Application of Exercise/Training Models to Evaluate Food Functionality with Special Focus on Preventing Inflammation and Oxidative Stress and Enhancing Exercise Performance
Abstract
1. Introduction
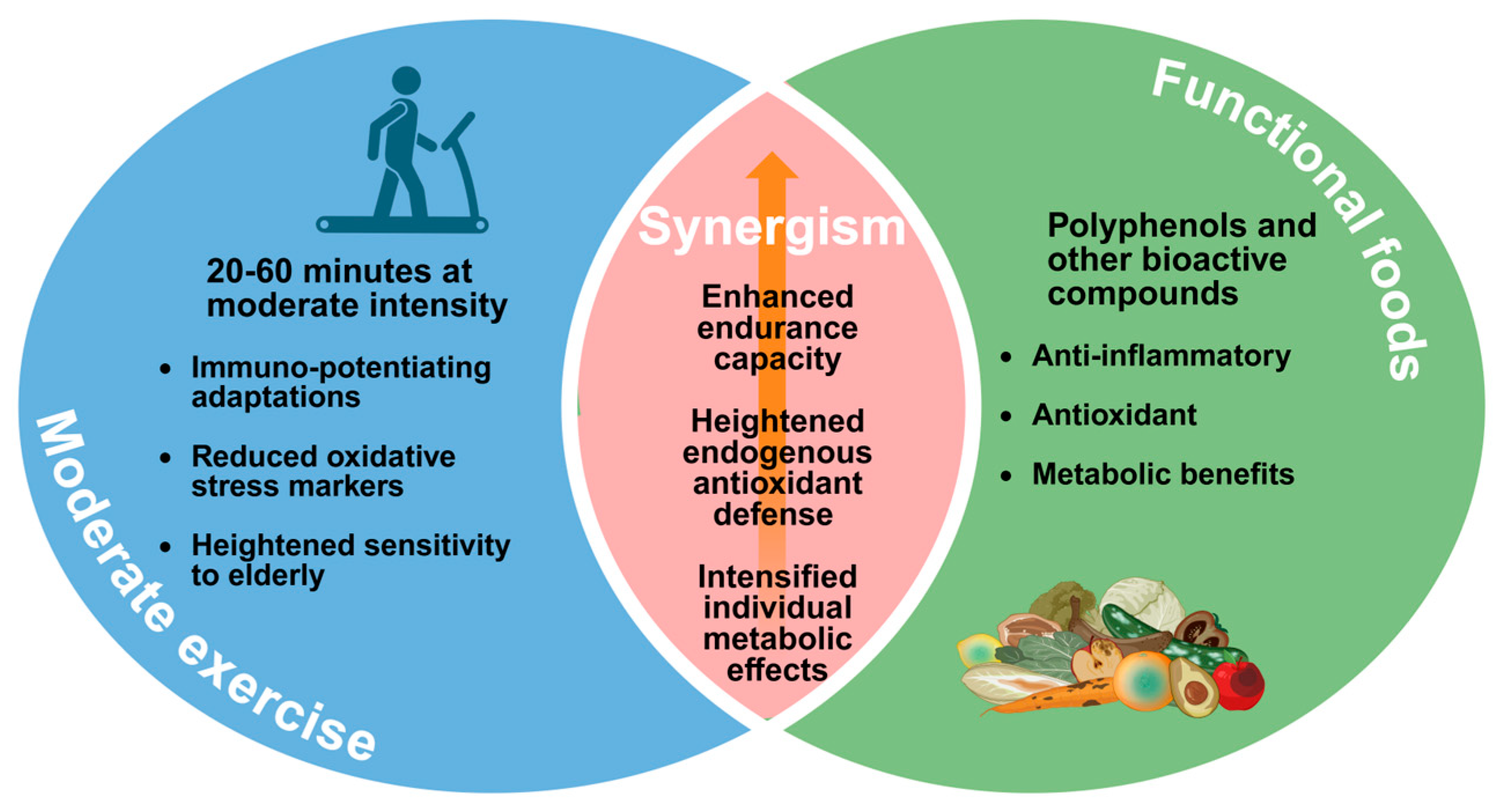
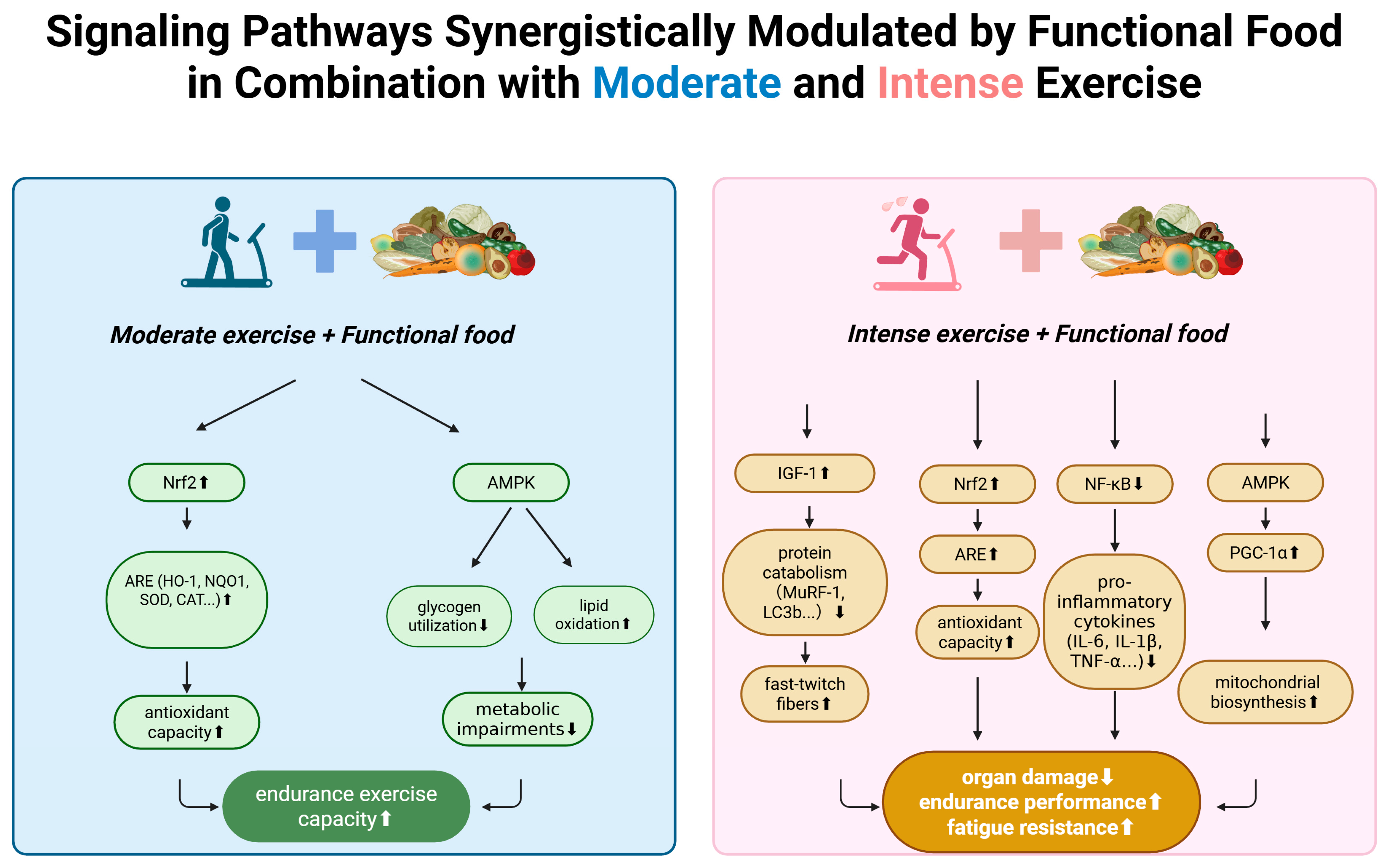
2. Moderate Exercise
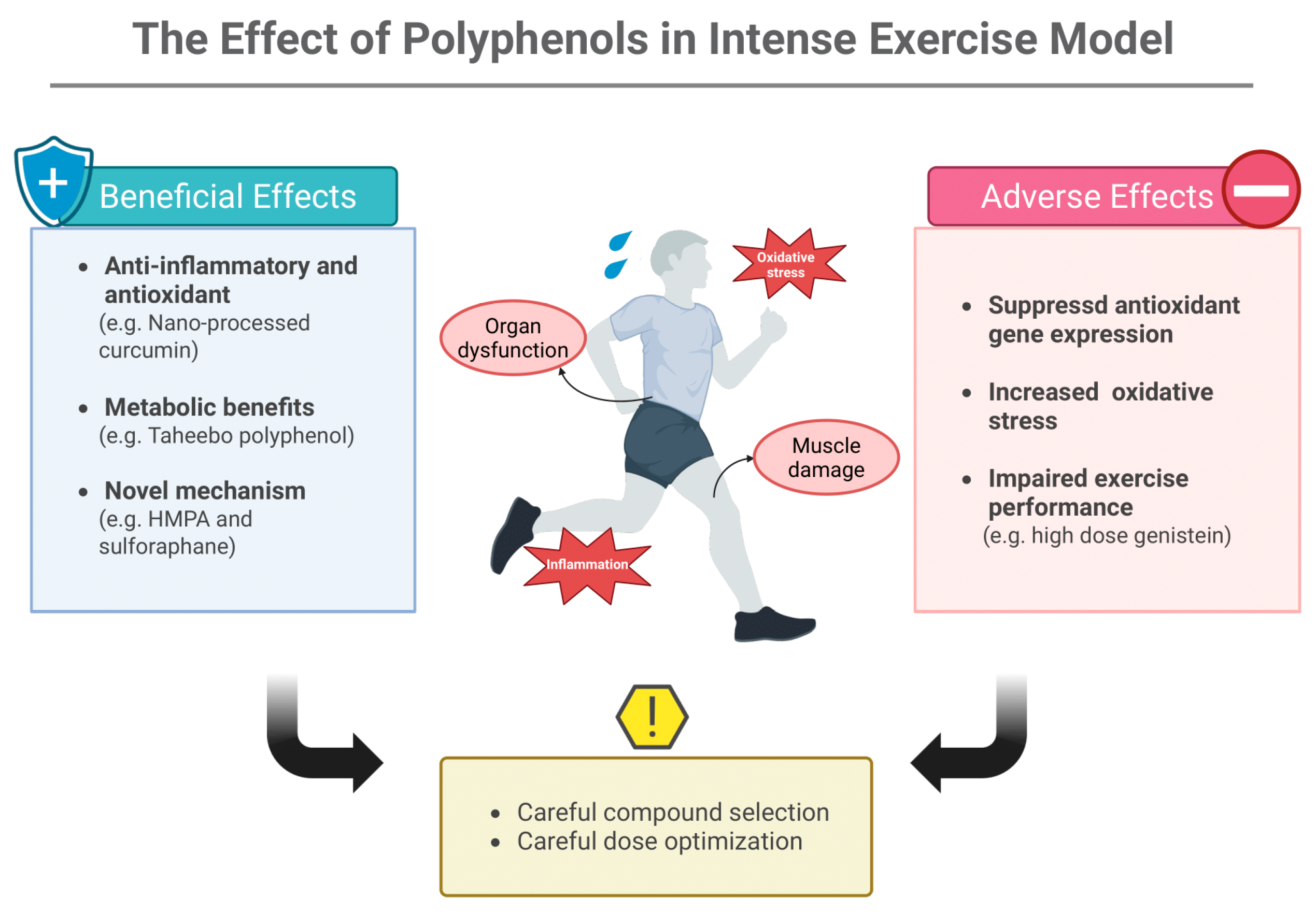
3. Intense Exercise
3.1. Polyphenols and Other Bioactive Compounds
3.2. Timing Strategies for Intense Exercise
3.3. Environmental Stressors and Intense Exercise Models
4. Training Models: Optimizing Adaptation and Recovery
4.1. Resistance Training and Muscle Adaptation
4.2. Endurance Training and Gut–Immune Interactions
5. Future Perspectives: Toward Personalized and Synergistic Approaches
5.1. Technological Advances in Bioavailability
5.2. Microbiome-Targeted Interventions
5.3. Combination Strategies—Interactions of Functional Foods and Drugs
5.4. Precision Nutrition
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, K. Recent progress in applicability of exercise immunology and inflammation research to sports nutrition. Nutrients 2021, 13, 4299. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef]
- La Gerche, A.; Rakhit, D.J.; Claessen, G. Exercise and the right ventricle: A potential Achilles’ heel. Cardiovasc Res. 2017, 113, 1499–1508. [Google Scholar] [CrossRef]
- Terada, O.; Suzuki, K.; Kurihara, Y.; Moriguchi, S. Effects of low-intensity brief exercise and training on cell-mediated immunity. Jpn. J. Complement. Altern. Med. 2007, 4, 71–77. [Google Scholar] [CrossRef]
- Matsuda, T.; Ogata, H.; Kanno, M.; Ishikawa, A.; Yamada, M.; Sakamaki-Sunaga, M. Effects of the menstrual cycle on oxidative stress and antioxidant response to high-intensity intermittent exercise until exhaustion in healthy women. J. Sports Med. Phys. Fit. 2020, 60, 1335–1341. [Google Scholar] [CrossRef]
- Neteca, J.; Veseta, U.; Liepina, I.; Volgemute, K.; Dzintare, M.; Babarykin, D. Effect of Beetroot Juice Supplementation on Aerobic Capacity in Female Athletes: A Randomized Controlled Study. Nutrients 2024, 17, 63. [Google Scholar] [CrossRef]
- Henderson, G.C. Sexual dimorphism in the effects of exercise on metabolism of lipids to support resting metabolism. Front. Endocrinol. 2014, 5, 162. [Google Scholar]
- Takahashi, M.; Miyashita, M.; Kawanishi, N.; Park, J.H.; Hayashida, H.; Kim, H.S.; Nakamura, Y.; Sakamoto, S.; Suzuki, K. Low-volume exercise training attenuates oxidative stress and neutrophils activation in older adults. Eur. J. Appl. Physiol. 2013, 113, 1117–1126. [Google Scholar] [CrossRef]
- Huang, J.; Tong, Y.; Wang, S.; Tagawa, T.; Seki, Y.; Ma, S.; Zhang, Z.; Cao, T.; Kobori, H.; Suzuki, K. 8-week Kaempferia parviflora extract administration improves submaximal exercise capacity in mice by enhancing skeletal muscle antioxidant gene expression and plasma antioxidant capacity. Antioxidants 2024, 13, 1147. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.; Hokayem, M.; Thomas, C.; Fabre, O.; Cassan, C.; Bourret, A.; Bernex, F.; Feuillet-Coudray, C.; Notarnicola, C.; Mercier, J.; et al. Combination of nutritional polyphenols supplementation with exercise training counteracts insulin resistance and improves endurance in high-fat diet-induced obese rats. Sci. Rep. 2018, 8, 2885. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.-M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Woodhead, J.S.; Zeng, N.; Blenkiron, C.; Merry, T.L.; Cameron-Smith, D.; Mitchell, C.J. Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E723–E733. [Google Scholar] [CrossRef]
- Rojano-Ortega, D.; Peña-Amaro, J.; Berral-Aguilar, A.; Berral-de la Rosa, F. Quercetin supplementation promotes recovery after exercise-induced muscle damage: A systematic review and meta-analysis of randomized controlled trials. Biol. Sport 2023, 40, 813–825. [Google Scholar] [CrossRef]
