Anti-Skin Aging Potential of Methoxyflavones from Kaempferia parviflora Against TNF-α-Induced Oxidative Stress and Photoaging in Normal Human Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation of Compounds
2.3. Cell Culture and Sample Preparation
2.4. Cell Viability Measurement
2.5. Measurement of Intracellular ROS Activity
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
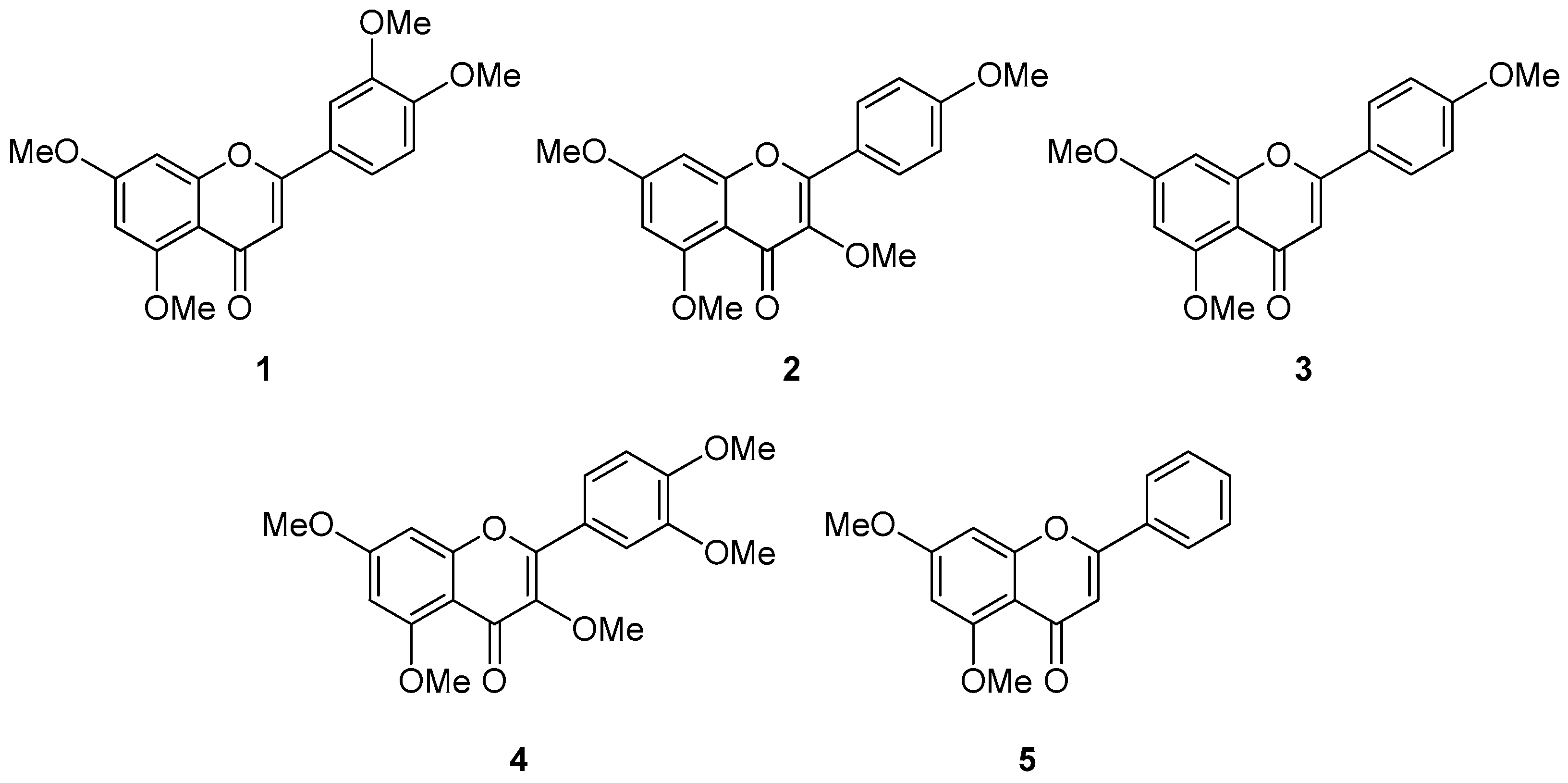
3.1. Isolation and Structural Identification of Methoxyflavones
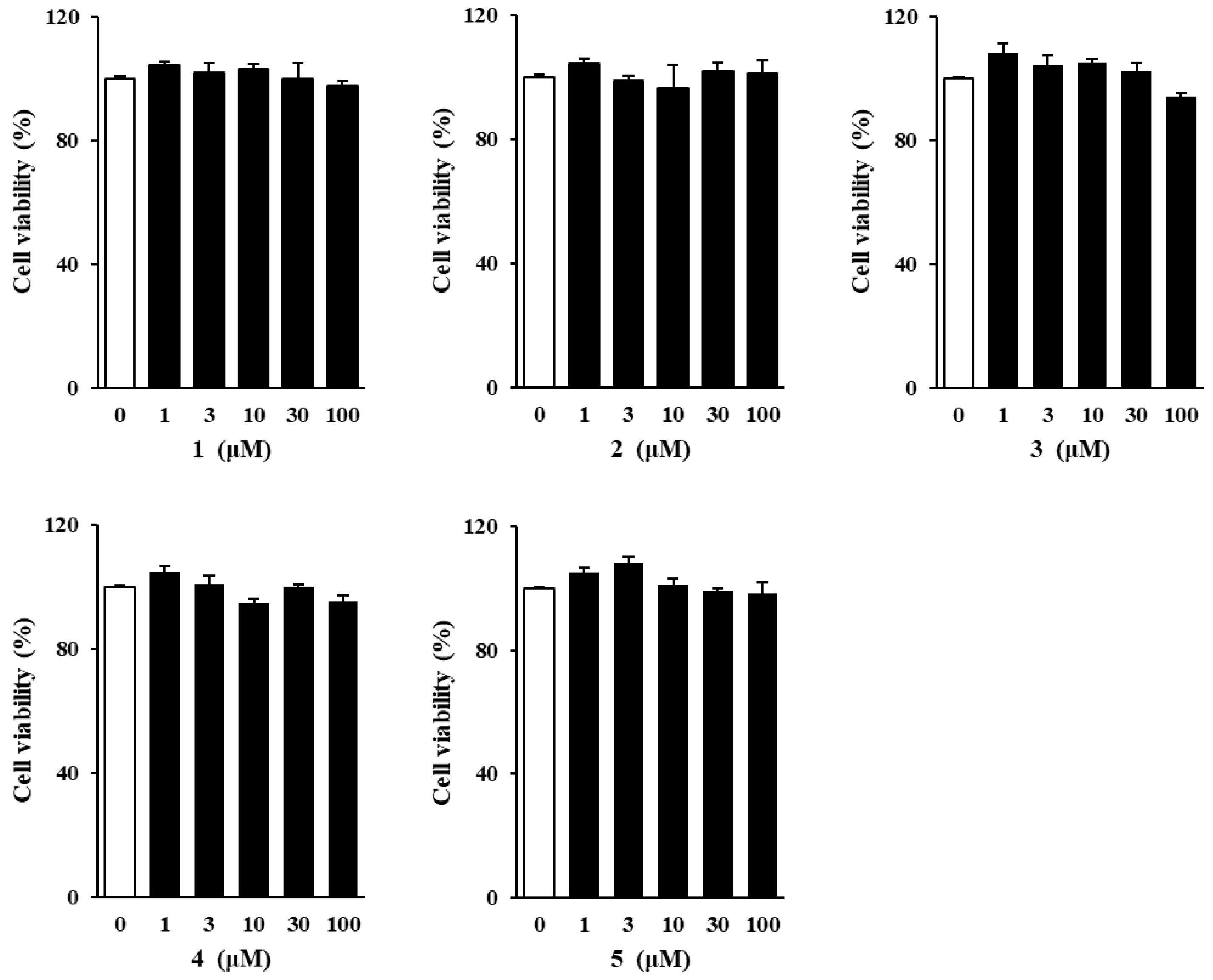
3.2. Effects of Methoxyflavones 1–5 on NHDF Viability
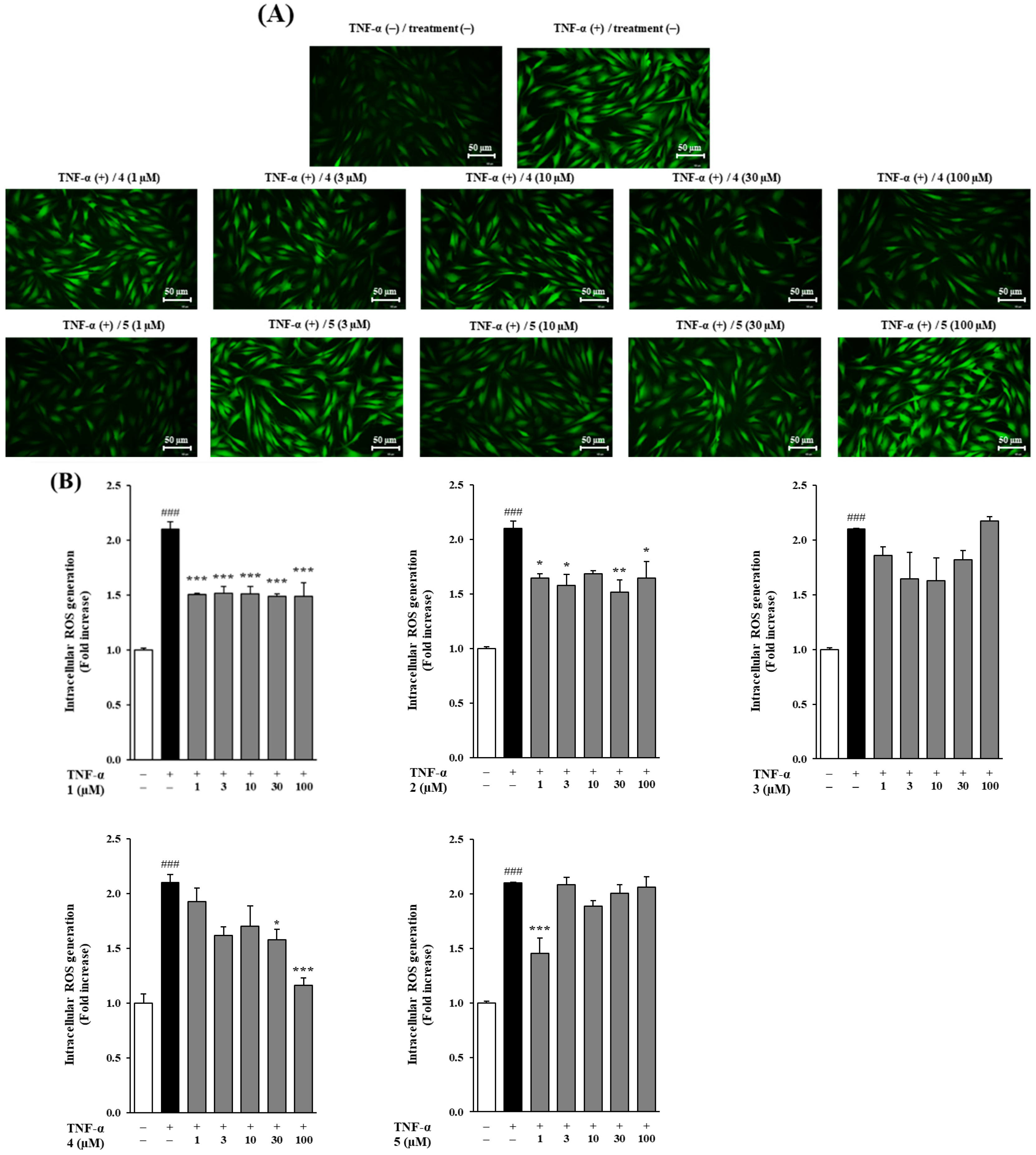
3.3. Effects of Methoxyflavones 1–5 on Intracellular ROS Generation in TNF-α-Stimulated NHDFs
3.4. Effects of Methoxyflavones 1–5 on MMP-1 and COLIA1 Protein Secretion in TNF-α-Treated NHDFs
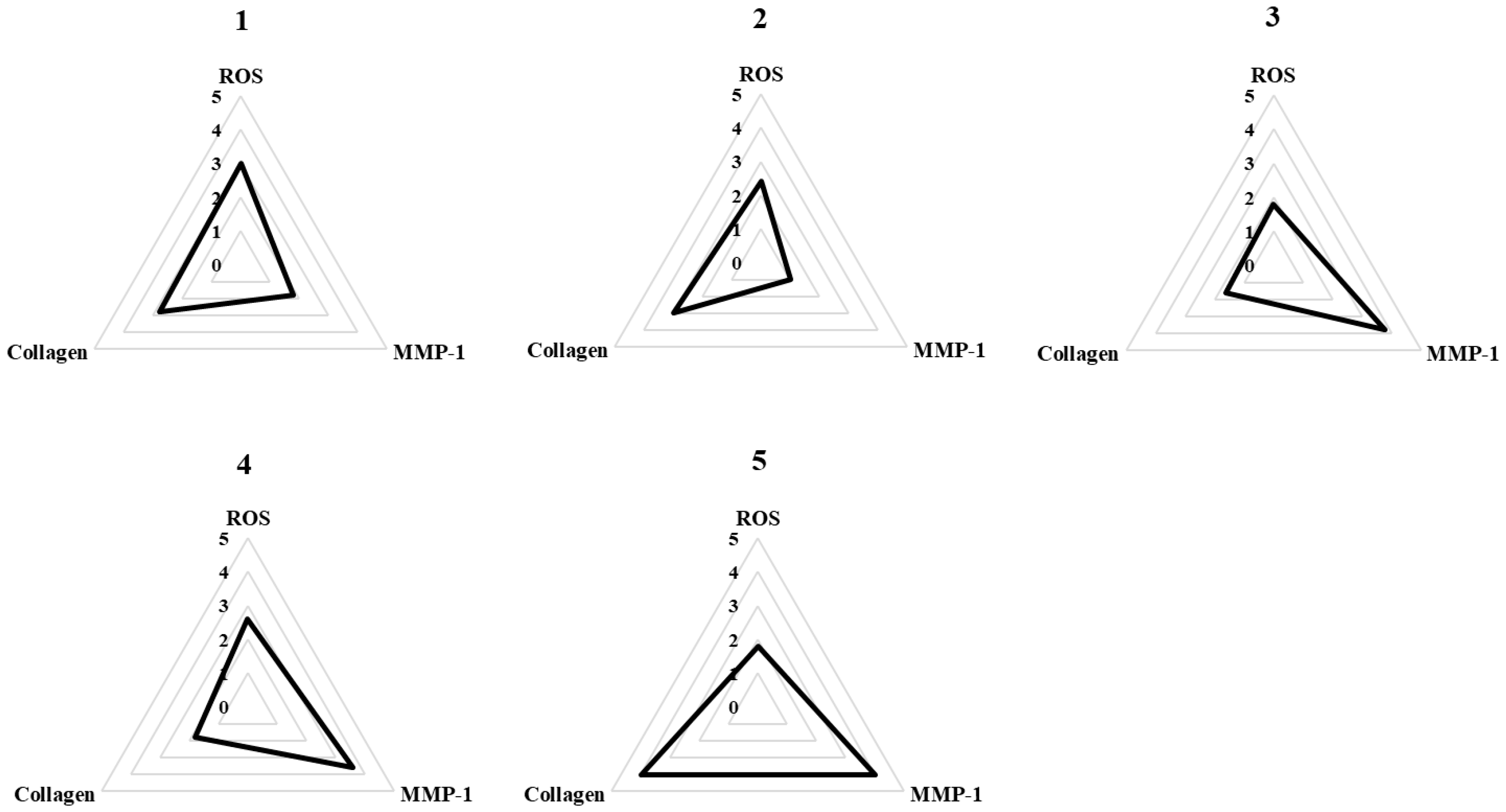
3.5. Spider Chart to Compare the Efficacy of Methoxyflavones 1–5
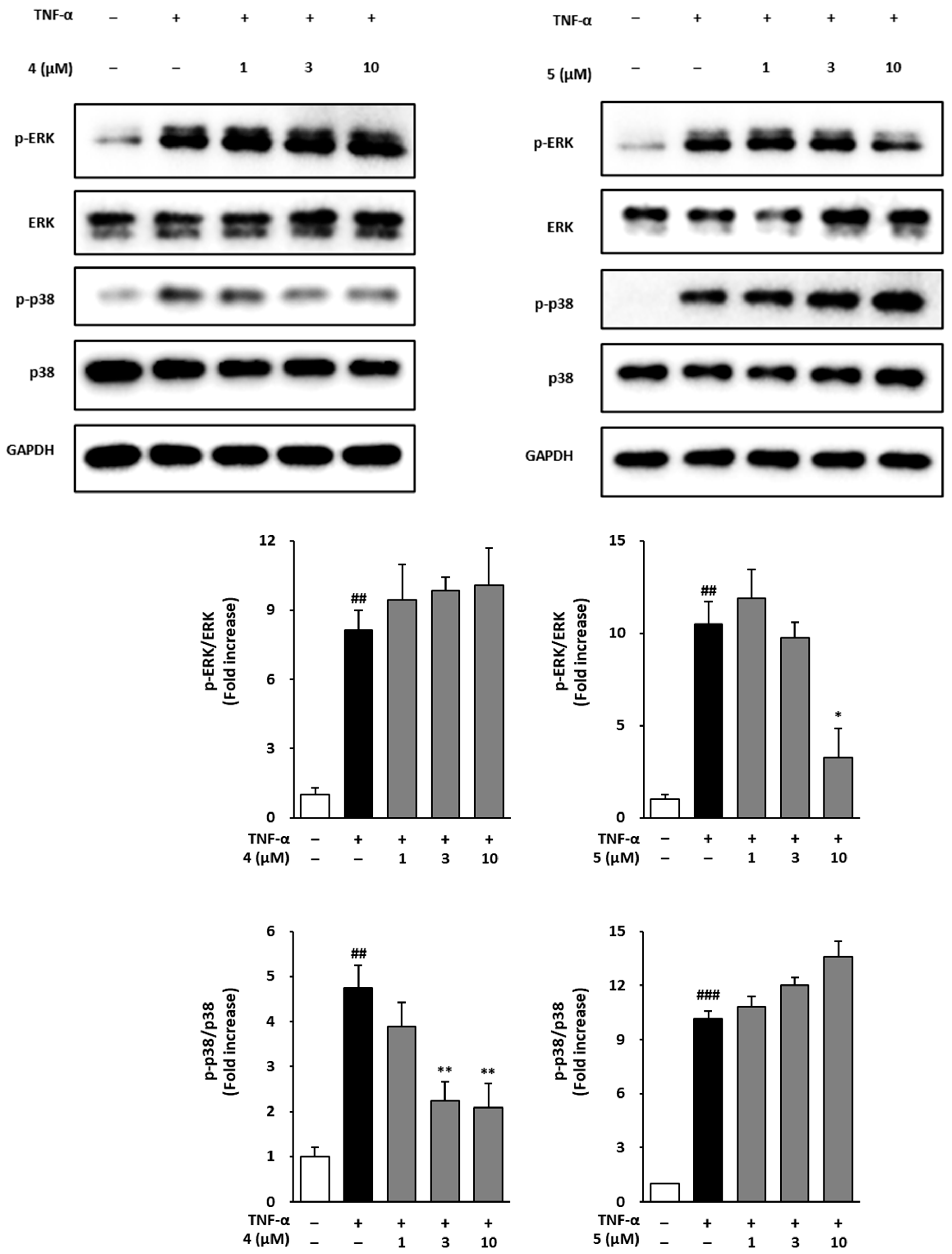
3.6. Effects of Methoxyflavones 4 and 5 on MAPK Phosphorylation in TNF-α-Treated NHDFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Chapter 1—Anatomy and Function of the Skin. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 1–14. ISBN 9780128029268. [Google Scholar]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-p.; Bi, B.; Chen, L.; Yang, P.; Guo, Y.; Zhou, Y.-q.; Liu, T.-y. Repeated exposure of mouse dermal fibroblasts at a sub-cytotoxic dose of UVB leads to premature senescence: A robust model of cellular photoaging. J. Dermatol. Sci. 2014, 73, 49–56. [Google Scholar] [CrossRef]
- Bergfeld, W.F. The aging skin. Int. J. Fertil. Womens Med. 1997, 42, 57–66. [Google Scholar]
