3.1. Fatty Acid Profile Analysis
The fatty acid compositions of two freeze-dried coconut meat samples and two freeze-dried coconut water samples were analyzed by GC–MS following lipid extraction and methyl esterification. A total of nine fatty acids were identified across all samples: caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), stearic acid (C18:0), oleic acid (C18:1), and linoleic acid (C18:2). The representative total ion GC-MS chromatograms of the four types of samples were demonstrated in
Supplementary Material Figure S1. Among the identified fatty acids, five (C6–C14) were classified as medium- and short-chain saturated fatty acids, while the remaining four (C16–C18) were long-chain fatty acids. All nine fatty acids were detected in coconut meat samples (MCM and TCM); however, caproic acid was absent in mature coconut water (MCW), and both caproic and caprylic acids were undetected in tender coconut water (TCW).
Quantitative analysis based on authentic standards revealed marked differences in total fatty acid content between tender and mature coconuts (
Table 1). On a dry-weight basis, MCM contained 299.70 ± 16.98 g/kg of total fatty acids, more than three times higher than the 90.87 ± 16.86 g/kg observed in TCM. Despite these quantitative differences, the overall fatty acid profiles were broadly similar across maturity stages, with lauric, myristic, and palmitic acids being the predominant components. Lauric acid was the most abundant, with a concentration of 142.97 ± 4.99 g/kg in MCM, accounting for 48% of the total fatty acids, compared with 35.11 ± 5.49 g/kg (39%) in TCM. Thus, lauric acid levels in MCM were approximately 4.1 times higher than in TCM. Myristic acid ranked second, with concentrations of 57.39 ± 2.08 g/kg (19%) in MCM and 18.17 ± 0.61 g/kg (20%) in TCM, representing a 3.2-fold difference. Palmitic acid, the third most abundant fatty acid, was also significantly higher in MCM (29.79 ± 1.89 g/kg) than in TCM (12.53 ± 2.22 g/kg), a 2.4-fold increase. It has also been revealed by Guo et al. that multiple lipid biosynthesis-related genes, such as WRI3, WIN1, and SHN2, accumulated with the maturation of coconut meat [
8]. By contrast, coconut water samples contained only trace levels of fatty acids, with concentrations of 3.6 ± 0.20 g/100 mL in MCW and 1.2 ± 0.20 g/100 mL in TCW, values negligible compared with those of coconut meat.
Given the nutritional relevance of medium-chain fatty acids (MCFAs) as rapidly metabolizable energy sources, their relative abundance was further examined. In MCM, MCFAs accounted for 59.51% of total fatty acids, compared with 45.49% in TCM. In coconut water, MCFA proportions were comparable between maturity stages, at ~50% in both MCW and TCW. These results suggest that coconut maturation strongly enhances the relative abundance of MCFAs in coconut meat, whereas the compositional profile of coconut water remains largely unaffected. This observation is consistent with previous studies. For example, Mahayothee et al. [
10] reported significant increases in caproic, caprylic, capric, and lauric acids as coconuts matured from 180–190 days to 225 days after pollination in Thailand. Similarly, Kumar et al. [
18] found that coconuts harvested at 11–12 months contained substantially higher proportions of capric, caprylic, and lauric acids than those harvested at 7–8 months, while the proportions of palmitic, oleic, and linoleic acids decreased. Together, these findings confirm that the compositional shift toward MCFA enrichment is a characteristic feature of coconut maturation.
The ratio between saturated fatty acids (SFAs) and unsaturated fatty acids (UFAs) also serves as an important indicator of lipid functionality. In the present study, SFAs dominated the fatty acid profile of all samples. Their relative proportions were 93.89% in MCM, 82.91% in TCM, 90.13% in MCW, and 86.19% in TCW. Therefore, SFAs are the major lipid constituents of both coconut milk and coconut water.
Additionally, the nutritional and health-related lipid indices derived from the fatty acid profiles showed distinct differences across coconut tissues and maturity stages. The hypocholesterolemic/hypercholesterolemic ratio (H/H) was highest in tender coconut meat and water (0.24 and 0.20, respectively), indicating relatively more favorable fatty acid compositions compared with mature samples. In contrast, MCM exhibited the lowest H/H value (0.08), reflecting its high proportion of hypercholesterolemic saturated fatty acids such as C14:0 and C16:0. The atherogenicity (IA) and thrombogenicity (IT) indices showed similar trends. MCM displayed markedly higher IA (20.81) and the lowest IT (433), while TCM had the lowest IA (7.75). TCW showed the highest IT (1650), suggesting that although TCW contains a comparatively healthier balance of unsaturated to saturated fatty acids, its specific fatty acid profile increases the thrombogenic potential relative to other tissues. Overall, these indices highlight that tender coconut tissues, particularly TCM, present a more favorable lipid-related nutritional profile, whereas mature coconut meat demonstrates less desirable characteristics due to its elevated levels of atherogenic and hypercholesterolemic fatty acids.
In summary, both coconut maturity and tissue type significantly influence fatty acid composition. As coconuts develop from tender to mature stages, the total lipid content increases markedly, accompanied by a relative enrichment of MCFAs, including lauric, caprylic, and capric acids, and a corresponding reduction in long-chain and unsaturated fatty acids. Consequently, the proportion of SFAs increases while that of UFAs declines. This developmental shift is likely associated with the upregulation of specific genes involved in medium-chain fatty acid biosynthesis during coconut maturation.
