Revisiting the Conventional Extraction of Protein Isolates from Faba Beans: Recovering Lost Protein from Sustainable Side Streams
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
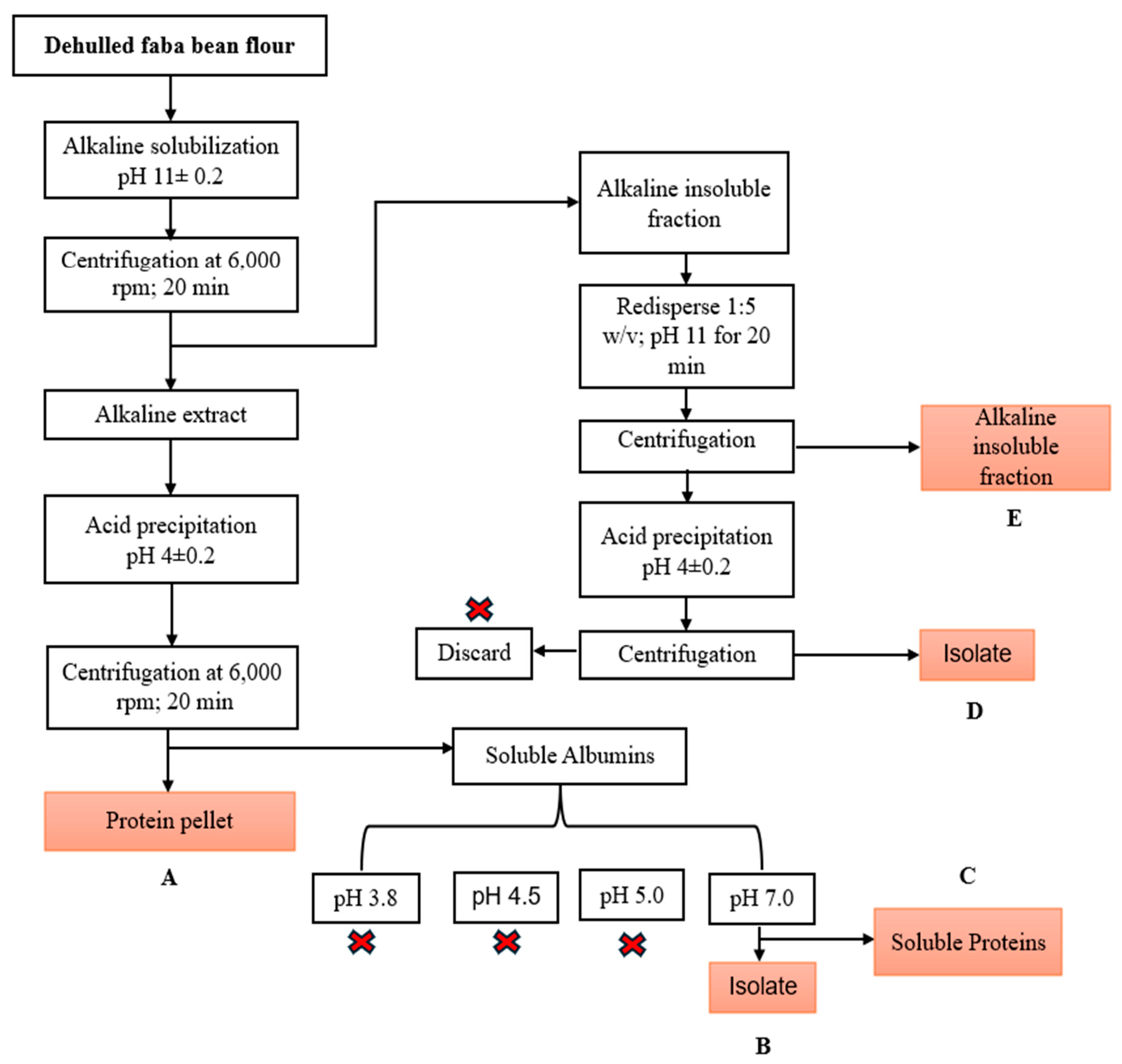
2.2. Alkaline–Isoelectric Precipitation of Faba Bean Protein Isolates
2.3. Protein Content and Extraction Yield
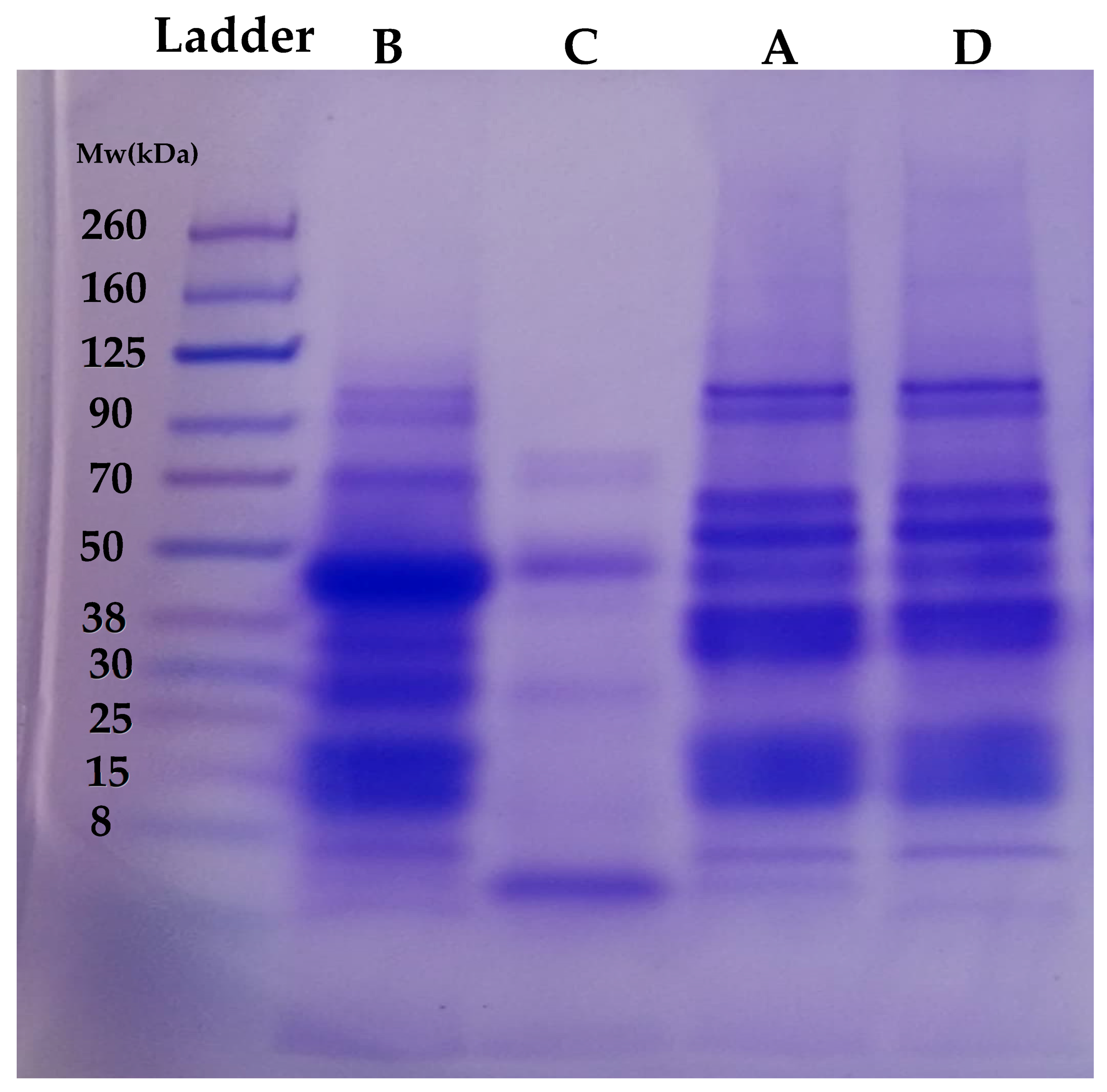
2.4. Qualitative Analysis of Proteins Using Electrophoresis (SDS-PAGE)
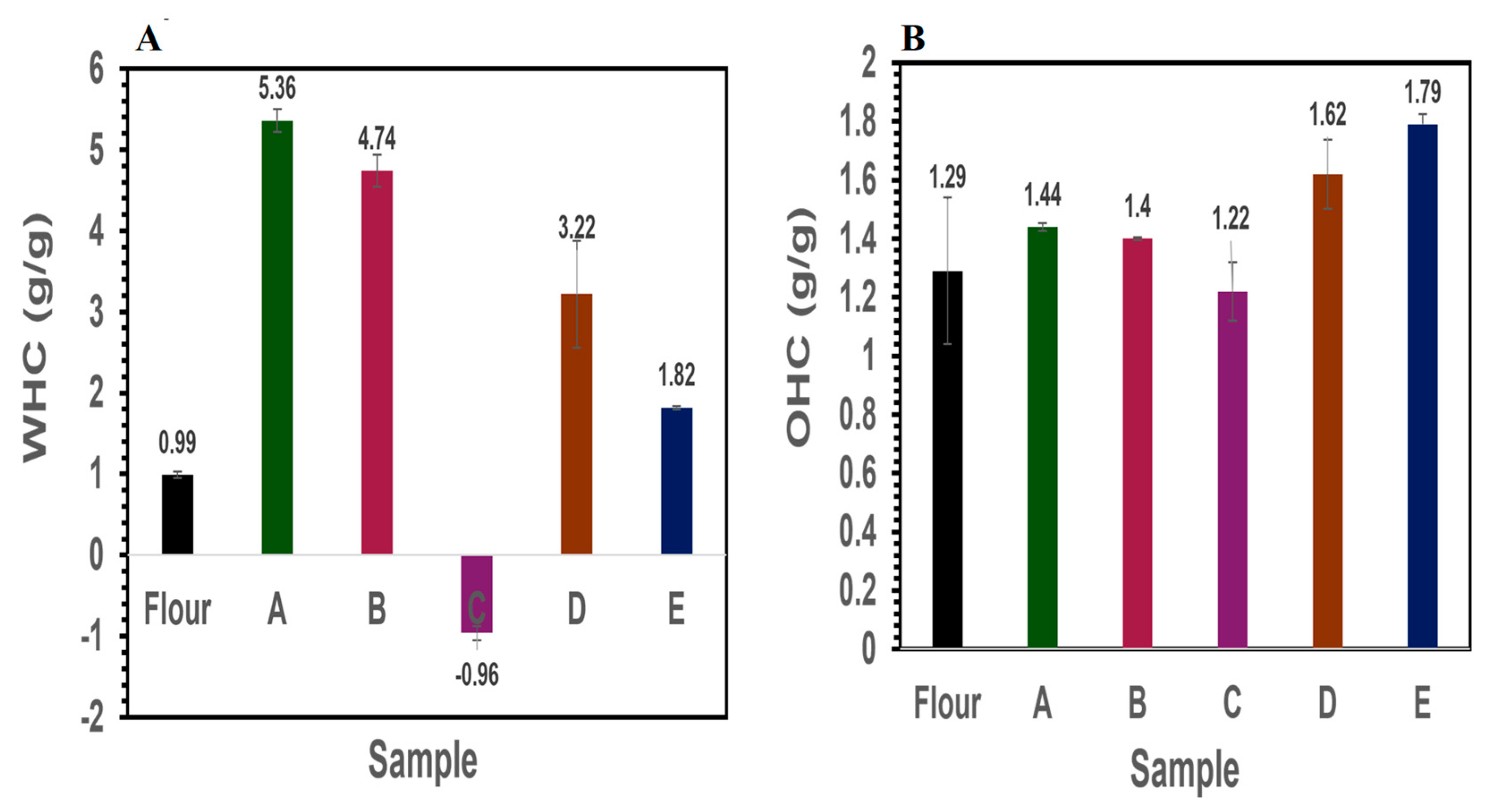
2.5. Protein Oil- and Water-Holding Capacity
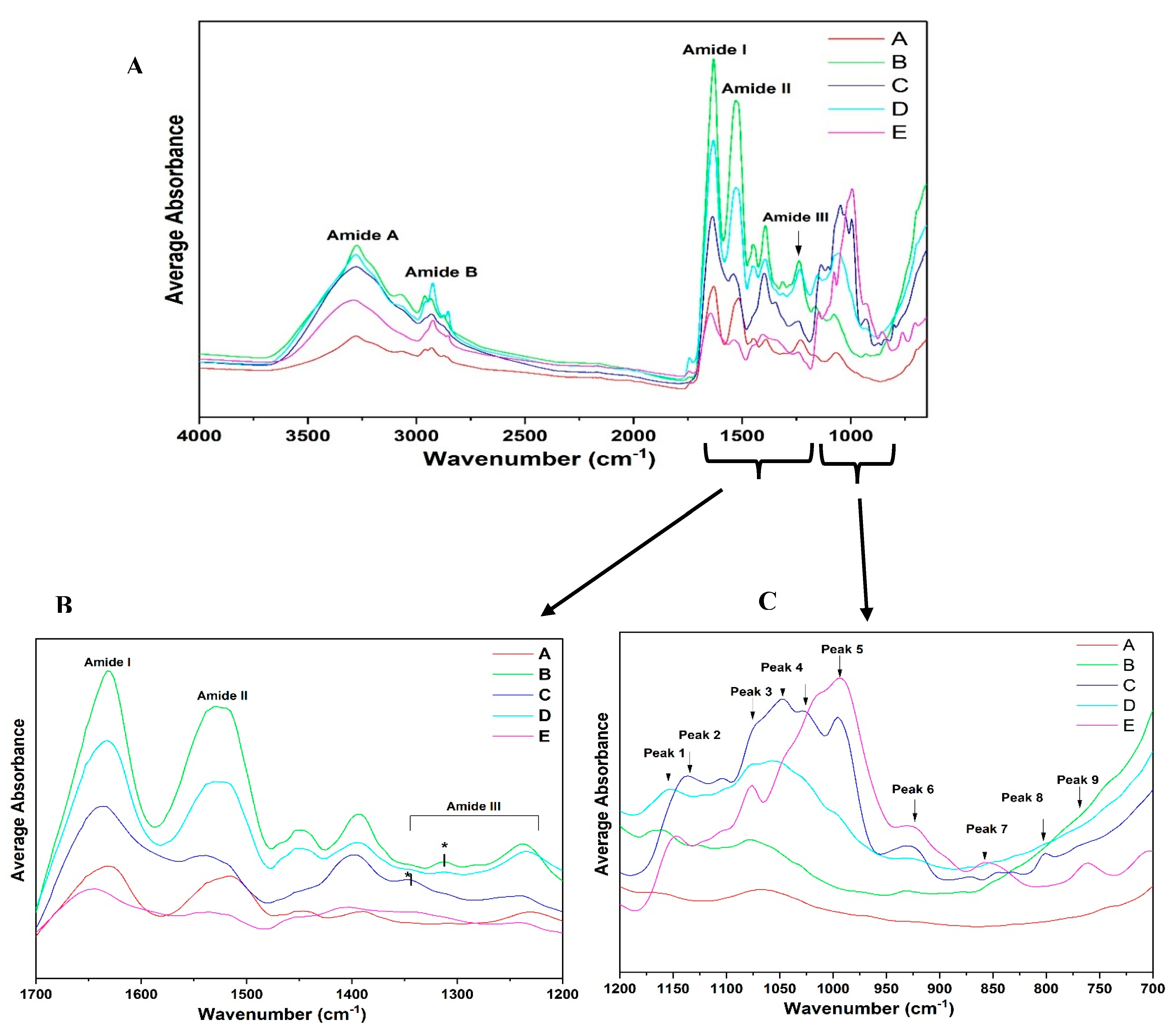
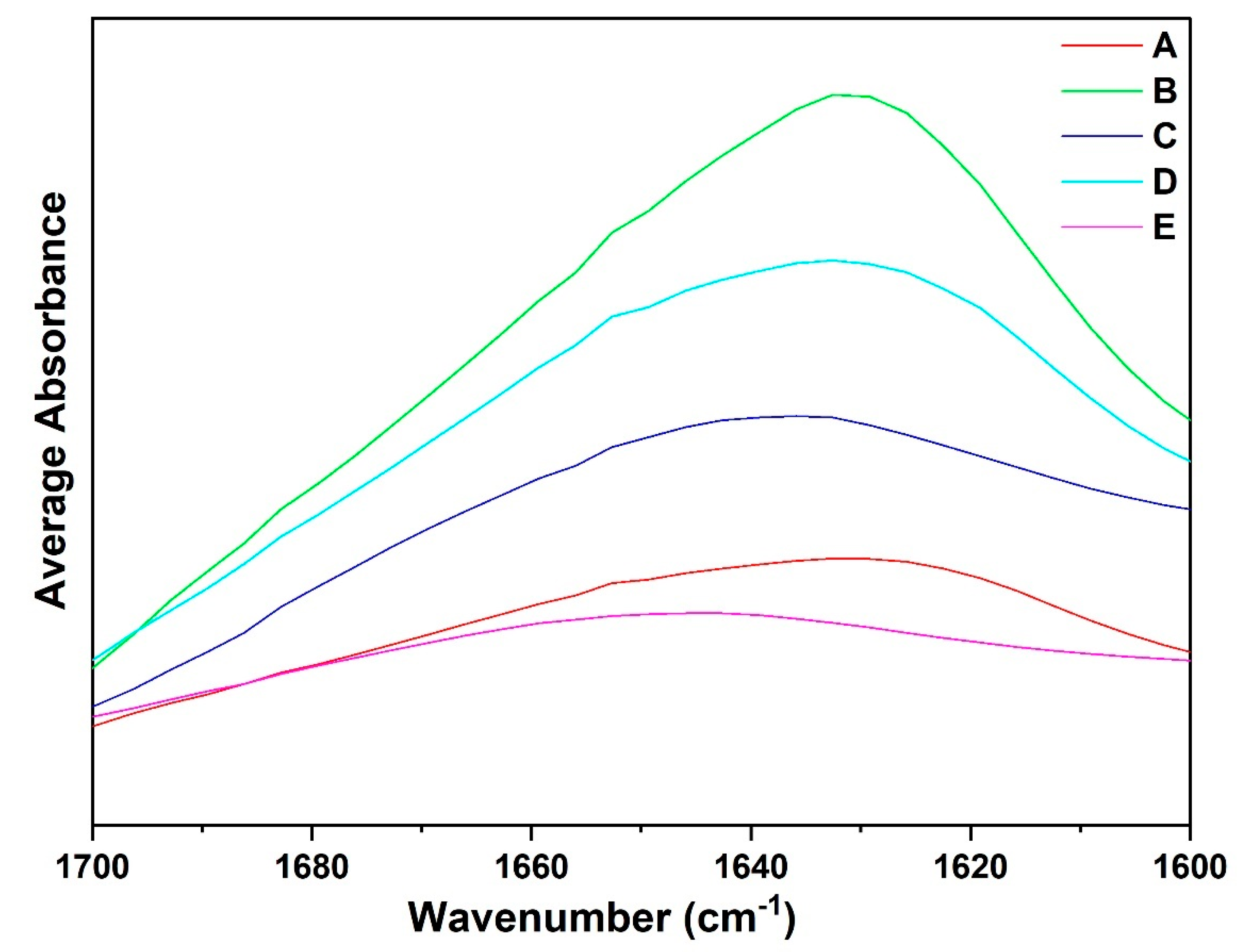
2.6. Fourier-Transform Infrared Spectroscopy Analysis (FTIR)
2.7. Statistical Analysis
3. Results and Discussion
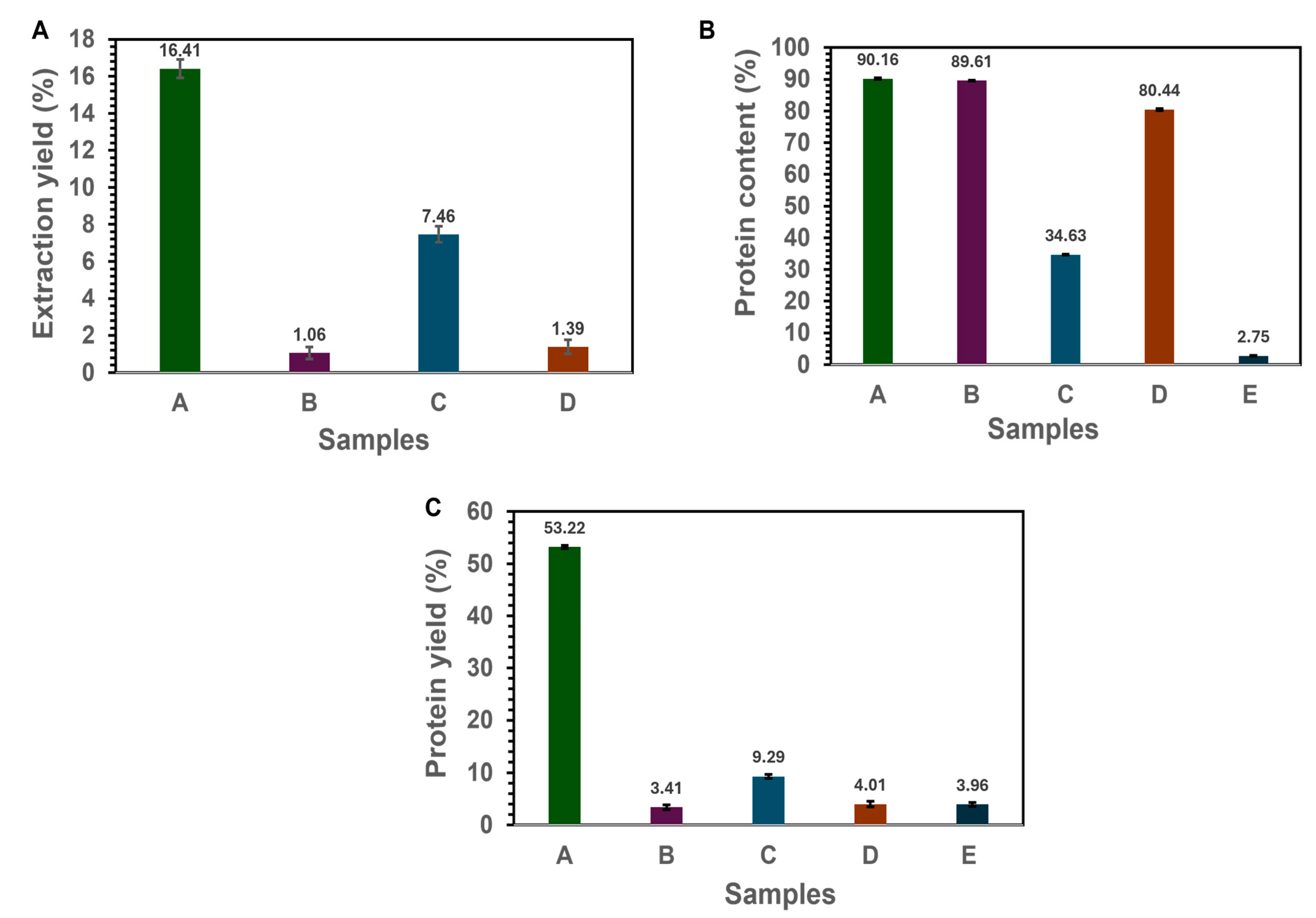
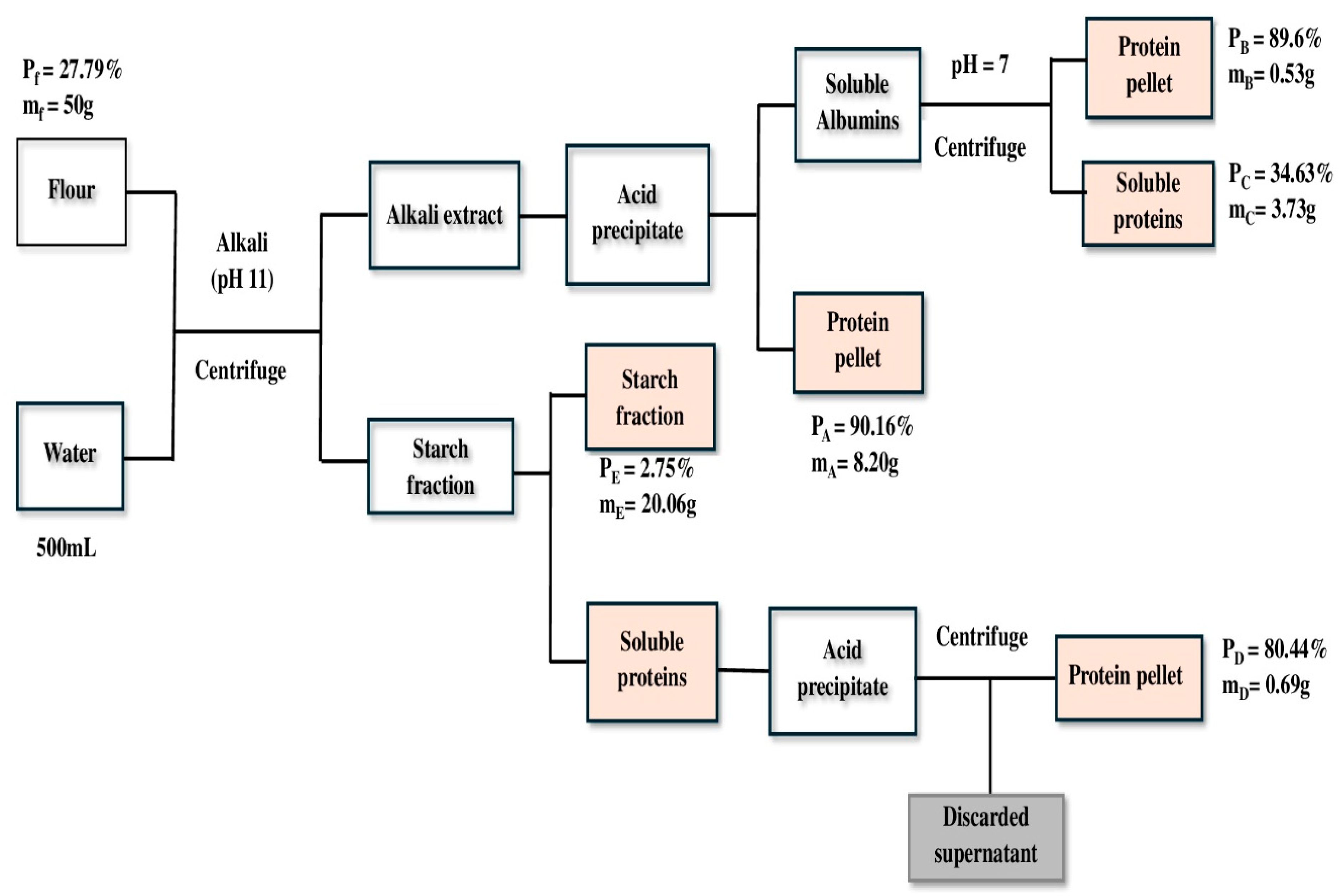
3.1. Extraction Yield
(50 × 0.2779) = (8.2023 × 0.9016) + (0.52825 × 0.896) + (3.72695 × 0.3463) +
(0.6932 × 0.8044) + (20.0626 × 0.0275) + Lp
13. 895 = 10.2681 + Lp
Lp = 3.63 g
3.2. Qualitative Analysis of Proteins Using Electrophoresis (SDS-PAGE)
3.3. Functional Properties
3.3.1. Water- and Oil-Holding Capacity
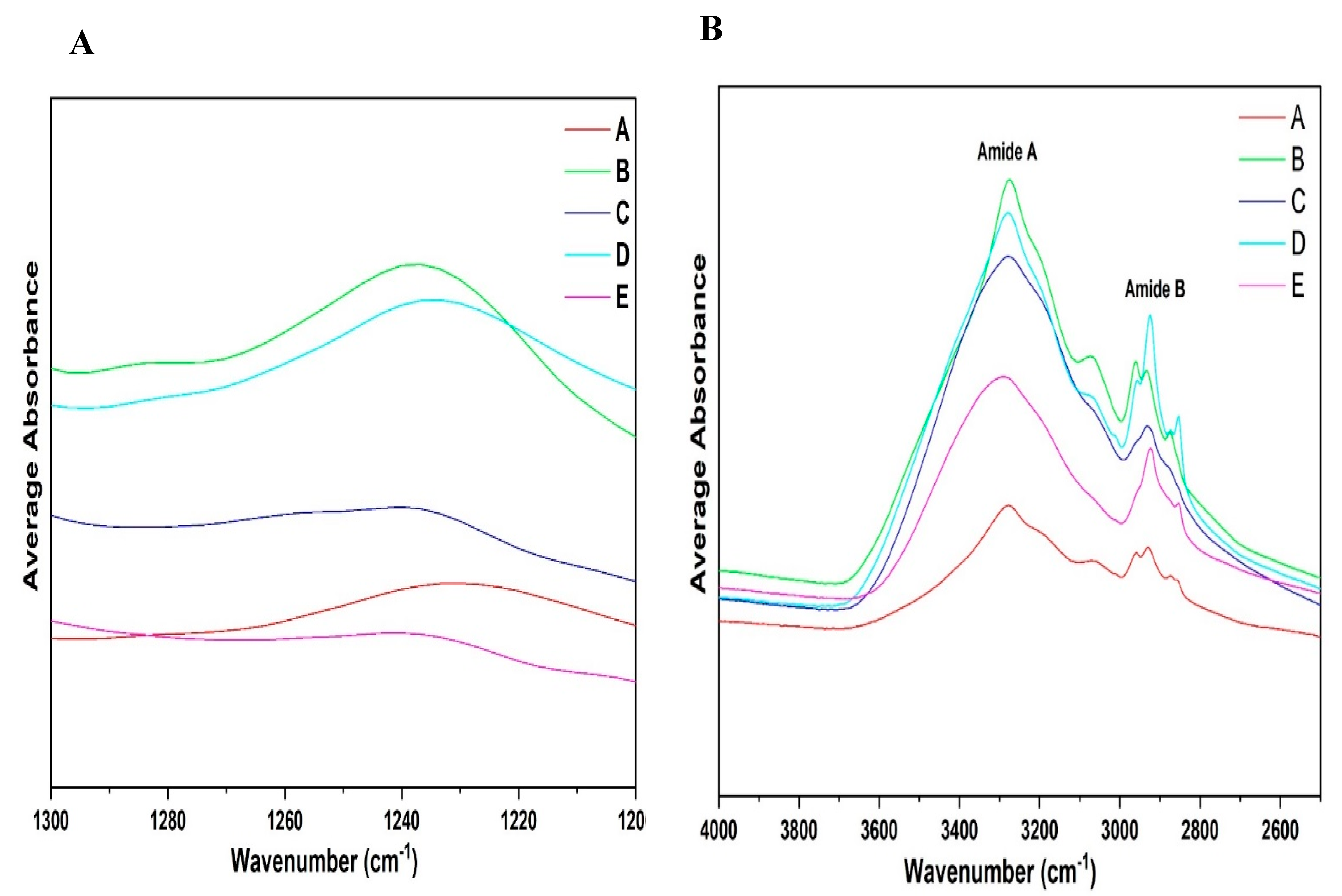
3.3.2. ATR-FTIR Spectroscopy
3.4. Qualitative Analysis of ATR-FTIR Spectra: Amide I Region
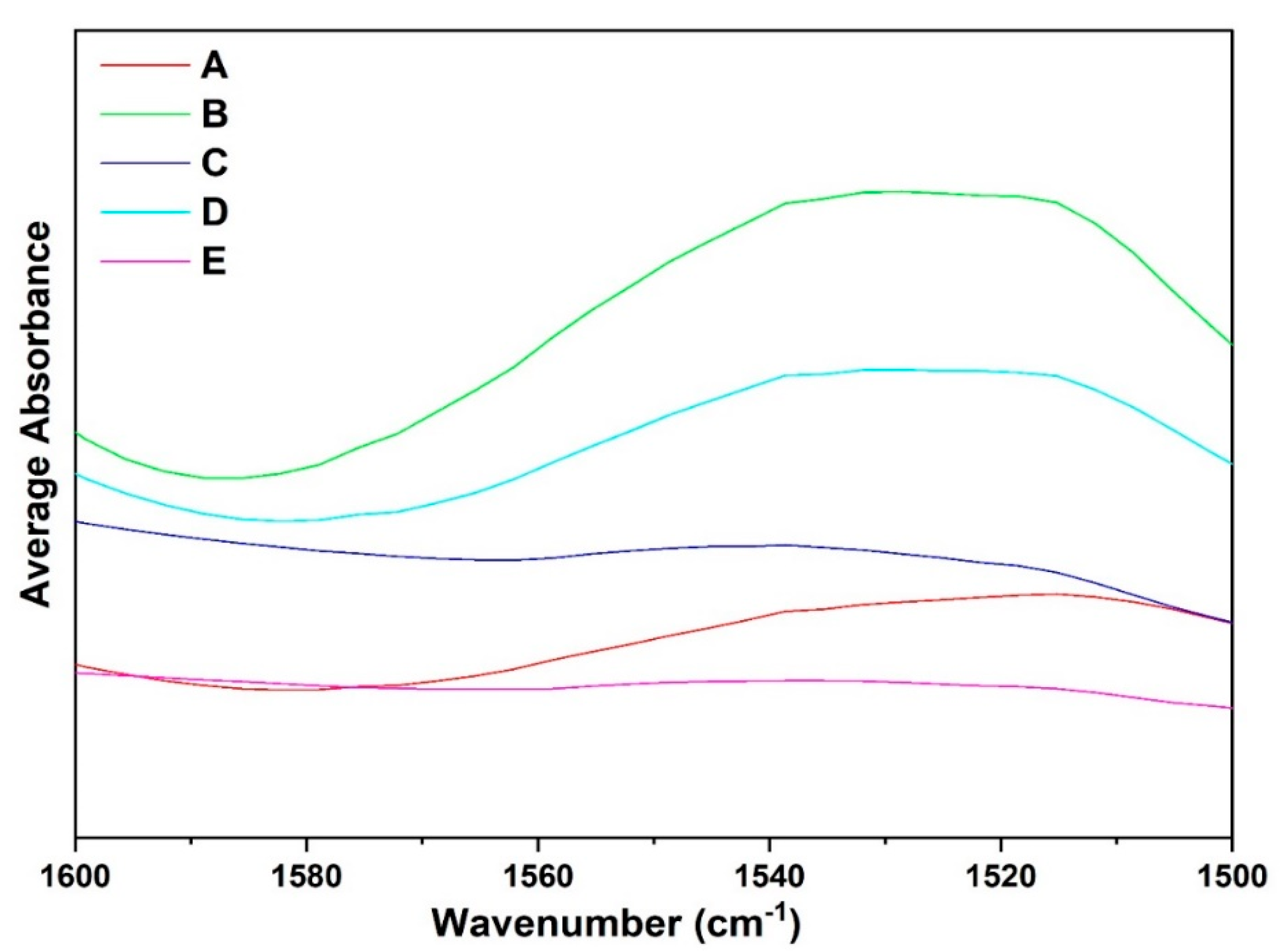
3.5. Qualitative Analysis of FTIR Spectra: Amide II Region
3.6. Qualitative Analysis of ATR-FTIR Spectra: Amide III and A–B Region
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wudil, A.H.; Usman, M.; Rosak-Szyrocka, J.; Pilař, L.; Boye, M. Reversing Years for Global Food Security: A Review of the Food Security Situation in Sub-Saharan Africa (SSA). Int. J. Environ. Res. Public Health 2022, 19, 14836. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, L.; Jha, H.; Sarkar, T.; Sarangi, P.K. Food Waste Utilization for Reducing Carbon Footprints towards Sustainable and Cleaner Environment: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2318. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, J.; Dugmore, T.I.J.; Matharu, A.; Martinez-Hernandez, E.; Aburto, J.; Rahman, P.K.S.M.; Lynch, J. Perspectives on “Game Changer” Global Challenges for Sustainable 21st Century: Plant-Based Diet, Unavoidable Food Waste Biorefining, and Circular Economy. Sustainability 2020, 12, 1976. [Google Scholar] [CrossRef]
- Muhie, S.H. Novel Approaches and Practices to Sustainable Agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Cheng, F.; Brewer, C.E. Conversion of Protein-Rich Lignocellulosic Wastes to Bio-Energy: Review and Recommendations for Hydrolysis + Fermentation and Anaerobic Digestion. Renew. Sustain. Energy Rev. 2021, 146, 111167. [Google Scholar] [CrossRef]
- Parlasca, M.C.; Qaim, M. Meat Consumption and Sustainability. Annu. Rev. Resour. Econ. 2022, 14, 17–41. [Google Scholar] [CrossRef]
- Chai, B.C.; van der Voort, J.R.; Grofelnik, K.; Eliasdottir, H.G.; Klöss, I.; Perez-Cueto, F.J.A. Which Diet Has the Least Environmental Impact on Our Planet? A Systematic Review of Vegan, Vegetarian and Omnivorous Diets. Sustainability 2019, 11, 4110. [Google Scholar] [CrossRef]
- Raak, N.; Corredig, M. Towards Creating Sustainable Foods from Side Streams: Heat-Induced Structure Formation in Blends of Sunflower Seed Press Cakes and Cheese Whey under Moderate Shear. Food Hydrocoll. 2023, 144, 108932. [Google Scholar] [CrossRef]
- Karefyllakis, D.; Octaviana, H.; van der Goot, A.J.; Nikiforidis, C.V. The Emulsifying Performance of Mildly Derived Mixtures from Sunflower Seeds. Food Hydrocoll. 2019, 88, 75–85. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Optimization of Ultrasound-Assisted Extraction of Faba Bean Protein Isolate: Structural, Functional, and Thermal Properties. Part 2/2. Ultrason. Sonochem. 2024, 110, 107030. [Google Scholar] [CrossRef]
