Highly Sensitive SERS Detection of Food Colorants via Charge Transfer of Metal and Semiconductor in Ag/TiO2/Ti Foam
Abstract
1. Introduction
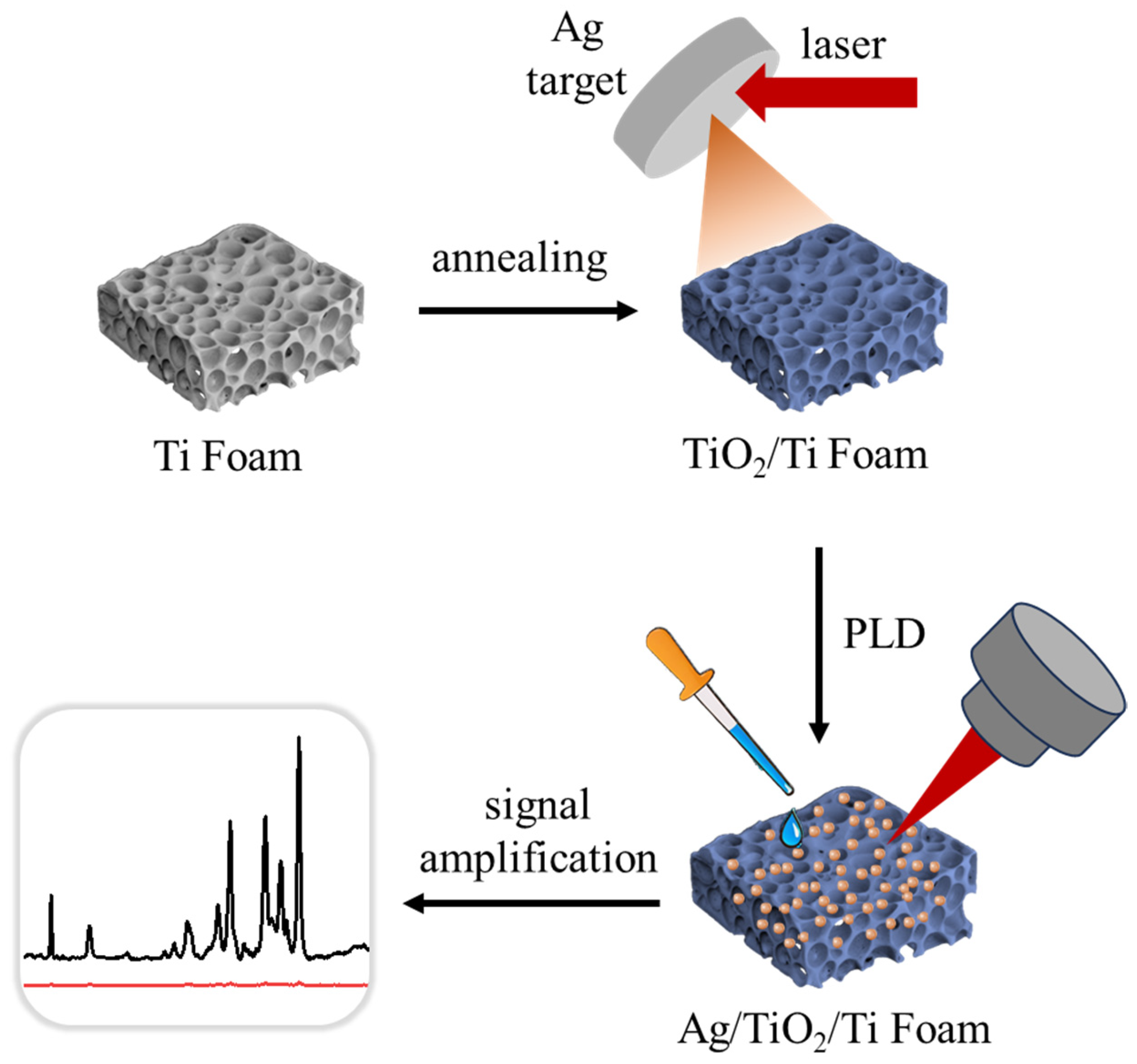
2. Experimental Section
2.1. Deposition of Ag NPs
2.2. Characterization
3. Results and Discussion
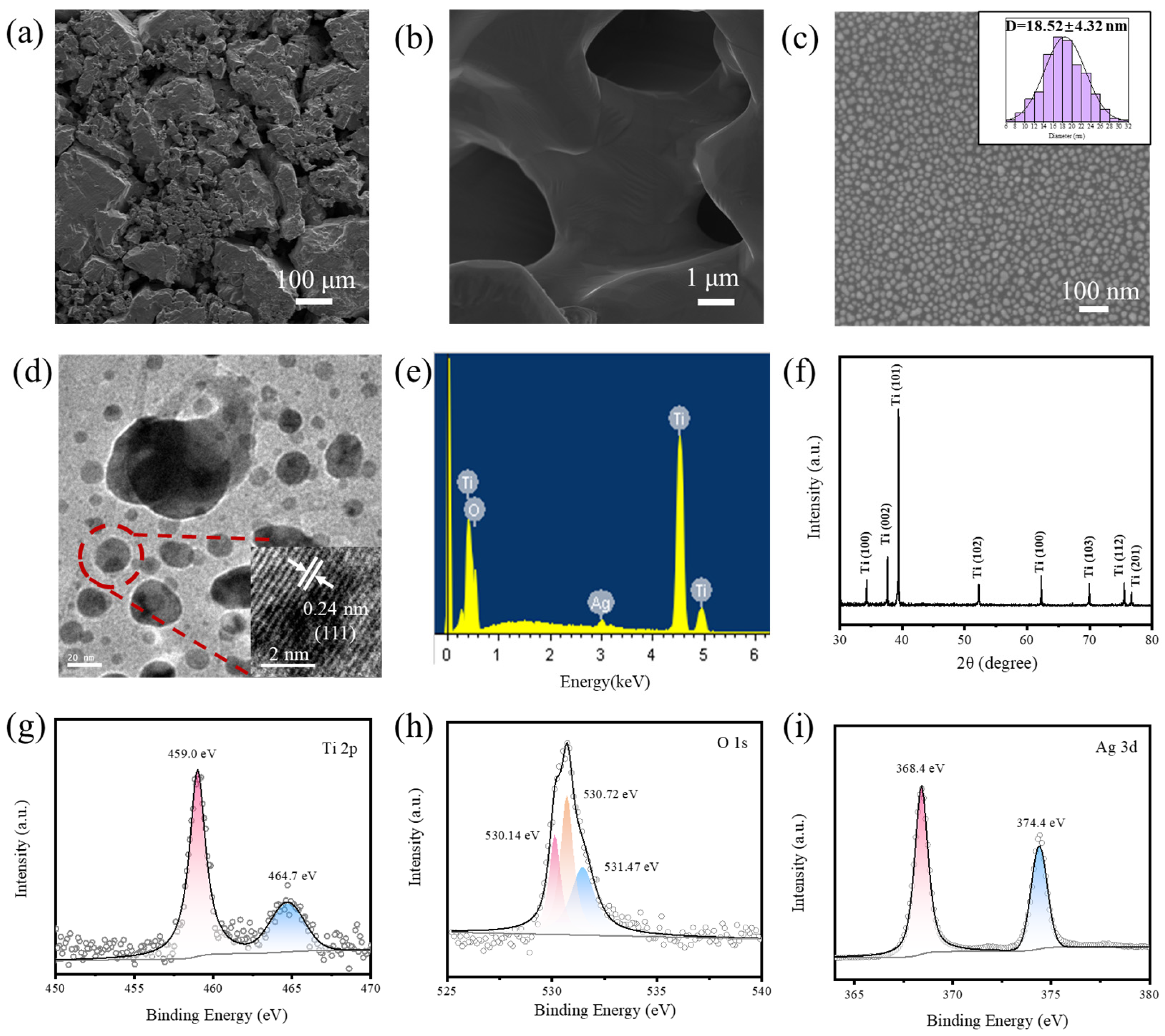
3.1. Characterization of Ag/TiO2/Ti Foam
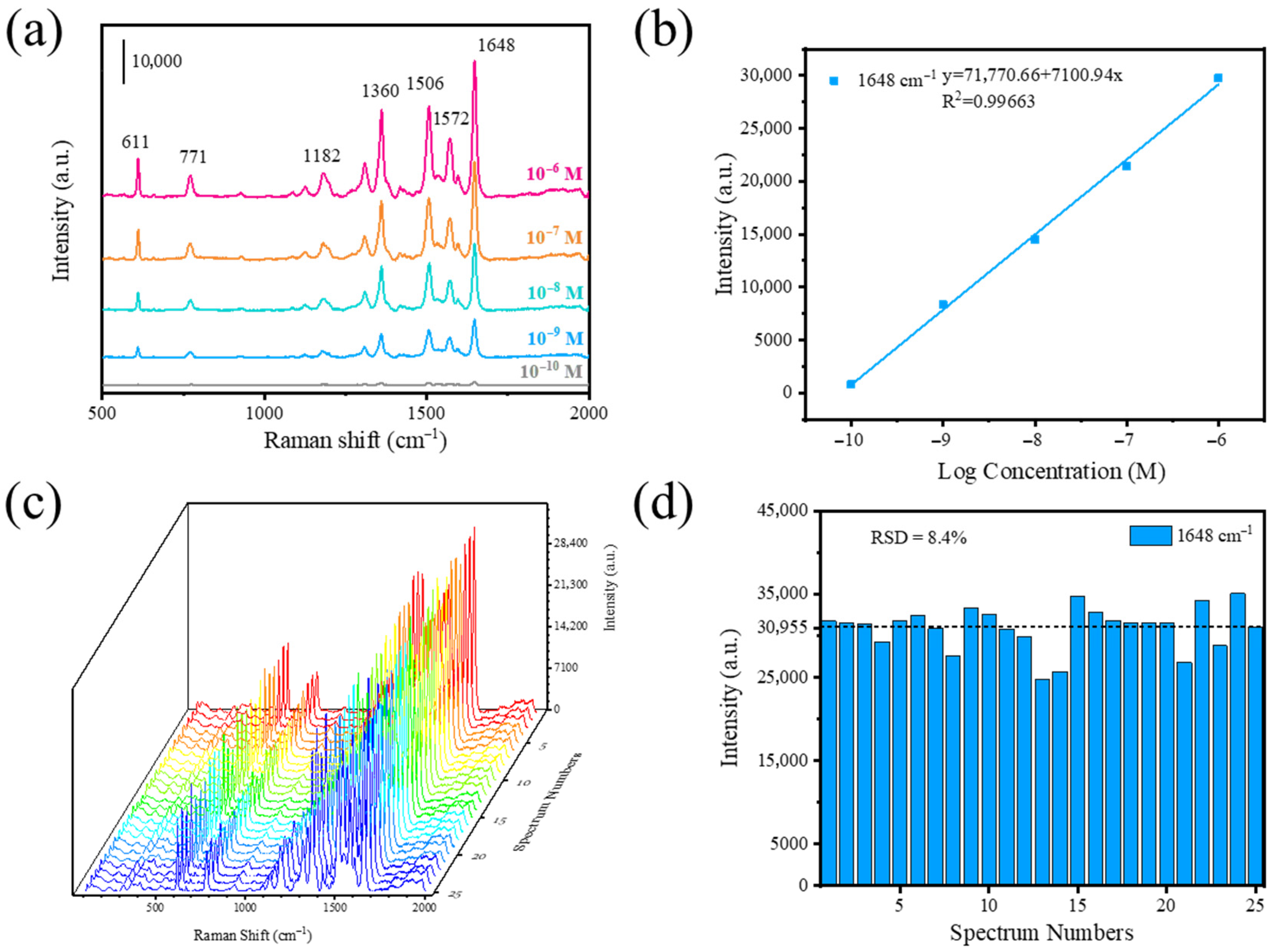
3.2. SERS of Ag/TiO2/Ti Foam
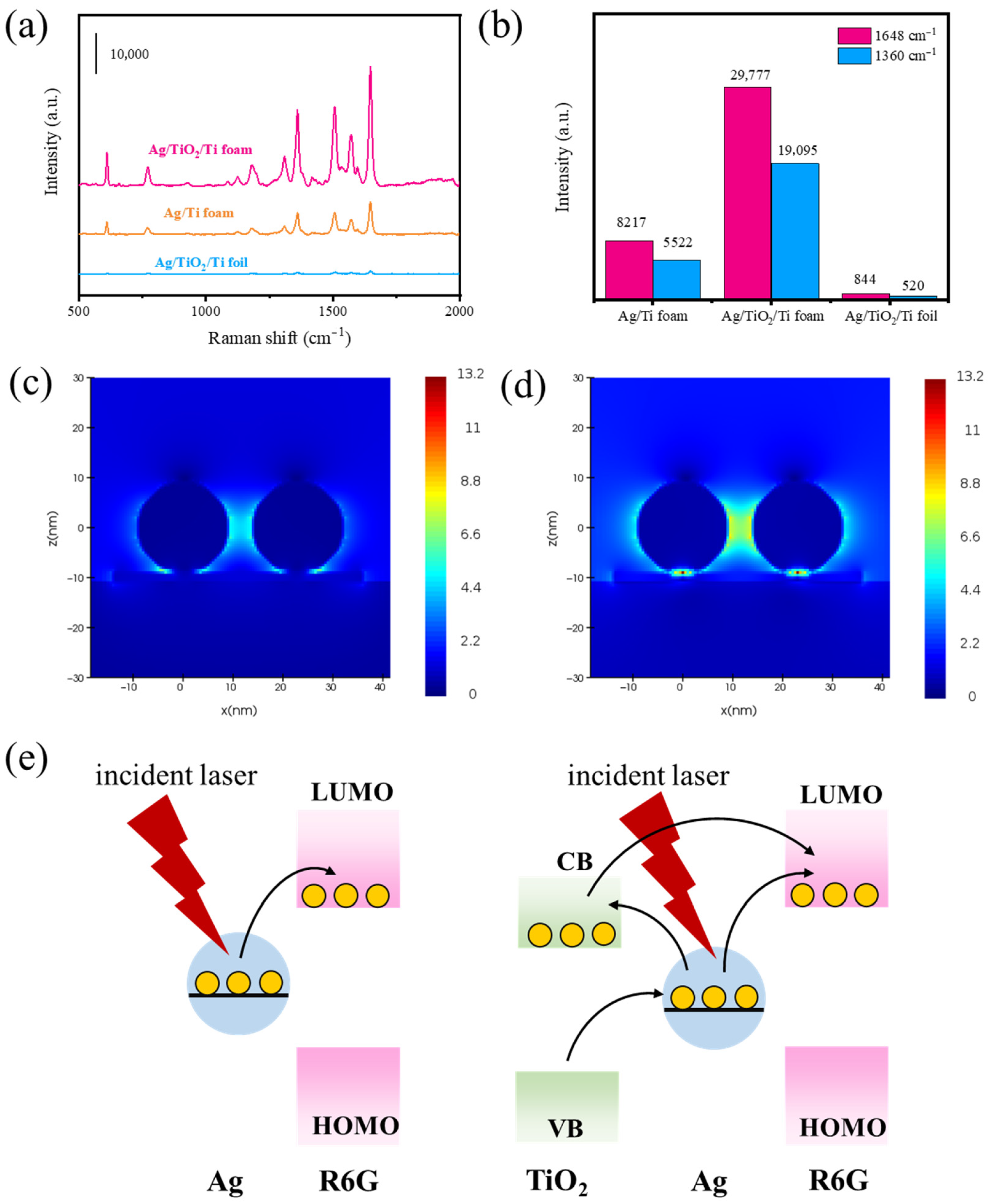
3.3. Mechanism
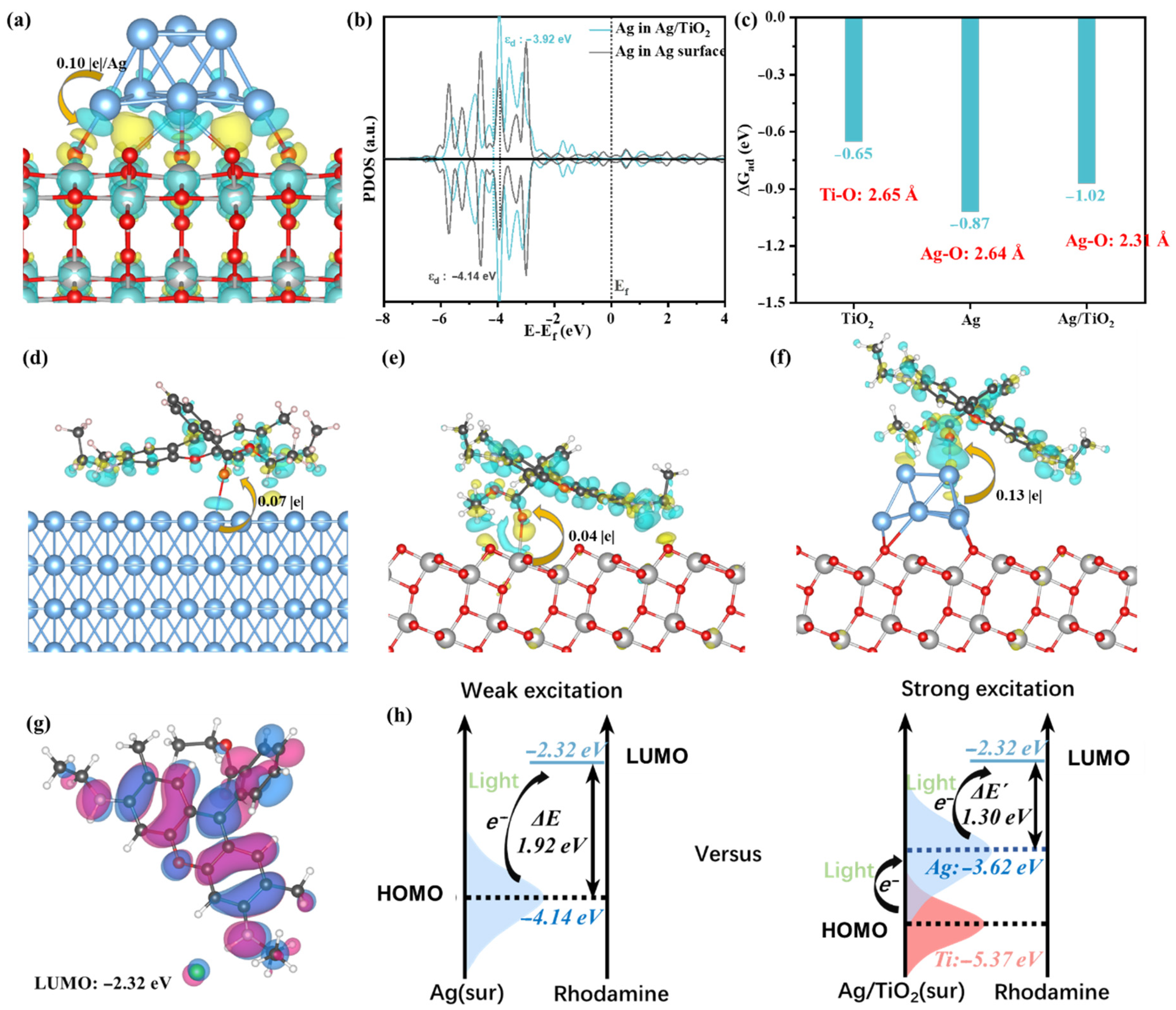
3.4. DFT Calculations
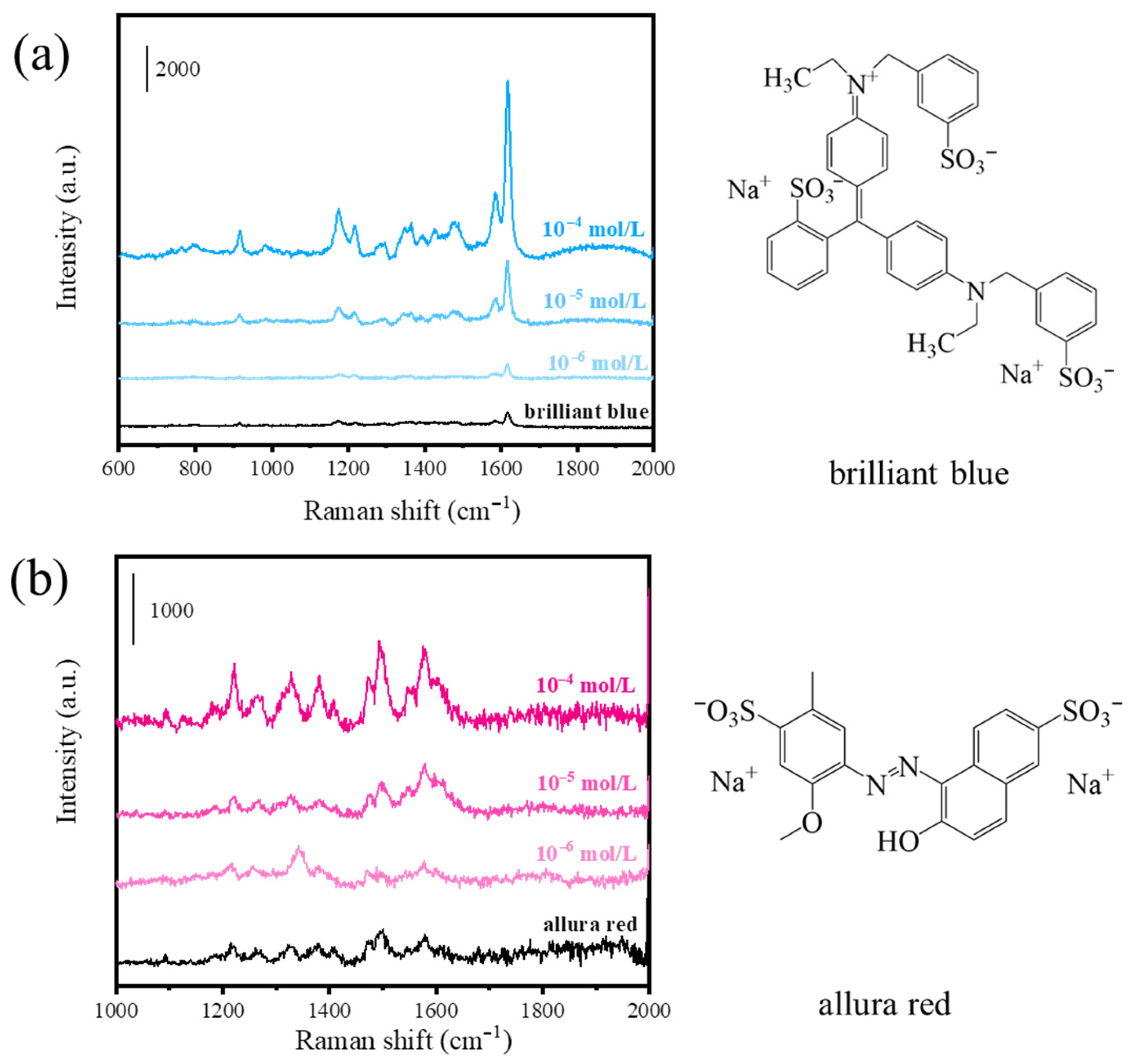
3.5. Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leleu, C.; Boulitrop, C.; Bel, B.; Jeudy, G.; Vabres, P.; Collet, E. Quinoline Yellow dye-induced fixed food-and-drug eruption. Contact Dermat. 