Mulberry Leaf Glutelin: Physicochemical, Functional, and Pancreatic Lipase Inhibitory Activity of Seven Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Mulberry Leaf Glutelins
2.3. Amino Acid Analysis
2.4. Scanning Electron Microscopy
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Functional Properties of Different Mulberry Leaf Glutelins
2.6.1. Protein Solubility
2.6.2. Water-Holding Capability (WHC) and Oil Absorption Capability (OAC)
2.6.3. Foaming Capability (FC) and Foam Stability (FS)
2.6.4. Emulsifying Activity Index (EAI) and Emulsion Stability Index (ESI)
2.7. In Vitro Protein Digestibility (IVPD)
2.8. Determination of Pancreatic Lipase Inhibitory Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Extraction of Seven Different Varieties of Mulberry Leaf Glutelins
3.1.1. Protein Yield and Content
3.1.2. Amino Acid Content and Composition
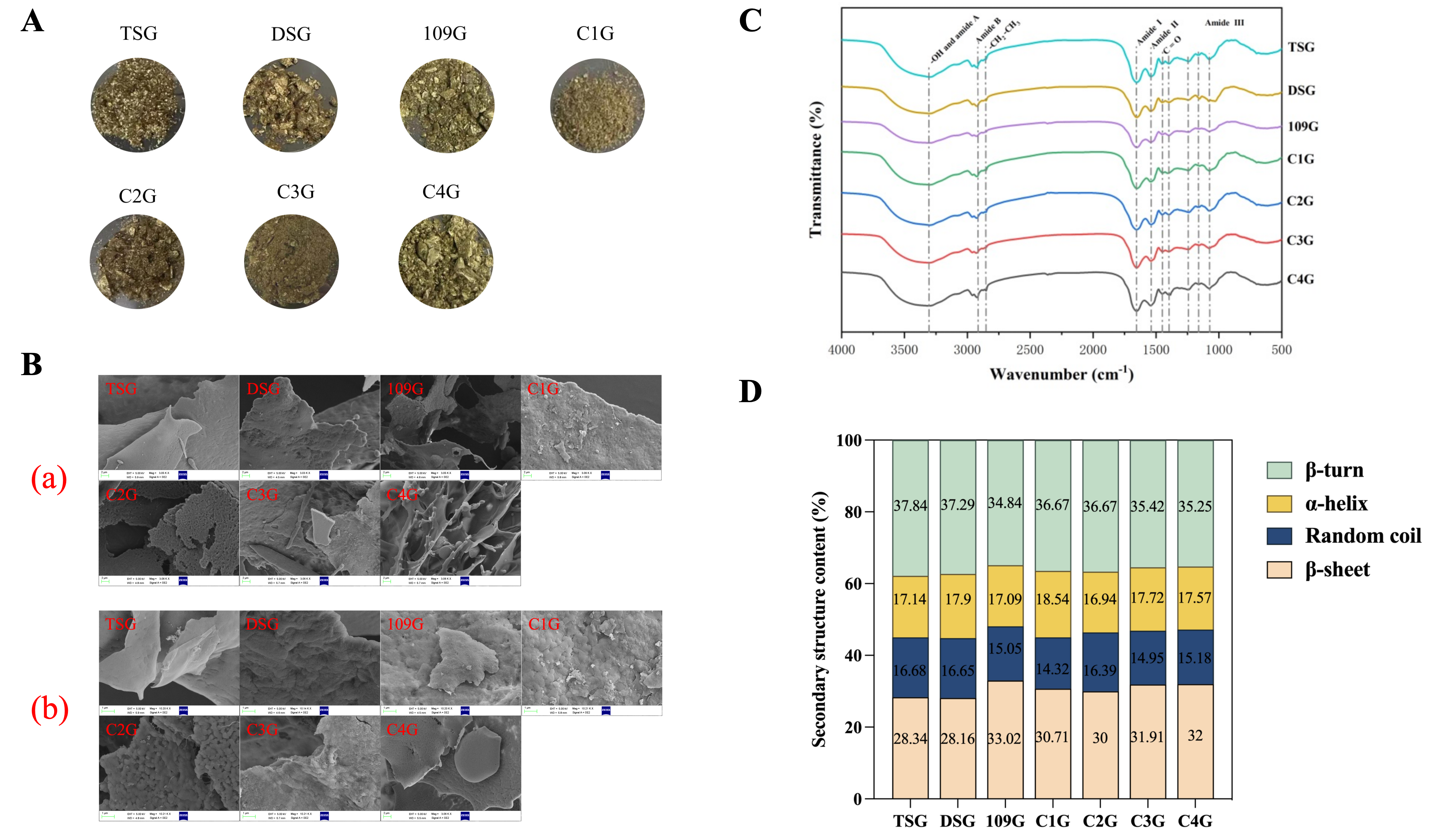
3.1.3. Surface Morphology
3.1.4. FTIR Analysis
3.2. Functional Properties of Seven Mulberry Leaf Glutelins
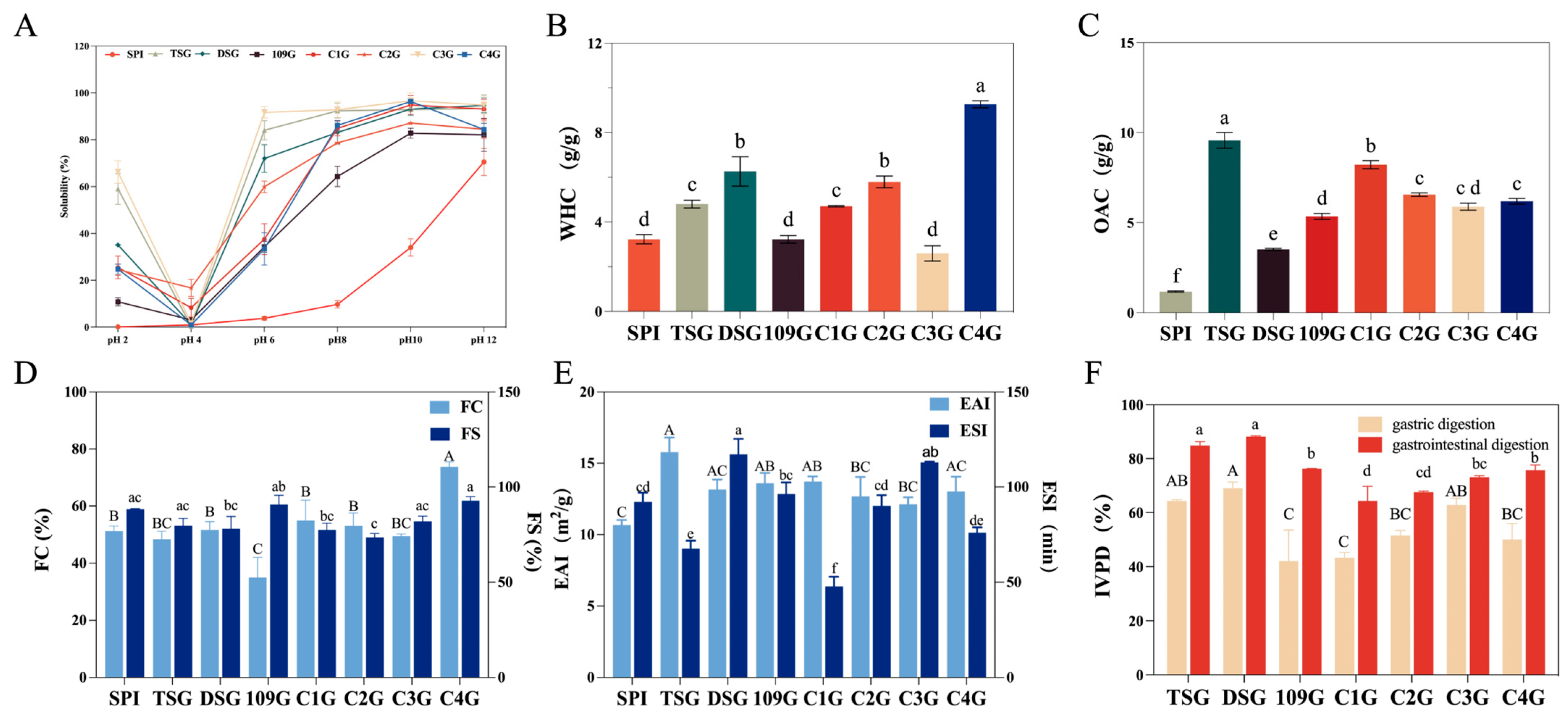
Solubility
3.2.2. Water-Holding Capability (WHC) and Oil Absorption Capability (OAC)
3.2.3. Foaming Properties
3.2.4. Emulsifying Properties
3.3. Digestibility
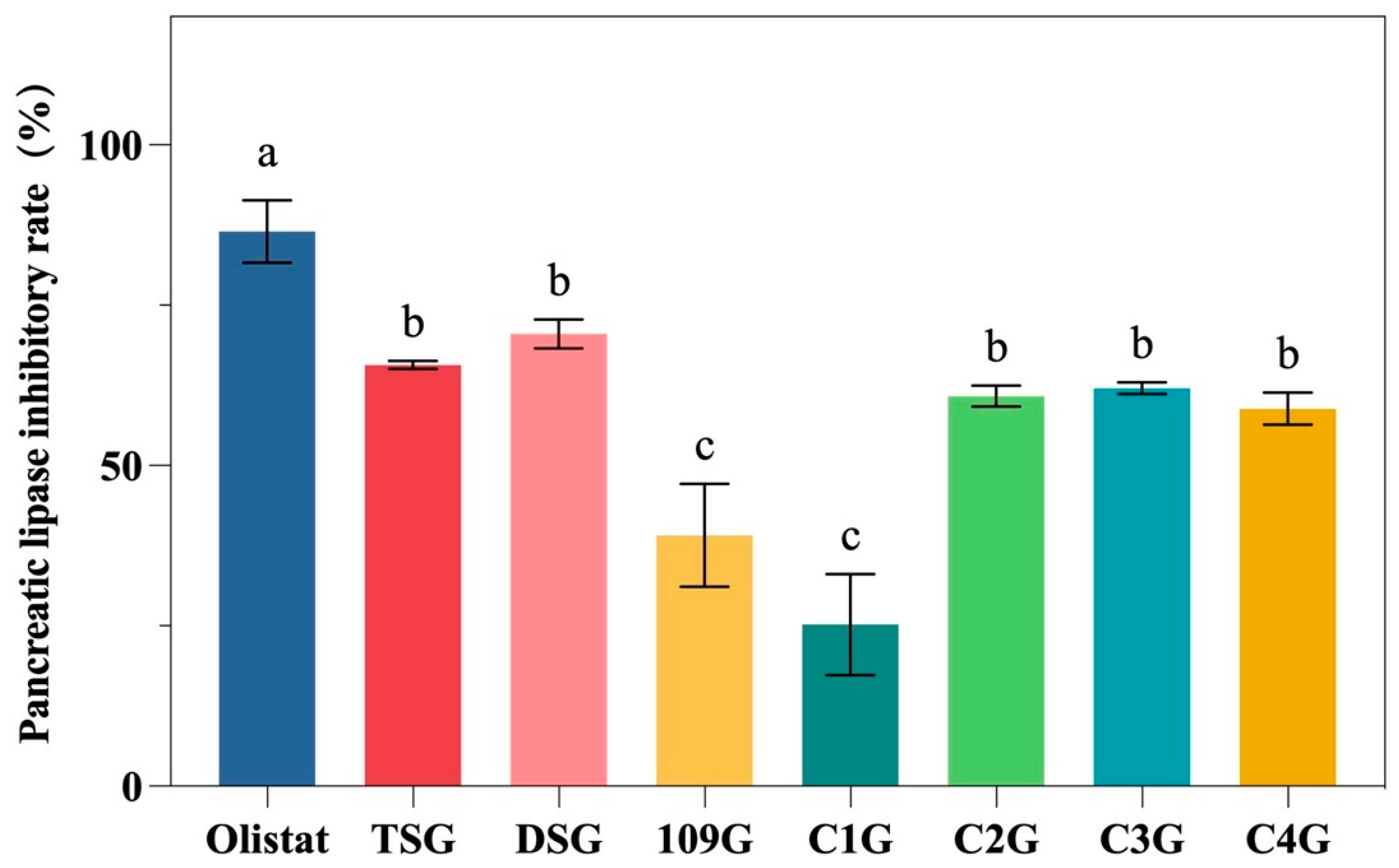
3.4. Pancreatic Lipase Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, R.; Chen, L.; Sun, C.; Muhammad, A.; Shao, Y. Mulberry Leaf Protein: Extraction Technologies, Functional Attributes and Food Applications. Foods 2025, 14, 2602. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Duan, H.Y.; Ren, X.L.; Guo, X.Y.; Zhang, Y.C.; Li, J.S.; Zhang, F.Y.; Chen, J.; Yang, X. Optimization of ultrasound-assisted cellulase degradation method on the extraction of mulberry leaf protein and its effect on the functional characteristics. Ultrason. Sonochemistry 2023, 99, 106561. