Comprehensive Profiling of Free Amino Acids in Litchi (Litchi chinensis Sonn.) Germplasm and Their Implications for Flavor Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical Reagents and Standards
2.3. Quantification of Free Amino Acids
2.3.1. Sample Preparation
2.3.2. Derivatization Procedure
2.3.3. Mobile Phase Preparation
2.3.4. Chromatographic Conditions
2.4. Data Analysis
3. Results and Discussion
3.1. Variation in Free Amino Acid Content Across 148 Litchi Germplasm Accessions
3.2. Correlation Analysis of Free Amino Acid Contents
3.3. Nutritional and Taste Profile Evaluation of Amino Acids in Litchi Germplasm Accessions
3.4. Variation in Free Amino Acid Profiles Across Different Types
3.5. Hierarchical Cluster Analysis of 148 Litchi Germplasm Accessions Based on Free Amino Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, G.B.; Feng, J.T.; Xiang, X.; Wang, J.B.; Salojärvi, J.; Liu, C.M.; Wu, Z.X.; Zhang, J.S.; Liang, X.M.; Jiang, Z.D.; et al. Two divergent haplotypes from a highly heterozygous lychee genome suggest independent domestication events for early and late-maturing cultivars. Nat. Genet. 2022, 54, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, K.; Wang, K.; Zhu, J.; Hu, Z.Y. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2139–2163. [Google Scholar] [CrossRef]
- Jiang, N.H.; Liu, W.; Xiao, Z.D.; Xiang, X.; Zhong, Y. Metabolomics unveiled the accumulation characteristics of taste compounds during the development and maturation of litchi fruit. Foods 2025, 14, 144. [Google Scholar] [CrossRef]
- Wen, Y.J.; Ou, L.X.; Shi, F.C.; Yan, Q.; Cai, C.H.; Jiang, Y.H.; Liu, H.L.; Chen, J.Z. Conservation status and innovative utilization of litchi resources in the National Litchi and Banana Germplasm Repository (Guangzhou). J. Plant Genet. Resour. 2023, 24, 1205–1214. [Google Scholar] [CrossRef]
