Survey of Tetrodotoxins (TTXs) in Gastropods, Sea Urchins, and Blue Crabs from the Adriatic Sea: First Report in Paracentrotus lividus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Design
2.2. Sample Treatment
2.3. Chemical Analysis of TTXs
2.3.1. Chemicals and Standards
2.3.2. TTX Extraction
2.3.3. HILIC-MS/MS Analysis
2.3.4. HILIC-MS/MS Method Validation/Optimization
2.3.5. Statistical Analysis
3. Results and Discussion
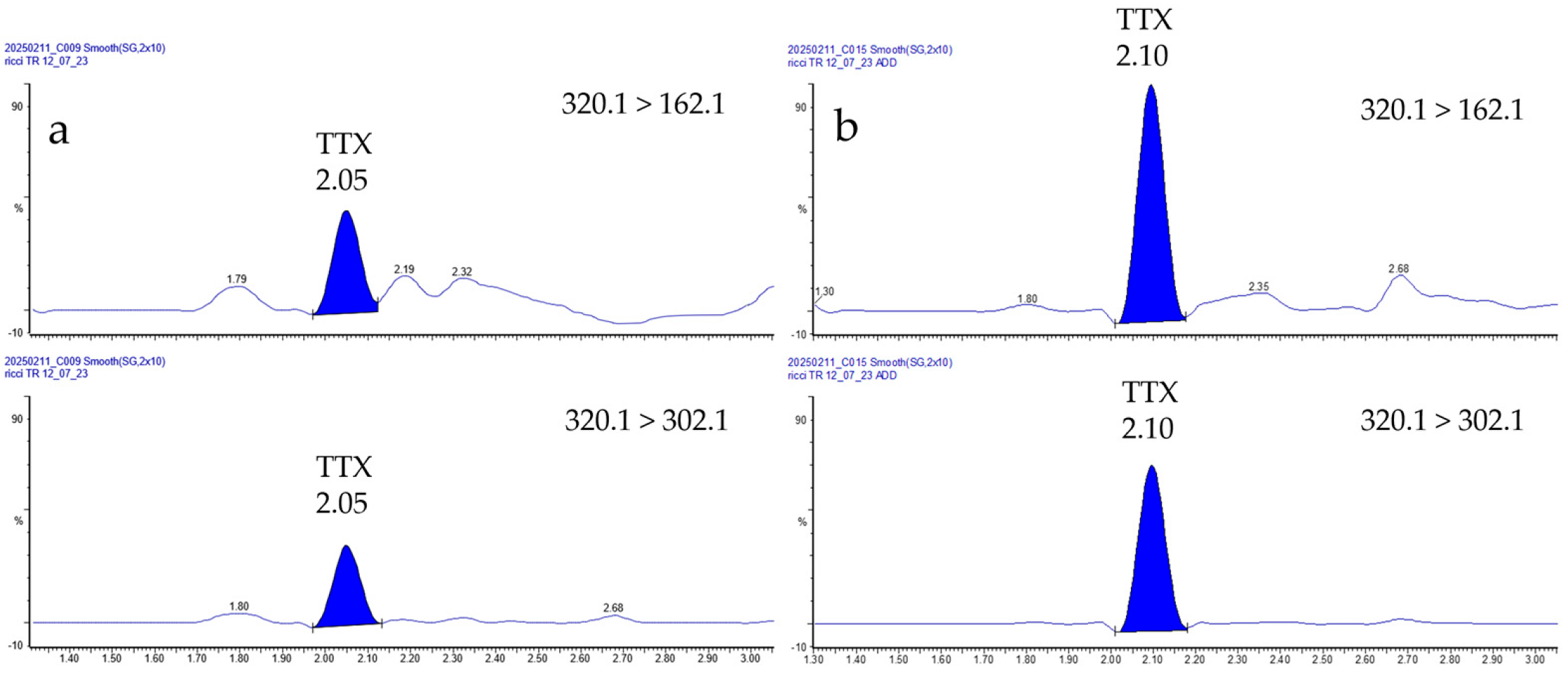
3.1. Method Performances
3.2. TTXs Survey in North–Central Adriatic Sea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, K.; Barnes, P.; Haughey, S.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C. Development and Single Laboratory Validation of an Optical Biosensor Assay for Tetrodotoxin Detection as a Tool to Combat Emerging Risks in European Seafood Rapid Detection in Food and Feed. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef]
- Kodama, M.; Noguchi, T.; Maruyama, J.; Ogata, T.; Hashimoto, K. Release of Tetrodotoxin and Paralytic Shellfish Poison from Puffer Liver by RNase. J. Biochem. 1983, 93, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX Accumulation in Pufferfish. Comp. Biochem. Physiol. D Genom. Proteom. 2006, 1, 145–152. [Google Scholar] [CrossRef]
- Chau, R.; Kalaitzis, J.A.; Neilan, B.A. On the Origins and Biosynthesis of Tetrodotoxin. Aquat. Toxicol. 2011, 104, 61–72. [Google Scholar] [CrossRef]
- Denac, H.; Mevissen, M.; Scholtysik, G. Structure, function and pharmacology of voltage-gated sodium channels. Naunyn Schmiedeberg’s Arch. Pharmacol. 2000, 362, 453–479. [Google Scholar] [CrossRef]
- Scheib, H.; McLay, I.; Guex, N.; Clare, J.J.; Blaney, F.E.; Dale, T.J.; Tate, S.N.; Robertson, G.M. Modeling the pore structure of voltage-gated sodium channels in closed, open, and fast-inactivated conformation reveals details of site 1 toxin and local anesthetic binding. J. Mol. Model. 2006, 12, 813–822. [Google Scholar] [CrossRef]
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of tetrodotoxin in Alexandrium tamarense, a causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996, 34, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First Detection of Tetrodotoxin in the Bivalve Paphies australis by Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry With and Without Precolumn Reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef] [PubMed]
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, L.A.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; et al. Detection of tetrodotoxin from the grey side-gilled sea slug—Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473. [Google Scholar] [CrossRef]
