50 80 °C: 32 | |||
| Dried Mixed with water (1:20 w/v), ultrasound (frequency 37 kHz, power 100%,20min,30 °C) | 40 °C: 24.1 60 °C: 40.9 80 °C: 53.8 | 40 °C: 66 60 °C: 46 80 °C: 25.2 | ||||
| - | Adjusted pH to 12, reacted for 60 min, centrifuged to collect the supernatant, and freeze-dried to obtain protein | 66.41 | - | [30] | ||
| Stirred for 1 min; Mixed with distilled water (1:4, w/v), heated to 50 °C, pH 4.5, added cellulase (1:50), incubated for 60 min, inactivated at 85 °C for 20 min, centrifuged, and collected the precipitate | 50.18 | - | ||||
| Dried and milled to a particle size of 10–200 μm | pH11, 60 °C, SSR (solid/solvent ratio) 1:17, 3 h | 87 | 44.37 | [15] | ||
| - | Mixed with 0.1 M NaOH, pH > 11, SSR 1:10 (w/w), 40 °C, 2 h, centrifuged, and collected the supernatant | 21.4 | 60.2 | [28] | ||
| Protein from the liquid phase | ||||||
| Ethanol extraction | - | Extracted with ethanol-sodium sulfite (pH 9) at 60 °C for 2 h. Centrifuged, precipitated protein with HCl, removed ethanol by rotary evaporation, centrifuged, washed, and freeze-dried | - | 60.7 | [31] | |
| BSG fermented with Rhizopus oligosporus (7 log10 cfu/g, 10:1 w/v) at 37 °C for 72 h. Dried and milled | 66.2 | |||||
| Enzymatic extraction | Air-dried, milled, and sieved to 300 μm, treated with Depol 740 L (xylanase activity) at 50 °C for 5 h. Centrifuged, collected the precipitate | Mixed the precipitate with pH 9.5 sodium carbonate. Incubated at 50 °C for 4 h, centrifuged, and extracted protein from the liquid phase | 53 | - | [11] | |
| Mixed the precipitate with water (1:10, m/v). Incubated with Alcalase 2.4 L, Promod 144 GL, and Acid Protease A at pH 9.5, 6.5, and 3.5 (40 °C, 4 h), centrifuged, and extracted | Alcalase 2.4 L: 86 Promod 144 GL: 31 Acid Protease A: 40 | - | ||||
| Mixed with water (1:10, m/v), sheared at 24,000 rpm for 10 min, incubated with β-glucosidase (75 μg/BSG) at 50 °C, pH 5.0, for 4 h, inactivated at 80 °C for 20 min, and centrifuged (2700× g, 10 °C, 10 min) | The solid was treated with Alcalase 2.4 L (1:50) and Flavourzyme 500 L (1:100) at 50 °C for 4 h, inactivated at 80 °C for 20 min, and centrifuged. Remaining solid was mixed with water (7:100, m/v), stirred at 50 °C for 30 min, centrifuged, and freeze-dried | 63 | 44 | [32] | ||
| Dissolved at 4 °C and milled | Mixed with deionized water to prepare a 5% (w/w) slurry. Added Alcalase protease (20 μg/g dry BSG), incubated at 60 °C for 4 h. Then vibrated for 15 min. Collected the filtrate, dried at 60 °C for 24 h, and stored at −20 °C | 43.7% | 42.8% | [33] | ||
| Novel Techniques | Ultrasound-assisted extraction | Mixed with 110 mM NaOH (1:20, w/v), ultrasound parameters: 70% amplitude, 15 min × 2.60 °C. Centrifuged to collect the supernatant, adjusted pH to 3.8, centrifuged to collect the precipitate, resuspended the particles, and freeze-dried | 43% | - | [34] | |
| Dried and milled | Extracted at room temperature for 81.4 min with ultrasonic power of 88.2 W/100 mL, using 2.0 g BSG/100 mL pH 10 sodium carbonate solution | 96.4 mg/g (dry BSG) Yield Approximately 45 | - | [35] | ||
| Dried at 50 °C, milled, and filtered through a 335 μm sieve | Mixed with 110 mM NaOH (1:15, w/v). Ultrasound-treated (20–25 kHz, 250 W, 25 °C, 20 min, 60% duty). Centrifuged, adjusted to pH 3.8, centrifuged, dissolved in 2 M NaOH (pH 7), dialyzed (1000 Da, 4 °C, overnight), and freeze-dried | 86.16% | 57.84% | [26] | ||
| - | Mixed BSG with pH 10 sodium carbonate buffer, ultrasound-treated for 1 h, filtered, and centrifuged (10,000× g, 4 °C). Concentrated using 5 and 30 kDa membranes (25 psi, 25 °C), then freeze-dried | 30 kDa: 10.01% 5 kDa: 14.09% | 30 kDa: 15.98% 5 kDa: 20.09% | [36] | ||
| Microwave-assisted extraction | BSG dried at 60 °C to <3% moisture and milled to <1 mm particle size | Mixed with 0.5 M NaOH solution (1:10, w/v), microwaved to 110 °C, and extracted for 10 min. Centrifuged to separate and collect the supernatant | 93.99% | - | [37] | |