- Badjona, A.; Millman, C.; Howarth, M.; Dubey, B.; Bradshaw, R. Response Surface Methodology Guided Approach for Optimization of Protein Isolate from Faba Bean. Part 1/2. Ultrason. Sonochem. 2024, 109, 107012. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.; Rao, J.; Chen, B. Faba Bean Protein: Chemical Composition, Functionality, Volatile Compounds, and Applications in Food Production. Trends Food Sci. Technol. 2025, 156, 104863. [Google Scholar] [CrossRef]
- Osborne, T.B. The Vegetable Proteins; Longmans, Green and Company: London, UK, 1924. [Google Scholar]
- Chandran, A.S.; Suri, S.; Choudhary, P. Sustainable Plant Protein: An up-to-Date Overview of Sources, Extraction Techniques and Utilization. Sustain. Food Technol. 2023, 1, 466–483. [Google Scholar] [CrossRef]
- Messina, V.; Skylas, D.J.; Roberts, T.H.; Valtchev, P.; Whiteway, C.; Li, Z.; Hopf, A.; Dehghani, F.; Quail, K.J.; Kaiser, B.N. Pulse Proteins: Processing, Nutrition, and Functionality in Foods. Foods 2025, 14, 1151. [Google Scholar] [CrossRef] [PubMed]
- Eze, C.R.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. Advances in Legume Protein Extraction Technologies: A Review. Innov. Food Sci. Emerg. Technol. 2022, 82, 103199. [Google Scholar] [CrossRef]
- Yang, J.; Kornet, R.; Diedericks, C.F.; Yang, Q.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Venema, P.; van der Linden, E.; Sagis, L.M.C. Rethinking Plant Protein Extraction: Albumin—From Side Stream to an Excellent Foaming Ingredient. Food Struct. 2022, 31, 100254. [Google Scholar] [CrossRef]