3.2. Total Phenolic Content and DPPH Scavenging Activities Analysis
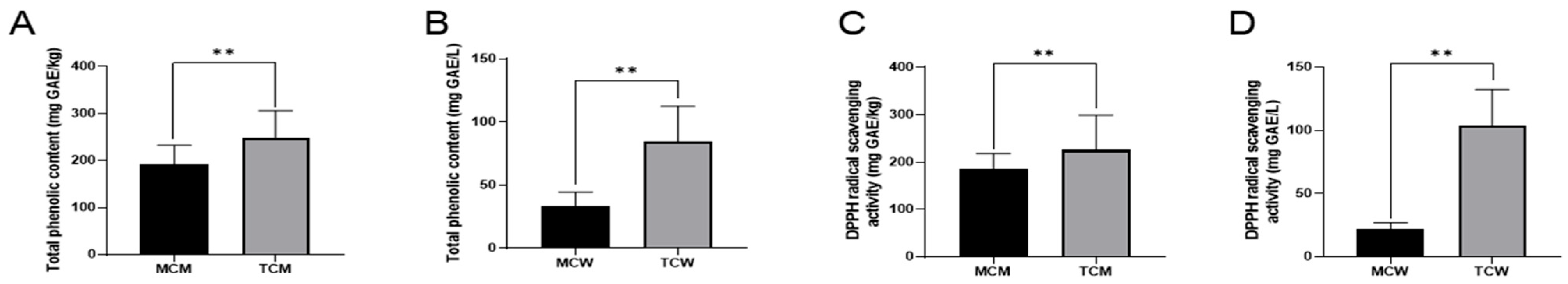
Phenolic compounds are widely considered as key contributors to the functional properties of coconut, and their concentration can serve as an important indicator of nutritional and bioactive potential. In this study, TPC of all samples was determined using the Folin–Ciocalteu method. As shown in
Figure 1A, distinct differences were observed between tender and mature coconuts. In coconut meat, TCM exhibited a significantly higher TPC (248.19 ± 35.89 mg GAE/kg) compared with MCM (190.90 ± 6.94 mg GAE/kg). A similar pattern was evident in coconut water, where TCW contained 92.41 ± 14.83 mg GAE/L and MCW contained 33.26 ± 6.99 mg GAE/L. Statistical analysis revealed that the total phenolic content of mature coconuts, both milk and water, was significantly lower than that of tender coconuts (
p < 0.01). Specifically, the TPC of TCM was approximately 1.3 times higher than that of MCM, while the TPC of TCW was about 2.7 times higher than that of MCW. These results clearly highlight the significant influence of maturity on the phenolic composition of coconut products. Previous studies support these findings. Li et al. [
19] demonstrated that coconut maturity strongly affects its internal constituents, noting that coconuts harvested at around 8 months after flowering contain abundant water and higher sugar levels, making them more suitable for fresh consumption or beverage processing. By contrast, at the mature stage (12 months), coconuts accumulate more fat, protein, and carbohydrates, with a marked thickening of the endosperm and a simultaneous decline in both water content and soluble sugars. This developmental transition is accompanied by a reduction in phenolic compounds, which may be due to the partial utilization of phenolic metabolites in physiological processes such as cell wall fortification. From a functional food perspective, these results suggest that incorporating tender coconut meat or water into the coconut milk production could enhance the phenolic content and bioactivity of coconut-based products.
Given the increasing consumer interest in natural functional foods, the antioxidant capacity of coconut is of particular relevance. The DPPH radical scavenging assay, a widely used method for assessing antioxidant activity, was employed to evaluate the samples (
Figure 1B). The results were consistent with TPC values. DPPH scavenging activity was 184.14 ± 7.47 mg GAE/kg in MCM and 225.00 ± 33.25 mg GAE/kg in TCM. For coconut water, the activity was markedly lower, with MCW containing 22.23 ± 2.12 mg GAE/L compared to 106.96 ± 0.80 mg GAE/L in TCW. Again, tender coconuts exhibited significantly stronger antioxidant activity than mature ones (
p < 0.01), a difference that can largely be attributed to their higher phenolic content. Phenolic compounds exhibit their antioxidant activity through several mechanisms, such as scavenging radicals, chelating metal ions, and inhibiting pro-oxidant enzymes [
20]. Therefore, the observed variations in phenolic content between tender and mature coconuts directly translated into differences in antioxidant potential.
Collectively, these findings confirm that maturity exerts a decisive influence on both phenolic levels and antioxidant activity in coconuts. From an application standpoint, supplementation with tender coconut water during product development may provide an effective strategy to enhance the antioxidant functionality of coconut milk–based foods and beverages.
3.3. Phytochemical Profile Analysis
The phytochemical composition of coconut milk contributes substantially to its nutritional and functional properties. To gain a comprehensive understanding of these constituents, an untargeted UHPLC-Q-Orbitrap-MS analysis was performed on coconut meat at two developmental stages and their corresponding coconut water samples. The representative chromatograms of the four types of samples are demonstrated in the
Supplementary Material Figure S2. Component identification was achieved based on accurate parent ion and fragment ion
m/
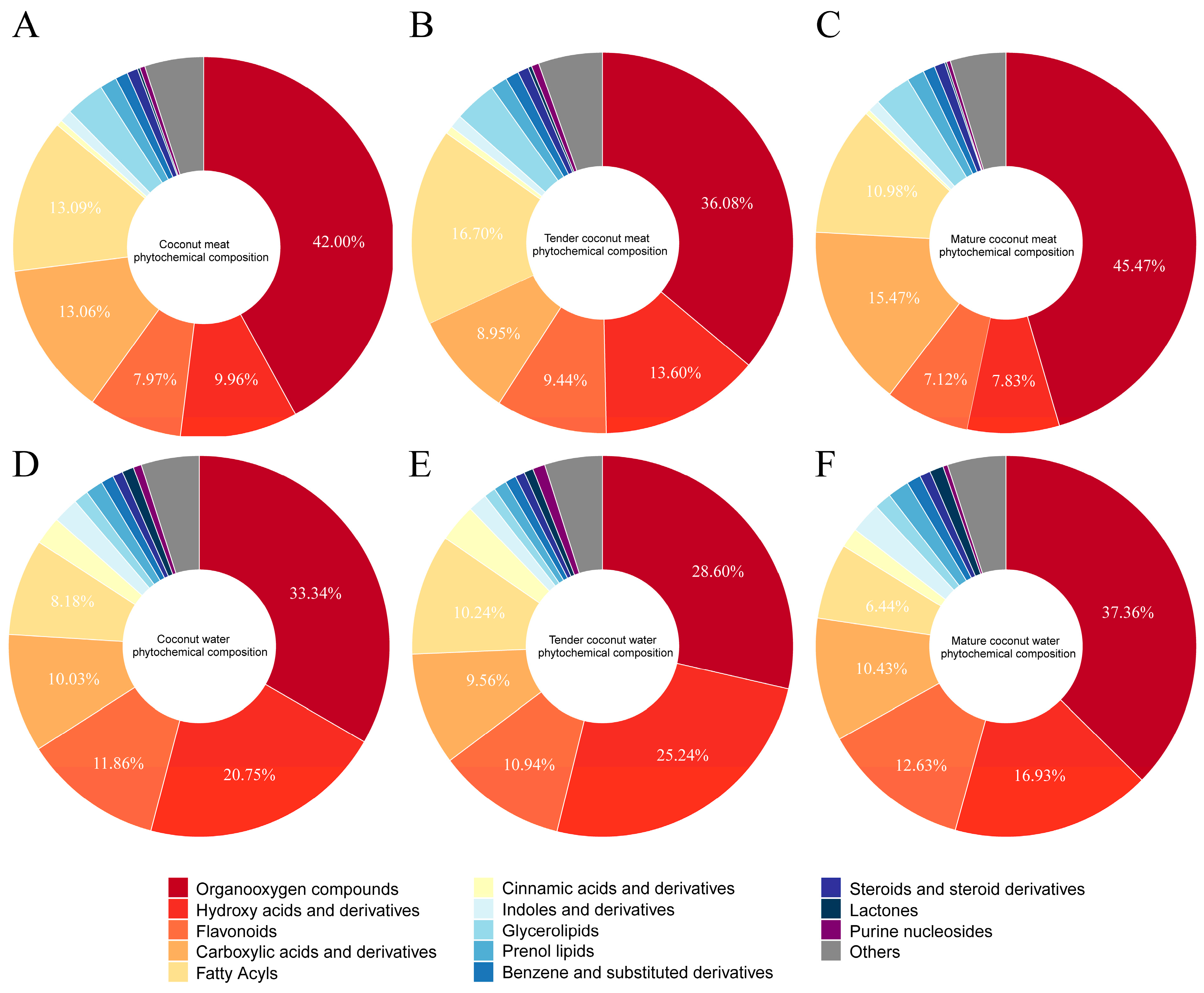
z information under both positive and negative ionization modes. In total, 1065 phytochemicals were tentatively identified and classified into 13 major categories, as demonstrated in the pie charts of
Figure 2. In coconut meat (
Figure 2A), the predominant categories (>1% relative abundance) included organooxygen compounds (42.00%, 199 compounds), fatty acyls (13.09%, 130 compounds), carboxylic acids and derivatives (13.06%, 231 compounds), hydroxy acids and derivatives (9.96%, 12 compounds), flavonoids (7.97%, 58 compounds), followed by glycerolipids (3.30%), prenol lipids (1.45%), unsaturated hydrocarbons (1.12%), and benzene derivatives (1.06%). Collectively, the top five categories accounted for approximately 86% of the phytochemical profile. Notably, phenolic compounds (flavonoids, hydroxycinnamic acids and derivatives, and benzoic acids and derivatives) represented 8.58% of total constituents, underscoring their potential nutritional relevance. The phytochemical profile of coconut water exhibited a similar compositional framework, although the relative abundance of key categories differed (
Figure 2D). The five most abundant groups were organooxygen compounds (33.34%), hydroxy acids and derivatives (20.75%), flavonoids (11.86%), carboxylic acids and derivatives (10.03%), and fatty acyls (8.18%). Importantly, the overall proportion of phenolic compounds in coconut water reached 14.44%, exceeding that of coconut meat. This suggests that while coconut meat is richer in lipid-related phytochemicals, coconut water has significantly higher content of phenolics.