- Solomon, T.P. Sources of inter-individual variability in the therapeutic response of blood glucose control to exercise in type 2 diabetes: Going beyond exercise dose. Front. Physiol. 2018, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Wang, C.K. Functional Foods: Role in Endurance Sports. In Extreme and Rare Sports: Performance Demands, Drivers, Functional Foods, and Nutrition; CRC Press: Boca Raton, FL, USA, 2019; pp. 73–113. [Google Scholar]
- Sugama, K.; Suzuki, K.; Yoshitani, K.; Shiraishi, K.; Miura, S.; Yoshioka, H.; Mori, Y.; Kometani, T. Changes of thioredoxin, oxidative stress markers, inflammation and muscle/renal damage following intensive endurance exercise. Exerc. Immunol. Rev. 2015, 21, 130–142. [Google Scholar]
- Tiller, N.B.; Millet, G.Y. Decoding ultramarathon: Muscle damage as the main impediment to performance. Sports Med. 2025, 55, 535–543. [Google Scholar] [CrossRef]
- Niemelä, M.; Kangastupa, P.; Niemelä, O.; Bloigu, R.; Juvonen, T. Acute changes in inflammatory biomarker levels in recreational runners participating in a marathon or half-marathon. Sports Med. Open 2016, 2, 21. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021, 33, 957–970.e6. [Google Scholar] [CrossRef]
- Larsen, S. Acute antioxidant supplementation and performance—Should this be considered. Free Radic. Biol. Med. 2024, 224, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Coombes, J.S. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Suzuki, K.; Hayashida, H. Effect of Exercise Intensity on Cell-Mediated Immunity. Sports 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Roberts, L.A.; Oginome, N.; Suzuki, K. Effect of acacia polyphenol supplementation on exercise-induced oxidative stress in mice liver and skeletal muscle. Antioxidants 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Suzuki, K. The effects of flavonoids on skeletal muscle mass, muscle function, and physical performance in individuals with sarcopenia: A systematic review of randomized controlled trials. Nutrients 2023, 15, 3897. [Google Scholar] [CrossRef]
- Rahman Mazumder, M.A.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in in vivo and in vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Rajabi, S. Polyphenols and post-exercise muscle damage: A comprehensive review of literature. Eur. J. Med. Res. 2025, 30, 260. [Google Scholar] [CrossRef]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef]
- Roberts, J.D.; Lillis, J.B.; Pinto, J.M.; Chichger, H.; López-Samanes, Á.; Coso, J.D.; Zacca, R.; Willmott, A.G.B. The effect of a hydroxytyrosol-rich, olive-derived phytocomplex on aerobic exercise and acute recovery. Nutrients 2023, 15, 421. [Google Scholar] [CrossRef]
- Howatson, G.; Snaith, G.C.; Kimble, R.; Cowper, G.; Keane, K.M. Improved endurance running performance following Haskap berry (Lonicera caerulea L.) ingestion. Nutrients 2022, 14, 780. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of exercise performance by 48 hours, and 15-day supplementation with mangiferin and luteolin in men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Noguera, F.J.; Marín-Pagán, C.; Carlos-Vivas, J.; Alcaraz, P.E. 8-week supplementation of 2S-hesperidin modulates antioxidant and inflammatory status after exercise until exhaustion in amateur cyclists. Antioxidants 2021, 10, 432. [Google Scholar] [CrossRef]
- Deley, G.; Guillemet, D.; Allaert, F.A.; Babault, N. An acute dose of specific grape and apple polyphenols improves endurance performance: A randomized, crossover, double-blind versus placebo controlled study. Nutrients 2017, 9, 917. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, S.; Ma, S.; Suzuki, K. Effect of genistein supplementation on exercise-induced inflammation and oxidative stress in mice liver and skeletal muscle. Medicina 2021, 57, 1028. [Google Scholar] [CrossRef] [PubMed]