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, W.; Ma, Y.; Min, T.; Lai, F.; Wu, H. Physicochemical, functional properties, and antioxidant activities of protein fractions obtained from mulberry (Morus atropurpurea roxb.) leaf. Int. J. Food Prop. 2017, 20, S3311–S3325. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.H.; Chen, F.H.; Yang, Y.L.; Shen, L.W.; Xun, X.M.; Zhang, Z.A.; Zhan, Y.F.; You, S.; Wang, J. Biochemical and protein nutritional potential of mulberry (Morus alba L.) leaf: Partial substitution improves the nutrition of conventional protein. J. Sci. Food Agric. 2024, 104, 2204–2214. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-assisted extraction and modification of plant-based proteins: Impact on physicochemical, functional, and nutritional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef]
- Huang, D.; Li, W.; Li, G.; Zhang, W.; Chen, H.; Jiang, Y.; Li, D. Effect of high-intensity ultrasound on the physicochemical properties of Tenebrio Molitor Protein. Food Hydrocoll. 2023, 134, 108056. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Ma, M.; Li, Z.; Zhang, X.; Jiang, H.; Yuan, C. Investigating the impact of ultrasound-assisted treatment on the crafting of mulberry leaf protein and whey isolate complex: A comprehensive analysis of structure and functionality. Ultrason. Sonochemistry 2024, 108, 106983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ouyang, D.; Cheng, X.; Zhou, X.; Lin, L.; Wang, J.; Wu, Q.; Jia, J. Multi-frequency ultrasound-assisted cellulase extraction of protein from mulberry leaf: Kinetic, thermodynamic, and structural properties. Ultrason. Sonochemistry 2023, 99, 106554. [Google Scholar] [CrossRef]
- Phakthawat, K.; Shen, P.; Ranathunga, A.; Klinkesorn, U.; Suwannaporn, P. Hydrolyzed rice glutelin as plant-based emulsifier to reduce tween in emulsion facing process stresses and its application in coconut milk. Food Hydrocoll. 2023, 145, 109070. [Google Scholar] [CrossRef]