A comparison between tender and mature coconut meat samples further revealed maturity-dependent compositional differences (
Figure 2B,C). In TCM, the top contributors were organooxygen compounds (36.08%), fatty acyls (16.70%), hydroxy acids and derivatives (13.60%), flavonoids (9.44%), and carboxylic acids and derivatives (8.95%). By contrast, MCM was dominated by organooxygen compounds (45.47%), followed by carboxylic acids and derivatives (15.47%), fatty acyls (10.98%), hydroxy acids and derivatives (7.83%), and flavonoids (7.12%). Similar changes were observed in coconut water (
Figure 2E,F). These shifts suggest that as coconuts mature, organooxygen compounds and carboxylic acids accumulate, while fatty acyls, hydroxy acids, and flavonoids decline proportionally.
Overall, these findings demonstrate that both the tissue type (meat vs. water) and developmental stage (tender vs. mature) exert significant influences on the phytochemical profile of coconut. Coconut meat is characterized by a higher abundance of lipid-derived metabolites, whereas coconut water contains a greater share of phenolic constituents. Moreover, tender coconut meat retains relatively higher proportions of fatty acyls and flavonoids compared with mature meat, highlighting the impact of maturation on nutritional quality. These compositional insights provide a foundation for evaluating the functional value of coconut-derived products and optimizing their use in plant-based nutrition and functional food development. Notably, 96 phenolic compounds were tentatively identified, accounting for ~7.16% of the total detected phytochemicals. These widespread plant compounds are known to confer numerous health benefits, including antioxidant, anti-obesity, anti-inflammatory, and antibacterial activities. Their presence in both coconut meat and water is therefore of particular nutritional and functional significance. The next section focuses specifically on these phenolic constituents to further elucidate their potential health-promoting properties in coconut-derived products.
3.4. Phenolic Profile Analysis
Given the relatively high proportion and biological relevance of phenolic compounds in coconut, this section focuses specifically on their detailed characterization in both coconut meat and coconut water. A total of 96 phenolic compounds were tentatively identified, including 58 flavonoids, 25 hydroxycinnamic acids and derivatives, and 13 benzoic acids and derivatives. Detailed information on these phenolic compounds is shown in
Supplementary Table S2. Comparative analyses were subsequently performed to investigate differences between coconut meat and coconut water, as well as between tender and mature stages.
3.4.1. General Comparison of the Phenolic Profile
Flavonoids represented the most abundant phenolic class across all samples. The major flavonoids identified included gardenin B, epicatechin, catechin, procyanidin B5, naringin, and procyanidin B2. Overall, flavonoids accounted for 10.84% of total metabolites, with relative contributions of 7.97% in coconut meat and 11.86% in coconut water. A maturity-dependent decline was observed: flavonoids comprised 9.44% of TCM but only 7.12% of MCM. This decreasing pattern suggests that flavonoid accumulation is more prominent in earlier developmental stages, consistent with their potential role in stress defense and growth regulation.
Hydroxycinnamic acids and derivatives were the second major group of phenolics, with key representatives including 2-hydroxycinnamic acid, 3-caffeoyl-1,5-quinolactone, trans-p-feruloyl-β-D-glucopyranoside, N1,N10-diferuloylspermidine, and caffeoylferuloylspermidine. This class accounted for 1.87% of total metabolites, with a markedly higher abundance in coconut water (2.36%) compared to coconut meat (0.49%). Within meat samples, their proportion declined from 0.66% in TCM to 0.39% in MCM, again indicating a maturity-related reduction. Given their well-established antioxidant and antimicrobial properties, the relatively higher concentration of hydroxycinnamic derivatives in tender coconuts suggests enhanced functional potency at early developmental stages.
Benzoic acids and derivatives were less abundant, with major compounds including benzocaine, ethyl benzoate, bis(2-ethylhexyl) phthalate, (+)-zeylenol, and vanillic acid. They accounted for only 0.19% of total metabolites, with relative levels of 0.22% in coconut water and 0.12% in coconut meat. Within meat samples, benzoic derivatives were slightly higher in TCM (0.13%) than in MCM (0.11%). Despite their low abundance, certain members, such as vanillic acid, are bioactive antioxidants and anti-inflammatory agents, likely contributing to the overall health-promoting attributes of coconut products.
Overall, the phenolic profile revealed clear influences of both tissue type and developmental stage. Coconut water contained a higher proportion of phenolics than coconut meat, particularly flavonoids and hydroxycinnamic acids, highlighting its role as a polyphenol-rich beverage. In addition, tender samples consistently exhibited greater phenolic abundance than mature ones, indicating that phenolic richness declines with fruit maturation as metabolic resources shift toward lipid accumulation in the kernel. Importantly, the identified flavonoids such as catechin, epicatechin, and procyanidins, which are well known for their antioxidant and cardioprotective properties, underscore the nutritional and functional potential of coconut-derived products, especially in their tender stage.