- Wood, S.A.; Casas, M.; Taylor, D.I.; McNabb, P.; Salvitti, L.; Ogilvie, S.; Cary, S.C. Depuration of Tetrodotoxin and Changes in Bacterial Communities in Pleurobranchea maculata Adults and Egg Masses Maintained in Captivity. J. Chem. Ecol. 2012, 38, 1342–1350. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New Gastropod Vectors and Tetrodotoxin Potential Expansion in Temperate Waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 2000, 38, 763–773. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-Producing Bacteria: Detection, Distribution and Migration of the Toxin in Aquatic Systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef]
- Lee, M.-J.; Jeong, D.Y.; Kim, W.S.; Kim, H.D.; Kim, C.H.; Park, W.W.; Park, Y.H.; Kim, K.S.; Kim, H.M.; Kim, D.S. A Tetrodotoxin-Producing Vibrio strain, LM-1, from the Puffer Fish Fugu vermicularis radiatus. Appl. Environ. Microbiol. 2000, 66, 1698–1701. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Y.; Xie, L.; Xia, G.; Hu, J.; Wang, S.; Zhang, R. Toxicity and distribution of tetrodotoxin-producing bacteria in puffer fish Fugu rubripes collected from the Bohai Sea of China. Toxicon 2005, 46, 471–476. [Google Scholar] [CrossRef]
- Do, H.K.; Kogure, K.; Simidu, U. Identification of Deep-Sea-Sediment Bacteria Which Produce Tetrodotoxin. Appl. Environ. Microbiol. 1993, 56, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef] [PubMed]
- Akyol, O.; Ünal, V.; Ceyhan, T.; Bilecenoglu, M. First confirmed record of Lagocephalus sceleratus (Gmelin, 1789) in the Mediterranean Sea. J. Fish Biol. 2005, 66, 1183–1186. [Google Scholar] [CrossRef]
- Golani, D.; Levy, Y. New records and rare occurrences of fish species from the Mediterranean coast of Israel. Zool. Middle East. 2005, 36, 27–32. [Google Scholar] [CrossRef]
- Corsini, M.; Margies, P.; Kondilatos, G.; Economidis, P.S. Three new exotic fish records from the SE Aegean Greek waters. Sci. Mar. 2006, 70, 319–323. [Google Scholar] [CrossRef]
- Kasapidis, P. First record of the Lessepsian migrant Lagocephalus sceleratus (Gmelin 1789) (Osteichthyes: Tetraodontidae) in the Cretan Sea (Aegean, Greece). Aquat. Invasions 2007, 2, 71–73. [Google Scholar] [CrossRef]
- Mutlu, E.; De Meo, I.; Miglietta, C. Spatio-temporal distribution of pufferfish (Tetraodontidae) along the Turkish coast of the Mediterranean Sea. Mediterr. Mar. Sci. 2021, 22, 1–19. [Google Scholar] [CrossRef]
- Coro, G.; Gonzalez Vilas, L.; Magliozzi, C.; Ellenbroek, A.; Scarponi, P.; Pagano, P. Forecasting the ongoing invasion of Lagocephalus sceleratus in the Mediterranean Sea. Ecol. Model. 2018, 371, 37–49. [Google Scholar] [CrossRef]
- Azzurro, E.; Bariche, M.; Cerri, J.; Garrabou, J. The long reach of the Suez Canal: Lagocephalus sceleratus (Gmelin, 1789) an unwanted Indo-Pacific pest at the Atlantic gate. Bioinvasions Rec. 2020, 9, 204–208. [Google Scholar] [CrossRef]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin. Tetrodotoxin in European Bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009. [Google Scholar] [CrossRef] [PubMed]