- Yazar, G.; Kokini, J.L.; Smith, B. Comparison of mixing and non-linear viscoelastic properties of carob germ glutelins and wheat glutenin. Food Hydrocoll. 2023, 143, 108922. [Google Scholar] [CrossRef]
- Tao, H.; Li, Y.N.; Zhou, H.Y.; Sun, J.Y.; Fang, M.J.; Cai, W.H.; Wang, H.L. Unveiling the binding mechanism between wheat arabinoxylan and different molecular weights of wheat glutenins during the dough mixing process. Food Hydrocoll. 2025, 160, 110762. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Feng, L.; Wang, F.; Liu, T.; Gu, F.; Wang, F.; Huang, Q.; Zheng, J. Mechanistic study of synergetic stabilization of Pickering emulsions by corn glutelin and starch complexes. Food Chem. 2025, 463, 141558. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Ouyang, K.; Xie, H.; Xiong, H.; Zhao, Q. Enhancing mechanical and thermal properties of rice glutelin films through the interaction with pectin valorized from Akebia trifoliata var. australis waste. Sustain. Chem. Pharm. 2024, 41, 101713. [Google Scholar] [CrossRef]
- Sun, C.Z.; Shan, Y.W.; Tang, X.; Han, D.; Wu, X.Y.; Wu, H.; Hosseininezhad, M. Effects of enzymatic hydrolysis on physicochemical property and antioxidant activity of mulberry (Morus atropurpurea roxb.) leaf protein. Food Sci. Nutr. 2021, 9, 5379–5390. [Google Scholar] [CrossRef]
- Cao, J.; Liang, K.; Lai, L.; Wang, Y.; Wang, J.; Yu, P.; Cao, F.; Su, E. Investigation of the physicochemical and functional properties of protein concentrate and glutelin derived from an underutilized green leaf plant: Edible dock (Rumexpatientia L. × Rumextianshanicus A Los). Food Hydrocoll. 2024, 156, 110349. [Google Scholar] [CrossRef]
- Standard’s GB 5009.124-2016; Food safety national standards—Determination of amino acids in foods. National Health and Family Planning Commission of the People’s Republic of China (NHFPC) & China Food and Drug Administration (CFDA): Beijing, China, 2016.