- Patil, N.D.; Bains, A.; Sridhar, K.; Bhaswant, M.; Kaur, S.; Tripathi, M.; Lanterbecq, D.; Chawla, P.; Sharma, M. Extraction, Modification, Biofunctionality, and Food Applications of Chickpea (Cicer Arietinum) Protein: An Up-to-Date Review. Foods 2024, 13, 1398. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Zhao, B.; Liu, W.; Gao, Y. Enhancing the Adsorption of the Proteins in the Soy Whey Wastewater Using Foam Separation Column Fitted with Internal Baffles. J. Food Eng. 2013, 119, 377–384. [Google Scholar] [CrossRef]
- Lie-Piang, A.; Braconi, N.; Boom, R.M.; van der Padt, A. Less Refined Ingredients Have Lower Environmental Impact—A Life Cycle Assessment of Protein-Rich Ingredients from Oil- and Starch-Bearing Crops. J. Clean. Prod. 2021, 292, 126046. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Produced Using Dry Fractionation and Isoelectric Precipitation: Techno-Functional, Nutritional and Environmental Performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba Beans Protein as an Unconventional Protein Source for the Food Industry: Processing Influence on Nutritional, Techno-Functionality, and Bioactivity. Food Rev. Int. 2024, 40, 1999–2023. [Google Scholar] [CrossRef]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Structural, Thermal, and Physicochemical Properties of Ultrasound-Assisted Extraction of Faba Bean Protein Isolate (FPI). J. Food Eng. 2024, 377, 112082. [Google Scholar] [CrossRef]
- Iylia Fatinee Mohd Yusree, F.; Paul Peter, A.; Zuhair Mohd Nor, M.; Loke Show, P.; Noriznan Mokhtar, M. Latest Advances in Protein-Recovery Technologies from Agricultural Waste. Foods 2021, 10, 2748. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Zaky, A.A.; Lorenzo, J.M.; Camiña, M.; Franco, D. A Review on Bioactive Peptides Derived from Meat and By-Products: Extraction Methods, Biological Activities, Applications and Limitations. Meat Sci. 2023, 204, 109278. [Google Scholar] [CrossRef]