- Heck, D.E.; Vetrano, A.M.; Mariano, T.M.; Laskin, J.D. UVB Light Stimulates Production of Reactive Oxygen Species: UNEXPECTED ROLE FOR CATALASE. J. Biol. Chem. 2003, 278, 22432–22436. [Google Scholar] [CrossRef]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet Radiations: Skin Defense-Damage Mechanism. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 71–87. ISBN 978-3-319-56017-5. [Google Scholar]
- Wood, L.C.; Elias, P.M.; Calhoun, C.; Tsai, J.C.; Grunfeld, C.; Feingold, K.R. Barrier Disruption Stimulates Interleukin-1α Expression and Release from a Pre-Formed Pool in Murine Epidermis. J. Investig. Dermatol. 1996, 106, 397–403. [Google Scholar] [CrossRef]
- Berneburg, M.; Gattermann, N.; Stege, H.; Grewe, M.; Vogelsang, K.; Ruzicka, T.; Krutmann, J. Chronically Ultraviolet-exposed Human Skin Shows a Higher Mutation Frequency of Mitochondrial DNA as Compared to Unexposed Skin and the Hematopoietic System. Photochem. Photobiol. 1997, 66, 271–275. [Google Scholar] [CrossRef]
- Squier, T.C. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001, 36, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H.; Moore, W.G.I.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix Metalloproteinases: A Review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [PubMed]
- Catalgol, B.; Ziaja, I.; Breusing, N.; Jung, T.; Höhn, A.; Alpertunga, B.; Schroeder, P.; Chondrogianni, N.; Gonos, E.S.; Petropoulos, I.; et al. The Proteasome Is an Integral Part of Solar Ultraviolet A Radiation-induced Gene Expression. J. Biol. Chem. 2009, 284, 30076–30086. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B Biol. 2016, 165, 34–41. [Google Scholar]
