Development of New Red-Fleshed Seedless Table Grapes: In Vitro Insights on Glucose Absorption and Insulin Resistance Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape and Sample Preparation
2.2. Total Phenolic Content (TPC)
2.3. Total Anthocyanins
2.4. Total Tannins
2.5. Antioxidant Capacity
2.6. Determination of Phenolic Compounds by HPLC
2.6.1. Anthocyanins and Flavonols
2.6.2. Stilbenes
2.7. Simulation of In Vitro Gastrointestinal Digestion
2.8. Cells
2.9. Cell Viability Measurement
2.10. Transepithelial Intestinal Transport Studies and Glucose Levels
2.11. Liver Model to Study Modulation in Insulin Resistance Biomarkers
2.12. Intracellular Glycogen Level Determination
2.13. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.14. Statistical Analyses
3. Results and Discussion
3.1. Phenolic Compound Content and Antioxidant Activity
3.2. Concentration and Composition of Phenolic Compounds by HPLC
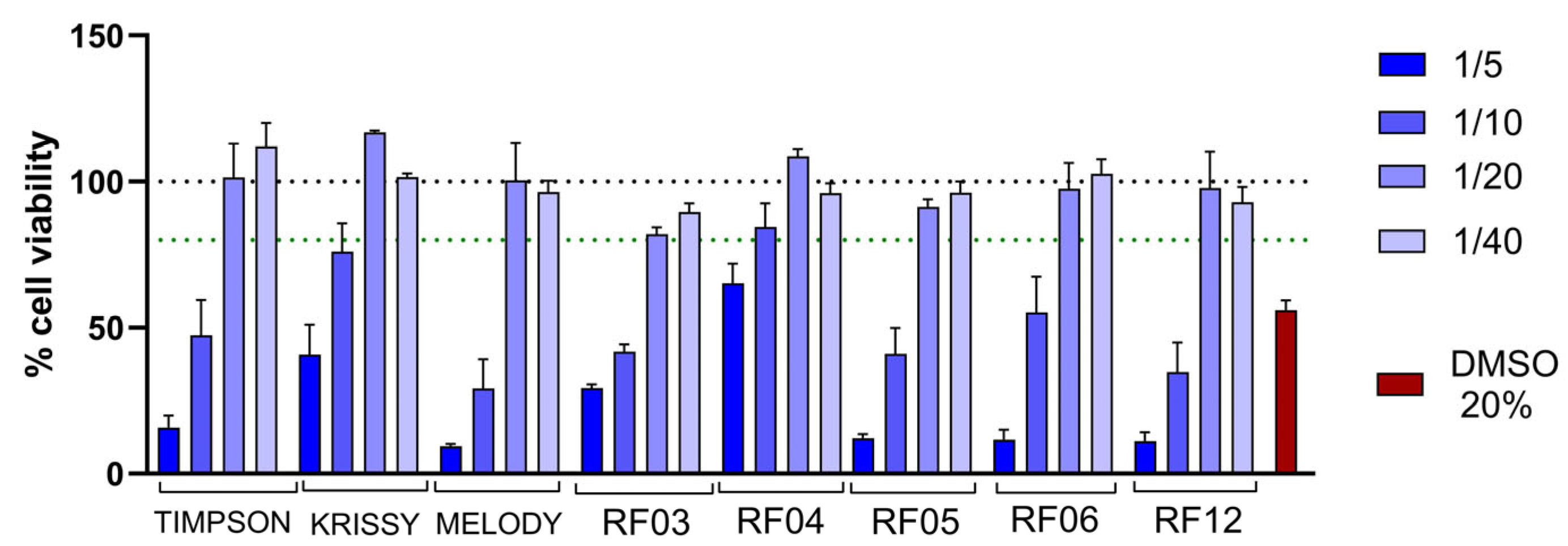
3.3. Biocompatibility of the Digests in the Intestinal Model to Study Intestinal Transport
3.4. Changes in Glucose Transport in the Intestinal Transport Model
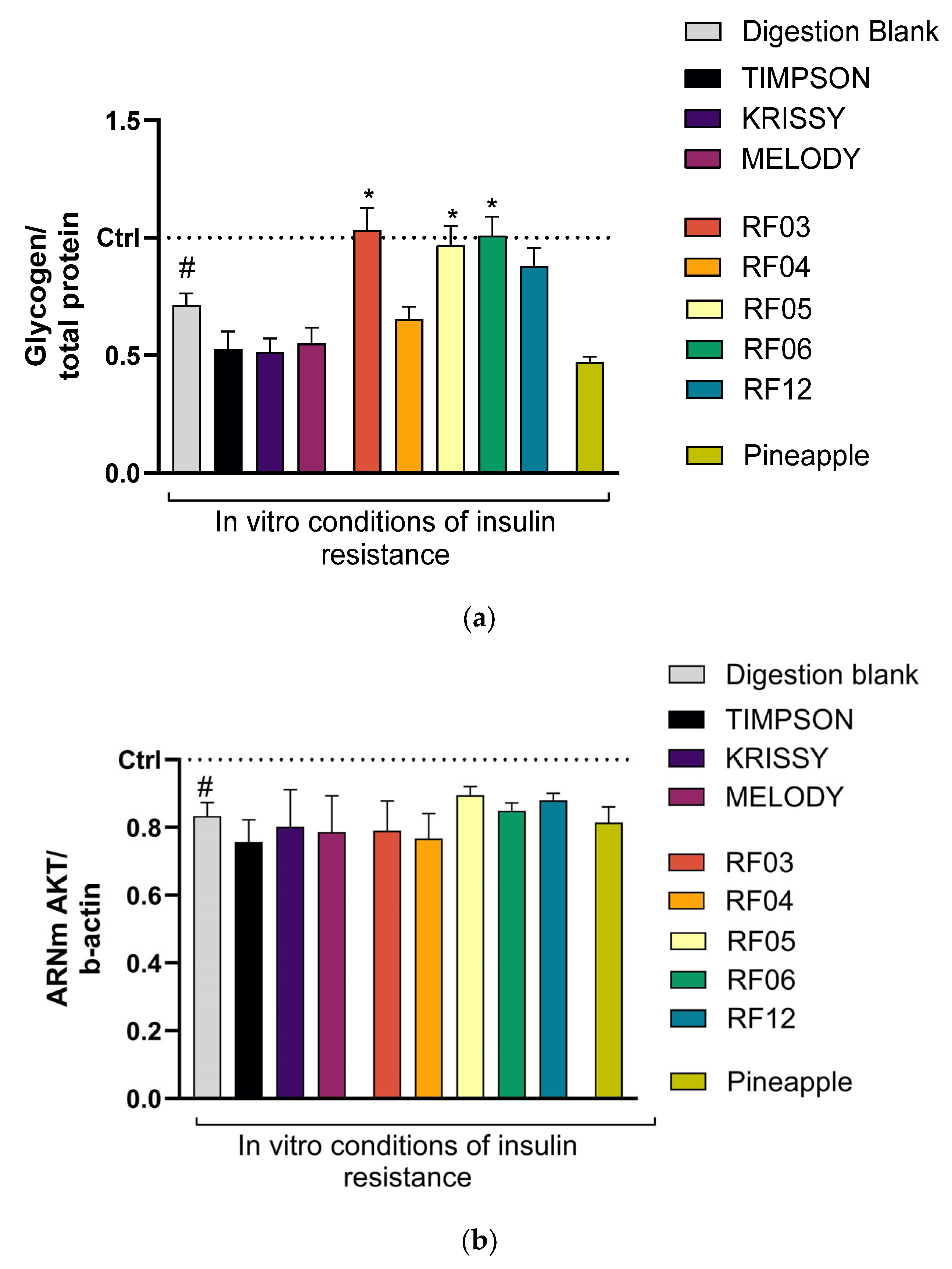
3.5. Changes in Insulin Resistance Biomarkers (Glycogen) in the Liver Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cerletti, C.; De Curtis, A.; Bracone, F.; Digesù, C.; Morganti, A.G.; Iacoviello, L.; Donati, M.B. Dietary anthocyanins and health: Data from FLORA and ATHENA EU projects. Br. J. Clin. Pharmacol. 2017, 83, 103–106. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Chaturvedi Bhargava, P.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbol. 2022, 381, 109890. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.T.; Maqsood, S.; Ikram, A.; Abdullahi, M.A. Recent Perspectives on the Role of Anthocyanins in Blueberries (Vaccinium spp.) Against Cardiovascular Diseases and Their Complications: An Updated Review. eFood 2025, 6, e70072. [Google Scholar] [CrossRef]
- Akram, M.T.; Qadri, R.; Khan, M.A.; Atak, A.; Liaquat, M.; Hussain, T.; Khan, M.M.; Azam, M.; ul Hasan, M. Comparative Assessment of Bioactive Compounds, Fruit Quality Attributes and Sugar Profiling in Early Maturing Table Grape (Vitis vinifera L.) Cultivars from Pothohar, Pakistan. Appl. Fruit Sci. 2024, 66, 983–995. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Li, H.B. Comparison of antioxidant activities of different grape varieties. Molecules 2018, 23, 2432. [Google Scholar] [CrossRef]
- Khalil, U.; Rajwana, I.A.; Razzaq, K.; Mustafa, G.; Rafique, R.; Jamil, A. Physicochemical Attributes and Bioactive Compounds of Grape Cultivars Grown Under Warm Climate. Appl. Fruit Sci. 2024, 66, 1559–1568. [Google Scholar] [CrossRef]