- Sheikh, M.A.; Saini, C.S.; Sharma, H.K. Structural Modification of Plum (Prunus Domestica L) Kernel Protein Isolate by Supercritical Carbon-Dioxide Treatment: Functional Properties and in-Vitro Protein Digestibility. Int. J. Biol. Macromol. 2023, 230, 123128. [Google Scholar] [CrossRef]
- Shen, X.; Fang, T.; Gao, F.; Guo, M. Effects of Ultrasound Treatment on Physicochemical and Emulsifying Properties of Whey Proteins Pre- and Post-Thermal Aggregation. Food Hydrocoll. 2017, 63, 668–676. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Pulkkinen, M.; Wang, Y.J.; Lampi, A.M.; Stoddard, F.L.; Salovaara, H.; Piironen, V.; Sontag-Strohm, T. Faba Bean Flavour and Technological Property Improvement by Thermal Pre-Treatments. LWT 2016, 68, 295–305. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Yang, J.; Lorenzetti, R.L.; Bing, D.; Zhang, S.; Lu, J.; Chen, L. Composition, Functionalities, and Digestibility of Proteins from High Protein and Normal Pea (Pisum Sativum) Genotypes. Sustain. Food Proteins 2023, 1, 4–15. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Boom, R.M.; Schutyser, M.A.I. Functional Analysis of Mildly Refined Fractions from Yellow Pea. Food Hydrocoll. 2015, 44, 12–22. [Google Scholar] [CrossRef]
- Pelgrom, P.J.M.; Vissers, A.M.; Boom, R.M.; Schutyser, M.A.I. Dry Fractionation for Production of Functional Pea Protein Concentrates. Food Res. Int. 2013, 53, 232–239. [Google Scholar] [CrossRef]
- De Angelis, D.; Pasqualone, A.; Costantini, M.; Ricciardi, L.; Lotti, C.; Pavan, S.; Summo, C. Data on the Proximate Composition, Bioactive Compounds, Physicochemical and Functional Properties of a Collection of Faba Beans (Vicia faba L.) and Lentils (Lens culinaris Medik.). Data Brief 2021, 34, 106660. [Google Scholar] [CrossRef] [PubMed]