- Krieg, T.; Hein, R.; Hatamochi, A.; Aumailley, M. Molecular and clinical aspects of connective tissue. Eur. J. Clin. Investig. 1988, 18, 105–123. [Google Scholar] [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.-Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Kohl, E.; Steinbauer, J.; Landthaler, M.; Szeimies, R.-M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 873–884. [Google Scholar] [CrossRef]
- Lavker, R.M.; Veres, D.A.; Irwin, C.J.; Kaidbey, K.H. Quantitative assessment of cumulative damage from repetitive exposures to suberythemogenic doses of UVA in human skin. Photochem. Photobiol. 1995, 62, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, T.W.; Watson, R.E.; Langton, A.K. Skin ageing and topical rejuvenation strategies. Br. J. Dermatol. 2023, 189, i17–i23. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.A.; Mikail, M.A.; Zamakshshari, N.; Abdullah, A.-S.H. Natural anti-aging skincare: Role and potential. Biogerontology 2020, 21, 293–310. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research progress on skin aging and active ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential anti-skin aging effect of (-)-Catechin isolated from the root bark of Ulmus davidiana var. japonica in tumor necrosis factor-α-stimulated normal human dermal fibroblasts. Antioxidants 2020, 9, 981. [Google Scholar]
- Devi, N.; Das, A.; Singh, P. Kaempferia parviflora (Zingiberaceae): A new record in the flora of Manipur. Int. J. Innov. Sci. Eng. Technol. 2016, 3, 661–665. [Google Scholar]
- Pham, N.K.; Nguyen, H.T.; Nguyen, Q.B. A review on the ethnomedicinal uses, phytochemistry and pharmacology of plant species belonging to Kaempferia L. genus (Zingiberaceae). Pharm. Sci. Asia 2021, 48, 1–24. [Google Scholar] [CrossRef]