- Tavano, L.O.; Neves, A.V.; Júnior, S.D.I.S. In vitro versus in vivo protein digestibility techniques for calculating PDCAAS (protein digestibility-corrected amino acid score) applied to chickpea fractions. Food Res. Int. 2016, 89, 756–763. [Google Scholar] [CrossRef]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino Acid Availability of a Dairy and Vegetable Protein Blend Compared to Single Casein, Whey, Soy, and Pea Proteins: A Double-Blind, Cross-Over Trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef]
- Xie, J.; Bi, J.; Nicolas, J.; Christophe, B.; Wang, F.; Lyu, J. Dysphagia food: Impact of soy protein isolate (SPI) addition on textural, physicochemical and microstructural properties of peach complex gels. Food Hydrocoll. 2024, 154, 110130. [Google Scholar] [CrossRef]
- Orellana-Palacios, J.C.; Garcia, S.R.; Rabanal-Ruiz, Y.; McClements, D.J.; Moreno, A.; Hadidi, M. Corn silk as an underutilized sustainable source of plant proteins: Extraction, fractionation, structure, and techno-functional properties. Food Hydrocoll. 2025, 158, 110550. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Jackfruit (Artocarpus heterophyllus Lam) leaf as a new source to obtain protein hydrolysates: Physicochemical characterization, techno-functional properties and antioxidant capacity. Food Hydrocoll. 2021, 112, 106319. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Enujiugha, V.N.; Aluko, R.E. In vitro digestibility, structural and functional properties of Moringa oleifera seed proteins. Food Hydrocoll. 2020, 101, 105574. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Yao, L.; Qin, F.; Hou, G.; Chen, B.; Jin, L.; Deng, J.; Shen, Y. Characterisation, physicochemical and functional properties of protein isolates from Amygdalus pedunculata Pall seeds. Food Chem. 2020, 311, 125888. [Google Scholar] [CrossRef]
- Rawiwan, P.; Quek, S.Y. Physicochemical and functional attributes of RuBisCo-enriched Brassicaceae leaf protein concentrates. Food Hydrocoll. 2024, 151, 109887. [Google Scholar] [CrossRef]
- Zhao, Q.; Xie, T.; Hong, X.; Zhou, Y.; Fan, L.; Liu, Y.; Li, J. Modification of functional properties of perilla protein isolate by high-intensity ultrasonic treatment and the stability of o/w emulsion. Food Chem. 2022, 368, 130848. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Chen, F.; Lin, L.; Zhao, M. Co-extraction of soy protein and polysaccharide with lipid-lowering activity: Characterization of functional property, nutritional property and colonic fermentation property through a metabolomics approach. Food Hydrocoll. 2023, 138, 108472. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Pulsed ultrasound assisted extraction of alternative plant protein from sugar maple leaves: Characterization of physical, structural, thermal, electrical, and techno-functional properties. Food Hydrocoll. 2024, 152, 109960. [Google Scholar] [CrossRef]
- Mutlu, C.; Korkmaz, F. Freeze, spray, and vacuum dried Camelina sativa protein powders and their physicochemical and functional properties. J. Food Process Pres. 2024, 148, 559–567. [Google Scholar] [CrossRef]
- Dietary protein quality evaluation in human nutrition. Report of an FAQ Expert Consultation. FAO Food Nutr. Pap. 2013, 92, 1–66. [Google Scholar]
- Mandal, P.K.; Roy, R.G.; Samkaria, A. Oxidative Stress: Glutathione and Its Potential to Protect Methionine-35 of Aβ Peptide from Oxidation. ACS Omega 2022, 7, 27052–27061. [Google Scholar] [CrossRef]
- Contardo, I.; Gutiérrez, S.; Hurtado-Murillo, J.; Escobar, N. Understanding the structural differences in chickpea globulins and their relationship with in vitro protein digestibility. Food Res. Int. 2025, 202, 115702. [Google Scholar] [CrossRef]