- Yan, Q.; Feng, J.T.; Chen, J.Z.; Wen, Y.J.; Jiang, Y.H.; Mai, Y.X.; Huang, K.; Liu, H.L.; Liu, H.S.; Shi, F.C.; et al. Comprehensive genomic and phenotypic analyses reveal the genetic basis of fruit quality in litchi. Genome Biol. 2025, 26, 222. [Google Scholar] [CrossRef]
- Lomax, J.; Ford, R.; Bar, I. Multi-omic applications for understanding and enhancing tropical fruit flavour. Plant Mol. Biol. 2024, 114, 83. [Google Scholar] [CrossRef] [PubMed]
- Başak, H.; Aydin, A.; Aydin, H.; Turan, M. Phytochemical profile analysis of tomato cultivars based on color and size. J. Food Compos. Anal. 2025, 146, 107992. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The genetics of fruit flavour preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Colantonio, V.; Ferrão, L.F.V.; Tieman, D.M.; Bliznyuk, N.; Sims, C.; Klee, H.J.; Munoz, P.; Resende, M.F.R., Jr. Metabolomic selection for enhanced fruit flavor. Proc. Natl. Acad. Sci. USA 2022, 119, e2115865119. [Google Scholar] [CrossRef]
- Yao, P.F.; Gao, Y.; Simal-Gandara, J.; Farag, M.A.; Chen, W.J.; Yao, D.N.; Delmas, D.; Chen, Z.J.; Liu, K.M.; Hu, H.; et al. Litchi (Litchi chinensis Sonn.): A comprehensive review of phytochemistry, medicinal properties, and product development. Food Funct. 2021, 12, 9527–9548. [Google Scholar] [CrossRef]
- Zheng, J.J.; Liu, S.; Chen, Y.; Zhang, X.; Yang, H.; Liu, X.X.; Wang, F.H. Research progress on quality evaluation of litchi. Food Nutr. China 2019, 25, 10–14. [Google Scholar] [CrossRef]
- Koláčková, T.; Sumczynski, D.; Zálešáková, L.; Šenkárová, L.; Orsavová, J.; Lanczová, N. Free and bound amino acids, minerals and trace elements in matcha (Camellia sinensis L.): A nutritional evaluation. J. Food Compos. Anal. 2020, 92, 103581. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.G.; Quek, S.Y.; Zhao, L.Y. Flavor–food ingredient interactions in fortified or reformulated novel food: Binding behaviors, manipulation strategies, sensory impacts, and future trends in delicious and healthy food design. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4004–4029. [Google Scholar] [CrossRef]
- Solano, F. Amino Acids in Nutrition and Health; Springer: Cham, Switzerland, 2020; Volume 11, pp. 187–199. ISBN 978-3-030-45328-2. [Google Scholar]
- Xie, Y.W.; Wang, T.; Guo, C.F.; Chu, C.Q.; Liu, Z.J.; Jiang, L.Z.; Deng, Y.S.; Yi, J.J. Metagenomic insights into the microorganisms responsible for producing amino acid nitrogen during sufu fermentation. Food Chem. 2025, 487, 144763. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ninomiya, K. Umami and food palatability. J. Nutr. 2000, 130, 921S–926S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Schieber, A.; Gänzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.J.; Liu, H.L.; Jiang, Y.H.; Shi, F.C.; Ou, L.X.; Yan, Q. Analysis and comprehensive evaluation of free amino acids in different varieties of lychee. Food Ferment. Ind. 2025, 51, 343–352. [Google Scholar] [CrossRef]
- GB/T 30987-2020; Determination of Free Amino Acids in Plant. Standards Press of China: Beijing, China, 2020.
- Zhang, X.A.; Su, M.S.; Du, J.H.; Zhou, H.J.; Li, X.W.; Zhang, M.H.; Hu, Y.; Ye, Z.W. Analysis of the free amino acid content and profile of 129 peach (Prunus persica (L.) Batsch) germplasms using LC-MS/MS without derivatization. J. Food Compos. Anal. 2022, 114, 104811. [Google Scholar] [CrossRef]
