Influence of Banana Genotype on Polyphenol Content and Starch Digestibility of Unripe Flours
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Sample Preparation
2.2. Materials
2.3. Chemical Composition
2.4. Extraction of Total Polyphenol Content
2.5. Total Polyphenol Content
2.6. Analysis of Antioxidant Capacity
2.6.1. ABTS Assay
2.6.2. DPPH Assay
2.6.3. FRAP Assay
2.7. In Vitro Starch Digestibility
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Polyphenol Content and Antioxidant Capacity
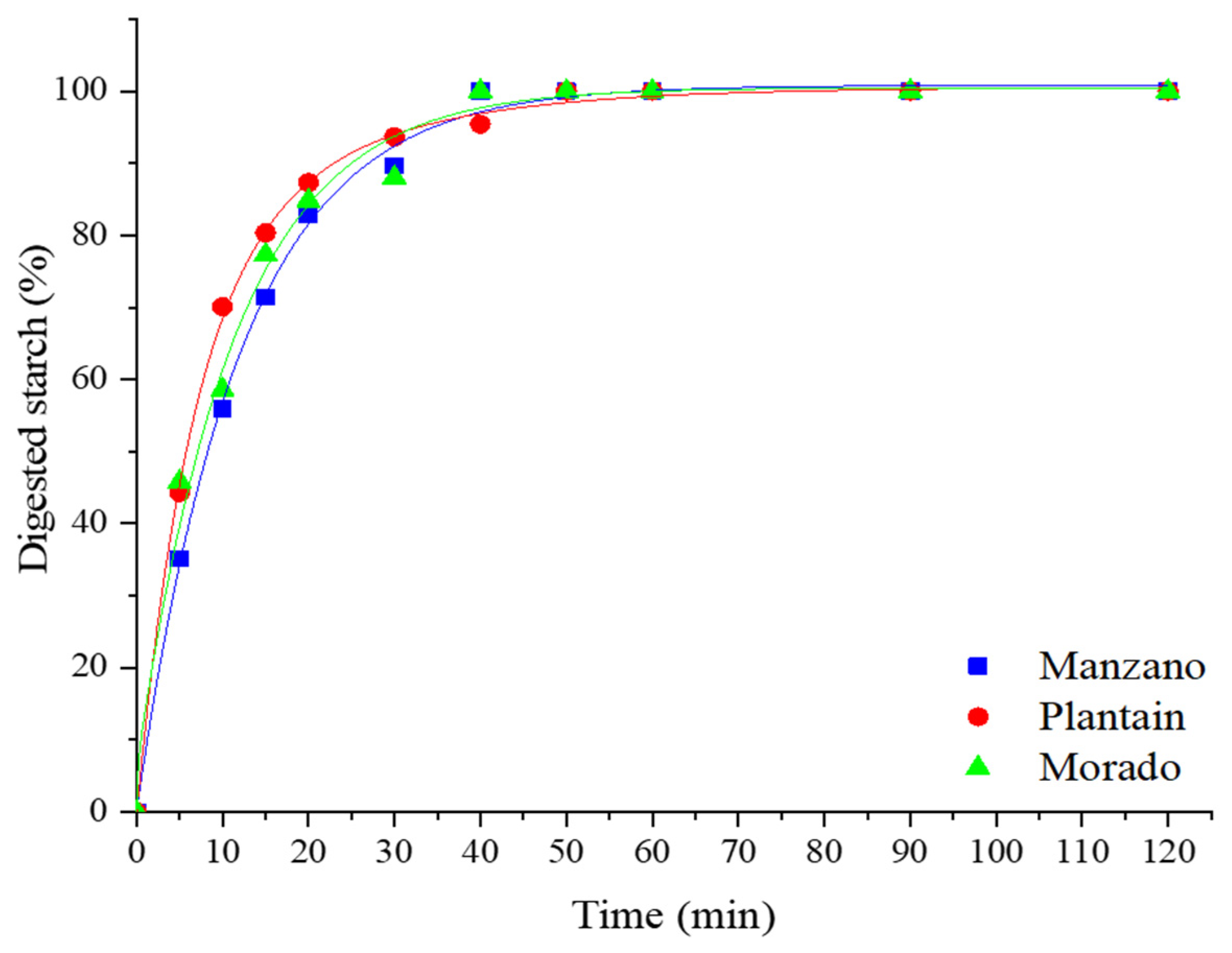
3.3. Starch Digestion Rate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ovando-Martinez, M.; Sáyago-Ayerdi, S.; Agama-Acevedo, E.; Goñi, I.; Bello-Pérez, L.A. Unripe banana flour as an ingredient to increase the undigestible carbohydrates of pasta. Food Chem. 2009, 113, 121–126. [Google Scholar] [CrossRef]
- Gibert, O.; Dufour, D.; Giraldo, A.; Sánchez, T.; Reynes, M.; Pain, J.P.; González, A.; Fernández, A.; Díaz, A. Differentiation between cooking bananas and dessert bananas. 1. Mand compositional characterization of cultivated Colombian Musaceae (Musa sp.) in relation to consumer preferences. J. Agric. Food Chem. 2009, 57, 7857–7869. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.C.; Galán-Saúco, V. Bananas and Plantains; CABI: Oxfordshire, UK, 2010. [Google Scholar]
- Pereira, A.; Maraschin, M. Banana (Musa spp.) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015, 160, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Magallanes-Cruz, P.A.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Tovar, J.; Carmona-Garcia, R. Effect of the addition of thermostable and non-thermostable type 2 resistant starch (RS2) in cake batters. LWT 2020, 118, 108834. [Google Scholar] [CrossRef]
- Yang, J.; Bi, Y.; Liang, S.; Gu, Z.; Cheng, L.; Li, C.; Li, Z.; Zhang, Y.; Hong, Y. The in vivo digestibility study of banana flour with high content of resistant starch at different ripening stages. Food Funct. 2020, 11, 10945–10953. [Google Scholar] [CrossRef]
- Ye, J.; Roman, L.; Pico, J.; Aguirre-Cruz, A.; Bello-Perez, L.A.; Bertoft, E.; Martinez, M.M. The molecular structure of starch fron different Musa genotypes: Higher branching density of amylose chains seems to promote enzyme-resistant structures. Foods Hydrocoll. 2021, 112, 106351. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 11th ed.; American Association of Cereal Chemist: St. Paul, MN, USA, 2000. [Google Scholar]
- Megazyme. Total Starch. Megazyme International Ireland. 2020. Available online: https://www.megazyme.com/search?sSearch=starch&p=2 (accessed on 30 September 2025).
- Mercado-Mercado, G.; Blancas-Benitez, F.J.; Velderrain-Rodríguez, G.R.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.). J. Funct. Foods 2015, 18, 171–181. [Google Scholar] [CrossRef]