- Alenazi, M.M.; Shafiq, M.; Alobeed, R.S.; Alsdon, A.A.; Abbasi, N.A.; Ali, I.; Javed, I. Application of abscisic acid at veraison improves red pigmentation and accumulation of dietary antioxidants in red table grapes cv. Red Globe Harvest. Sci. Hortic. 2019, 257, 108672. [Google Scholar] [CrossRef]
- Du, B.; He, B.J.; Shi, P.B.; Li, F.Y.; Li, J.; Zhu, F.M. Phenolic content and antioxidant activity of wine grapes and table grapes. J. Med. Plants Res. 2012, 6, 3381–3387. [Google Scholar] [CrossRef]
- Bakir, S. The antioxidant activity of grapes after different drying treatments. Int. J. Food Engin. 2025, 21, 437–446. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Boban, M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Ćurko, N.; Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Oliboni, L.S.; Vanderlinde, R.; Bonatto, D.; Salvador, M.; Henriques, J.A.P. Phenolic content and antioxidant activities of white and purple juices manufactured with organically-or conventionally-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, H.; Du, W.; Li, G.; Zhao, Y.; Song, K.; Qiu, J.; Liu, Y.; Fang, S. Effect of ultrasound-assisted extraction combined with enzymatic pretreatment on bioactive compounds, antioxidant capacity and flavor characteristics of grape pulp extracts. Ultrason. Sonochem. 2025, 121, 107572. [Google Scholar] [CrossRef] [PubMed]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Postharvest stilbene-enrichment of red and white table grape varieties using UV-C irradiation pulses. J. Agric. Food Chem. 2002, 50, 6322–6329. [Google Scholar] [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; de Andrés, M.T.; Arroyo, T.; Sierra, I. A comparative study of phenolic composition and antioxidant activity in commercial and experimental seedless table grapes cultivated in a Mediterranean climate. J. Food Meas. Charact. 2021, 15, 1916–1930. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Devi, R. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 873–886. [Google Scholar] [CrossRef]
- Rashmi, H.; Negi, P. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Bondonno, N.; Lewis, J.; Blekkenhorst, L.; Bondonno, C.; Shin, J.; Croft, K.; Woodman, R.; Wong, G.; Lim, W.; Gopinath, B.; et al. Association of flavonoids and flavonoid-rich foods with all-cause mortality: The Blue Mountains Eye Study. Clin. Nutr. 2020, 39, 141–150. [Google Scholar] [CrossRef]
- Nieto, J.A.; Jaime, L.; Arranz, E.; Reglero, G.; Santoyo, S. Winemaking by-products as anti-inflammatory food ingredients. Food Agric. Immunol. 2017, 28, 1507–1518. [Google Scholar] [CrossRef]
- Radziuk, J.; Pye, S. Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis. Diabetes Metab. Res. Rev. 2001, 17, 250–272. [Google Scholar] [CrossRef]