- He, Y.L.; Qin, H.Y.; Wen, J.L.; Wang, L.; Cao, W.Y.; Fan, S.T.; Lu, W.P.; Li, J.Q.; Li, C.Y. Characterization of amino acid composition, nutritional value, and taste of fruits from different Actinidia arguta resources. J. Food Qual. 2024, 2024, 1005194. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Cui, Z.X.; Xu, H.; Wei, Z.H.; Zhang, J.W.; Bai, C.H.; Yao, L.X. Comparison on fruit quality and flavor of new and fine litchi cultivars. J. Chin. Inst. Food Sci. Technol. 2023, 26, 327–338. [Google Scholar] [CrossRef]
- Liao, H.S.; Chung, Y.H.; Hsieh, M.H. Glutamate: A multifunctional amino acid in plants. Plant Sci. 2022, 318, 111238. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Signorelli, S.; Hofte, M. GABA and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric acid (GABA) signalling in plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Deng, Y.N.; Zhao, L.; Wang, K.; Wu, D.M.; Hu, Z.Y.; Liu, X.W. GABA and fermented litchi juice enriched with GABA promote the beneficial effects in ameliorating obesity by regulating the gut microbiota in HFD-induced mice. Food Funct. 2023, 14, 8170–8185. [Google Scholar] [CrossRef]
- Lee, K.T.; Liao, H.S.; Hsieh, M.H. Glutamine metabolism, sensing and signaling in plants. Plant Cell Physiol. 2023, 64, 1466–1481. [Google Scholar] [CrossRef]
- Tsai, H.H.; Tang, Y.J.; Jiang, L.M.; Xu, X.Y.; Dénervaud Tendon, V.; Pang, J.; Jia, Y.Y.; Wippel, K.; Vacheron, J.; Keel, C.; et al. Localized glutamine leakage drives the spatial structure of root microbial colonization. Science 2025, 390, eadu4235. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.A.; Lea, P.J. Lysine metabolism in higher plants. Amino Acids 2001, 20, 261–279. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhou, Y.J.; Jiang, Y. Amino acids in rice grains and their regulation by polyamines and phytohormones. Plants 2022, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Fenalti, G.; Law, R.H.P.; Buckle, A.M.; Langendorf, C.; Tuck, K.; Rosado, C.J.; Faux, N.G.; Mahmood, K.; Hampe, C.S.; Banga, J.P.; et al. GABA production by glutamic acid decarboxylase is regulated by a dynamic catalytic loop. Nat. Struct. Mol. Biol. 2007, 14, 280–286. [Google Scholar] [CrossRef]