- Leão, J.M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, Ó.; Gago-Martínez, A. Preliminary Results on the Evaluation of the Occurrence of Tetrodotoxin Associated to Marine Vibrio Spp. in Bivalves from the Galician Rias (Northwest of Spain). Mar. Drugs 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First Detection of Tetrodotoxin in Greek Shellfish by UPLC-MS/MS Potentially Linked to the Presence of the Dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef]
- Gerssen, A.; Bovee, T.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R. First Report on the Occurrence of Tetrodotoxins in Bivalve Mollusks in The Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First Toxicity Report of Tetrodotoxin and 5, 6,11-TrideoxyTTX in the Trumpet Shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–892. [Google Scholar] [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Leoni, F.; Tavoloni, T.; Accoroni, S.; Gorbi, S.; Giuliani, M.E.; Stramenga, A. Tetrodotoxins (TTXs) and Vibrio alginolyticus in Mussels from Central Adriatic Sea (Italy): Are They Closely Related? Mar. Drugs 2021, 19, 304. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. A Risks for public health related to the Presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, 47–52. [Google Scholar] [CrossRef]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A Global Retrospective Study on Human Cases of Tetrodotoxin (TTX) Poisoning after Seafood Consumption. Food Rev. Int. 2020, 36, 645–667. [Google Scholar] [CrossRef]
- Chiesa, S.; Petochi, T.; Brusà, R.B.; Raicevich, S.; Cacciatore, F.; Franceschini, G.; Antonini, C.; Vallini, C.; Bernarello, V.; Oselladore, F.; et al. Impacts of the blue crab invasion on Manila clam aquaculture in Po Delta coastal lagoons (Northern Adriatic Sea, Italy). Estuar. Coast. Shelf Sci. 2025, 312, 109037. [Google Scholar] [CrossRef]
- Ippoliti, D.; Lenzo, D.; Vollaro, T.M.; Cangini, M.; Dall’Ara, S.; Calfapietra, A.; Pino, F.; Milandri, S. Tetrodotossine nei molluschi bivalvi: Primi casi di positività in Emilia-Romagna. In Proceedings of the XI Convegno Nazionale SIRAM, Cattolica, Italy, 4–5 October 2024. [Google Scholar]
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Accoroni, S.; Romagnoli, T.; Campanelli, A.; Griffoni, F.; Tavoloni, T.; Gorbi, S.; et al. A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors. Mar. Drugs 2023, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Zore-Armanda, M.; Bone, M.; Dadić, V.; Morović, M.; Ratković, D.; Stojanoski, L.; Vukadin, I. Hydrographic properties of the adriatic sea in the period from 1971 through 1983. Acta Adriat. 1991, 32, 5–540. [Google Scholar]
- EURLMB. SOP for the Analysis of Paralytic Shellfish Toxins (PST) by Pre-Column HPLC-FLD According to OMA AOAC 2005.06—Version 1. 2020. Available online: https://www.aesan.gob.es/en/CRLMB/web/public_documents/seccion/crlmb_standard_operating_procedures.htm (accessed on 20 October 2025).