- Sakai, K.; Okada, M.; Yamaguchi, S. Umami and saltiness enhancements of vegetable soup by enzyme-produced glutamic acid and branched-chain amino acids. Front Nutr. 2024, 11, 1436. [Google Scholar] [CrossRef]
- GMN, J.A.F.; Gerardo, M. Effect of Processing on the in Vitro and in Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum). J. Agric. Food Chem. 2017, 65, 7790–7796. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; United Nations University. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007; Volume 935, pp. 1–265. [Google Scholar] [PubMed]
- Zhang, J.P.; Fan, M.C.; Wang, L.; Qian, H.F.; Li, Y. Unveiling the structural and physico-chemical properties of glutenin macropolymer under frozen storage: Studies on experiments and molecular dynamics simulation. Food Res. Int. 2024, 197, 115252. [Google Scholar] [CrossRef]
- Fang, B.C.; Chang, L.Y.; Ohm, J.B.; Chen, B.C.; Rao, J.J. Structural, functional properties, and volatile profile of hemp protein isolate as affected by extraction method: Alkaline extraction-isoelectric precipitation vs salt extraction. Food Chem. 2023, 405, 135001. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification in album (Chenopodium album) protein isolates due to controlled thermal modification and its relationship with protein digestibility and functionality. Food Hydrocoll. 2020, 103, 105708. [Google Scholar] [CrossRef]
- López-Monterrubio, D.I.; Lobato-Calleros, C.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Huauzontle (Chenopodium nuttalliae Saff.) protein: Composition, structure, physicochemical and functional properties. Food Hydrocoll. 2020, 108, 106043. [Google Scholar] [CrossRef]
- Campos Assumpção de Amarante, M.; Ong, L.; Spyropoulos, F.; Gras, S.; Wolf, B. Modulation of physico-chemical and technofunctional properties of quinoa protein isolate: Effect of precipitation acid. Food Chem. 2024, 457, 140399. [Google Scholar] [CrossRef]
- Liu, C.; Wang, N.; Li, L.; Wu, D.; Wang, L.; Zhang, N.; Yu, D. Effect of microwave plasma processing on the structure, physicochemical properties and functional properties of rice bran protein. Food Hydrocoll. 2025, 160, 110851. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C.G.; Almeida Junior, J.C.d.; Viana, C.C.R.; Neves, L.N.d.O.; Silva, P.H.F.d.; Bell, M.J.V.; Anjos, V.d.C.d. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Ortega, M.L.S.; Orellana-Palacios, J.C.; Garcia, S.R.; Rabanal-Ruiz, Y.; Moreno, A.; Hadidi, M. Olive leaf protein: Extraction optimization, in vitro digestibility, structural and techno-functional properties. Int. J. Biol. Macromol. 2024, 256, 128273. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zha, F.; Yang, Z.; Rao, J.; Chen, B. Structure characteristics and functionality of water-soluble fraction from high-intensity ultrasound treated pea protein isolate. Food Hydrocoll. 2022, 125, 107409. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef]
- Gélébart, P.; Maraval, G.; Kermarrec, A.; Birault, L.B.; Geairon, A.; Meynier, A.; Riaublanc, A.; Réau, A.; Carabin, C.B. Structure and solubility of pea seed-derived ingredients produced by air classification: Relevance of the seed milling process and endogenous lipid accumulation. Food Res. Int. 2025, 222, 117556. [Google Scholar] [CrossRef]