- Wei, W.L.; Zhang, T.T.; Chen, Y.P.; Zhou, Z.Q.; Su, W.B.; Xu, Q.Z.; Zhang, Y.L.; Zheng, S.Q.; Jiang, J.M.; Deng, C.J. Characterization of the glutamate decarboxylase (GAD) gene and functional analysis of DlGAD3 in the accumulation of γ-aminobutyric acid in longan (Dimocarpus longan Lour.) pulp. Horticulturae 2025, 11, 686. [Google Scholar] [CrossRef]
- Takayama, M.; Matsukura, C.; Ariizumi, T.; Ezura, H. Activating glutamate decarboxylase activity by removing the autoinhibitory domain leads to hyper γ-aminobutyric acid (GABA) accumulation in tomato fruit. Plant Cell Rep. 2017, 36, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, B.B.; Luo, Z.; Yuan, Y.; Zhao, Z.H.; Liu, M.J. Composition analysis and nutritional value evaluation of amino acids in the fruit of 161 jujube cultivars. Plants 2023, 12, 1744. [Google Scholar] [CrossRef]
- Sun, H.; Liu, F.B.; Sun, L.W.; Liu, J.Z.; Wang, M.Y.; Chen, X.N.; Xu, X.H.; Ma, R.; Feng, K.; Jiang, R. Proteomic analysis of amino acid metabolism differences between wild and cultivated Panax ginseng. J. Ginseng Res. 2016, 40, 113–120. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, L.L.; Sun, Y.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Determination of the free amino acid, organic acid, and nucleotide in commercial vinegars. J. Food Sci. 2017, 82, 1116–1123. [Google Scholar] [CrossRef]
- An, Y.Q.; Cai, X.W.; Cong, L.; Hu, Y.; Liu, R.; Xiong, S.B.; Hu, X.B. Quality improvement of Zhayu, a fermented fish product in China: Effects of inoculated fermentation with three kinds of lactic acid bacteria. Foods 2022, 11, 2756. [Google Scholar] [CrossRef]