- Shete, V.; Mahajan, N.M.; Shivhare, R.; Akkewar, A.; Gupta, A.; Gurav, S. Genistein: A promising phytoconstituent with reference to its bioactivities. Phytother. Res. 2024, 38, 3935–3953. [Google Scholar] [CrossRef]
- Yang, L.; Jia, L.; Li, X.; Zhang, K.; Wang, X.; He, Y.; Hao, M.; Rayman, M.P.; Zhang, J. Prooxidant activity-based guideline for a beneficial combination of (−)-epigallocatechin-3-gallate and chlorogenic acid. Food Chem. 2022, 386, 132812. [Google Scholar] [CrossRef] [PubMed]
- Ashtary-Larky, D.; Rezaei Kelishadi, M.; Bagheri, R.; Moosavian, S.P.; Wong, A.; Davoodi, S.H.; Khalili, P.; Dutheil, F.; Suzuki, K.; Asbaghi, O. The effects of nano-curcumin supplementation on risk factors for cardiovascular disease: A GRADE-assessed systematic review and meta-analysis of clinical trials. Antioxidants 2021, 10, 1015. [Google Scholar] [CrossRef]
- Tanabe, Y.; Fujii, N.; Suzuki, K. Dietary supplementation for attenuating exercise-induced muscle damage and delayed-onset muscle soreness in humans. Nutrients 2022, 14, 70. [Google Scholar] [CrossRef]
- Takahashi, M.; Suzuki, K.; Kim, H.K.; Otsuka, Y.; Imaizumi, A.; Miyashita, M.; Sakamoto, S. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 2014, 35, 469–475. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, K.; Takahashi, M.; Mizokami, T.; Otsuka, Y.; Imaizumi, A.; Shiva, D.; Yano, H.; Suzuki, K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013, 441, 573–578. [Google Scholar] [CrossRef]
- Tong, Y.; Ma, S.; Awa, R.; Tagawa, T.; Seki, Y.; Cao, T.; Kobori, H.; Suzuki, K. Effects of 3-(4-hydroxy-3-methoxyphenyl)propionic acid on regulating oxidative stress and muscle fiber composition. Nutrients 2025, 17, 668. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Roberts, L.A.; Ma, S.; Suzuki, K. Organosulfur compounds: A review of their anti-inflammatory effects in human health. Front. Nutr. 2020, 7, 64. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Effects of sulforaphane treatment on skeletal muscle from exhaustive exercise-induced inflammation and oxidative stress through the Nrf2/HO-1 signaling pathway. Antioxidants 2025, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, F.; Zhang, D.; Shi, D.; Zhou, J.; Guo, S.; Chang, X. Lonicera caerulea berry polyphenols extract alleviates exercise fatigue in mice by reducing oxidative stress, inflammation, skeletal muscle cell apoptosis, and by increasing cell proliferation. Front. Nutr. 2022, 9, 853225. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, A.; Koike, A.; Karasawa, T.; Tsutsui, M.; Kondo, S.; Terada, S. Effects of a ketogenic diet containing medium-chain triglycerides and endurance training on metabolic enzyme adaptations in rat skeletal muscle. Nutrients 2020, 12, 1269. [Google Scholar] [CrossRef]
- Wiciński, M.; Leis, K.; Szyperski, P.; Węclewicz, M.M.; Mazur, E.; Pawlak-Osińska, K. Impact of resveratrol on exercise performance: A review. Sci. Sports 2018, 33, 207–212. [Google Scholar] [CrossRef]
- Butler, C.A. The Effects of Acute Omega-3 Fatty Acid Supplementation on Delayed-Onset Muscle Soreness and Recovery. Master’s Thesis, Georgia Southern University, Statesboro, GA, USA, 2019. [Google Scholar]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef]
- Boorsma, R.K.; Whitfield, J.; Spriet, L.L. Beetroot juice supplementation does not improve performance of elite 1500-m runners. Med. Sci. Sports Exerc. 2014, 46, 2326–2334. [Google Scholar] [CrossRef]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Walters, J.A.; Baier, S.M.; Fuller, J.C., Jr.; Stout, J.R.; Norton, L.E.; Sikorski, E.M.; Wilson, S.M.C.; et al. β-Hydroxy-β-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. Br. J. Nutr. 2013, 110, 538–544. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef]