- Cao, G.S.; Shi, Y.X.; Li, J.; Zhao, Y.; Yu, Z.X.; Zhang, H.Y.; Yan, M.M. Plant proteins from Semen Astragali Complanati: Functional and physicochemical properties and antioxidant activity. LWT 2024, 212, 116919. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, D.H.; Tu, J.C.; Zhong, Y.J.; Zhang, D.; Wang, Z.M.; Tao, X.Q. Mechanisms of change in gel water-holding capacity of myofibrillar proteins affected by lipid oxidation: The role of protein unfolding and cross-linking. Food Chem. 2021, 344, 128587. [Google Scholar] [CrossRef]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef]
- Duan, X.; Li, M.; Shao, J.; Chen, H.Y.; Xu, X.M.; Jin, Z.Y.; Liu, X.B. Effect of oxidative modification on structural and foaming properties of egg white protein. Food Hydrocoll. 2018, 75, 223–228. [Google Scholar] [CrossRef]
- Raygoza Garay, J.A.; Turpin, W.; Lee, S.H.; Smith, M.I.; Goethel, A.; Griffiths, A.M.; Moayyedi, P.; Espin-Garcia, O.; Abreu, M.; Aumais, G.L.; et al. Gut Microbiome Composition Is Associated With Future Onset of Crohn’s Disease in Healthy First-Degree Relatives. Gastroenterology 2023, 165, 670–681. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Geng, H.; Zhang, X.; Chen, Z. Formation, structural characteristics, foaming and emulsifying properties of rice glutelin fibrils. Food Chem. 2021, 354, 129554. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, S.; Jin, Y.; Wu, X.; Liu, X.; Chen, J. Comparative efficacy of amino acid availability and peptidomic analysis of alternative proteins from different sources under dynamic in vitro protein digestion. Food Hydrocoll. 2025, 159, 110665. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Peng, N.; Li, Y.-Q.; Liang, Y.; Guo, Z.-W.; Wang, C.-Y.; Wang, Z.-Y.; Wang, C.; Ren, X. Physicochemical properties, structural characteristics and protein digestibility of pea protein-wheat gluten composited meat analogues prepared via high-moisture extrusion. Food Hydrocoll. 2024, 156, 110283. [Google Scholar] [CrossRef]
- Choudhury, D.B.; Gul, K.; Sehrawat, R. Physicochemical, functional, molecular, and surface properties of bean proteins grown in the high-altitude northern Himalayas. Food Hydrocoll. 2025, 162, 110957. [Google Scholar] [CrossRef]
- Ma, M.; Zhan, Y.; Xiao, X.; Ma, A.; Chen, Z.; Li, S.; Jia, Y. Pancreatic lipase-inhibiting polysaccharide from Stropharia rugosoannulata: Structural characterization and interaction mechanisms. Food Res. Int. 2025, 206, 116063. [Google Scholar] [CrossRef] [PubMed]
- Ruilin, L.; Zihan, X.; Yanan, J.; Yajie, W.; Shuqin, L.; Jingna, Z.; Junyu, L.; Min, Z.; Chengwei, H.; Haixia, C. Polysaccharides from mulberry (Morus alba L.) leaf prevents obesity by inhibiting pancreatic lipase in high-fat diet induced mice. Int. J. Biol. Macromol. 2021, 192, 452–460. [Google Scholar] [CrossRef]
| Sample | Yield (%) | Protein Content (%) | Polysaccharide (g/100 g) | Polyphenol (g GAE/100 g) |
|---|---|---|---|---|
| TSG | 4.64 ± 0.15 a | 61.36 ± 2.66 ab | 5.85 ± 0.08 f | 10.96 ± 0.48 d |
| DSG | 4.73 ± 0.24 a | 62.06 ± 2.10 ab | 13.21 ± 0.25 b | 19.32 ± 0.42 a |
| 109G | 3.92 ± 0.04 b | 53.59 ± 1.83 c | 7.59 ± 0.25 e | 14.17 ± 1.82 c |
| C1G | 3.78 ± 0.11 b | 55.94 ± 2.99 bc | 11.13 ± 0.36 c | 15.64 ± 0.36 bc |
| C2G | 4.08 ± 0.17 ab | 59.59 ± 5.77 abc | 9.33 ± 0.40 d | 13.41 ± 0.13 cd |
| C3G | 4.07 ± 0.26 ab | 55.61 ± 1.90 bc | 19.55 ± 1.49 a | 17.59 ± 0.85 ab |
| C4G | 4.68 ± 0.27 a | 66.36 ± 5.95 a | 7.88 ± 0.09 e | 13.86 ± 2.47 c |
| Protein Samples | FAO/WHO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TSG | DSG | 109G | C1G | C2G | C3G | C4G | Child | Adult | ||
| non-essential | Asp | 81.08 ± 1.45 b | 86.86 ± 0.55 a | 63.37 ± 0.03 d | 69.19 ± 1.14 c | 79.07 ± 1.02 b | 71.16 ± 0.69 c | 87.97 ± 0.23 a | ||
| amino acid | Ser | 37.60 ± 0.68 ab | 38.24 ± 0.40 ab | 29.73 ± 0.48 b | 32.27 ± 0.53 b | 34.94 ± 1.91 ab | 31.78 ± 0.16 b | 39.34 ± 0.12 a | ||