- Ruiz Canizales, J.; Heredia, J.B.; Domínguez Avila, J.A.; Madera Santana, T.J.; Villegas Ochoa, M.A.; Robles Sánchez, R.M.; González Aguilar, G.A. Microencapsulation of blue maize (Zea mays L.) polyphenols in two matrices: Their stability during storage and in vitro digestion release. J. Food Meas. Charact. 2019, 13, 892–900. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, A.; Proteggente, N.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Englyst, H.N.; Hudson, G.J. The classification and measurement of dietary carbohydrates. Food Chem. 1996, 57, 15–21. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Rodriguez-Ambriz, S.L.; García-Suarez, F.J.; Gutiérrez-Meraz, F.; Pacheco-Vargas, G.; Bello-Pérez, L.A. Starch isolation and partial characterization of commercial cooking and dessert banana cultivars growing in Mexico. Starch-Starke 2014, 66, 337–344. [Google Scholar] [CrossRef]
- Wu, H.; Sang, S.; Weng, P.; Pan, D.; Wu, Z.; Yang, J.; Liu, L.; Farak, M.A.; Xiao, J.; Liu, L. Structural, reological, and gelling characteristics of starch-based material in context to 3D food printing applications in precision nutrition. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4217–4241. [Google Scholar] [CrossRef]
- Jiali, L.; Wu, Z.; Liu, L.; Yang, J.; Wang, L.; Li, Z.; Liu, L. The research advance of resistant starch: Structural characteristics, modification method, immunomodulatory function, and its delivery systems application. Crit. Rev. Food Sci. Nutr. 2024, 64, 10885–10902. [Google Scholar] [CrossRef] [PubMed]
- Danielski, R.; Shahidi, F. How in vitro gastrointestinal digestion impacts the phenolic profile and bioactivities of North American sea buckthorn fruit and seeds. Food Funct. 2025, 16, 6249–6259. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, E.; Grȩda, A.; Adamus, W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Borges, C.V.; Maraschin, M.; Coelho, D.S.; Leonel, M.; Gomez, H.A.G.; Belin, M.A.F.; Diamante, M.S.; Amorim, E.P.; Gianeti, T.; Castro, G.R.; et al. Nutritional value and antioxidant compounds during the ripening and after domestic cooking of bananas and plantains. Food Res. Int. 2020, 132, 109061. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Li, Y.; Dong, L.; Liu, Y.; Liu, L.; Farag, M.A.; Liu, L. Interaction and binding mechanism of ovalbumin with cereal phenolic acids: Improved structure, antioxidant activity, emulsifying and digestion properties for potential targeted delivery systems. Food Res. Int. 2024, 175, 113726. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Liu, X.; Su, S.; Yao, J.; Zhang, X.; Wu, Z.; Jia, L.; Liu, L.; Hou, R.; Farag, M.A.; Lui, L. Research advance abouth plant polysaccharide prebiotics, Benefit for probiotics on gut homeostasis modulation. Food Biosci. 2024, 59, 103831. [Google Scholar] [CrossRef]