- Jeganathan, B.; Vasanthan, T.; Temelli, F. Isolation of Clean-Label Faba Bean (Vicia faba L.) Proteins: A Comparative Study of Mild Fractionation Methods against Traditional Technologies. Innov. Food Sci. Emerg. Technol. 2023, 84, 103285. [Google Scholar] [CrossRef]
- Soto-Madrid, D.; Pérez, N.; Gutiérrez-Cutiño, M.; Matiacevich, S.; Zúñiga, R.N. Structural and Physicochemical Characterization of Extracted Proteins Fractions from Chickpea (Cicer arietinum L.) as a Potential Food Ingredient to Replace Ovalbumin in Foams and Emulsions. Polymers 2023, 15, 110. [Google Scholar] [CrossRef]
- Keivaninahr, F.; Gadkari, P.; Benis, K.Z.; Tulbek, M.; Ghosh, S. Prediction of Emulsification Behaviour of Pea and Faba Bean Protein Concentrates and Isolates from Structure-Functionality Analysis. RSC Adv. 2021, 11, 12117–12135. [Google Scholar] [CrossRef]
- Higa, F.A.; Boyd, L.; Sopiwnyk, E.; Nickerson, M.T. Effect of Particle Size, Flour:Water Ratio and Type of Pulse on the Physicochemical and Functional Properties of Wet Protein Extraction. Cereal Chem. 2022, 99, 1049–1062. [Google Scholar] [CrossRef]
- Gao, Z.; Shen, P.; Lan, Y.; Cui, L.; Ohm, J.B.; Chen, B.; Rao, J. Effect of Alkaline Extraction PH on Structure Properties, Solubility, and Beany Flavor of Yellow Pea Protein Isolate. Food Res. Int. 2020, 131, 109045. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Shi, K.; Wang, S.; Chen, J.; Liu, Y.; Huang, Q. Composition, Secondary Structure, and Self-Assembly of Oat Protein Isolate. J. Agric. Food Chem. 2009, 57, 4552–4558. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea Protein Isolates: Structure, Extraction, and Functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, Physicochemical and Sensorial Properties of Commercial Plant-Based Yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- Cornet, S.H.V.; Snel, S.J.E.; Lesschen, J.; van der Goot, A.J.; van der Sman, R.G.M. Enhancing the Water Holding Capacity of Model Meat Analogues through Marinade Composition. J Food Eng 2021, 290, 110283. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Functionality and Digestibility of Albumins and Globulins from Lentil and Horse Gram and Their Effect on Starch Rheology. Food Hydrocoll. 2016, 61, 843–850. [Google Scholar] [CrossRef]
- Shen, P.; Ha, S.M.L.; Peng, J.; Landman, J.; Sagis, L.M.C. Role of Pulse Globulins and Albumins in Air-Water Interface and Foam Stabilization. Food Hydrocoll. 2025, 160, 110792. [Google Scholar] [CrossRef]
- Carbonaro, M.; Nucara, A. Secondary Structure of Food Proteins by Fourier Transform Spectroscopy in the Mid-Infrared Region. Amino Acids 2010, 38, 679–690. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C.G.; Ranquine, T.; Azarias, C.A.; Bell, M.J.V.; De Carvalho Dos Anjos, V. Long-Term Ripening Evaluation of Ewes’ Cheeses by Fourier-Transformed Infrared Spectroscopy under Real Industrial Conditions. J. Spectrosc. 2018, 2018, 1381864. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Pop, O.L.; Socaciu, C. Monitoring Lactic Acid Fermentation in Media Containing Dandelion (Taraxacum officinale) by FTIR Spectroscopy. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 65–68. [Google Scholar] [CrossRef]
- Milošević, J.; Prodanović, R.; Polović, N. On the Protein Fibrillation Pathway: Oligomer Intermediates Detection Using ATR-FTIR Spectroscopy. Molecules 2021, 26, 970. [Google Scholar] [CrossRef]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