| Glu | 109.43 ± 1.89 c | 114.91 ± 0.66 b | 80.07 ± 0.31 e | 87.49 ± 0.98 d | 105.12 ± 1.10 c | 89.85 ± 0.68 d | 120.35 ± 0.16 a | |||
| Pro | 30.42 ± 0.71 a | 34.98 ± 2.50 a | 24.84 ± 0.43 b | 27.12 ± 0.29 ab | 29.64 ± 0.44 ab | 27.58 ± 0.55 ab | 33.21 ± 0.11 a | |||
| Gly | 66.82 ± 1.17 b | 73.43 ± 0.31 a | 56.68 ± 0.15 c | 59.44 ± 0.35 c | 67.65 ± 1.80 b | 60.24 ± 0.17 c | 73.77 ± 0.04 a | |||
| Ala | 41.22 ± 0.29 a | 43.96 ± 0.02 a | 34.49 ± 0.01 b | 35.06 ± 0.89 b | 40.01 ± 2.45 ab | 37.17 ± 0.22 b | 44.78 ± 0.22 a | |||
| Arg | 45.75 ± 0.82 a | 44.35 ± 0.33 a | 33.05 ± 0.04 c | 34.30 ± 0.80 bc | 39.00 ± 1.36 b | 33.24 ± 0.29 c | 46.22 ± 0.30 a | |||
| Tyr | 34.85 ± 0.59 b | 37.51 ± 0.18 ab | 25.85 ± 0.19 c | 28.97 ± 0.89 c | 32.15 ± 0.68 bc | 28.75 ± 0.38 c | 40.51 ± 0.22 a | |||
| Cys | 3.26 ± 0.19 a | 3.11 ± 0.14 a | 1.94 ± 0.16 a | 2.80 ± 0.31 a | 2.98 ± 0.31 a | 1.86 ± 0.02 a | 3.23 ± 0.02 a | |||
| essential | Thr | 38.10 ± 0.65 b | 43.14 ± 0.25 ab | 31.41 ± 0.11 c | 34.03 ± 0.62 bc | 39.13 ± 0.97 b | 33.68 ± 0.02 bc | 43.97 ± 0.12 a | 34 | 9 |
| amino acid | Val | 47.65 ± 0.70 a | 52.12 ± 0.07 a | 39.83 ± 0.28 c | 41.85 ± 0.01 bc | 46.62 ± 0.99 ab | 40.18 ± 0.29 c | 52.80 ± 0.29 a | 35 | 13 |
| Met | 13.92 ± 0.25 a | 13.96 ± 0.14 a | 9.57 ± 0.00 b | 9.91 ± 0.59 b | 12.62 ± 0.65 b | 8.02 ± 0.02 b | 15.08 ± 0.04 a | |||
| Ile | 43.52 ± 0.72 a | 46.06 ± 0.24 a | 35.99 ± 0.10 b | 38.15 ± 0.34 b | 41.13 ± 1.08 a | 36.05 ± 0.32 ab | 46.24 ± 0.42 a | 28 | 13 | |
| Leu | 80.12 ± 1.14 a | 80.96 ± 0.18 a | 63.24 ± 0.25 c | 64.83 ± 0.13 c | 73.66 ± 1.46 b | 70.16 ± 0.56 b | 84.58 ± 0.08 a | 66 | 19 | |
| Phe | 50.08 ± 0.99 a | 51.21 ± 0.23 a | 39.87 ± 0.25 c | 42.03 ± 0.20 bc | 45.54 ± 0.26 b | 44.16 ± 0.43 bc | 52.77 ± 0.02 a | |||
| Lys | 40.47 ± 0.62 b | 40.33 ± 0.08 b | 41.46 ± 0.29 b | 41.58 ± 0.90 b | 42.73 ± 1.46 b | 43.61 ± 0.01 ab | 47.39 ± 0.04 a | 58 | 16 | |
| His | 23.81 ± 0.33 ab | 23.53 ± 0.08 ab | 19.17 ± 0.29 b | 19.80 ± 0.60 ab | 20.21 ± 0.37 ab | 20.74 ± 0.21 ab | 24.54 ± 0.05 a | 19 | 16 | |
| ΣEAA 1 | 337.66 ± 5.41 c | 351.31 ± 1.27 b | 280.54 ± 1.14 f | 292.19 ± 0.57 e | 321.66 ± 1.80 d | 296.59 ± 0.57 e | 367.38 ± 0.58 a | |||
| ΣNEAA 2 | 450.43 ± 7.79 b | 477.35 ± 0.56 a | 350.01 ± 0.63 e | 376.64 ± 3.26 d | 430.56 ± 6.88 c | 381.63 ± 3.12 d | 489.37 ± 0.81 a | |||
| ΣTAA 3 | 788.09 ± 13.20 c | 828.65 ± 0.72 b | 630.55 ± 0.51 f | 668.83 ± 2.69 e | 752.21 ± 8.67 d | 678.23 ± 3.69 e | 856.75 ± 0.24 a | |||
| ΣHAA 4 | 373.75 ± 5.98 b | 396.67 ± 1.31 a | 304.50 ± 0.94 e | 318.40 ± 1.14 d | 356.88 ± 3.69 c | 323.55 ± 1.33 d | 403.24 ± 0.24 a | |||
| ΣBCAA 5 | 171.28 ± 2.56 c | 179.13 ± 0.49 b | 139.06 ± 0.43 f | 144.84 ± 0.47 e | 161.42 ± 0.61 d | 146.38 ± 0.05 e | 183.63 ± 0.62 a | |||
| ΣSAA 6 | 17.19 ± 0.44 ab | 17.08 ± 0.01 ab | 11.51 ± 0.16 cd | 12.71 ± 0.28 c | 15.60 ± 0.96 b | 9.87 ± 0.00 d | 18.31 ± 0.05 a | 25 | 17 | |
| ΣAAA 7 | 84.94 ± 1.59 c | 88.72 ± 0.05 b | 65.72 ± 0.06 f | 71.00 ± 1.10 e | 77.68 ± 0.42 d | 72.91 ± 0.81 e | 93.28 ± 0.20 a | 63 | 19 | |
| ΣEAA/ TAA TAA | 0.43 ± 0.00 c | 0.42 ± 0.00 c | 0.45 ± 0.00 a | 0.44 ± 0.00 b | 0.43 ± 0.00 c | 0.44 ± 0.00 b | 0.43 ± 0.00 c | |||
| EAA/ NEAA | 0.75 ± 0.00 c | 0.74 ± 0.00 c | 0.8 ± 0.01 a | 0.78 ± 0.01 b | 0.75 ± 0.01 c | 0.78 ± 0.01 b | 0.75 ± 0.00 c | |||
| Protein Samples | |||||||
|---|---|---|---|---|---|---|---|
| TSG | DSG | 109G | C1G | C2G | C3G | C4G | |
| Thr | 1.66 | 1.88 | 1.37 | 1.48 | 1.70 | 1.46 | 1.91 |
| Val | 1.22 | 1.34 | 1.02 | 1.07 | 1.20 | 1.03 | 1.35 |
| Ile | 1.45 | 1.54 | 1.20 | 1.27 | 1.37 | 1.20 | 1.54 |
| Leu | 1.36 | 1.37 | 1.07 | 1.10 | 1.25 | 1.19 | 1.43 |