2013, 68, 187–188. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cerniglia, C.E.; Chen, H. Toxicological significance of azo dye metabolism by human intestinal microbiota. Front. Biosci.-Elite 2012, 4, 568–586. [Google Scholar] [CrossRef]
- Amchova, P.; Kotolova, H.; Ruda-Kucerova, J. Health safety issues of synthetic food colorants. Regul. Toxicol. Pharmacol. 2015, 73, 914–922. [Google Scholar] [CrossRef]
- Shi, J.; Huang, M.; Yang, Q.; Xu, Y.; Wu, J.; Liu, H.; Zhang, J.; Zheng, F.; Dong, W. Relatively reliable and rapid identification of colorant compounds in food matrices by HPLC-DAD-QTOF-MS combined with theoretical calculation. Food Chem. 2025, 463, 141133. [Google Scholar] [CrossRef]
- Deolindo, C.T.P.; Hoff, R.B.; Costa, A.C.O. Development and validation of an HPLC-DAD method for the determination of artificial colorants in açaí pulp and commercial products. Food Res. Int. 2025, 221, 117609. [Google Scholar] [CrossRef]
- Ragab, M.; Khaled, O.; Elgendy, N.; Eissa, F.; Medhat, M. High-throughput LC-MS/MS method for the determination of 14 illegal dyes in complex food matrices and its application in a large-scale survey in Egypt. J. Hazard. Mater. 2025, 491, 137905. [Google Scholar] [CrossRef]
- Long, P.; Li, Y.; Han, Z.; Zhu, M.; Zhai, X.; Jiang, Z.; Wen, M.; Ho, C.-T.; Zhang, L. Discovery of color compounds: Integrated multispectral omics on exploring critical colorant compounds of black tea infusion. Food Chem. 2024, 432, 137185. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Yao, Y.; Chen, L.; Lin, B.; Zheng, W.; Zeng, Y.; Li, L.; She, Y.; Guo, L. One-Step, On-Site Chemical Printing of a 3D Plasmon-Coupled Silver Nanocoral Substrate toward SERS-Based POCT. Anal. Chem. 2023, 95, 6836–6845. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Yu, J.; Yang, M.; Li, Z.; Liu, C.; Wei, Y.; Zhang, C.; Man, B.; Lei, F. Hierarchical Particle-In-Quasicavity Architecture for Ultratrace In Situ Raman Sensing and Its Application in Real-Time Monitoring of Toxic Pollutants. Anal. Chem. 2020, 92, 14754–14761. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, X.; Cai, N.; Yang, F.; Yao, W.; Huang, L. Application of a Novel Au@ZIF-8 Composite in the Detection of Bisphenol A by Surface-Enhanced Raman Spectroscopy. Foods 2023, 12, 813. [Google Scholar] [CrossRef]
- Li, H.; Zhang, K.-L.; Kou, Y.; Xu, S.; Guo, X.-M.; Fu, S.-Y.; Li, Z.; Zhang, Y.-J.; Chen, X.; Li, J.-F. Resonance SERS probe based on the bifunctional molecule IR808 combined with SA test strips for highly sensitive detection of monkeypox virus. Spectrochim. Acta A 2025, 331, 125761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; He, M.; Dai, Y.; Jiang, T. UCL-SERS dual-mode sensing of NaYF4: Yb3+, Er3+/NaYF4: Yb3+@ Au composite nanoparticles. J. Alloys Compd. 2025, 1036, 181774. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mahdi Mousavi, S.; Amiri, A.; Tian, J.; Cao, R.; Wang, X. Surface-Enhanced Raman Spectroscopy Substrates for Food Safety and Quality Analysis. J. Agric. Food Chem. 2022, 70, 5463–5476. [Google Scholar] [CrossRef]