- Ren, S.; Li, K.; Liu, Z. Research on the influences of five food-borne polyphenols on in vitro slow starch digestion and the mechanism of action. J. Agric. Food Chem. 2019, 67, 8617–8625. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Interactions between blackcurrant polyphenols and food macronutrients in model systems: In vitro digestion studies. Foods 2021, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J. Sci. Food Agric. 2002, 82, 1800–1805. [Google Scholar] [CrossRef]
- Suárez-Diéguez, T.; Palma-Morales, M.; Camacho Bernal, G.I.; Valdez López, E.N.; Rodríguez-Pérez, C.; Cruz-Cansino, N.D.S.; Nieto, J.A. Modulation of the Hyperglycemia Condition in Diabetic Lab Rats with Extracts of the Creole Jamaica Flower (Hibiscus sabdariffa L.). Antioxidants 2024, 13, 1010. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Garbetta, A.; Cardinali, A.; Bazzu, G.; D’Antuono, I.; Rocchitta, G.; Minervini, F. Real-time monitoring of glucose and phenols intestinal absorption through an integrated Caco-2TC7cells/biosensors telemetric device: Hypoglycemic effect of fruit phytochemicals. Biosens. Bioelectron. 2017, 88, 159–166. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Cayla, L.; Cottereau, P.; Renard, R. Estimation de la maturité phénolique des raisins rouges par la méthode ITV standard. Rev. Française d’Oenol. 2002, 193, 10–16. [Google Scholar]
- Smith, P.A. Precipitation of tannin with methyl cellulose allows tannin quantification in grape and wine samples. Tech. Rev. Aust. Wine Res. Inst. 2005, 158, 3–7. [Google Scholar]
- Miller, N.J.; Sampson, J.; Candelas, L.P.; Bramley, C.A.; Rice-Evans, C. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Bautista-Ortín, A.B.; Jurado, R.; Gómez-Plaza, E. Combining high-power ultrasound and enological enzymes during winemaking to improve the chromatic characteristics of red wine. LWT 2022, 156, 113032. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Pérez-Álvarez, E.; López-Urrea, R.; Intrigliolo, D.; González-Centeno, M.R.; Teissedre, P.-L.; Gil-Muñoz, R. Is Deficit Irrig. Saline Waters A Viable Altern. Winegrowers Semiarid Areas? OENO One 2022, 56, 101–116. [Google Scholar] [CrossRef]
- Nieto, J.A.; Hellín, P.; Pérez, B.; Viadel, B.; Alapont, A.; Agudelo, A. Fresh Brassicaceae sprouting broccoli (Bimi®) glucosinolates profile characterization and bioaccessibility through an in vitro dynamic digestion study. J. Food Compos. Anal. 2023, 115, 104941. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.; Noort, M.W.; Rouau, X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res. Int. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R.; Veld, J.H.H.I.T. A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar] [CrossRef]