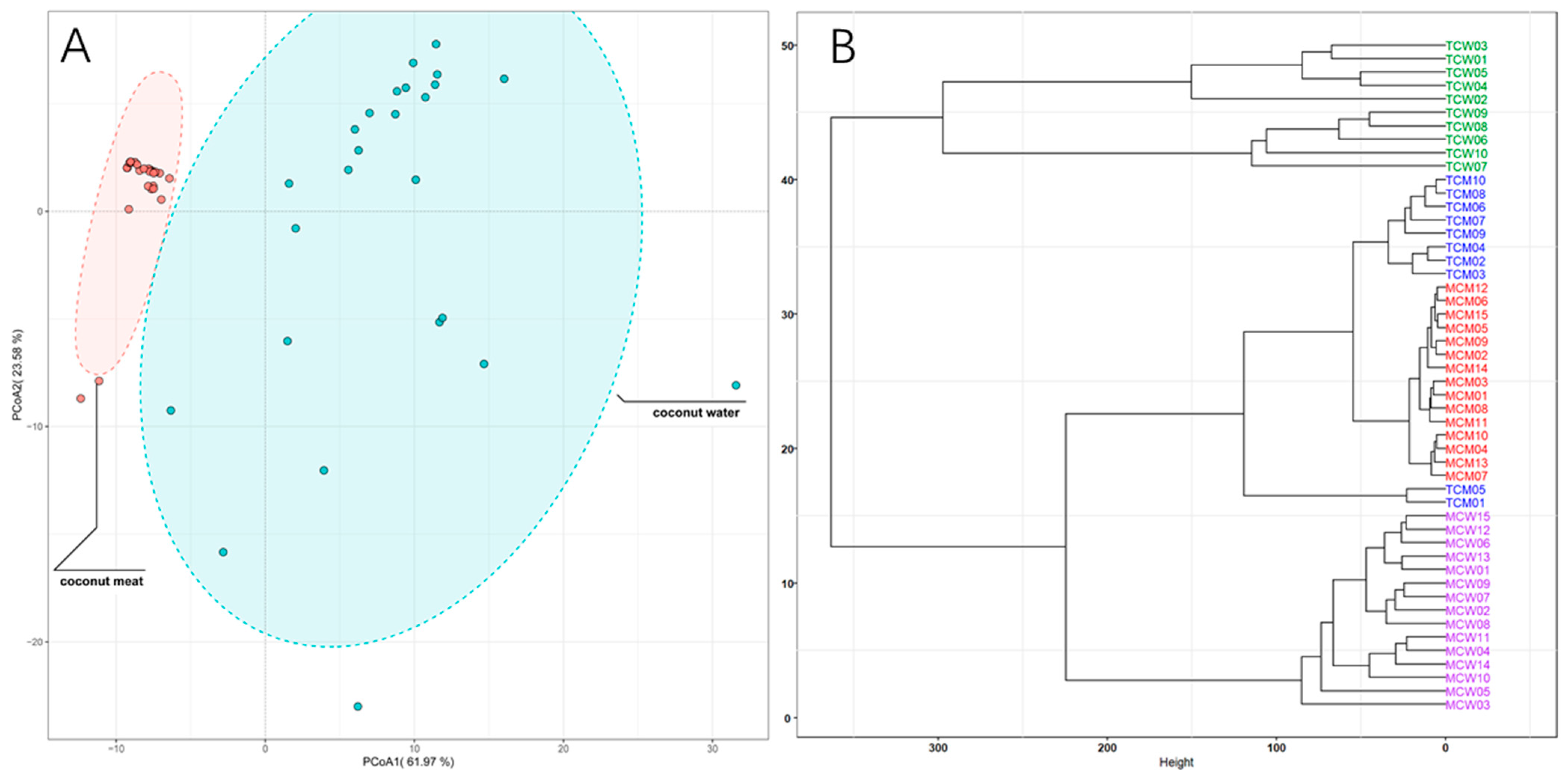
To further elucidate the overall distribution patterns of phenolic compounds across different sample types, principal coordinate analysis (PCoA) and hierarchical clustering analysis (HCA) were conducted (
Figure 3). The PCoA score plot (
Figure 3A) clearly separated coconut meat and coconut water, indicating a pronounced compositional divergence between these two matrices. Coconut meat samples clustered tightly within a small region, reflecting their relatively homogeneous phenolic composition. In contrast, coconut water samples were more widely dispersed, suggesting greater compositional variability, potentially due to environmental or physiological differences during fruit development. The HCA dendrogram (
Figure 3B) further supported these distinctions, revealing different clades corresponding to coconut water and coconut meat. Within the coconut meat group, tender (TCM) and mature (MCM) samples formed two adjacent but distinguishable subclusters, indicating maturity-dependent compositional shifts. For coconut water, tender (TCW) and mature (MCW) samples were also grouped separately, though with partially overlapping branches, reflecting both shared and distinct features in their phenolic profiles. Therefore, PCoA and HCA analyses reinforce that phenolic composition in coconut is strongly influenced by both tissue type and developmental stage. The tight clustering of coconut meat samples indicates greater biochemical stability in kernel-derived phenolics, whereas the wider variation in coconut water points to dynamic metabolite fluctuations during fruit maturation. These findings highlight the complementary contributions of meat and water to the overall phenolic profile of coconut, with coconut water serving as a more variable but phenolic-rich fraction, and coconut meat providing a more stable but lipid-associated phenolic pool.