- Macuh, M.; Knap, B. Effects of nitrate supplementation on exercise performance in humans: A narrative review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.A.; Vandusseldorp, T.A.; Uken, B.; Otis, J. Quercetin in sports and exercise: A review. Int. J. Exerc. Sci. 2023, 16, 1334. [Google Scholar] [CrossRef]
- Rangsimahariwong, W.; Kulaputana, O.; Tabtieang, T.; Sanguanrungsirikul, S.; Chuaypen, N. The effect of fluid replacement during 60 minutes of moderate-intensity running on splanchnic blood flow. Chulalongkorn Med. J. 2024, 68, 5. [Google Scholar] [CrossRef]
- Luan, C.; Wang, Y.; Li, J.; Zhou, N.; Song, G.; Ni, Z.; Xu, C.; Tang, C.; Fu, P.; Wang, X.; et al. Branched-chain amino acid supplementation enhances substrate metabolism, exercise efficiency and reduces post-exercise fatigue in active young males. Nutrients 2025, 17, 1290. [Google Scholar] [CrossRef]
- Aragon, A.A.; Schoenfeld, B.J. Nutrient timing revisited: Is there a post-exercise anabolic window? J. Int. Soc. Sports Nutr. 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Eijsvogels, T.M.; Daanen, H.A. Exercise under heat stress: Thermoregulation, hydration, performance implications, and mitigation strategies. Physiol. Rev. 2021, 101, 1873–1979. [Google Scholar] [CrossRef]
- Peel, J.S.; McNarry, M.A.; Heffernan, S.M.; Nevola, V.R.; Kilduff, L.P.; Waldron, M. The effect of dietary supplements on core temperature and sweating responses in hot environmental conditions: A meta-analysis and meta-regression. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2025, 328, R515–R555. [Google Scholar] [CrossRef]
- Martin, A.R.; Chung, S.; Koehler, K. Is exercise a match for cold exposure? Common molecular framework for adipose tissue browning. Int. J. Sports Med. 2020, 41, 427–442. [Google Scholar] [CrossRef]
- Saito, M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv. Food Nutr. Res. 2015, 76, 1–28. [Google Scholar]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef]
- Carriker, C. Effect of Acute Dietary Nitrate Consumption on Submaximal Oxygen Consumption and Oxidative Stress in Hypoxia. Master’s Thesis, University of New Mexico, Albuquerque, NM, USA, 2014. [Google Scholar]
- Verdon, F.; Burnand, B.; Fallab-Stubi, C.-L.; Bonard, C.; Graff, M.; Michaud, A.; Bischoff, T.; De Vevey, M.; Studer, J.-P.; Herzig, L.; et al. Iron supplementation for unexplained fatigue in non-anaemic women: Double blind randomised placebo controlled trial. BMJ 2003, 326, 1124. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.L.; Hurrie, D.; Graham, J.; Perija, B.; Rimmer, E.; Rabbani, R.; Bernstein, C.N.; Turgeon, A.F.; Fergusson, D.A.; Houston, D.S.; et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: Systematic review. BMJ Open 2018, 8, e019240. [Google Scholar] [CrossRef]
- Rathmacher, J.A.; Pitchford, L.M.; Stout, J.R.; Townsend, J.R.; Jäger, R.; Kreider, R.B.; Campbell, B.I.; Kerksick, C.M.; Harty, P.S.; Candow, D.G.; et al. International society of sports nutrition position stand: β-hydroxy-β-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2025, 22, 2434734. [Google Scholar] [CrossRef] [PubMed]
- Bideshki, M.V.; Behzadi, M.; Jamali, M.; Jamilian, P.; Zarezadeh, M.; Gargari, B.P. Ergogenic Benefits of β-Hydroxy-β-Methyl Butyrate (HMB) Supplementation on Body Composition and Muscle Strength: An Umbrella Review of Meta-Analyses. J. Cachexia Sarcopenia Muscle 2025, 16, e13671. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Belozo, F.L.; Micheletti, T.O.; Conrado, M.; Stout, J.R.; Pimentel, G.D.; Gonzalez, A.M. β-Hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: A systematic review. Nutr. Res. 2017, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Arazi, H.; Suzuki, K. Effects of β-hydroxy-β-methylbutyrate-free acid supplementation on strength, power and hormonal adaptations following resistance training. Nutrients 2017, 9, 1316. [Google Scholar] [CrossRef]