- Toda, K.; Hitoe, S.; Takeda, S.; Shimoda, H. Black ginger extract increases physical fitness performance and muscular endurance by improving inflammation and energy metabolism. Heliyon 2016, 2, e00115. [Google Scholar] [CrossRef] [PubMed]
- Phurailatpam, A.; Choudhury, A.; Yatung, T.; Momin, K.C. A review on the importance of two medicinal plants of North East India: Paris polyphylla Smith and Kaempheria parviflora Wall. ex Baker. Ann. Phytomed. 2022, 11, 214–223. [Google Scholar] [CrossRef]
- Azuma, T.; Tanaka, Y.; Kikuzaki, H. Phenolic glycosides from Kaempferia parviflora. Phytochemistry 2008, 69, 2743–2748. [Google Scholar] [CrossRef]
- Moon, H.-I.; Cho, S.-B.; Lee, J.-H.; Paik, H.-D.; Kim, S.-K. Immunotoxicity activity of sesquiterpenoids from black galingale (Kaempferia parviflora Wall. Ex. Baker) against Aedes aegypti L. Immunopharmacol. Immunotoxicol. 2011, 33, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Fuchino, H.; Fukui, N.; Iida, O.; Wada, H.; Kawahara, N. Inhibitory effect of black ginger (Kaempferia parviflora) constituents on nitric oxide production. Jpn. J. Food Chem. Saf. 2018, 25, 152–159. [Google Scholar]
- Chen, D.; Li, H.; Li, W.; Feng, S.; Deng, D. Kaempferia parviflora and its methoxyflavones: Chemistry and biological activities. Evid. Based Complement. Alternat. Med. 2018, 2018, 4057456. [Google Scholar] [CrossRef]
- Sawasdee, P.; Sabphon, C.; Sitthiwongwanit, D.; Kokpol, U. Anticholinesterase activity of 7-methoxyflavones isolated from Kaempferia parviflora. Phytother. Res. 2009, 23, 1792–1794. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kato, T.; Azuma, T.; Kikuzaki, H.; Abe, K. Anti-allergenic activity of polymethoxyflavones from Kaempferia parviflora. J. Funct. Foods. 2015, 13, 100–107. [Google Scholar] [CrossRef]