- EURLMB. Determination of Tetrodotoxin by HILIC-MS/MS. 2017. Available online: https://www.aesan.gob.es/en/CRLMB/web/public_documents/seccion/crlmb_standard_operating_procedures.htm (accessed on 20 October 2025).
- Turner, A.D.; Dean, K.J.; Dhanji-Rapkova, M.; Dall’Ara, S.; Pino, F.; McVey, C.; Haughey, S.; Logan, N.; Elliott, C.; Gago-Martinez, A.; et al. Interlaboratory Evaluation of Multiple LC–MS/MS Methods and a Commercial ELISA Method for Determination of Tetrodotoxin in Oysters and Mussels. J. AOAC Int. 2023, 106, 356–369. [Google Scholar] [CrossRef]
- Ferrer, C.; Martínez-Bueno, M.J.; Lozano, A.; Fernández-Alba, A.R. Pesticide residue analysis of fruit juices by LC-MS/MS direct injection. One year pilot survey. Talanta 2011, 83, 1552–1561. [Google Scholar] [CrossRef]
- Lage, S.; Afonso, I.I.; Reis Costa, P.; Canário, A.V.M.; Da Silva, J.P. Tissue accumulation of tetrodotoxin (TTX) and analogues in trumpet shell Charonia lampas. Food. Addit. Contam. Part A 2023, 40, 159–168. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T. Ultrahigh-Performance Hydrophilic Interaction Liquid Chromatography with Tandem Mass Spectrometry Method for the Determination of Paralytic Shellfish Toxins and Tetrodotoxin in Mussels, Oysters, Clams, Cockles, and Scallops: Collaborative Study. J. AOAC Int. 2020, 103, 533–562. [Google Scholar] [CrossRef]
- Maggiore, A.; Afonso, A.; Barrucci, F.; Sanctis, G.D. Climate change as a driver of emerging risks for food and feed safety, plant, animal health and nutritional quality. EFSA Support. Publ. 2020, 17, 1881E. [Google Scholar] [CrossRef]
- Hong, H.K.; Kajino, N.; Park, B.K.; Shin, J.S.; Lee, J.; Choi, K.S. Detection of tetrodotoxin (TTX) and its analogues in mud snails Nassarius livescens occurring on a sandy beach in Jeju Island, Korea, using liquid chromatography–tandem mass spectrometry (LC–MS/MS). Fish Sci. 2023, 89, 863–873. [Google Scholar] [CrossRef]