- Fu, N.; Wang, X.C. Research progress in interactions between taste-active components. Food Sci. 2014, 35, 269–275. [Google Scholar] [CrossRef]
- Lioe, H.N.; Apriyantono, A.; Takara, K.; Wada, K.; Yasuda, M. Umami taste enhancement of MSG/NaCl mixtures by subthreshold L-α-aromatic amino acids. J. Food Sci. 2005, 70, S401–S405. [Google Scholar] [CrossRef]
- Fan, S.Y.; Wang, D.; Xie, H.H.; Wang, H.C.; Qin, Y.H.; Hu, G.B.; Zhao, J.T. Sugar transport, metabolism and signaling in fruit development of Litchi chinensis Sonn: A review. Int. J. Mol. Sci. 2021, 22, 11231. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wang, T.D.; Wang, H.C.; Huang, X.M.; Qin, Y.H.; Hu, G.B. Patterns of enzyme activities and gene expressions in sucrose metabolism in relation to sugar accumulation and composition in the aril of Litchi chinensis Sonn. J. Plant Physiol. 2013, 170, 731–740. [Google Scholar] [CrossRef]
- Wu, J.Y.; Fan, J.B.; Li, Q.H.; Jia, L.T.; Xu, L.L.; Wu, X.; Wang, Z.W.; Li, H.X.; Qi, K.J.; Qiao, X.; et al. Variation of organic acids in mature fruits of 193 pear (Pyrus spp.) cultivars. J. Food Compos. Anal. 2022, 109, 104483. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.W. Metabolomic profiling of litchi cultivars reveals key metabolites associated with fruit development and quality. Sci. Hortic. 2025, 344, 114103. [Google Scholar] [CrossRef]
- Chen, L.M.; Yan, J.N.; Miu, Y.W.; Huang, R.; Wei, H.; Wang, L.L.; Zhang, C.; Yuan, L.Y.; Tong, H.R. The spatiotemporal variations of L-glutamic acid and catechins during the development of etiolated tea leaves in ‘Huangjinye’. Sci. Hortic. 2024, 328, 112888. [Google Scholar] [CrossRef]
- Hu, W.S.; Wang, B.Y.; Ali, M.M.; Chen, X.P.; Zhang, J.S.; Zheng, S.Q.; Chen, F.X. Free amino acids profile and expression analysis of core genes involved in branched-chain amino acids metabolism during fruit development of longan (Dimocarpus longan Lour.) cultivars with different aroma types. Biology 2021, 10, 807. [Google Scholar] [CrossRef] [PubMed]