- Gao, F.; Jian, L.; Zafar, M.I.; Du, W.; Cai, Q.; Shafqat, R.A.; Lu, F. 4-Hydroxyisoleucine improves insulin resistance in HepG2 cells by decreasing TNF-α and regulating the mechanism. Mol. Med. Rep. 2015, 12, 6555–6560. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, S.; Ni, L.; Huang, L.; Wang, K.; Zhou, Y. Dexmedetomidine alleviates insulin resistance in hepatocytes by reducing endoplasmic reticulum stress. Endocrine 2020, 67, 87–94. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roca, J.M.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B. Effect of different enological practices on skin and seed proanthocyanidins in three varietal wines. J. Agric. Food Chem. 2010, 58, 11333–11339. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Xia, N.; Sun, Q.; Duan, C.; Pan, Q. Evolution of seed-soluble and insoluble tannins during grape berry maturation. Molecules 2023, 28, 3050. [Google Scholar] [CrossRef]
- Hasanaliyeva, G.; Chatzidimitrou, E.; Wang, J.; Baranski, M.; Volakakis, N.; Seal, C.; Rempelos, L. Effects of production region, production systems and grape type/variety on nutritional quality parameters of table grapes; results from a UK retail survey. Foods 2020, 9, 1874. [Google Scholar] [CrossRef]
- Lutz, M.; Jorquera, K.; Cancino, B.; Ruby, R.; Henriquez, C. Phenolics and antioxidant capacity of table grape (Vitis vinifera L.) cultivars grown in Chile. J. Food Sci. 2011, 76, C1088–C1093. [Google Scholar] [CrossRef]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M.E.; Xiao, D.; Weinstein, I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Am. Assoc. Cancer Res. 2002, 8, 893–903. [Google Scholar]
- Bautista-Ortín, A.B.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. The effect of grape ripening stage on red wine color. OENO One 2006, 40, 15–24. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; García, J.M.R.; Bautista-Ortín, A.B.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Anthocyanins and tannins in four grape varieties (Vitis vinifera L.). Evolution of their content and extractability. OENO One 2008, 42, 147–156. [Google Scholar] [CrossRef]
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.; Chalot, G. Chemical composition, bioactive compounds, and volatiles of six table grape varieties (Vitis vinifera L.). Food Chem. 2018, 240, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.G.; Lean, J.; Crozier, A. Extraction of phenolics and changes in antioxidant activity of red wines during vinification. J. Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef]
- Colombo, F.; Di Lorenzo, C.; Regazzoni, L.; Fumagalli, M.; Sangiovanni, E.; de Sousa, L.P.; Dell’Agli, M. Phenolic profiles and anti-inflammatory activities of sixteen table grape (Vitis vinifera L.) varieties. Food Funct. 2019, 10, 1797–1807. [Google Scholar] [CrossRef]