- Horigome, S.; Yoshida, I.; Ito, S.; Inohana, S.; Fushimi, K.; Nagai, T.; Yamaguchi, A.; Fujita, K.; Satoyama, T.; Katsuda, S.-I. Inhibitory effects of Kaempferia parviflora extract on monocyte adhesion and cellular reactive oxygen species production in human umbilical vein endothelial cells. Eur. J. Nutr. 2017, 56, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Klinngam, W.; Rungkamoltip, P.; Thongin, S.; Joothamongkhon, J.; Khumkhrong, P.; Khongkow, M.; Namdee, K.; Tepaamorndech, S.; Chaikul, P.; Kanlayavattanakul, M. Polymethoxyflavones from Kaempferia parviflora ameliorate skin aging in primary human dermal fibroblasts and ex vivo human skin. Biomed. Pharmacother. 2022, 145, 112461. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jang, T.; Kim, K.H.; Kang, K.S. Improvement of damage in human dermal fibroblasts by 3, 5, 7-trimethoxyflavone from black ginger (Kaempferia parviflora). Antioxidants 2022, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Phung, H.M.; Lee, S.; Hong, S.; Lee, S.; Jung, K.; Kang, K.S. Protective effect of polymethoxyflavones isolated from Kaempferia parviflora against TNF-α-induced human dermal fibroblast damage. Antioxidants 2021, 10, 1609. [Google Scholar] [CrossRef]
- Lee, D.E.; Park, K.H.; Hong, J.-H.; Kim, S.H.; Park, K.-M.; Kim, K.H. Anti-osteoporosis effects of triterpenoids from the fruit of sea buckthorn (Hippophae rhamnoides) through the promotion of osteoblast differentiation in mesenchymal stem cells, C3H10T1/2. Arch. Pharm. Res. 2023, 46, 771–781. [Google Scholar] [CrossRef]