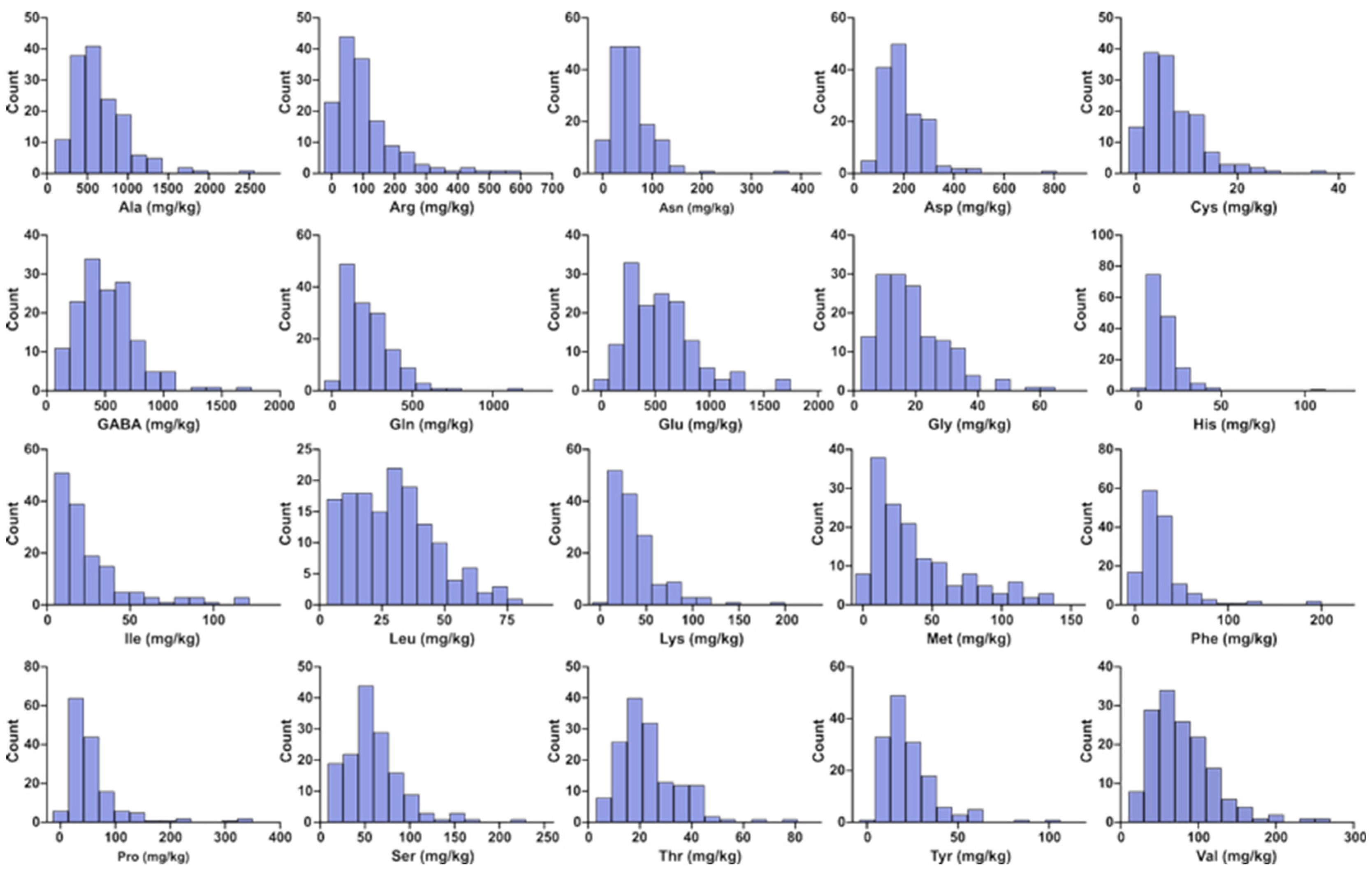
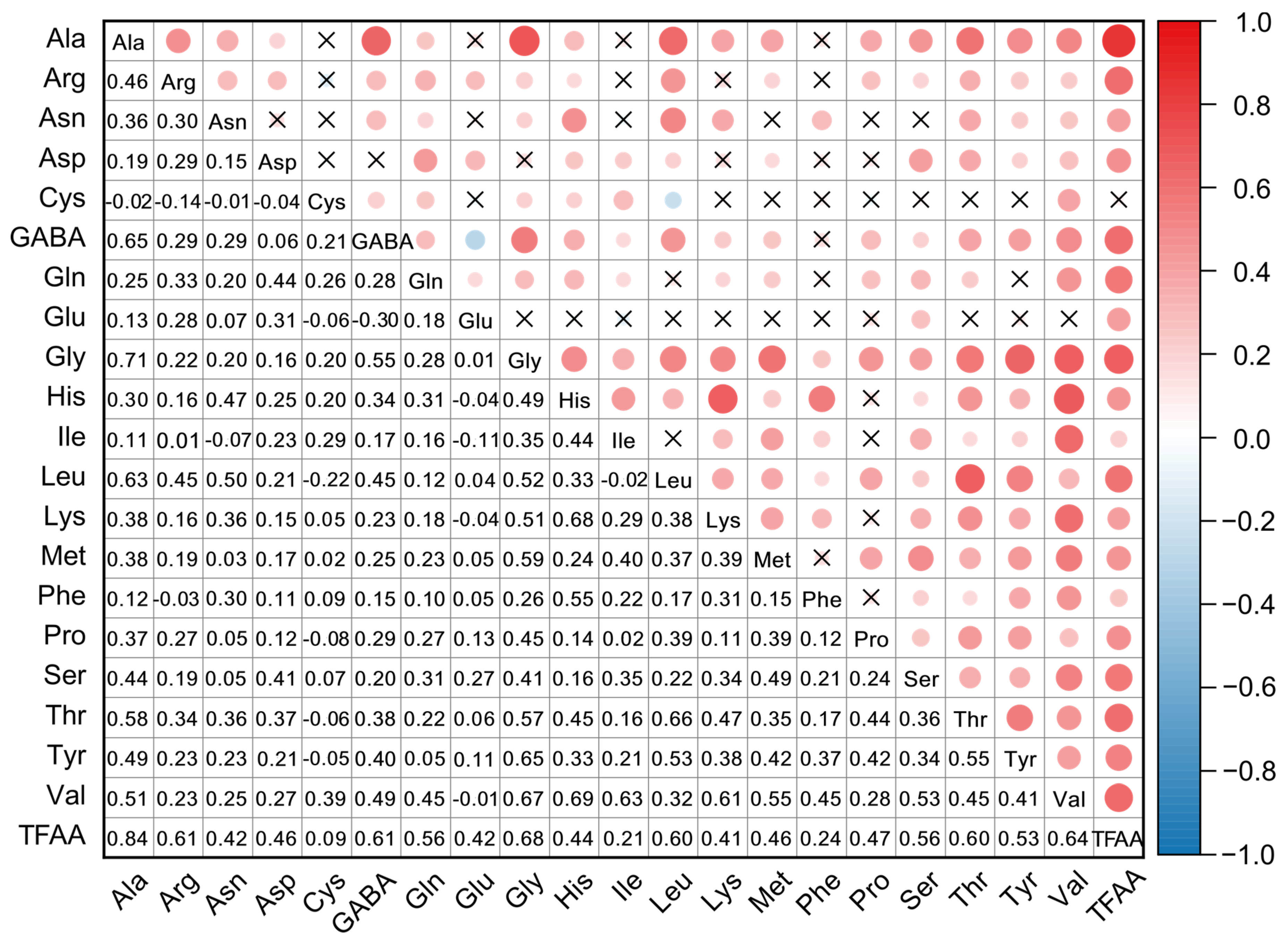
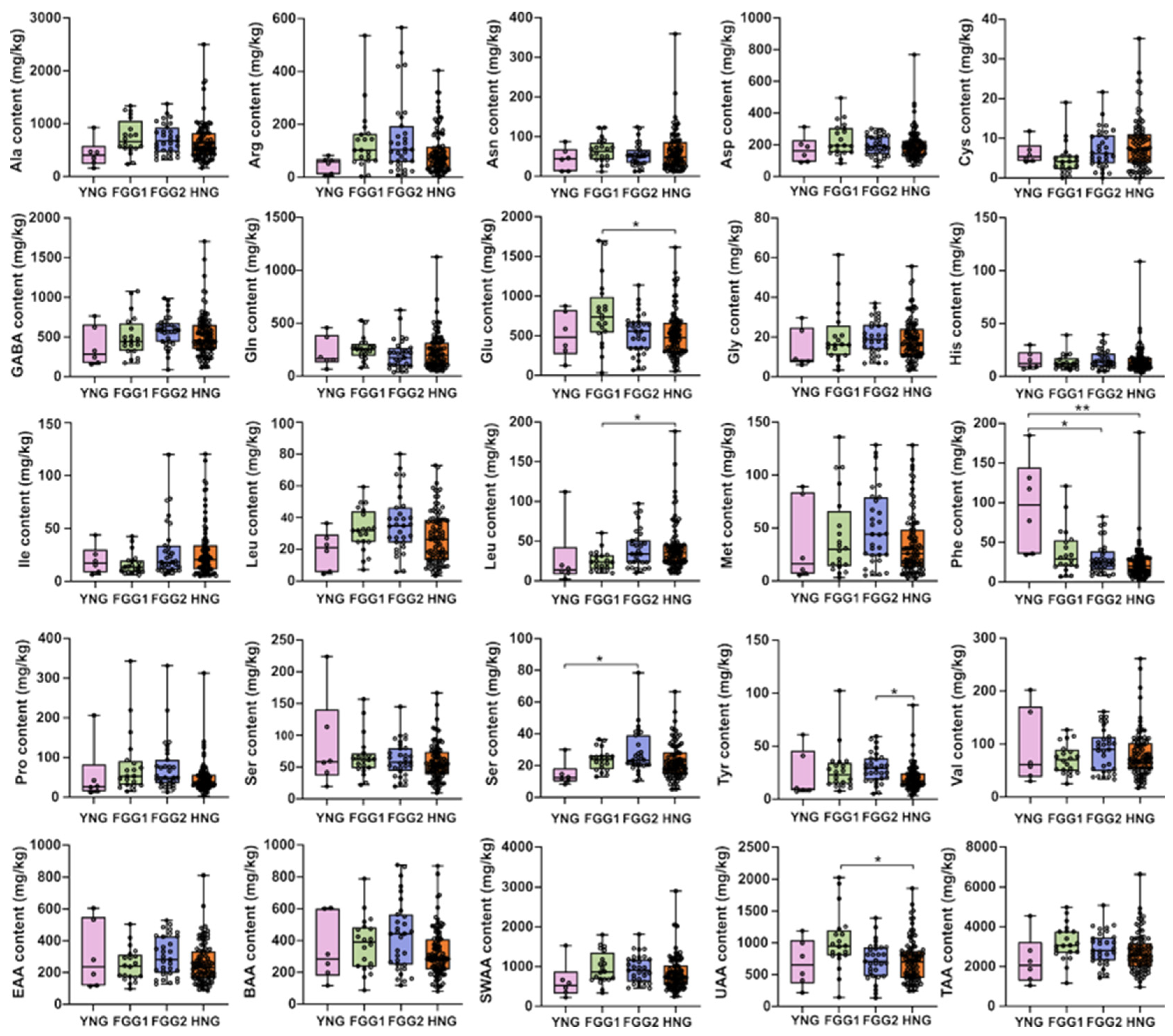
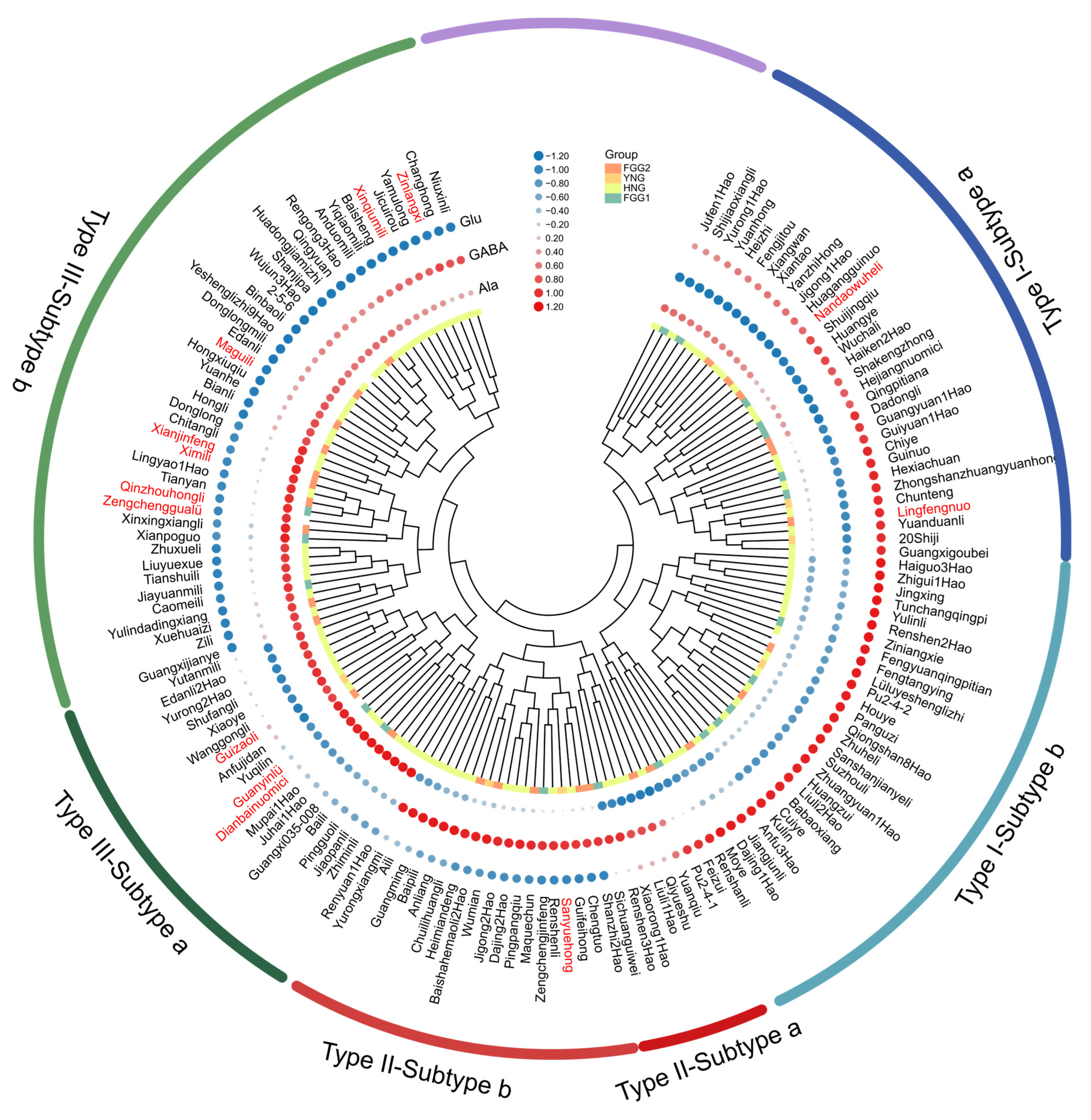
| Amino Acids | Mean (mg/kg) | SD (mg/kg) | Median (mg/kg) | Min (mg/kg) | Max (mg/kg) | CV (%) |
|---|---|---|---|---|---|---|
| Ala | 659.35 | 351.60 | 566.80 | 141.62 | 2497.77 | 53.33 |
| Arg | 109.03 | 103.93 | 77.54 | 2.55 | 566.06 | 95.32 |
| Asn | 59.59 | 43.34 | 51.21 | 11.15 | 359.16 | 72.73 |
| Asp | 202.46 | 91.81 | 188.64 | 64.05 | 768.39 | 45.35 |
| Cys | 7.40 | 5.87 | 6.06 | <LOD 1 | 35.19 | 79.27 |
| GABA | 520.47 | 262.86 | 493.76 | 87.13 | 1703.36 | 50.50 |
| Gln | 228.20 | 158.03 | 202.38 | 9.89 | 1126.07 | 69.25 |
| Glu | 551.16 | 321.47 | 523.97 | 33.89 | 1699.27 | 58.33 |
| Gly | 18.93 | 10.68 | 16.65 | 3.06 | 61.42 | 56.41 |
| His | 15.28 | 11.18 | 12.85 | 1.56 | 108.60 | 73.14 |
| Ile | 26.02 | 23.58 | 18.45 | 3.65 | 120.48 | 90.64 |
| Leu | 29.46 | 16.92 | 27.51 | 3.30 | 80.05 | 57.43 |
| Lys | 38.15 | 27.61 | 31.43 | 2.45 | 188.42 | 72.38 |
| Met | 38.75 | 33.05 | 29.27 | 2.65 | 136.06 | 85.29 |
| Phe | 29.35 | 28.57 | 22.61 | 3.59 | 188.63 | 97.34 |
| Pro | 58.50 | 54.48 | 43.21 | 2.68 | 342.77 | 93.13 |
| Ser | 60.54 | 32.39 | 55.11 | 9.18 | 223.93 | 53.50 |
| Thr | 23.48 | 11.58 | 20.99 | 5.06 | 78.43 | 49.33 |
| Tyr | 22.90 | 14.97 | 19.49 | 3.19 | 102.25 | 65.37 |
| Val | 80.26 | 42.67 | 72.76 | 16.77 | 261.17 | 53.16 |
| TFAA | 2778.45 | 960.22 | 2693.42 | 847.11 | 6636.28 | 34.56 |
| Taste | FAA | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|
| umami | Asp | 0.20 | 0.09 | 0.19 | 0.06 | 0.77 |
| umami | Glu | 1.84 | 1.07 | 1.76 | 0.11 | 5.66 |