- Retzepis, N.O.; Avloniti, A.; Kokkotis, C.; Stampoulis, T.; Balampanos, D.; Gkachtsou, A.; Aggelakis, P.; Kelaraki, D.; Protopapa, M.; Pantazis, D.; et al. The effect of peak height velocity on strength and power development of young athletes: A scoping review. J. Funct. Morphol. Kinesiol. 2025, 10, 168. [Google Scholar] [CrossRef]
- Colecchia, F.P.; Di Padova, M.; Mancini, S.; Polito, R.; Basta, A.; Grosu, V.T.; Limone, P.; Messina, G.; Monda, M.; Monda, A.; et al. Protein intake in adolescent athletes: Nutritional requirements and performance implications. J. Phys. Educ. Sport 2025, 25, 773–784. [Google Scholar]
- Wentz, L.M. Calcium Intake and the Incidence of Stress Fractures. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2011. [Google Scholar]
- Korovljev, D.; Stajer, V.; Ostojic, S.M. Relationship between dietary creatine and growth indicators in children and adolescents aged 2–19 years: A cross-sectional study. Nutrients 2021, 13, 1027. [Google Scholar] [CrossRef]
- Aragon, A.A.; Tipton, K.D.; Schoenfeld, B.J. Age-related muscle anabolic resistance: Inevitable or preventable? Nutr. Rev. 2023, 81, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.; Willoughby, D.S. Mechanisms behind pyrroloquinoline quinone supplementation on skeletal muscle mitochondrial biogenesis: Possible synergistic effects with exercise. J. Am. Coll. Nutr. 2018, 37, 738–748. [Google Scholar] [CrossRef]
- Kviatkovsky, S.A.; Hickner, R.C.; Cabre, H.E.; Small, S.D.; Ormsbee, M.J. Collagen peptides supplementation improves function, pain, and physical and mental outcomes in active adults. J. Int. Soc. Sports Nutr. 2023, 20, 2243252. [Google Scholar] [CrossRef]
- Ramírez-Campillo, R.; Andrade, D.C.; Izquierdo, M. Effects of plyometric training volume and training surface on explosive strength. J. Strength Cond. Res. 2013, 27, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Holland, B.M.; Roberts, B.M.; Krieger, J.W.; Schoenfeld, B.J. Does HMB enhance body composition in athletes? A systematic review and meta-analysis. J. Strength Cond. Res. 2022, 36, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Rashki, M.; Hemmatinafar, M.; Safari, K.; Imanian, B.; Rezaei, R.; Koushkie Jahromi, M.; Suzuki, K. Capsaicin’s role in mitigating muscle soreness and enhancing futsal players’ recovery after exercise-induced muscle damage. Nutrients 2025, 17, 813. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Surendran, S.; Khaitan, J.; Patil, M.; Shathi, M.A. The relationship between gut health and sports performance. In Evaluating the Effectiveness of Functional Ingredients in Sports Nutrition; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 307–342. [Google Scholar]
- Li, Q.; Wen, X.; Wang, G. Gut microbiota and exercise-induced fatigue: Unraveling the connections. Food Sci. Anim. Prod. 2024, 2, 9240061. [Google Scholar] [CrossRef]
- Mohr, A.E.; Jäger, R.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Townsend, J.R.; West, N.P.; Black, K.; Gleeson, M.; Pyne, D.B.; et al. The athletic gut microbiota. J. Int. Soc. Sports Nutr. 2020, 17, 24. [Google Scholar] [CrossRef]
- Razazan, R.; Hemmatinafar, M.; Imanian, B.; Jahaniboushehri, N.; Rezaei, R.; Nazemzadegan, G. Performance-enhancing effects of caffeine and L-theanine among Iranian elite wrestlers: A focus on cognitive and specific physical performance. J. Int. Soc. Sports Nutr. 2025, 22, 2564238. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. Physical activity and neurocognitive functioning in aging—A condensed updated review. Eur. Rev. Aging Phys. Act. 2016, 13, 1. [Google Scholar] [CrossRef]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of probiotic (Bifidobacterium longum 35624) supplementation on exercise performance, immune modulation, and cognitive outlook in Division I female swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.; Soltani, S. Probiotics supplementation and brain-derived neurotrophic factor (BDNF): A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2023, 26, 942–952. [Google Scholar] [CrossRef]