3.4.2. Differential Phenolics Between Coconut Meat and Coconut Water
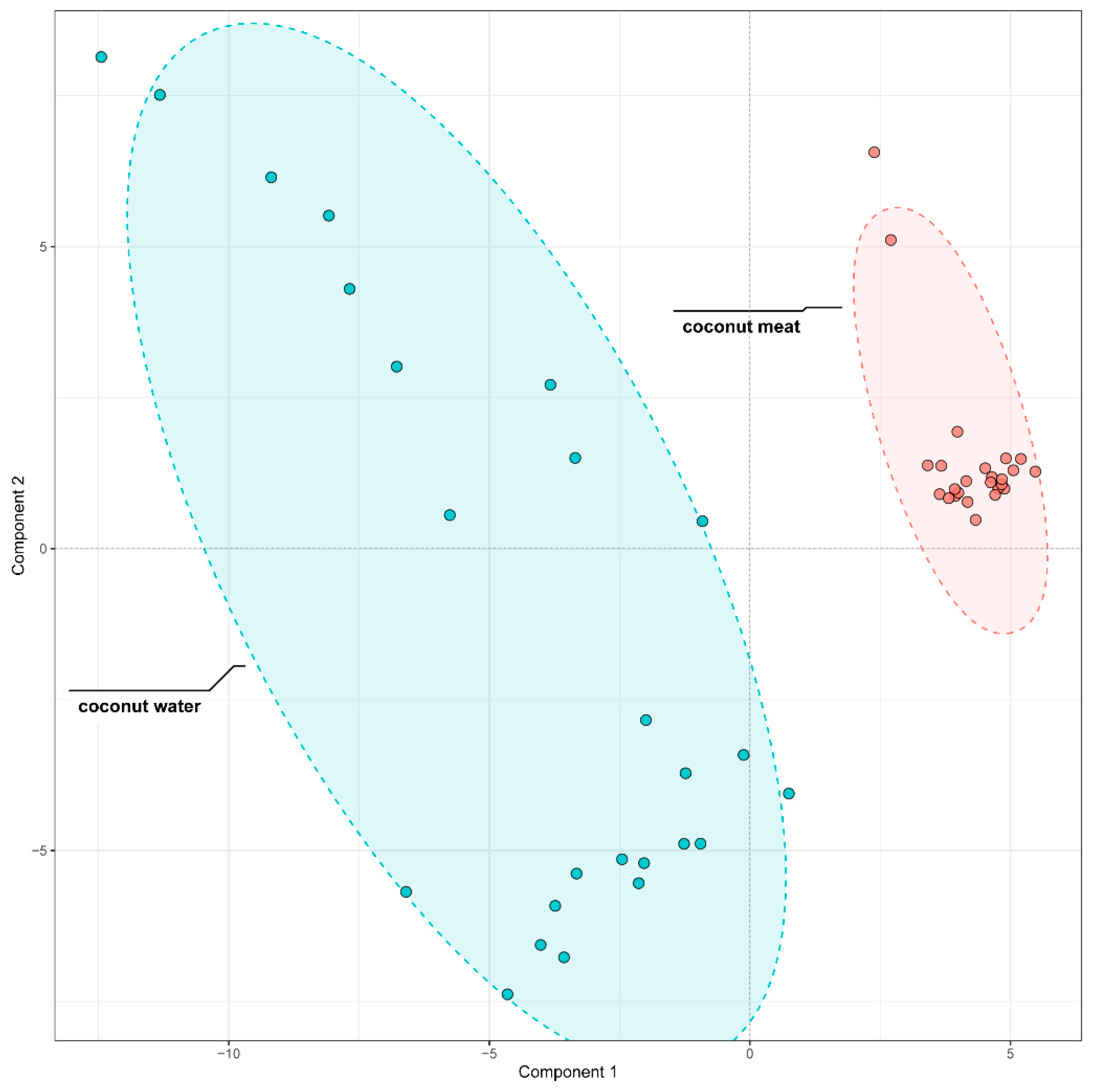
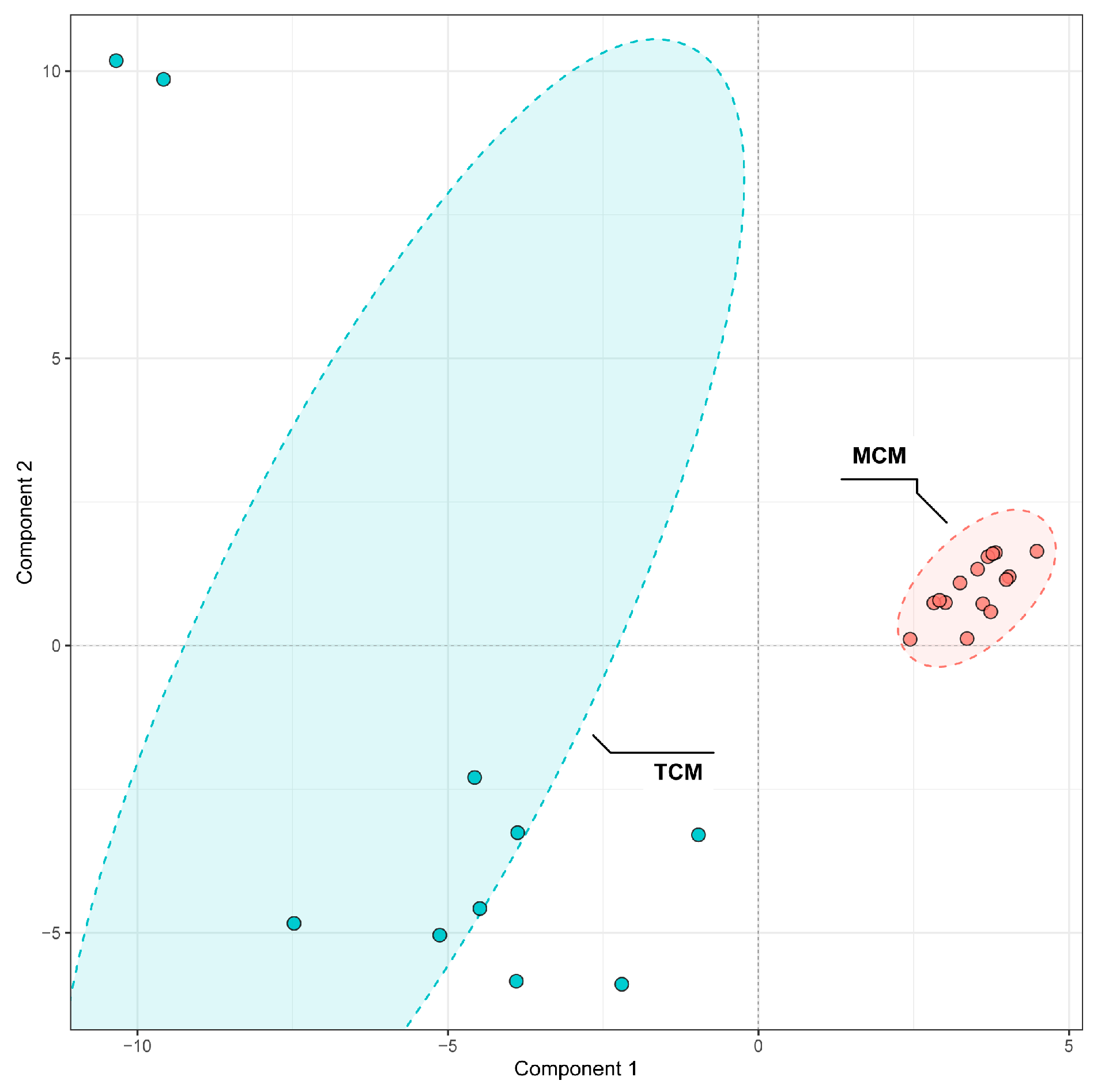
To further discriminate the phenolic composition of coconut meat and coconut water, we employed partial least squares-discriminant analysis (PLS-DA) on the 96 identified phenolic compounds. The model demonstrated excellent performance, with both its fitting (R
2) and predictive (Q
2) values reaching 0.99 during cross-validation, confirming its high reliability and predictive ability. The score plot revealed a distinct separation between the two sample types (
Figure 4), confirming that coconut meat and coconut water exhibit markedly different phenolic profiles. The coconut meat samples clustered tightly together, indicating a relatively homogeneous phenolic composition, whereas the coconut water samples exhibited broader dispersion, reflecting greater variability in their phenolic profiles.