| BSG fermented with Rhizopus oligosporus (7 log10 cfu/g, 10:1 w/v) at 37 °C for 72 h. Dried and milled | MATPP process: Mixed BSG with water, stirred, and microwaved in a covered beaker. Filtered through fine cloth to obtain crude extract. Added saturated ammonium sulfate and t-butanol to the extract, vortexed for 3 min, left at room temperature for 30 min, and centrifuged (1000× g, 15 min). Separated the organic upper layer and aqueous middle phase, then freeze-dried | 82.2% | - | [17] | ||
| Subcritical Water Extraction | Washed, dried at 45 °C for 3 h | 12 g BSG in a fixed-bed reactor (20.6 cm length, 2.8 cm diameter) at 5 MPa; water flow rate 4 mL/min; extracted at 185 °C for 150 min | 78% | - | [21] | |
| 0.5 mm Washed, dried at 45 °C to 8% moisture; ground to <0.5 mm particle size | Laboratory scale: 0.5 L reactor, BSG-water ratio 1:20 (w/v), 170 °C, stirred at 500 rpm, 5 MPa for 22 min. Pilot scale: 20 L reactor, BSG-water ratio 1:20 (w/v), 170 °C, 2 MPa for 22 min | Laboratory scale: 63% Pilot scale: 64% | Laboratory scale: 6.5 g/L Pilot scale: Not reported | [38] | ||
| Pressurized Solvent Extraction | 1.5 g BSG,9 g sand→10 mL extraction cell, preheat for 6 min at 1500 psi, 4.7% ethanol, 155 °C, 10 min, 5 cycles | 69% | - | [34] | ||
| Deep eutectic solvent Extraction | Defatted with supercritical CO2 | Extracted using a solvent of 90 wt.% sodium acetate (NaAcO):urea (molar ratio 1:2) with 10 wt.% water. Solid-to-solvent ratio was 1:9 (w/w). Stirred at 80 °C for 2 h, filtered, washed, dialyzed using a 3.5 kDa membrane, then concentrated and dried | 79% | 52–54.7% | [39] |
| Techno-Functional Properties | Extraction | Treatment | Key Findings | Reference |
|---|---|---|---|---|
| Solubility | Alkaline | Extraction at varying pH values and temperatures | higher extraction pH → surface hydrophobicity ↑, →intermolecular hydrophobic interactions → solubility ↓ increasing temperature → promoting molecular motion and interactions with the solvent → solubility ↓ | [15] |
| Enzymatic, fungal fermentation | Enzymatic hydrolysis, Rhizopus oligosporus ATCC 64,063 fermentation | Peptides with better solubility, proteins with more charged amino acids → solubility ↑ | [13,31,58] | |
| Microwave-assisted | Microwave-assisted extraction with three-phase partitioning for fungal-fermented BSG | Microwave treatment did not change the molecular weight, and solubility slightly ↑ | [17] | |
| WHC, OHC | Alkaline | Extraction at varying pH values and temperatures | pH from 8 to 12 → WHC ↑ (3.2 g/g to 5 g/g), due to compositional changes in the protein extracts. Temperatures ranging from 40 °C to 80 °C did not significantly affect the WHC, no significant effect on OHC | [15] |
| Alkaline and alcohol extraction | Extraction via alkaline and alcohol | Alkali-extracted BSGP → exposed polar amino acid side chains contain high-molecular-weight glutenins, which form a network capable of retaining more water → higher WHC no significant effect on OHC | [12] | |
| fungal fermentation | Rhizopus oligosporus ATCC 64,063 fermentation | solid-state fermentation → increased number of polar groups → better WHC, OHC | [31] | |
| Ultrasound-assisted | 110 mM NaOH as solvent, ultrasound treatment | Ultrasound treatment → exposure of hydrophobic groups buried within the protein molecules, α-helix content↓, protein unfolding, allows for better adsorption at water-oil interfaces → better OHC(3.1 g/g) | [26] | |
| Microwave-assisted | Microwave-assisted extraction with three-phase partitioning for fungal-fermented BSG | Microwave treatment enhances both WHC (4.4–4.9 g/g) and OHC (7.3–8.3 g/g) | [17] | |
| Emulsifying Properties | Alkaline | Extraction at varying pH values and temperatures | Mild conditions(pH 8 and 60 °C) resulted in the best EAI (81.97 m2/g) and ESI (approximately 90 m2/g) | [15] |
| Alkaline and alcohol extraction | Extraction via alkaline and alcohol | Alkali-extracted BSGP → the exposure of polar amino acids and the presence of glutenin proteins that formed a stable protein network → better emulsifying properties | [12] | |