- Hernández-Jiménez, A.; Gil-Muñoz, R.; Ruiz-García, Y.; López-Roca, J.M.; Martinez-Cutillas, A.; Gómez-Plaza, E. Evaluating the polyphenol profile in three segregating grape (Vitis vinifera L.) populations. J. Anal. Methods Chem. 2013, 2013, 572896. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E.; Puertas, B.; Richard, T. Daily preharvest UV-C light maintains the high stilbenoid concentration in grapes. J. Agric. Food Chem. 2016, 64, 5139–5147. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bae, H. An overview of stress-induced resveratrol synthesis in grapes: Perspectives for resveratrol-enriched grape products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin-rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Diaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Yuan, Q.; Su, D.; Liu, W. Phytochemical properties and antioxidant capacities of various colored berries. J. Sci. Food Agric. 2014, 94, 180–188. [Google Scholar] [CrossRef]
- Ogawa, K.; Sakakibara, H.; Iwata, R.; Ishii, T.; Sato, T.; Goda, T.; Kumazawa, S. Anthocyanin composition and antioxidant activity of the crowberry (Empetrum nigrum) and other berries. J. Agric. Food Chem. 2008, 56, 4457–4462. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Palmieri, L.; Mattivi, F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J. Food Compos. Anal. 2012, 25, 9–16. [Google Scholar] [CrossRef]
- Moze, S.; Polak, T.; Gasperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef] [PubMed]
- Yuca, H. An approach throughout in vitro studies on natural compounds as α-glucosidase inhibitors: A review. Stud. Nat. Prod. Chem. 2024, 83, 249–292. [Google Scholar]
- Barrett, A.H.; Farhadi, N.F.; Smith, T.J. Slowing starch digestion and inhibiting digestive enzyme activity using plant flavanols/tannins—A review of efficacy and mechanisms. LWT 2018, 87, 394–399. [Google Scholar] [CrossRef]
- Barrett, A.; Ndou, T.; Hughey, C.A.; Straut, C.; Howell, A.; Dai, Z.; Kaletunc, G. Inhibition of α-amylase and glucoamylase by tannins extracted from cocoa, pomegranates, cranberries, and grapes. J. Agric. Food Chem. 2013, 61, 1477–1486. [Google Scholar] [CrossRef]
- Oliveira, H.; Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as antidiabetic agents—In vitro and in silico approaches of preventive and therapeutic effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 2. α-Glucosidase inhibition by isolated acylated anthocyanins. J. Agric. Food Chem. 2001, 49, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells—Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Kim, K.H.; Park, Y. Piceatannol reduces fat accumulation in Caenorhabditis elegans. J. Med. Food 2017, 20, 887–894. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1145–1154. [Google Scholar] [CrossRef]