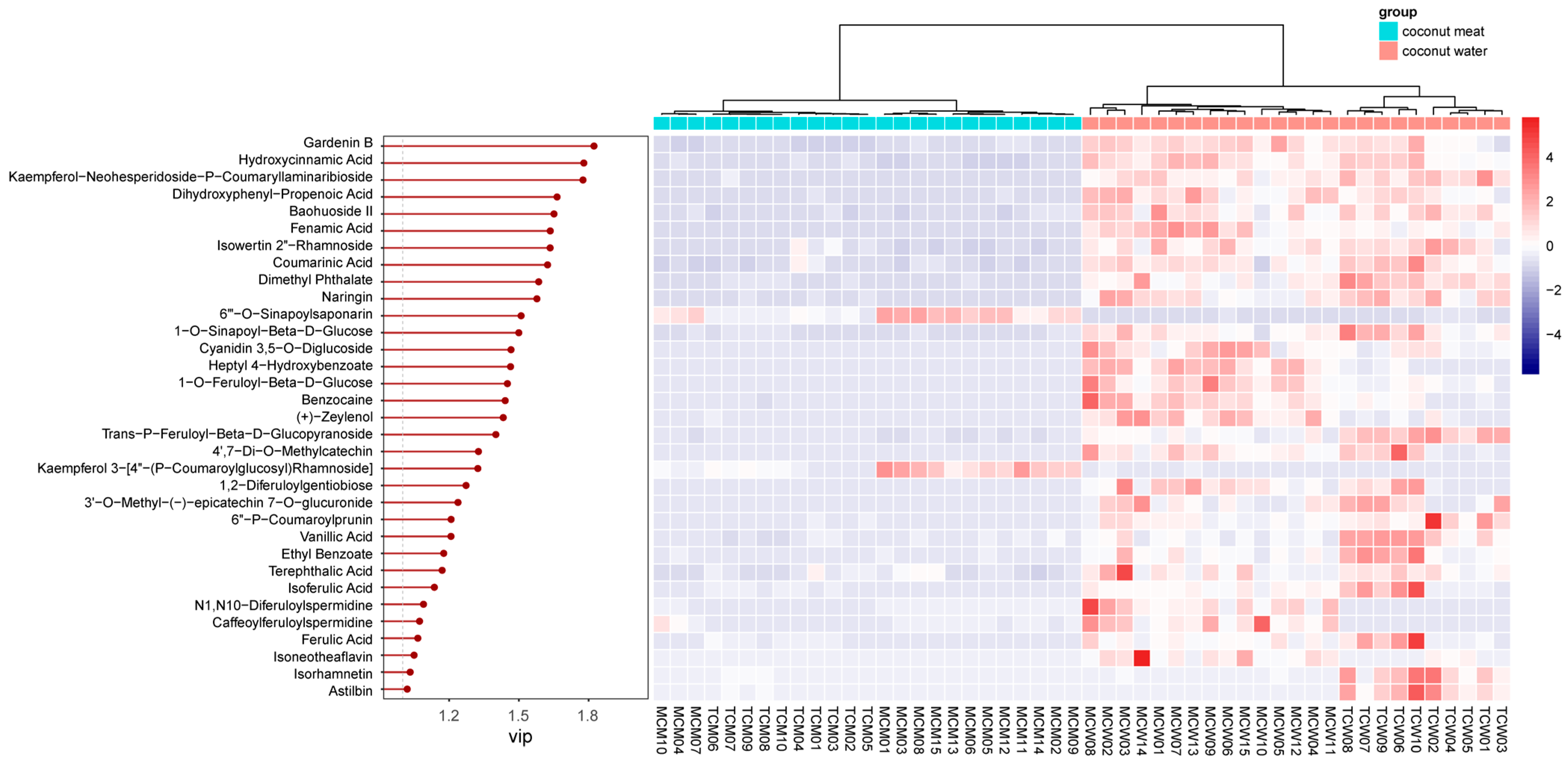
To identify the specific compounds driving this separation, variable importance in projection (VIP) scores were calculated. A total of 33 compounds showed VIP values greater than 1, indicating their strong contribution to group discrimination. These compounds were further visualized through a heatmap analysis (
Figure 5), which highlighted contrasting abundance patterns between coconut meat and coconut water.
Among these, the top five discriminant phenolics were gardenin B (VIP = 1.82), hydroxycinnamic acid (VIP = 1.78), kaempferol-neohesperidoside-p-coumaryllaminaribioside (VIP = 1.78), dihydroxyphenyl-propenoic acid (VIP = 1.66), and baohuoside II (VIP = 1.65). Gardenin B, a polymethoxyflavone, was markedly enriched in coconut water, suggesting a potential role in antioxidant defense and plant–environment interactions [
21]. Hydroxycinnamic acid and its derivatives, also more abundant in coconut water, are well known for their antioxidant, antimicrobial, and anti-inflammatory properties [
22], further emphasizing the functional potential of water compared with meat. Similarly, kaempferol glycosides and baohuoside II (a prenylated flavonoid) were more prominent in coconut water, indicating that glycosylated and specialized flavonoids preferentially accumulate in the liquid endosperm.
3.4.3. Differential Phenolics Between Tender and Mature Coconut Meat
To investigate the influence of developmental stage on the phenolic composition of coconut meat, PLS-DA was conducted to differentiate TCM and MCM. The PLS-DA score plot showed a clear separation between TCM and MCM (
Figure 6), indicating distinct phenolic signatures associated with the developmental stage. Notably, MCM samples clustered more tightly, indicating a relatively uniform phenolic composition at the mature stage, whereas TCM samples displayed broader dispersion, reflecting greater variability in phenolic accumulation during the earlier developmental stage. This suggests that phenolic biosynthesis and metabolism are more dynamic in tender coconuts but stabilize as the fruit matures.