- Yin, H.L.; Lin, H.S.; Huang, C.C.; Hwang, D.F.; Liu, J.S.; Chen, W.H. Tetrodotoxication with Nassauris glans: A possibility of tetrodotoxin spreading in marine products near Pratas Island. Am. J Trop. Med. Hyg. 2005, 73, 985–990. [Google Scholar] [CrossRef]
- Silva, M.; Rodríguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. Tetrodotoxins Occurrence in Non-Traditional Vectors of the North Atlantic Waters (Portuguese Maritime Territory, and Morocco Coast). Toxins 2019, 11, 306. [Google Scholar] [CrossRef]
- Biessy, A.; Novinscak, A.; Blom, J.; Léger, G.; Thomashow, L.S.; Cazorla, F.M.; Josic, D.; Filion, M. Diversity of phytobeneficial traits revealed by whole-genome analysis of worldwide-isolated phenazine-producing Pseudomonas spp. Environ. Microbiol. 2019, 21, 437–455. [Google Scholar] [CrossRef]
- Hwang, D.F.; Chueh, C.H.; Jeng, S.S. Tetrodotoxin secretion from the lined moon shell Natica lineata in response to external stimulation. Toxicon 1990, 28, 1133–1136. [Google Scholar] [CrossRef]
- Hwang, D.F.; Tai, K.P.; Chueh, C.H.; Lin, L.C.; Jeng, S.S. Tetrodotoxin and derivatives in several species of the gastropod Naticidae. Toxicon 1991, 29, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Han, J.L.; Zhang, L.; Zhou, P.P.; Xu, J.J.; Pan, X.D.; Cao, P.; Xu, X.M. Analytical Method Optimization of Tetrodotoxin and Its Contamination in Gastropods. Foods 2023, 12, 3103. [Google Scholar] [CrossRef]
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef]
- Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Harada, T. Occurrence of tetrodotoxin in the Japanese ivory shell Babylonia japonica. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 901–913. [Google Scholar] [CrossRef]
- Noguchi, T.; Maruyama, J.; Narita, H.; Hashimoto, K. Occurrence of tetrodotoxin in the gastropod mollusk Tutufa lissostoma (frog shell). Toxicon 1984, 22, 219–226. [Google Scholar] [CrossRef]