- Da Costa, G.F.; Santos, I.B.; de Bem, G.F.; Cordeiro, V.S.C.; da Costa, C.A.; de Carvalho, L.C.R.M.; de Moura, R.S. The beneficial effect of anthocyanidin-rich Vitis vinifera L. grape skin extract on metabolic changes induced by high-fat diet in mice involves antiinflammatory and antioxidant actions. Phytother. Res. 2017, 31, 1621–1632. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure–activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Regazzoni, L.; Redaelli, V.; Grandi, S.; Riva, A.; Aldini, G. Pharmacokinetic profile of bilberry anthocyanins in rats and the role of glucose transporters: LC–MS/MS and computational studies. J. Pharm. Biomed. Anal. 2017, 144, 112–121. [Google Scholar] [CrossRef]
- Cahyana, Y.; Mills, C.E.; Huda, S.; Gordon, M.H. Factors affecting cellular uptake of anthocyanins: The role of pH, glucose and anthocyanin structure. Nutrients 2022, 14, 4807. [Google Scholar] [CrossRef]
- Walgren, R.A.; Lin, J.T.; Kinne, R.K.H.; Walle, T. Cellular uptake of dietary flavonoid quercetin 4′-β-glucoside by sodium-dependent glucose transporter SGLT1. J. Pharmacol. Exp. Ther. 2000, 294, 837–843. [Google Scholar] [CrossRef]
- Wolffram, S.; Blöck, M.; Ader, P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Role of red grape polyphenols as antidiabetic agents. Integr. Med. Res. 2014, 3, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Nunes, S.; Vieira, P.; Gomes, P.; Viana, S.D.; Reis, F. Blueberry as an attractive functional fruit to prevent (pre) diabetes progression. Antioxidants 2021, 10, 1162. [Google Scholar] [CrossRef]
- Sharma, K.; Malathi, V.M.; Thappa, C.; Kour, N.; Abhimannue, A.P. Bioactive Compounds in Blueberry Fruit and Their Antidiabetic Activity. Curr. Food Sci. Technol. Rep. 2024, 2, 309–318. [Google Scholar] [CrossRef]
- Crescenti, A.; del Bas, J.M.; Arola-Arnal, A.; Oms-Oliu, G.; Arola, L.; Caimari, A. Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up-regulate AMPK in the muscle of male offspring in adulthood. J. Nutr. Biochem. 2015, 26, 912–920. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Weirauch, M.T. The human transcription factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Peng, J.; Lu, C.; Luo, Y.; Su, X.; Li, S.; Ho, C.T. Hypoglycemic effects and associated mechanisms of resveratrol and related stilbenes in diet. Food Funct. 2024, 15, 2381–2405. [Google Scholar] [CrossRef]
- Moore, W.T.; Luo, J.; Liu, D. Kaempferol improves glucose uptake in skeletal muscle via an AMPK-dependent mechanism. Food Sci. Hum. Wellness 2023, 12, 2087–2094. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the paradox of hepatic insulin resistance. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Meeprom, A.; Sompong, W.; Suwannaphet, W.; Yibchok-anun, S.; Adisakwattana, S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signalling pathways. Br. J. Nutr. 2011, 106, 1173–1181. [Google Scholar] [CrossRef]
- Dhital, S.; Gidley, M.J.; Warren, F.J. Inhibition of α-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydr. Polym. 2015, 123, 305–312. [Google Scholar] [CrossRef]