- Ma, S.; Tominaga, T.; Kanda, K.; Sugama, K.; Omae, C.; Hashimoto, S.; Aoyama, K.; Yoshikai, Y.; Suzuki, K. Effects of an 8-week protein supplementation regimen with hyperimmunized cow milk on exercise-induced organ damage and inflammation in male runners: A randomized, placebo-controlled, cross-over study. Biomedicines 2020, 8, 51. [Google Scholar] [CrossRef]
- Gaamouri, N.; Zouhal, H.; Suzuki, K.; Hammami, M.; Ghaith, A.; El Mouhab, E.H.; Hackney, A.C.; Laher, I.; Ben Ounis, O. Effects of carob rich-polyphenols on oxidative stress markers and physical performance in taekwondo athletes. Biol. Sport 2024, 41, 277–284. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of beetroot juice supplementation on muscle soreness and performance recovery after exercise-induced muscle damage in female volleyball players. Nutrients 2023, 15, 3763. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.B.; Chow, L.S.; Stagg, D.B.; Gillingham, J.R.; Evans, M.D.; Pan, M.; Hughey, C.C.; Myers, C.L.; Han, X.; Crawford, P.A.; et al. Acute aerobic exercise reveals that FAHFAs distinguish the metabolomes of overweight and normal-weight runners. JCI Insight 2022, 7, e158037. [Google Scholar] [CrossRef]
- Yang, G.; Hong, J.; Park, S.B. Wearable device for continuous sweat lactate monitoring in sports: A narrative review. Front. Physiol. 2024, 15, 1376801. [Google Scholar] [CrossRef]
- Huang, C.; Clayton, E.A.; Matyunina, L.V.; McDonald, L.D.; Benigno, B.B.; Vannberg, F.; McDonald, J.F. Machine learning predicts individual cancer patient responses to therapeutic drugs with high accuracy. Sci. Rep. 2018, 8, 16444. [Google Scholar] [CrossRef]
- Subasi, A.; Subasi, M.E. Digital twins in healthcare and biomedicine. In Artificial Intelligence, Big Data, Blockchain and 5G for the Digital Transformation of the Healthcare Industry; Academic Press: Cambridge, MA, USA, 2024; pp. 365–401. [Google Scholar]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Yu, C.H.; Lai, C.C.; Chen, J.H.; Chen, I.C.; Tai, H.L.; Fu, S.K. Effect of Lactobacillus plantarum PS128 on neuromuscular efficiency after a half-marathon. Front. Physiol. 2023, 14, 1254985. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Stone, J.D.; Turner, S.M.; Anzalone, A.J.; Eimerbrink, M.J.; Pane, M.; Amoruso, A.; Rowlands, D.S.; Oliver, J.M. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef]
- Verbeke, K.A.; Boobis, A.R.; Chiodini, A.; Edwards, C.A.; Franck, A.; Kleerebezem, M.; Nauta, A.; Raes, J.; Van Tol, E.A.F.; Tuohy, K.M. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr. Res. Rev. 2015, 28, 42–66. [Google Scholar] [CrossRef]
- Luo, C.; Wei, X.; Song, J.; Xu, X.; Huang, H.; Fan, S.; Zhang, D.; Han, L.; Lin, J. Interactions between gut microbiota and polyphenols: New insights into the treatment of fatigue. Molecules 2022, 27, 7377. [Google Scholar] [CrossRef]
- Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Huang, C.C. Live and heat-killed probiotic Lactobacillus paracasei PS23 accelerated the improvement and recovery of strength and damage biomarkers after exercise-induced muscle damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- Pang, Y.; Ermann Lundberg, L.; Mata Forsberg, M.; Ahl, D.; Bysell, H.; Pallin, A.; Sverremark-Ekström, E.; Karlsson, R.; Jonsson, H.; Roos, S. Extracellular membrane vesicles from Limosilactobacillus reuteri strengthen the intestinal epithelial integrity, modulate cytokine responses and antagonize activation of TRPV1. Front. Microbiol. 2022, 13, 1032202. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.M.; Reimer, R.A. Unlocking a novel determinant of athletic performance: The role of the gut microbiota, short-chain fatty acids, and “biotics” in exercise. J. Sport Health Sci. 2023, 12, 36–44. [Google Scholar] [CrossRef]