- Badjona, A.; Cherono, B.; Bradshaw, R.; Dubey, B. Gelation and Rheological Properties of Ultrasound-Extracted Faba Bean Protein: A Comparative Study with Commercial Plant Proteins. Food Hydrocoll. 2025, 162, 110997. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R.; Singh, B.R. Identification Of-Turn and Random Coil Amide III Infrared Bands for Secondary Structure Estimation of Proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Zhu, Q.; Chen, F.; Zhao, X.; Ao, Q. Determination of the Domain Structure of the 7S and 11S Globulins from Soy Proteins by XRD and FTIR. J. Sci. Food Agric. 2013, 93, 1687–1691. [Google Scholar] [CrossRef]
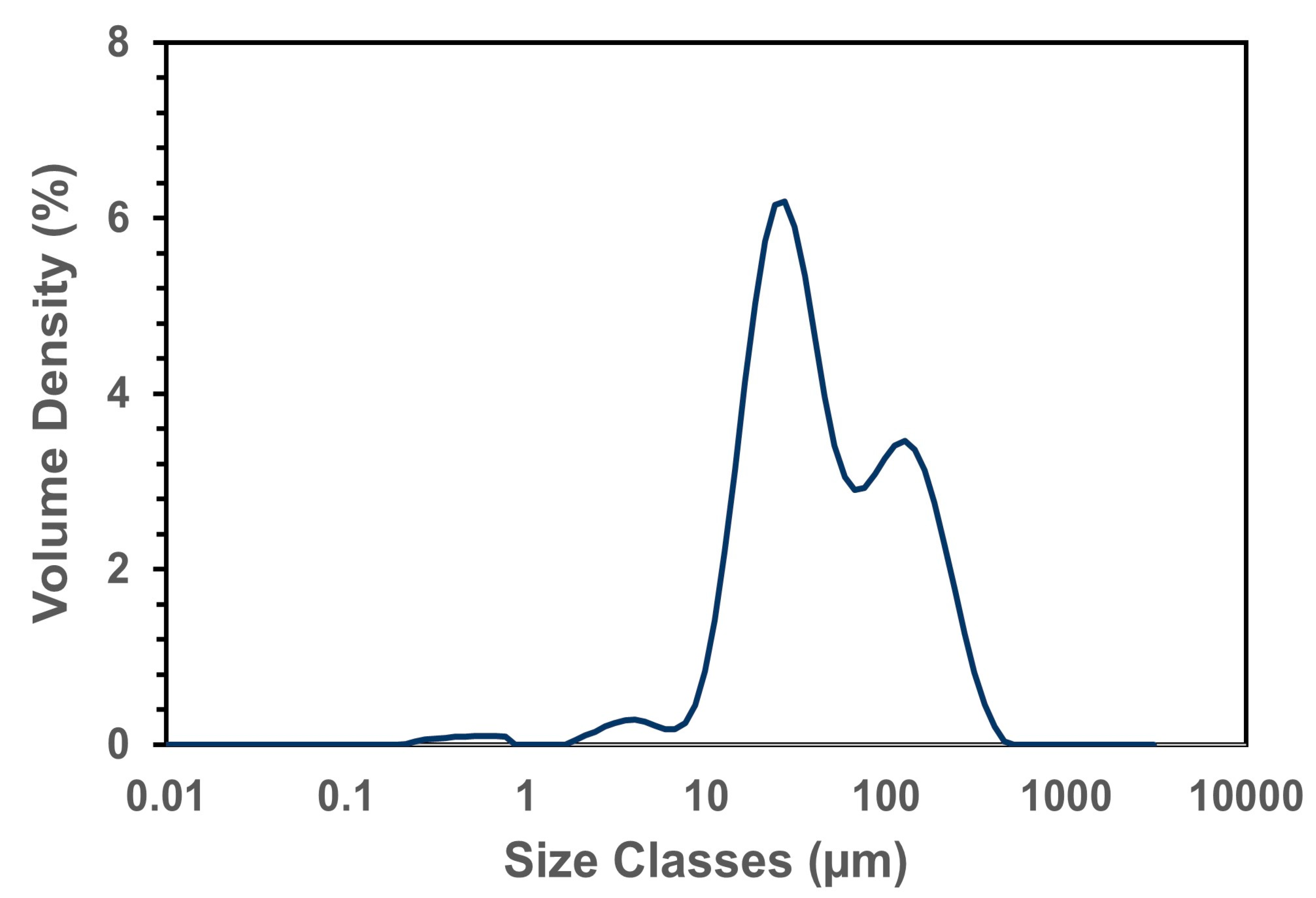
| Particle Size of Flour | ||
|---|---|---|
| Dx [10] | 4.68 ± 0.1 | μm |
| Dx [50] | 29.4 ± 0.54 | μm |
| Dx [90] | 243.4 ± 2.61 | μm |
| Span: (D90 − D10)/D50 | 8.12 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Revisiting the Conventional Extraction of Protein Isolates from Faba Beans: Recovering Lost Protein from Sustainable Side Streams. Foods 2025, 14, 1906. https://doi.org/10.3390/foods14111906
Badjona A, Bradshaw R, Millman C, Howarth M, Dubey B. Revisiting the Conventional Extraction of Protein Isolates from Faba Beans: Recovering Lost Protein from Sustainable Side Streams. Foods. 2025; 14(11):1906. https://doi.org/10.3390/foods14111906
Chicago/Turabian StyleBadjona, Abraham, Robert Bradshaw, Caroline Millman, Martin Howarth, and Bipro Dubey. 2025. "Revisiting the Conventional Extraction of Protein Isolates from Faba Beans: Recovering Lost Protein from Sustainable Side Streams" Foods 14, no. 11: 1906. https://doi.org/10.3390/foods14111906
APA StyleBadjona, A., Bradshaw, R., Millman, C., Howarth, M., & Dubey, B. (2025). Revisiting the Conventional Extraction of Protein Isolates from Faba Beans: Recovering Lost Protein from Sustainable Side Streams. Foods, 14(11), 1906. https://doi.org/10.3390/foods14111906