- Wang, J.; Xie, B.; Sun, Z. Anion carboxymethylated β-glucan alleviates undesirable binding between procyanidins and β-galactosidase. Food Chem. 2021, 344, 128686. [Google Scholar] [CrossRef] [PubMed]
- Kanagarla, N.S.S.A.V.; Kuppast, I.J.; Veerashekar, T.; Reddy, C.L. A Review on benefits and uses of Vitis vinifera (Grape). Res. Rev. BioSci. 2013, 7, 175–180. [Google Scholar]
- Hussain, S.Z.; Naseer, B.; Qadri, T.; Fatima, T.; Bhat, T.A. Pecan Nut (Carya illinoensis)—Morphology, Taxonomy, Composition and Health Benefits. In Fruits Grown in Highland Regions of the Himalayas; Srivastava, R.P., Ed.; Springer: Cham, Switzerland, 2021; pp. 345–372. [Google Scholar]
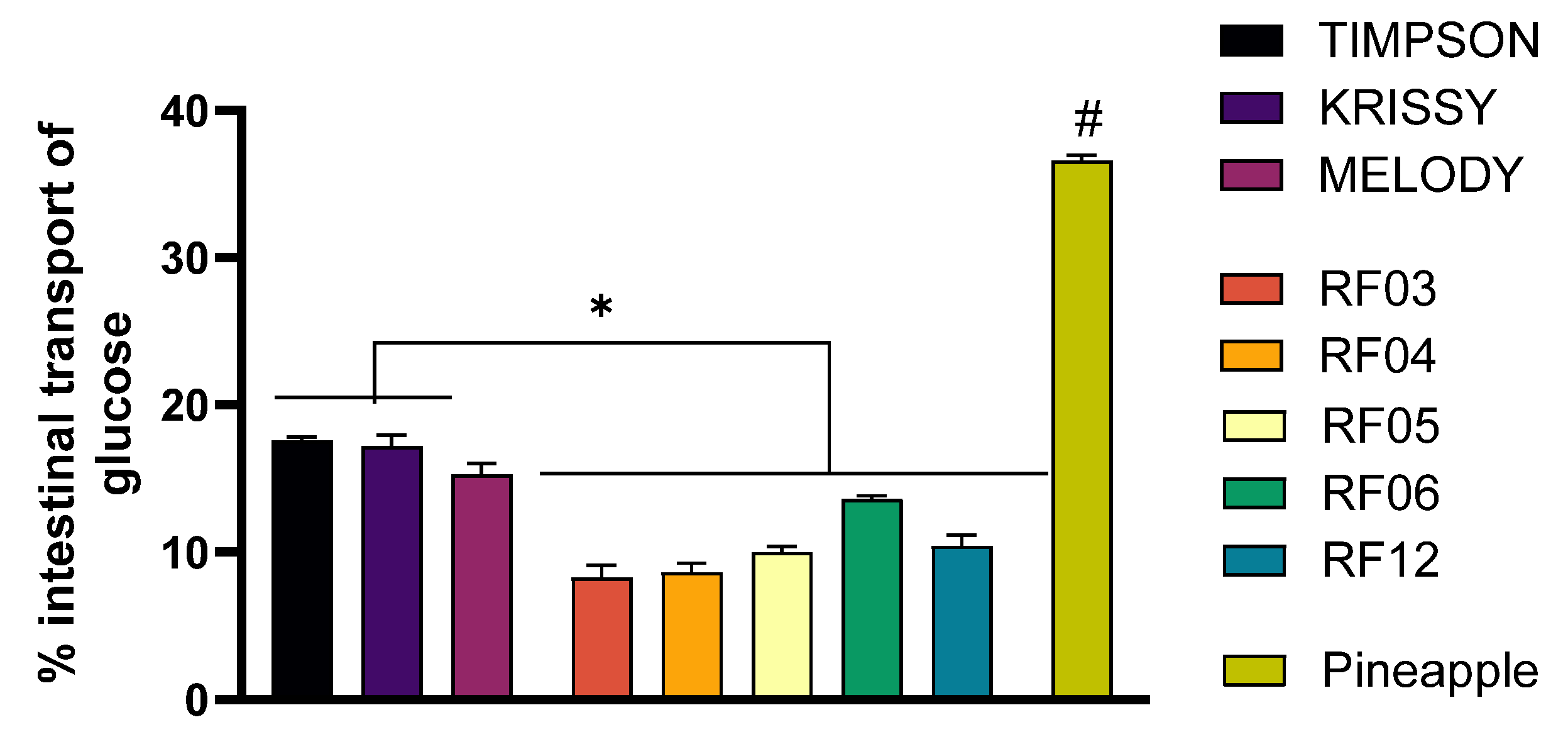
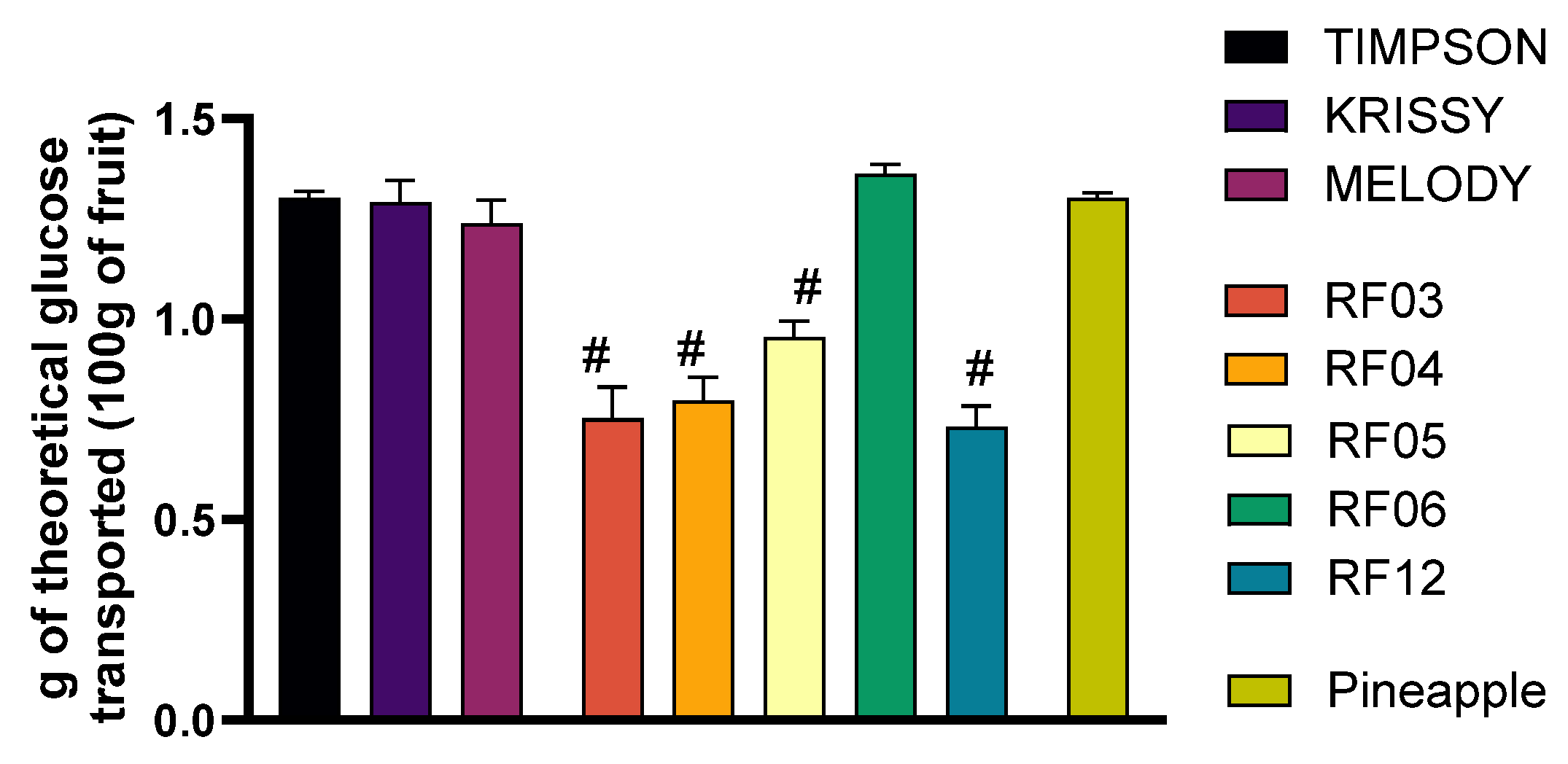
| Samples | TEAC | TFC | TA | TT |
|---|---|---|---|---|
| TimpsonTM | 58.3 ± 2.9 a | 21.1 ± 1.0 a | 0.0 a | 38.6 ± 1.9 a |
| KrissyTM | 62.4 ± 3.1 a | 29.8 ± 1.5 b | 9.2 ± 0.5 b | 33.6 ± 1.7 a |
| MelodyTM | 80.9 ± 4.0 a | 61.0 ± 3.1 d | 30.1 ± 1.5 c | 109.5 ± 5.5 cd |
| RF01 | 660.6 ± 33.0 h | 161.6± 8.1 i | 169.6 ± 8.5 h | 250.8 ±12.5 h |
| RF02 | 762.7 ± 38.1 i | 164.1 ± 8.2 i | 261.3 ±13.1 k | 216.5 ± 10.8 g |
| RF03 | 259.3 ± 12.9 c | 113.5 ± 5.7 h | 180.6 ± 9.0 ij | 181.6 ± 9.1 f |
| RF04 | 295.0 ± 14.7 d | 187.3 ± 9.3 k | 337.7 ± 16.9 l | 55.3 ± 2.7 b |
| RF05 | 195.3 ± 9.7 b | 52.4 ± 2.6 c | 78.2 ± 3.9 d | 92.6 ± 4.6 c |
| RF06 | 261.2 ± 13.1 c | 73.8 ± 3.7 e | 150.2 ± 7.5 g | 111.3 ± 5.5 d |
| RF07 | 329.6 ± 16.5 e | 84.0 ± 4.2 j | 123.8 ± 6.2 f | 154.1 ± 7.7 e |
| RF08 | 333.4 ± 16.7 e | 75.0 ± 3.8 e | 189.3 ± 9.4 j | 119.9 ± 5.9 d |
| RF09 | 230.8 ± 11.5 c | 74.5 ± 3.7 e | 177.0 ± 8.8 hi | 114.3 ± 5.7 d |
| RF10 | 435.5 ± 21.8 g | 99.3 ± 4.9 g | 187.7 ± 9.4 j | 249.3 ± 12.4 i |
| RF11 | 372.0 ± 8.6 f | 88.4 ± 4.4 f | 101.1 ± 5.0 e | 240.1 ± 12.0 i |
| RF12 | 257.6 ± 12.9 c | 69.2 ± 3.5 de | 123.8 ± 6.2 f | 194.5 ± 9.7 g |
| Compounds | RF03 | RF04 | RF05 | RF06 | RF12 |
|---|---|---|---|---|---|
| Anthocyanins (mg/100 g FW) | |||||
| Delphinidin-3-Glu | 1.9 ± 0.1 c | 11.± 0.6 d | 0.4 ± 0.0 ab | 0.1 ± 0.0 a | 0.9 ± 0.0 b |
| Cianidin-3-Glu | 0.5 ± 0.0 a | 8.0 ± 0.4 c | 0.4 ± 0.0 a | 0.9 ± 0.0 b | 0.3 ± 0.0 a |
| Petunidin-3-Glu | 3.0 ± 0.1 c | 10.9 ± 0.5 d | 0.6 ± 0.0 a | 0.4 ± 0.0 a | 1.7 ± 0.1 b |
| Peonidin-3-Glu | 9.9 ± 0.5 c | 47.4 ± 2.4 d | 10.7 ± 0.5 c | 2.0 ± 0.1 a | 5.9 ± 0.3 b |
| Malvidin-3-Glu | 20.8 ± 1.0 c | 42.3 ± 2.1 d | 6.2 ± 0.3 a | 12.1 ± 0.6 b | 13.7 ± 0.7 b |
| TA-Acylated | 40.3 ± 2.0 cd | 45.9 ± 2.3 d | 15.1 ± 0.7 a | 36.6 ± 1.8 c | 23.3 ± 1.1 b |
| ƩTotal | 76.4 ± 3.8 c | 166.4 ± 8.3 d | 33.3 ± 1.6 a | 52.1 ± 2.6 b | 45.7 ± 2.3 ab |
| Flavonols (mg/100 g FW) | |||||
| Myricetin-3-Glu | 0.92 ± 0.04 c | 2.71 ± 0.13 d | 0.23 ± 0.01 a | 0.65 ± 0.03 b | 0.52 ± 0.02 b |
| Quercetin-3-Glur | 0.41 ± 0.02 c | 0.24 ± 0.01 b | 0.17 ± 0.01 a | 0.28 ± 0.01 b | 0.92 ± 0.04 d |