- Batatinha, H.; Tavares-Silva, E.; Leite, G.S.; Resende, A.S.; Albuquerque, J.A.; Arslanian, C.; Fock, R.A.; Lancha, A.H.; Lira, F.S.; Krüger, K.; et al. Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon: A randomized placebo-controlled double-blind study. Sci. Rep. 2020, 10, 18777. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, S.; Yang, T.; Guo, J.; Zhu, X.; Dong, Y. Impact of exercise-induced alterations on gut microbiota diversity and composition: Comparing effects of different training modalities. Cell Regen. 2025, 14, 28. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Saragiotto, G.K.; de Oliveira, L.F.V.; Scharlack, N.K.; de Oliveira, M.M.; Freire, F.C.; Simabuco, F.M.; Sivieri, K.; Sartoratto, A.; Belli, T.; Antunes, A.E.C. Effects of a 217-km mountain ultramarathon on the gut microbiota of an obese runner: A case report. Physiol. Rep. 2024, 12, e70017. [Google Scholar] [CrossRef]
- O’Brien, M.T.; O’Sullivan, O.; Claesson, M.J.; Cotter, P.D. The athlete gut microbiome and its relevance to health and performance: A review. Sports Med. 2022, 52 (Suppl. S1), 119–128. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef]
- Whittaker, P. Iron and zinc interactions in humans. Am. J. Clin. Nutr. 1998, 68, 442S–446S. [Google Scholar] [CrossRef]
- Koleini, N.; Shapiro, J.S.; Geier, J.; Ardehali, H. Ironing out mechanisms of iron homeostasis and disorders of iron deficiency. J. Clin. Investig. 2021, 131, e148201. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Tang, N. Unraveling the intricate interplay: Iron and zinc’s regulatory mechanisms on calcium bioavailability during intestinal absorption. Food Rev. Int. 2025, 41, 345–372. [Google Scholar] [CrossRef]
- Hallberg, L.; Brune, M.; Erlandsson, M.; Sandberg, A.S.; Rossander-Hultén, L. Calcium and iron absorption. Am. J. Clin. Nutr. 1991, 53, 112–119. [Google Scholar] [CrossRef]
- Haller, C.A.; Benowitz, N.L. Enhanced stimulant and metabolic effects of combined ephedrine and caffeine. Clin. Pharmacol. Ther. 2005, 77, 312–321. [Google Scholar] [CrossRef]
- McCarty, M.F. “Iatrogenic Gilbert syndrome”—A strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med. Hypotheses 2007, 69, 974–994. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, A.C.; Blanchfield, J.T.; Toth, I.; Fassett, R.G.; Coombes, J.S. Improved resistance to serum oxidation in Gilbert’s syndrome: A mechanism for cardiovascular protection. Atherosclerosis 2008, 199, 390–396. [Google Scholar] [CrossRef]
- Ledowsky, C.J.; Schloss, J.; Steel, A. Variations in folate prescriptions for patients with the MTHFR genetic polymorphisms: A case series study. J. Australas. Coll. Nutr. Environ. Med. 2025, 44, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Margulis, D. Interactions Between Food Supplements in Sport: A Systematic Review. Master’s Thesis, Lithuanian University of Health Sciences, Kaunas, Lithuania, 2019. [Google Scholar]
- BaHammam, A.S.; Pirzada, A. Timing matters: The interplay between early mealtime, circadian rhythms, gene expression, circadian hormones, and metabolism—A narrative review. Clocks Sleep 2023, 5, 507–535. [Google Scholar] [CrossRef] [PubMed]
| Model (Species) | Advantages | Limitations | Typical Endpoints/Biomarkers |
|---|---|---|---|