- Lee, S.; Jang, M.; Ryoo, R.; Roh, J.; Ko, S.-K.; Kim, K.H. New autophagy-modulating lanostane-type triterpenoids from a hallucinogenic poisonous mushroom Gymnopilus orientispectabilis. Arch. Pharm. Res. 2024, 47, 272–287. [Google Scholar] [CrossRef]
- Sutthanut, K.; Sripanidkulchai, B.; Yenjai, C.; Jay, M. Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J. Chromatogr. A 2007, 1143, 227–233. [Google Scholar] [CrossRef]
- Ryu, G.; Ma, C.J. Neuroprotective compounds isolated from Lysimachia christinae. Nat. Prod. Sci. 2023, 29, 10–16. [Google Scholar] [CrossRef]
- Klotz, L.; Holbrook, N.J.; Sies, H. UVA and singlet oxygen as inducers of cutaneous signaling events. Curr. Probl. Dermatol. 2001, 29, 95–113. [Google Scholar] [PubMed]
- Kang, D.-M.; Kim, H.-J.; Park, W.S.; Bae, J.-Y.; Akter, K.-M.; Kim, Y.; Khalil, A.A.K.; Ahn, M.-J. Antioxidant and anti-inflammatory activities of Rumex acetosa. Nat. Prod. Sci. 2023, 29, 330–336. [Google Scholar] [CrossRef]
- Kang, D.-M.; Kwon, J.-M.; Jeong, W.-J.; Neupane, B.D.; Ahn, M.-J. Antioxidant compounds of Sambucus pendula Stem. Nat. Prod. Sci. 2024, 30, 275–281. [Google Scholar] [CrossRef]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar]
- Zouboulis, C.C.; Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Elewa, R.; Makrantonaki, E. Aesthetic aspects of skin aging, prevention, and local treatment. Clin. Dermatol. 2019, 37, 365–372. [Google Scholar] [CrossRef]
- Chen, X.; Andresen, B.T.; Hill, M.; Zhang, J.; Booth, F.; Zhang, C. Role of reactive oxygen species in tumor necrosis factor-alpha induced endothelial dysfunction. Curr. Hypertens. Rev. 2008, 4, 245–255. [Google Scholar] [CrossRef]