- Lin, S.J.; Hwang, D.F. Possible source of tetrodotoxin in the starfish Astropecten scoparius. Toxicon 2001, 39, 573–579. [Google Scholar] [CrossRef]
- Mazza, S.; Bacchiocchi, S.; Siracusa, M.; Diomedi, G.; Barchiesi, F.; Calandri, E.; Piersanti, A. TTXs in molluscs and blue crabs from Adriatic Sea: Do these species represent a risk for human consumption? In Proceedings of the 12th MS J-Day, Pisa, Italy, 22–23 May 2025. [Google Scholar]
- Ghisotti, F. Rapana venosa (Valenciennes), nuova ospite Adriatica? Conchiglie 1974, 10, 125–126. [Google Scholar]
- Khor, S.; Wood, S.A.; Salvitti, L.; Taylor, D.I.; Adamson, J.; McNabb, P.; Cary, S.C. Investigating Diet as the Source of Tetrodotoxin in Pleurobranchaea maculata. Mar. Drugs 2014, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- FishStatJ; Software for Fishery and Aquaculture; Statistical Time Series; Food and Agriculture Organization of the United Nations, Fishery Division: Rome, Italy, 2020.
- Yasumura, D.; Oshima, Y.; Yasumoto, T.; Alcala, A.C.; Alcala, L.C. Tetrodotoxin and Paralytic Shellfish Toxins in Philippine Crabs. Agric. Biol. Chem. 1986, 50, 593–598. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Occurrence of tetrodotoxin and paralytic shellfish poison in the Taiwanese crab Lophozozymus pictor. Toxicon 1995, 33, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Toxicity and toxic components of two xanthid crabs, atergatis floridus and demania reynaudi, in taiwan. Toxicon 1997, 35, 1327–1335. [Google Scholar] [CrossRef]
- Asakawa, M.; Gomez-Delan, G.; Tsuruda, S.; Shimomura, M.; Shida, Y.; Taniyama, S.; Barte-Quilantang, M.; Shindo, J. Toxicity Assessment of the Xanthid Crab Demania cultripes from Cebu Island, Philippines. J. Toxicol. 2010, 2010, 172367. [Google Scholar] [CrossRef]