- Eroglu, E.C.; Arslan, R.; Unlu, M.; Arslan, R. Determination of total phenolic, antioxidant, sugar content, minerals and pomological characteristics of some banana varieties and local types. Appl. Fruit Sci. 2025, 67, 305. [Google Scholar] [CrossRef]

| Parameters | Manzano (AAB) | Plantain (AAB) | Morado (AAA) |
|---|---|---|---|
| Moisture | 8.6 ± 0.1 a | 5.3 ± 0.1 c | 7.4 ± 0.4 b |
| Protein | 4.3 ± 0.2 b | 4.5 ± 0.2 b | 4.9 ± 0. 4 a |
| Lipids | 1.5 ± 0.2 b | 1.2 ± 0.0 b | 2.1 ± 0.0 a |
| Ash | 3.4 ± 0.1 b | 3.7 ± 0.0 b | 4.4 ± 0.2 a |
| Soluble carbohydrates * | 12.2 ± 0.3 c | 22.7 ± 0.2 b | 29.5 ± 0.4 a |
| Total dietary fiber | 12.8 ± 0.1 b | 8.0 ± 0.6 c | 15.4 ± 0.0 a |
| Total starch | 63.71 ± 1.7 a | 59.82 ± 0.8 a | 43.66 ± 1.9 b |
| Manzano (AAB) | Plantain (AAB) | Morado (AAA) | ||
|---|---|---|---|---|
| Polyphenols | TSPs (mg GAE/g db) | 5.36 ± 0.1 b | 6.25 ± 0.2 a | 6.78 ± 0.3 a |
| Flavonoids (mg CE/g db) | 0.40 ± 0.02 a | 0.29 ± 0.03 b | 0.40 ± 0.04 a | |
| Antioxidant capacity | ABTS+ (µmol TE/g db) | 6.20 ± 0.1 a | 5.21 ± 0.7 b | 5.07 ± 0.8 b |
| DPPH (µmol TE/g db) | 52.79 ± 0.4 a | 47.78 ± 1.1 b | 54.97± 0.3 a | |
| FRAP (µmol TE/g db) | 6.33 ± 0.5 b | 17.30 ± 0.2 a | 17.36 ± 0.4 a |
| Sample | RDS | SDS | RS | K × 10−2 (min−1) |
|---|---|---|---|---|
| Manzano | 74.59 ±0.37 c | 17.11 ± 0.41 a | 8.28 ± 0.04 c | 40.56 ± 0.253 c |
| Plantain | 78.56 ± 0.25 a | 12.71 ± 0.28 c | 8.72 ± 0.03 a | 68.38 ± 0.329 b |
| Morado | 76.32 ± 0.50 b | 15.18 ± 0.55 b | 8.48 ± 0.05 b | 71.46 ± 0.046 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agama-Acevedo, E.; Solis-Mariano, A.L.; Altamirano-Monico, S.M.; Zárate-Córdova, V.L.; Bello-Perez, L.A. Influence of Banana Genotype on Polyphenol Content and Starch Digestibility of Unripe Flours. Foods 2025, 14, 4048. https://doi.org/10.3390/foods14234048
Agama-Acevedo E, Solis-Mariano AL, Altamirano-Monico SM, Zárate-Córdova VL, Bello-Perez LA. Influence of Banana Genotype on Polyphenol Content and Starch Digestibility of Unripe Flours. Foods. 2025; 14(23):4048. https://doi.org/10.3390/foods14234048
Chicago/Turabian StyleAgama-Acevedo, Edith, Ana L. Solis-Mariano, Sherlin M. Altamirano-Monico, Vareska L. Zárate-Córdova, and Luis A. Bello-Perez. 2025. "Influence of Banana Genotype on Polyphenol Content and Starch Digestibility of Unripe Flours" Foods 14, no. 23: 4048. https://doi.org/10.3390/foods14234048
APA StyleAgama-Acevedo, E., Solis-Mariano, A. L., Altamirano-Monico, S. M., Zárate-Córdova, V. L., & Bello-Perez, L. A. (2025). Influence of Banana Genotype on Polyphenol Content and Starch Digestibility of Unripe Flours. Foods, 14(23), 4048. https://doi.org/10.3390/foods14234048