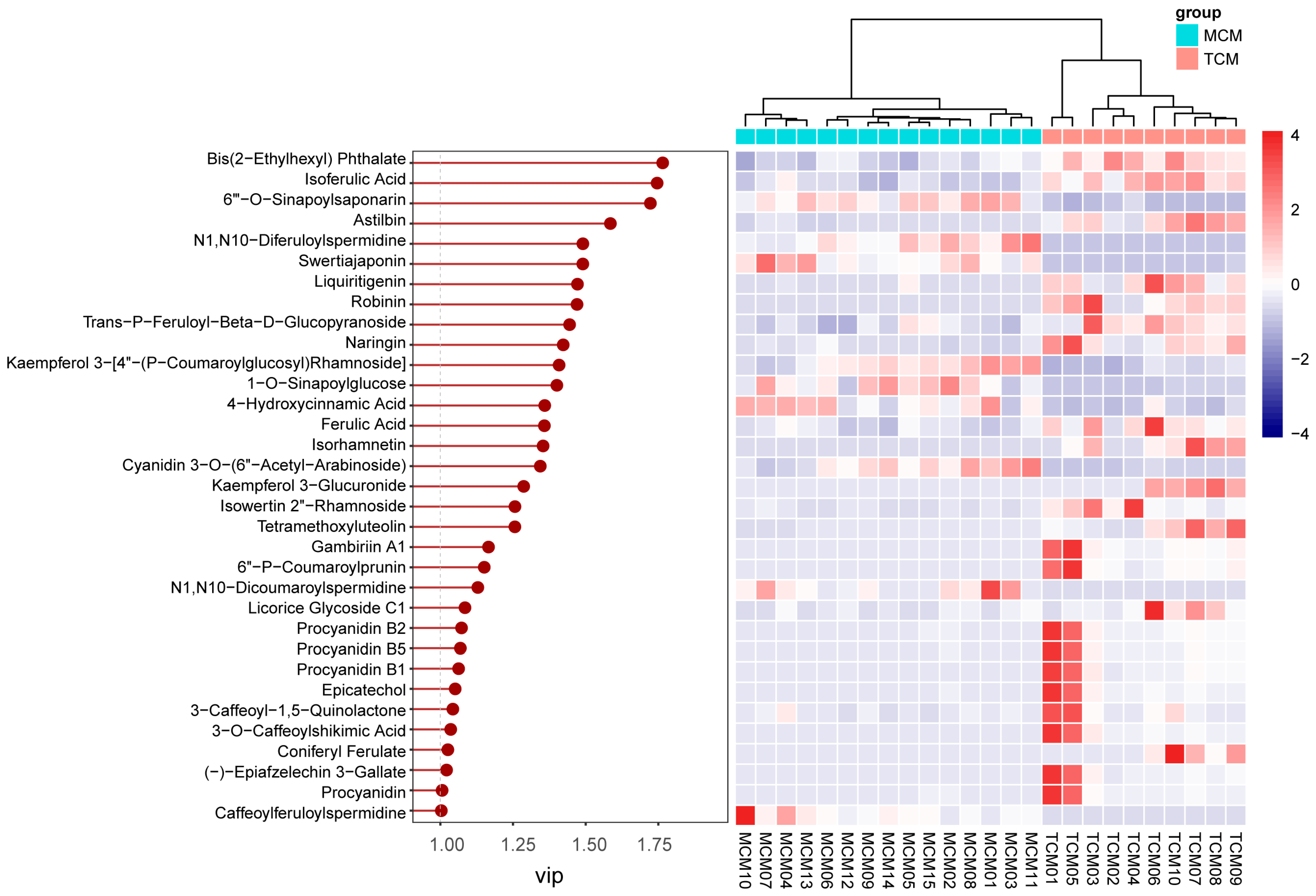
VIP analysis identified 33 discriminant compounds with VIP values greater than 1, highlighting their significant contribution to the observed separation. These discriminant phenolics were further visualized in a heatmap (
Figure 7), which clearly illustrated maturity-dependent accumulation patterns.
The top five discriminant compounds were bis(2-ethylhexyl) phthalate (VIP = 1.77), isoferulic acid (VIP = 1.75), 6′′′-O-sinapoylsaponarin (VIP = 1.72), astilbin (VIP = 1.58), and N1,N10-diferuloylspermidine (VIP = 1.49). Among these, isoferulic acid, a hydroxycinnamic acid derivative, was more abundant in TCM, indicating stronger accumulation of antioxidant and antimicrobial phenolics in the early developmental stage [
23,
24]. Similarly, astilbin, a flavonoid with notable anti-inflammatory and hepatoprotective properties [
25], was enriched in TCM, further reinforcing its higher functional potential. In contrast, 6′′′-O-sinapoylsaponarin, a phenolic glycoside, and N1,N10-diferuloylspermidine, a hydroxycinnamic acid derivative, were more enriched in MCM, suggesting compositional shifts toward more complex or storage-related phenolics with fruit maturation.
3.4.4. Differential Phenolics Between Tender and Mature Coconut Water
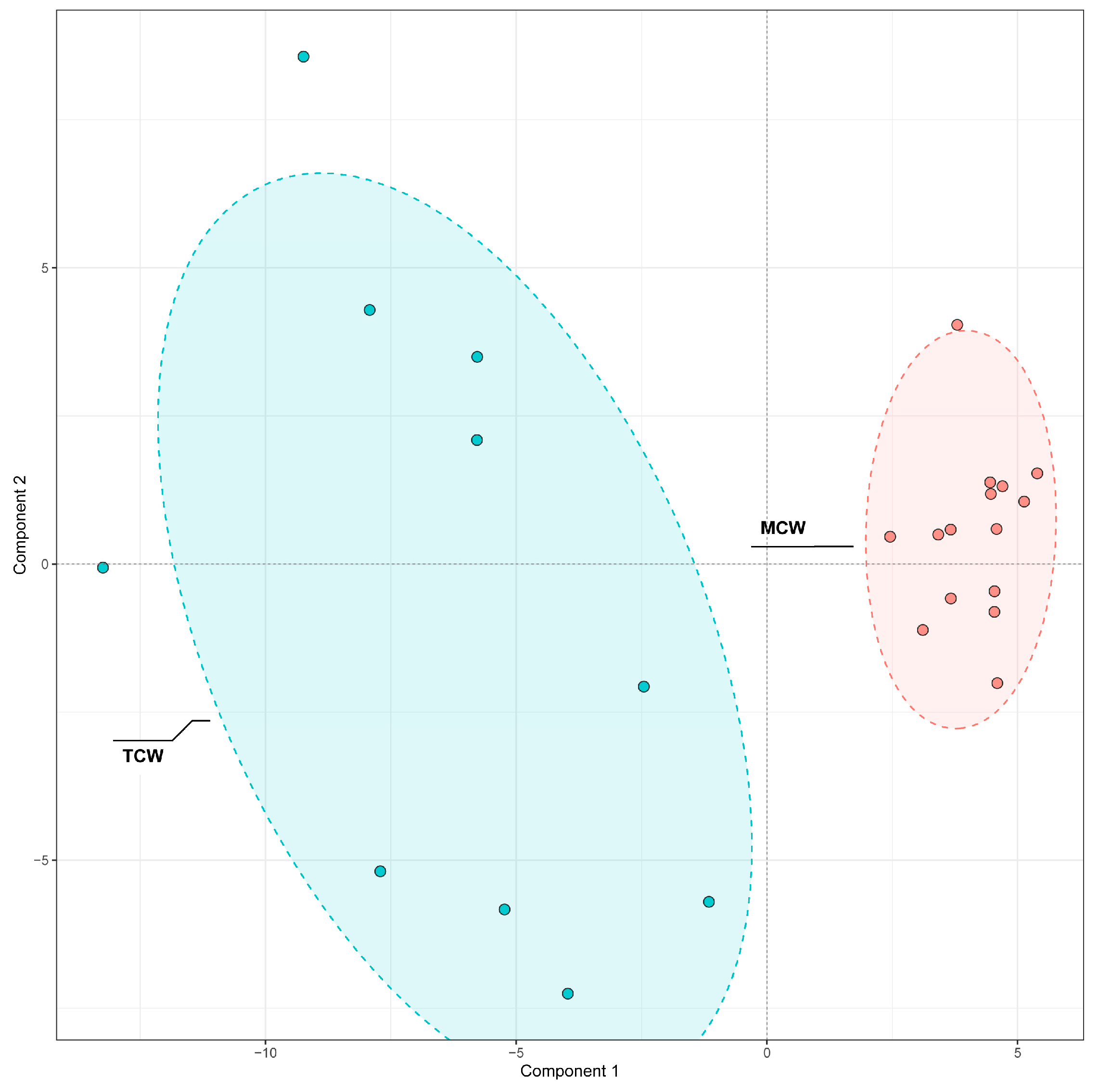
A similar PLS-DA approach was used to examine differences between TCW and MCW (
Figure 8). The clear separation along the first principal component indicated maturity-dependent variation in phenolic composition.