- Lage, S.; ten Brink, F.; Canário, A.V.M.; Da Silva, J.P. New Vectors of TTX Analogues in the North Atlantic Coast: The Edible Crabs Afruca tangeri and Carcinus maenas. Mar. Drugs 2023, 21, 320. [Google Scholar] [CrossRef] [PubMed]
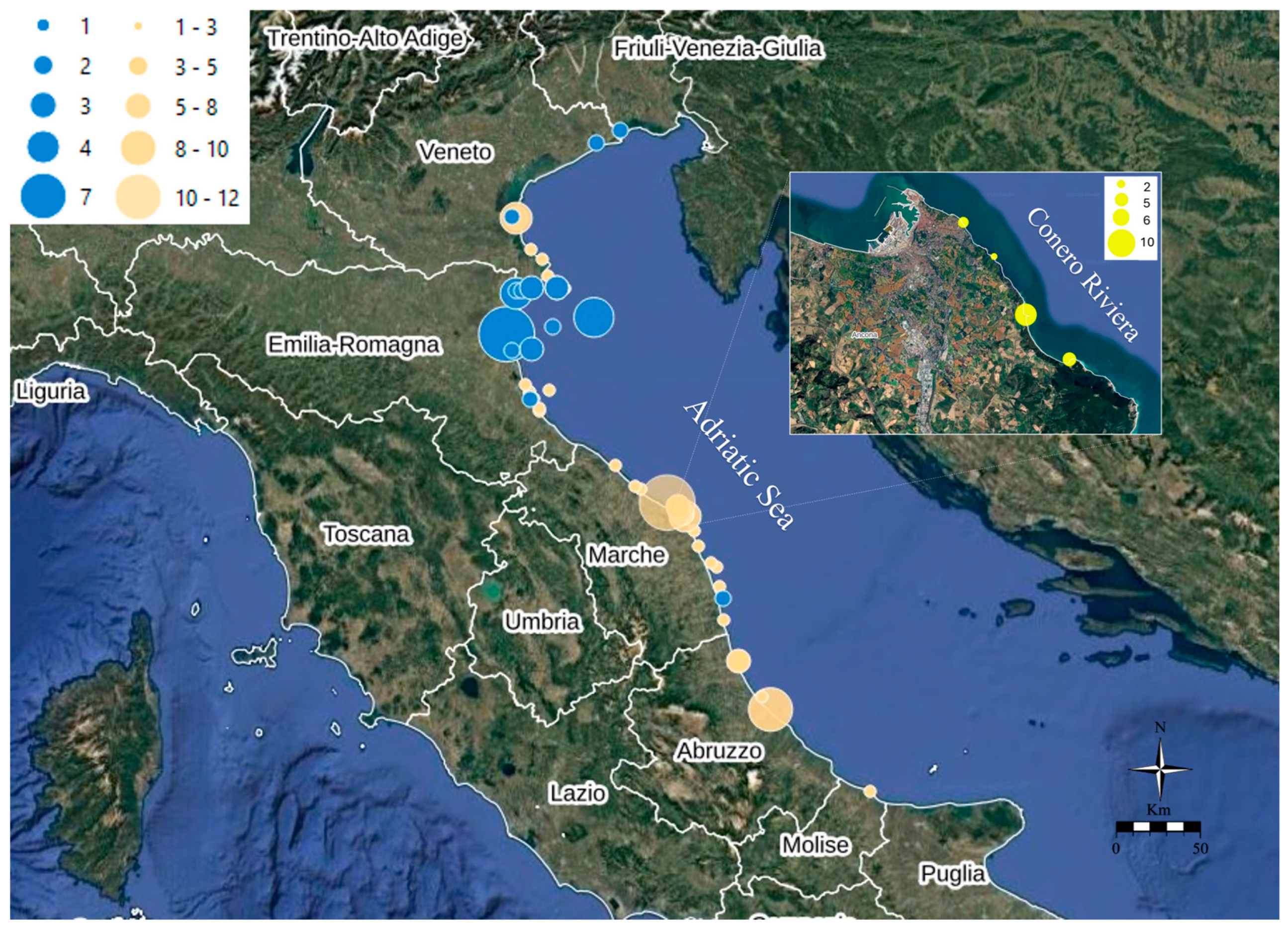
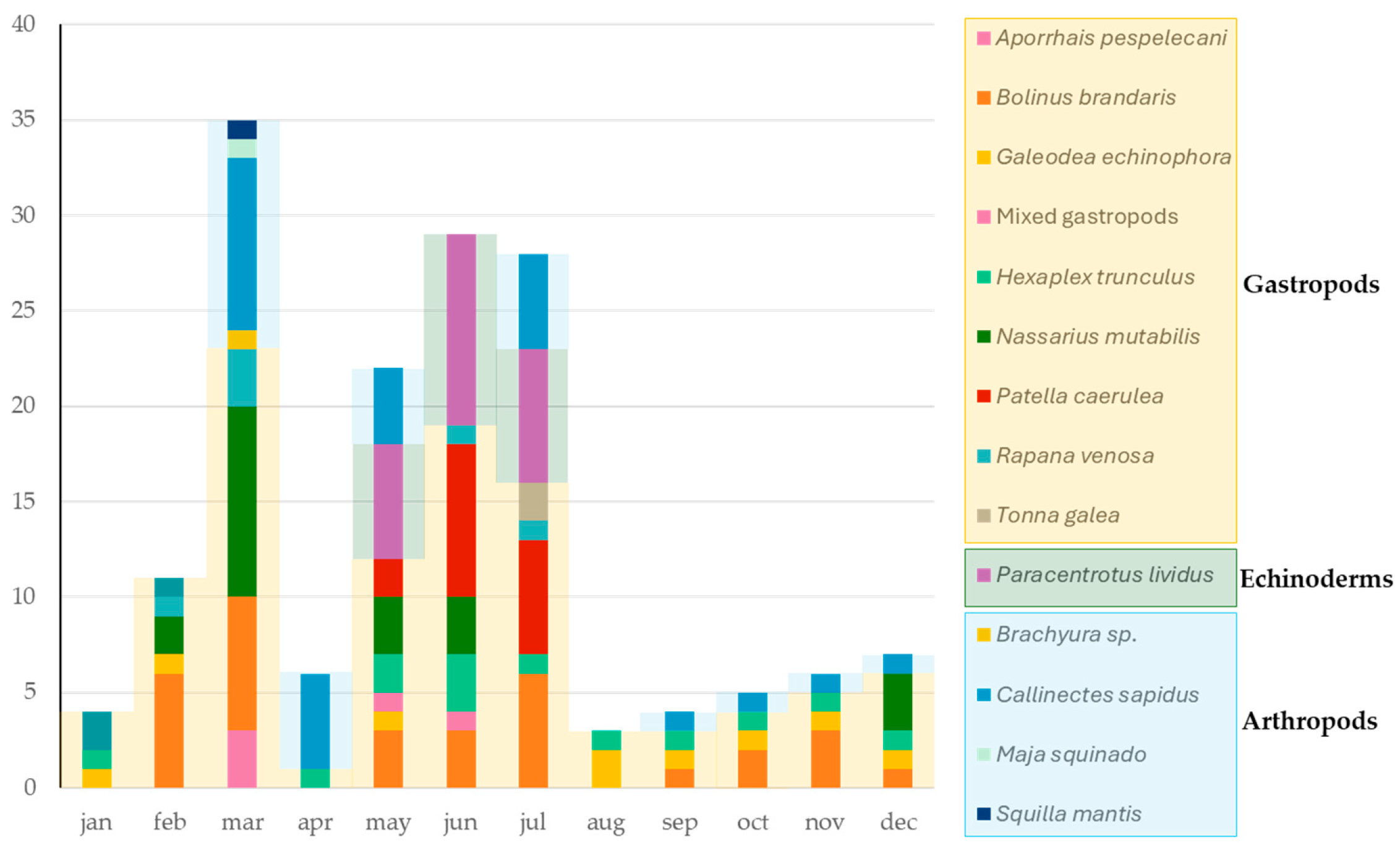
| Phylum | Species | N° of Samples | Sample Size (Specimens per Sample) | Sampling Area * | Sampling Period | Analyzed Sample (Pooled Specimens) |
|---|---|---|---|---|---|---|
| Mollusks (Gastropods) | Bolinus brandaris | 32 | 50 | Ve, ER, Ma, Ab | January 2023– March 2025 | 15 |
| Nassarius mutabilis | 21 | 100 | ER, Ma, Ab | 30 | ||
| Patella caerulea | 16 | 20 | CR (Ma) | 10 | ||
| Hexaplex trunculus | 13 | 50 | Ve, Ma | 15 | ||
| Galeodea echinophora | 9 | 10 | Ab, Mo | 5 | ||
| Rapana venosa | 6 | 5 | ER | 5 | ||
| Aporrhais pespelecani | 3 | 50 | ER | 30 | ||
| Tonna galea | 2 | 3 | Ab, Mo | 3 | ||
| Mixed gastropods species | 2 | 30 | Ma, Mo | 20 | ||
| Tot. | 104 | |||||
| Arthropods | Callinectes sapidus | 30 | 10 | FVG, Ve, ER, Ma | January 2023– March 2025 | 5 |
| Brachyura spp. | 1 | 3 | ER | 3 | ||
| Maja squinado | 1 | 2 | ER | 2 | ||
| Squilla mantis | 1 | 1 | ER | 1 | ||
| Tot. | 33 | |||||
| Echinoderms | Paracentrotus lividus | 23 | 10 | CR (Ma) | May–July 2023–2024 | 10 |
| Tot. | 23 |
| Element | Unit | Sample ID | Sample Type | Reported Value (µg kg−1) | NDA Mean (µg kg−1) | NDA Sd | N° Obs | Total Error (µg kg−1) | Z’-Score |
|---|---|---|---|---|---|---|---|---|---|
| TTXs | µg L−1 | QTT012SS | Standard solution | 81.85 | 76.6 | 4.4 | 12 | 9.8 | 0.5 |
| µg kg−1 | QTT014BT | Mollusk homogenate | 225.4 | 254 | 81 | 13 | 44 | −0.7 | |
| µg kg−1 | QTT013BT | Mollusk homogenate | n.d. * | n.d. * | / | / | / | / |
| Matrix | Curve | R2 | ME (%) * | EQM (%) ** | tcalc *** | ttable *** |
|---|---|---|---|---|---|---|