| Quercetin-3-Glu | 0.43 ± 0.02 a | 2.15 ± 0.01 d | 0.88 ± 0.04 c | 0.55 ± 0.03 b | 0.51 ± 0.02 b |
| Laricitrin-3-Glu | 0.38 ± 0.02 c | 0.46 ± 0.02 d | 0.14 ± 0.01 a | 0.29 ± 0.01 b | 0.35 ± 0.02 c |
| Isorhamnetin-3-Glu | 0.41 ± 0.02 b | 1.39±0.07 d | 0.53 ± 0.02 c | 0.48 ± 0.02 c | 0.32 ± 0.01 a |
| Siringetin-3-Glu | 0.55 ± 0.03 d | 0.52 ± 0.02 d | 0.16 ± 0.01 a | 0.28 ± 0.01 b | 0.49 ± 0.02 c |
| ƩTotal | 3.09 ± 0.15 c | 7.58 ± 0.38 d | 2.09 ± 0.10 a | 2.54 ± 0.13 b | 3.11 ± 0.15 c |
| Stilbenes (µg/100 g FW) | |||||
| Trans-Piceid | 12.2 ± 0.6 ab | 35.7 ± 1.8 c | 10.1 ± 0.5 a | 14.5 ± 0.7 b | 8.8 ± 0.4 a |
| Piceatannol | 59.1 ± 2.9 b | 190.1 ± 9.5 c | 1.5 ± 0.1 a | 4.9 ± 0.2 a | 4.1 ± 0.2 a |
| Cis-Piceid | 269.4 ± 13.5 c | 316.2 ± 15.8 d | 55.5 ± 2.8 a | 112.2 ± 5.6 b | 88.4 ± 4.4 ab |
| Trans-Resveratrol | 243.9 ± 12.2 a | 384.5 ± 19.2 a | 1081.6 ± 54.1 b | 2480.2 ± 124.0 d | 2017.8 ± 100.9 c |
| Cis-Resveratrol | 0.0 a | 0.0 a | 535.2 ± 26.7 b | 1220.8 ± 61.0 d | 994.2 ± 49.7 c |
| Viniferin | 197.2 ± 9.8 c | 204.3 ± 10.2 c | 62.0 ± 3.1 a | 80.8 ± 4.0 a | 144.7 ± 7.2 b |
| ƩTotal | 781.8 ± 39.1 a | 1130.8 ± 56.5 b | 1745.8 ± 87.3 c | 3913.4 ± 195.7 e | 3258.0 ± 162.9 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Ortín, A.B.; Martínez-Moreno, A.; Pérez-Mendoza, A.L.; Carrasco-Palazón, M.J.; Osete-Alcaraz, L.; Soriano-Romaní, L.; Díez-Sánchez, E.; Nieto, J.A.; Soto-Jover, S.; Gómez-Plaza, E. Development of New Red-Fleshed Seedless Table Grapes: In Vitro Insights on Glucose Absorption and Insulin Resistance Biomarkers. Foods 2025, 14, 4035. https://doi.org/10.3390/foods14234035
Bautista-Ortín AB, Martínez-Moreno A, Pérez-Mendoza AL, Carrasco-Palazón MJ, Osete-Alcaraz L, Soriano-Romaní L, Díez-Sánchez E, Nieto JA, Soto-Jover S, Gómez-Plaza E. Development of New Red-Fleshed Seedless Table Grapes: In Vitro Insights on Glucose Absorption and Insulin Resistance Biomarkers. Foods. 2025; 14(23):4035. https://doi.org/10.3390/foods14234035
Chicago/Turabian StyleBautista-Ortín, Ana Belén, Alejandro Martínez-Moreno, Ana Leticia Pérez-Mendoza, María José Carrasco-Palazón, Lucía Osete-Alcaraz, Laura Soriano-Romaní, Elena Díez-Sánchez, Juan Antonio Nieto, Sonia Soto-Jover, and Encarna Gómez-Plaza. 2025. "Development of New Red-Fleshed Seedless Table Grapes: In Vitro Insights on Glucose Absorption and Insulin Resistance Biomarkers" Foods 14, no. 23: 4035. https://doi.org/10.3390/foods14234035
APA StyleBautista-Ortín, A. B., Martínez-Moreno, A., Pérez-Mendoza, A. L., Carrasco-Palazón, M. J., Osete-Alcaraz, L., Soriano-Romaní, L., Díez-Sánchez, E., Nieto, J. A., Soto-Jover, S., & Gómez-Plaza, E. (2025). Development of New Red-Fleshed Seedless Table Grapes: In Vitro Insights on Glucose Absorption and Insulin Resistance Biomarkers. Foods, 14(23), 4035. https://doi.org/10.3390/foods14234035