- Lee, Y.H.; Seo, E.K.; Lee, S.-T. Skullcapflavone II inhibits degradation of type I collagen by suppressing MMP-1 transcription in human skin fibroblasts. Int. J. Mol. Sci. 2019, 20, 2734. [Google Scholar] [CrossRef]
- Tanaka, H.; Okada, T.; Konishi, H.; Tsuji, T. The effect of reactive oxygen species on the biosynthesis of collagen and glycosaminoglycans in cultured human dermal fibroblasts. Arch. Dermatol. Res. 1993, 285, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Cortez, D.M.; Feldman, M.D.; Mummidi, S.; Valente, A.J.; Steffensen, B.; Vincenti, M.; Barnes, J.L.; Chandrasekar, B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK-and ERK1/2-dependent C/EBP-β, NF-κB, and AP-1 activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3356–H3365. [Google Scholar] [CrossRef]
- Kushibiki, T.; Tu, Y.; Abu-Yousif, A.O.; Hasan, T. Photodynamic activation as a molecular switch to promote osteoblast cell differentiation via AP-1 activation. Sci. Rep. 2015, 5, 13114. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-g. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Qin, Z.; Robichaud, P.; He, T.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Oxidant exposure induces cysteine-rich protein 61 (CCN1) via c-Jun/AP-1 to reduce collagen expression in human dermal fibroblasts. PLoS ONE 2014, 9, e115402. [Google Scholar] [CrossRef] [PubMed]
- Volanti, C.; Matroule, J.Y.; Piette, J. Involvement of Oxidative Stress in NF-κB Activation in Endothelial Cells Treated by Photodynamic Therapy. Photochem. Photobiol. 2002, 75, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Kang, S.; Varani, J.; Lin, J.; Fisher, G.J.; Voorhees, J.J. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J. Invest. Dermatol. 