| Solvent | y = 593.07x + 675.93 | 0.9949 | / | / | / | |
| Mussels | y = 180.20x − 35.85 | 0.9942 | 30 | / | / | |
| Gastropods | y = 178.74x + 48.41 | 0.9944 | 30 | 99 | 0.41 | 2.10 |
| Echinoderms | y = 174.35x − 41.28 | 0.9986 | 29 | 97 | 1.87 | |
| Arthropods | y = 171.17x − 139.30 | 0.9990 | 29 | 95 | 1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacchiocchi, S.; Siracusa, M.; Diomedi, G.; Mazza, S.; Calandri, E.; Tavoloni, T.; Vivani, V.; Cangini, M.; Arcangeli, G.; Losasso, C.; et al. Survey of Tetrodotoxins (TTXs) in Gastropods, Sea Urchins, and Blue Crabs from the Adriatic Sea: First Report in Paracentrotus lividus. Foods 2025, 14, 4036. https://doi.org/10.3390/foods14234036
Bacchiocchi S, Siracusa M, Diomedi G, Mazza S, Calandri E, Tavoloni T, Vivani V, Cangini M, Arcangeli G, Losasso C, et al. Survey of Tetrodotoxins (TTXs) in Gastropods, Sea Urchins, and Blue Crabs from the Adriatic Sea: First Report in Paracentrotus lividus. Foods. 2025; 14(23):4036. https://doi.org/10.3390/foods14234036
Chicago/Turabian StyleBacchiocchi, Simone, Melania Siracusa, Giulia Diomedi, Simone Mazza, Erica Calandri, Tamara Tavoloni, Veronica Vivani, Monica Cangini, Giuseppe Arcangeli, Carmen Losasso, and et al. 2025. "Survey of Tetrodotoxins (TTXs) in Gastropods, Sea Urchins, and Blue Crabs from the Adriatic Sea: First Report in Paracentrotus lividus" Foods 14, no. 23: 4036. https://doi.org/10.3390/foods14234036
APA StyleBacchiocchi, S., Siracusa, M., Diomedi, G., Mazza, S., Calandri, E., Tavoloni, T., Vivani, V., Cangini, M., Arcangeli, G., Losasso, C., Rubini, S., Di Francesco, G., Leoni, F., Piersanti, A., & Barchiesi, F. (2025). Survey of Tetrodotoxins (TTXs) in Gastropods, Sea Urchins, and Blue Crabs from the Adriatic Sea: First Report in Paracentrotus lividus. Foods, 14(23), 4036. https://doi.org/10.3390/foods14234036