| Treadmill running (rodent) | Controlled speed, incline and duration; reproducible workload; suitable for VO2 max and metabolic rate measurement | Forced exercise induces stress; unnatural gait; requires training and motivators (e.g., shocks) | VO2 max, lactate, cortisol, cytokines, antioxidant enzymes |
| Voluntary wheel running (rodent) | Low stress; reflects habitual physical activity; useful for long-term interventions | Low control over intensity; high variability; nocturnal running patterns | Distance run, activity patterns, basal cytokines, body weight |
| Endurance training (human) | Widely used protocols; enhances aerobic fitness and mitochondrial biogenesis; sensitive to antioxidant interventions | Requires long duration; may not address muscle hypertrophy; adaptation reduces inflammatory responses over time | VO2 max, lactate threshold, antioxidant enzymes, cytokines |
| Resistance training (human) | Increases muscle mass and strength; stimulates mTOR–IGF-1 pathway; relevant for sarcopenia | Skill-dependent; difficult to standardize across participants; limited improvements in cardiovascular fitness | 1-RM, muscle cross-sectional area, IGF-1, mTOR phosphorylation, creatine kinase |
| High-intensity interval/eccentric exercise (human) | Elicits pronounced oxidative stress and inflammation; useful for testing anti-inflammatory compounds | May cause muscle damage and soreness; not sustainable for certain populations | IL-6, TNF-α, creatine kinase, DOMS, oxidized glutathione |
| Functional Compound | Major Molecular Target(s) | Evidence and Effects |
|---|---|---|
| Resveratrol | Activate Nrf2–Keap1 pathway; inhibit NF-κB; promote mitochondrial biogenesis via SIRT1/PGC-1α | Resveratrol enhances mitochondrial function and insulin sensitivity, is associated with a beneficial effect on the sports performance [47]. |
| Curcumin | Suppresses NF-κB and COX-2; activates Nrf2/HO-1; reduces NLRP3 inflammasome | Curcumin supplementation attenuates exercise-induced increases in IL-6 and lipid peroxidation; combining curcumin with endurance training reduced muscle soreness and oxidative stress [38,39]. |
| Omega-3 PUFAs | Incorporate into membranes; reduce proinflammatory eicosanoids | n-3 PUFAs mitigate delayed-onset muscle soreness and reduce free radical production [48]. |
| Dietary nitrate (beetroot juice) | Increases nitric oxide via nitrate–nitrite–NO pathway; improves mitochondrial efficiency; lowers VO2 cost | At altitude, nitrate supplementation improved 16.1 km cycling performance, but results are inconsistent [49]. |
| HMB | β-hydroxy-β-methylbutyrate (HMB) activates mTOR and suppresses proteolysis | HMB reduces markers of muscle damage and enhances recovery after intense training [50]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, K.; Wu, C.; Ma, S. Application of Exercise/Training Models to Evaluate Food Functionality with Special Focus on Preventing Inflammation and Oxidative Stress and Enhancing Exercise Performance. Foods 2025, 14, 4025. https://doi.org/10.3390/foods14234025
Suzuki K, Wu C, Ma S. Application of Exercise/Training Models to Evaluate Food Functionality with Special Focus on Preventing Inflammation and Oxidative Stress and Enhancing Exercise Performance. Foods. 2025; 14(23):4025. https://doi.org/10.3390/foods14234025
Chicago/Turabian StyleSuzuki, Katsuhiko, Cong Wu, and Sihui Ma. 2025. "Application of Exercise/Training Models to Evaluate Food Functionality with Special Focus on Preventing Inflammation and Oxidative Stress and Enhancing Exercise Performance" Foods 14, no. 23: 4025. https://doi.org/10.3390/foods14234025
APA StyleSuzuki, K., Wu, C., & Ma, S. (2025). Application of Exercise/Training Models to Evaluate Food Functionality with Special Focus on Preventing Inflammation and Oxidative Stress and Enhancing Exercise Performance. Foods, 14(23), 4025. https://doi.org/10.3390/foods14234025