2000, 115, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Leu, Y.L.; Al-Suwayeh, S.A.; Ku, M.C.; Hwang, T.L.; Fang, J.Y. Anti-inflammatory activity and percutaneous absorption of quercetin and its polymethoxylated compound and glycosides: The relationships to chemical structures. Eur. J. Pharm. Sci. 2012, 47, 857–864. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.-y.; Jeong, S.Y.; Lee, B.S.; Joh, Y.S.; Hamishehkar, H.; Lee, S.; Kim, K.H. Anti-Skin Aging Potential of Methoxyflavones from Kaempferia parviflora Against TNF-α-Induced Oxidative Stress and Photoaging in Normal Human Dermal Fibroblasts. Foods 2025, 14, 4012. https://doi.org/10.3390/foods14234012
Ahn S-y, Jeong SY, Lee BS, Joh YS, Hamishehkar H, Lee S, Kim KH. Anti-Skin Aging Potential of Methoxyflavones from Kaempferia parviflora Against TNF-α-Induced Oxidative Stress and Photoaging in Normal Human Dermal Fibroblasts. Foods. 2025; 14(23):4012. https://doi.org/10.3390/foods14234012
Chicago/Turabian StyleAhn, Si-young, Se Yun Jeong, Bum Soo Lee, Yun Seok Joh, Hamed Hamishehkar, Sullim Lee, and Ki Hyun Kim. 2025. "Anti-Skin Aging Potential of Methoxyflavones from Kaempferia parviflora Against TNF-α-Induced Oxidative Stress and Photoaging in Normal Human Dermal Fibroblasts" Foods 14, no. 23: 4012. https://doi.org/10.3390/foods14234012
APA StyleAhn, S.-y., Jeong, S. Y., Lee, B. S., Joh, Y. S., Hamishehkar, H., Lee, S., & Kim, K. H. (2025). Anti-Skin Aging Potential of Methoxyflavones from Kaempferia parviflora Against TNF-α-Induced Oxidative Stress and Photoaging in Normal Human Dermal Fibroblasts. Foods, 14(23), 4012. https://doi.org/10.3390/foods14234012