| Subcritical water, alkaline, and alcohol extraction | Extraction via subcritical water, alkaline, and alcohol | subcritical water extraction → lower oil-water interfacial tension → stronger adsorption at the oil-water interface | [28] | |
| Ultrasound-assisted | 110 mM NaOH as solvent, ultrasound treatment | Ultrasound treatment → the reduction of protein size and the exposure of hydrophobic groups → protein adsorption at the oil-water interface ↑ → improved emulsifying properties | [26] | |
| Microwave-assisted | Microwave-assisted extraction with three-phase partitioning for fungal-fermented BSG | Microwave treatment enhances both EAI and ESI | [17] | |
| fungal fermentation | Rhizopus oligosporus ATCC 64,063 fermentation | solid-state fermentation → peptides with better hydrophobic interactions → better emulsifying properties | [31] | |
| Enzymatic treatment | Enzymatic hydrolysis(Alcalase and Pepsin/Flavourzyme) | Alcalase and Pepsin → EAI ↓ as the DH increased; Flavourzyme-hydrolyzed proteins→maintained good emulsifying properties regardless of the DH, likely due to the enzyme’s ability to generate larger peptides with high surface hydrophobicity that adsorb well at the oil-water interface | [9] | |
| Foaming Properties | Alkaline and alcohol extraction | Extraction via alkaline and alcohol | Alkali-extracted BSGP → the exposure of hydrophobic amino acid side chains and the presence of larger glutenin proteins → stronger and more stable foams | [12] |
| Enzymatic treatment | Enzymatic hydrolysis(Alcalase and Pepsin/Flavourzyme) | Enzymatic hydrolysis → broke proteins into smaller peptides, which have a smaller size and increased hydrophobicity → better foaming properties Flavourzyme-treated proteins showed better foam stability compared to those hydrolyzed by Alcalase or Pepsin | [9] | |
| Ultrasound-assisted | 110 mM NaOH as solvent, ultrasound treatment | Ultrasound treatment → better protein adsorption at the gas–liquid interface → improved foaming properties | [26] | |
| Microwave-assisted | Microwave-assisted extraction with three-phase partitioning for fungal-fermented BSG | Microwave treatment enhances both FC and FS by inducing partial protein unfolding and increasing protein flexibility | [17] | |
| Gelation Properties | Alkaline | Extraction at varying pH values and temperatures | Higher pH and temperature → partial protein denaturation and exposure of hydrophobic regions → promoted intermolecular interactions and the formation of a stronger elastic network → enhanced gelation | [15] |
| - | Heat treatment | Heat treatment can facilitate gel formation by promoting the aggregation of proteins into structured networks | [77] | |
| Alkaline and alcohol extraction | Extraction via alkaline and alcohol | Alkaline extraction → hemicellulose being co-extracted → ability to retain water → better gelation properties | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, H.; Zhang, P.; Zhang, L.; Zhou, W.; Lin, Z.; Yu, T.; Liu, G.; Liu, D. Extraction Techniques for Brewer’s Spent Grain Protein: A Comparative Review of Efficiency, Purity, and Functionality. Foods 2025, 14, 4058. https://doi.org/10.3390/foods14234058
Tong H, Zhang P, Zhang L, Zhou W, Lin Z, Yu T, Liu G, Liu D. Extraction Techniques for Brewer’s Spent Grain Protein: A Comparative Review of Efficiency, Purity, and Functionality. Foods. 2025; 14(23):4058. https://doi.org/10.3390/foods14234058
Chicago/Turabian StyleTong, Haocheng, Puxuan Zhang, Liang Zhang, Wei Zhou, Zhengte Lin, Tengfei Yu, Guanchen Liu, and Donghong Liu. 2025. "Extraction Techniques for Brewer’s Spent Grain Protein: A Comparative Review of Efficiency, Purity, and Functionality" Foods 14, no. 23: 4058. https://doi.org/10.3390/foods14234058
APA StyleTong, H., Zhang, P., Zhang, L., Zhou, W., Lin, Z., Yu, T., Liu, G., & Liu, D. (2025). Extraction Techniques for Brewer’s Spent Grain Protein: A Comparative Review of Efficiency, Purity, and Functionality. Foods, 14(23), 4058. https://doi.org/10.3390/foods14234058