| sweet | Ala | 1.10 | 0.58 | 0.95 | 0.27 | 4.16 |
| sweet | Gly | 0.01 | 0.01 | 0.01 | 0.00 | 0.05 |
| sweet | Ser | 0.04 | 0.02 | 0.04 | 0.01 | 0.15 |
| sweet | Thr | 0.01 | 0.00 | 0.01 | 0.01 | 0.03 |
| sweet/bitter | Lys | 0.08 | 0.06 | 0.06 | 0.00 | 0.38 |
| sweet/bitter | Pro | 0.02 | 0.02 | 0.01 | 0.00 | 0.11 |
| bitter | Arg | 0.22 | 0.21 | 0.16 | 0.01 | 1.13 |
| bitter | His | 0.08 | 0.06 | 0.06 | 0.02 | 0.54 |
| bitter | Ile | 0.03 | 0.03 | 0.02 | 0.01 | 0.13 |
| bitter | Leu | 0.02 | 0.01 | 0.01 | 0.00 | 0.04 |
| bitter | Met | 0.13 | 0.11 | 0.10 | 0.01 | 0.45 |
| bitter | Phe | 0.03 | 0.03 | 0.03 | 0.00 | 0.21 |
| bitter | Tyr | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| bitter | Val | 0.20 | 0.11 | 0.18 | 0.04 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Shi, F.; Jiang, Y.; Liu, H.; Yan, Q. Comprehensive Profiling of Free Amino Acids in Litchi (Litchi chinensis Sonn.) Germplasm and Their Implications for Flavor Quality. Foods 2025, 14, 4051. https://doi.org/10.3390/foods14234051
Wen Y, Shi F, Jiang Y, Liu H, Yan Q. Comprehensive Profiling of Free Amino Acids in Litchi (Litchi chinensis Sonn.) Germplasm and Their Implications for Flavor Quality. Foods. 2025; 14(23):4051. https://doi.org/10.3390/foods14234051
Chicago/Turabian StyleWen, Yingjie, Fachao Shi, Yonghua Jiang, Hailun Liu, and Qian Yan. 2025. "Comprehensive Profiling of Free Amino Acids in Litchi (Litchi chinensis Sonn.) Germplasm and Their Implications for Flavor Quality" Foods 14, no. 23: 4051. https://doi.org/10.3390/foods14234051
APA StyleWen, Y., Shi, F., Jiang, Y., Liu, H., & Yan, Q. (2025). Comprehensive Profiling of Free Amino Acids in Litchi (Litchi chinensis Sonn.) Germplasm and Their Implications for Flavor Quality. Foods, 14(23), 4051. https://doi.org/10.3390/foods14234051