| Lys | 0.90 | 0.90 | 0.89 | 0.93 | 1.02 | 0.98 | 1.05 |
| His | 1.59 | 1.57 | 1.28 | 1.32 | 1.35 | 1.38 | 1.64 |
| Met + Cys | 0.78 | 0.78 | 0.52 | 0.58 | 0.65 | 0.45 | 0.83 |
| Phe + Tyr | 2.24 | 2.33 | 1.73 | 1.87 | 2.03 | 1.92 | 2.45 |
| AAS | 0.78 | 0.78 | 0.52 | 0.58 | 0.65 | 0.45 | 0.83 |
| Protein Source | Protein Characteristic Value | Recommended Composition of Essential Amino Acids of FAO/WHO | SRC | PDCAAS (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thr | Val | Ile | Leu | Lys | Met + Cys | Phe + Tyr | ||||
| TSG | RAA | 1.12 | 1.36 | 1.55 | 1.21 | 0.70 | 0.69 | 1.35 | 70.70 | 66.40 |
| RCAA | 0.98 | 1.19 | 1.36 | 1.06 | 0.61 | 0.60 | 1.18 | |||
| DSG | RAA | 1.27 | 1.49 | 1.64 | 1.23 | 0.70 | 0.68 | 1.41 | 68.65 | 68.30 |
| RCAA | 1.06 | 1.24 | 1.37 | 1.02 | 0.58 | 0.57 | 1.17 | |||
| 109G | RAA | 0.92 | 1.14 | 1.29 | 0.96 | 0.69 | 0.46 | 0.82 | 69.37 | 39.70 |
| RCAA | 1.03 | 1.27 | 1.43 | 1.07 | 0.77 | 0.51 | 0.92 | |||
| C1G | RAA | 1.00 | 1.20 | 1.36 | 0.98 | 0.72 | 0.51 | 0.92 | 70.49 | 37.00 |
| RCAA | 1.05 | 1.25 | 1.42 | 1.03 | 0.76 | 0.53 | 0.96 | |||
| C2G | RAA | 1.18 | 1.31 | 1.43 | 0.57 | 0.79 | 0.57 | 1.01 | 64.31 | 43.90 |
| RCAA | 1.20 | 1.34 | 1.47 | 0.58 | 0.81 | 0.58 | 1.03 | |||
| C3G | RAA | 0.99 | 1.15 | 1.29 | 1.06 | 0.76 | 0.39 | 0.91 | 68.77 | 32.80 |
| RCAA | 1.06 | 1.23 | 1.37 | 1.13 | 0.81 | 0.42 | 0.97 | |||
| C4G | RAA | 1.29 | 1.51 | 1.65 | 1.28 | 0.82 | 0.73 | 1.48 | 71.97 | 63.30 |
| RCAA | 1.03 | 1.20 | 1.32 | 1.02 | 0.65 | 0.59 | 1.18 | |||
| pH | Protein Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| SPI | TSG | DSG | 109G | C1G | C2G | C3G | C4G | |
| 2 | 0.10 ± 0.00 e | 59.00 ± 6.61 a | 35.10 ± 0.11 b | 10.81 ± 1.67 d | 25.49 ± 4.86 c | 24.55 ± 1.69 c | 66.22 ± 3.41 a | 24.69 ± 2.15 c |
| 4 | 0.92 ± 0.28 c | 0.38 ± 0.14 c | 1.15 ± 0.45 c | 3.07 ± 0.08 c | 8.29 ± 2.82 b | 16.74 ± 2.57 a | 1.60 ± 0.30 c | 0.94 ± 0.29 c |
| 6 | 3.77 ± 0.71e | 84.04 ± 4.05 a | 71.97 ± 5.91 b | 34.30 ± 2.31 d | 37.56 ± 6.56 d | 59.91 ± 2.48 a | 91.65 ± 1.72 a | 33.38 ± 6.88 d |
| 8 | 9.75 ± 1.56 e | 92.37 ± 3.12 a | 83.11 ± 4.91 bc | 64.34 ± 4.29 d | 84.89 ± 2.26 bc | 78.69 ± 0.15 c | 92.84 ± 2.33 a | 86.11 ± 2.08 ab |
| 10 | 34.03 ± 3.70 d | 92.89 ± 0.59 ab | 93.08 ± 2.67 ab | 82.92 ± 1.14 c | 94.86 ± 2.86 a | 87.13 ± 0.04 bc | 96.65 ± 2.37 a | 92.36 ± 0.86 a |
| 12 | 70.50 ± 5.77 c | 93.32 ± 5.78 a | 94.68 ± 3.17 a | 82.10 ± 7.00 b | 93.09 ± 2.95 a | 84.46 ± 2.75 ab | 94.82 ± 2.70 a | 84.35 ± 2.58 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; He, D.; Zhang, X.; Liu, Z.; Li, M.; Shen, T.; Wei, S.; Wu, X.; Sun, C. Mulberry Leaf Glutelin: Physicochemical, Functional, and Pancreatic Lipase Inhibitory Activity of Seven Varieties. Foods 2025, 14, 4004. https://doi.org/10.3390/foods14234004
Li H, He D, Zhang X, Liu Z, Li M, Shen T, Wei S, Wu X, Sun C. Mulberry Leaf Glutelin: Physicochemical, Functional, and Pancreatic Lipase Inhibitory Activity of Seven Varieties. Foods. 2025; 14(23):4004. https://doi.org/10.3390/foods14234004
Chicago/Turabian StyleLi, Hongyan, Dongjun He, Xiaomin Zhang, Zhenpeng Liu, Mingxi Li, Tianran Shen, Shuang Wei, Xiyang Wu, and Chongzhen Sun. 2025. "Mulberry Leaf Glutelin: Physicochemical, Functional, and Pancreatic Lipase Inhibitory Activity of Seven Varieties" Foods 14, no. 23: 4004. https://doi.org/10.3390/foods14234004
APA StyleLi, H., He, D., Zhang, X., Liu, Z., Li, M., Shen, T., Wei, S., Wu, X., & Sun, C. (2025). Mulberry Leaf Glutelin: Physicochemical, Functional, and Pancreatic Lipase Inhibitory Activity of Seven Varieties. Foods, 14(23), 4004. https://doi.org/10.3390/foods14234004