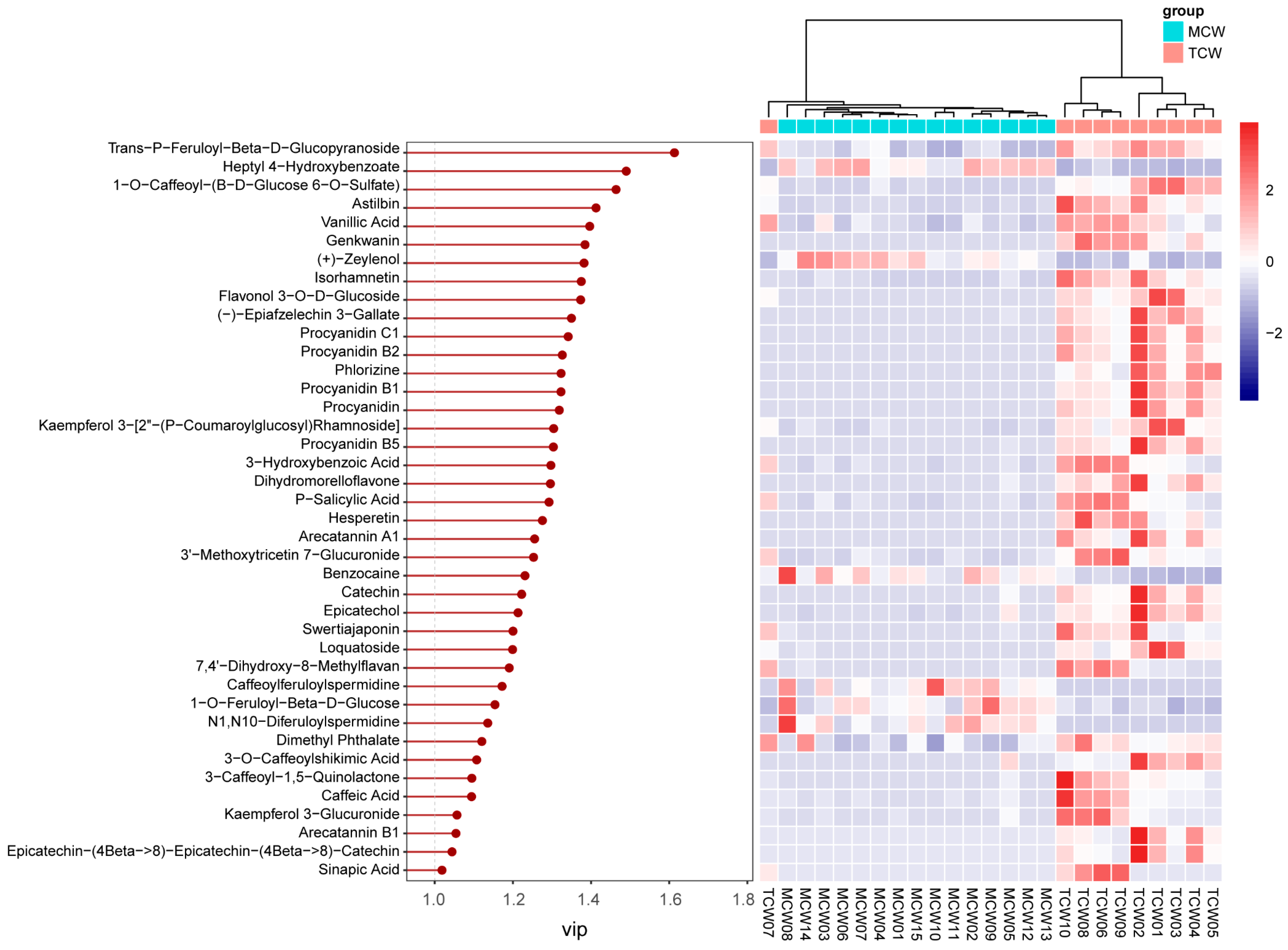
VIP analysis identified 40 discriminant compounds (VIP > 1), whose abundance patterns were displayed in the heatmap (
Figure 9). The differential abundance patterns revealed that several procyanidins (B1, B2, B5, C1) and hydroxybenzoic acid derivatives were more enriched in MCW, whereas TCW was characterized by higher levels of catechins, caffeoyl derivatives, and certain flavonoids such as hesperetin and loquatoside. The five top VIP compounds were trans-p-feruloyl-β-D-glucopyranoside (VIP = 1.61), heptyl 4-hydroxybenzoate (VIP = 1.49), 1-O-caffeoyl-(β-D-glucose 6-O-sulfate) (VIP = 1.46), astilbin (VIP = 1.41), and vanillic acid (VIP = 1.40). These compounds collectively contributed to the clear separation observed between TCW and MCW. Their distribution patterns suggest that the maturation of coconut water is associated with an accumulation of benzoic and cinnamic acid derivatives, possibly reflecting enhanced phenolic metabolism and oxidative polymerization during fruit development.
Overall, the phenolic composition of coconut exhibits remarkable tissue- and maturity-dependent diversity. Coconut water is a richer and more dynamic reservoir of phenolics, particularly flavonoids and hydroxycinnamic acids, while coconut meat has a more stable but lipid-associated matrix. The tender stage of development is characterized by higher concentrations of bioactive phenolics such as catechins, epicatechin, and astilbin, conferring stronger antioxidant potential, whereas the mature stage features a transition toward structurally complex or storage-related phenolics. These compositional transitions reflect the metabolic reprogramming accompanying coconut maturation and underline the nutritional and functional significance of phenolic compounds, particularly in tender coconut products.