- Zhai, W.; Cao, M.; Xiao, Z.; Li, D.; Wang, M. Rapid Detection of Malathion, Phoxim and Thiram on Orange Surfaces Using Ag Nanoparticle Modified PDMS as Surface-Enhanced Raman Spectroscopy Substrate. Foods 2022, 11, 3597. [Google Scholar] [CrossRef]
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Ghafoor, A.; Zhang, Y.-F.; Zhang, X.-B.; Li, H.; Dong, X.-R.; Wang, R.-P.; Zhang, Y.; Zhang, Y.; et al. Chemical Enhancement and Quenching in Single-Molecule Tip-Enhanced Raman Spectroscopy. Angew. Chem. Int. Ed. 2023, 62, e202218799. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wang, R.; Wang, Q.; Xiang, Z.; Li, Z.; Gu, H.; Wang, X. An overview of surface-enhanced Raman scattering substrates by pulsed laser deposition technique: Fundamentals and applications. Adv. Compos. Hybrid Mater. 2021, 4, 885–905. [Google Scholar] [CrossRef] [PubMed]
- Atta, S.; Watcharawittayakul, T.; Vo-Dinh, T. Ultra-high SERS detection of consumable coloring agents using plasmonic gold nanostars with high aspect-ratio spikes. Analyst 2022, 147, 3340–3349. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qin, X.; Jiang, X.; Gong, M.; Yin, D.; Zhang, Y.; Zhao, B. SERS investigation of ciprofloxacin drug molecules on TiO2 nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 17809–17815. [Google Scholar] [CrossRef]
- Liu, W.; He, X.; Wang, Z.; Yuan, M.; Zhao, Z.; Ye, X.; Shang, S.; Song, Z.; Huang, L.; Liu, Y.; et al. Geometric and Electronic Structure Modulation to Optimize the Charge Transfer of TiO2 for Ultrasensitive and Stable SERS Sensing. Inorg. Chem. 2024, 63, 17608–17616. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, A.; Liu, X.; Han, Y.; Chen, J.; Zada, A.; Sun, Y.; Yuan, Z.; Luo, F.; Li, T.; et al. Synergistic Resonances and Charge Transfer in Double-Shelled ZnO Hollow Microspheres for High-Performance Semiconductor-Based SERS Substrates. ACS Appl. Nano Mater. 2024, 7, 10104–10113. [Google Scholar] [CrossRef]
- Chowdhury, J. How the Charge Transfer (CT) Contributions Influence the SERS Spectra of Molecules? A Retrospective from the View of Albrecht’s “A” and Herzberg-Teller Contributions. Appl. Spectrosc. Rev. 2015, 50, 240–260. [Google Scholar] [CrossRef]
- Han, X.X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Dong, Y.; Xie, Q.; Wu, S.; Li, J.; Sun, L.; Ji, W. Revealing Adsorption Mechanism of p-Mercaptobenzoic Acid with TiO2 Surfaces Using Electric Field-Enhanced Semiconductor SERS. J. Phys. Chem. C 2023, 127, 9418–9424. [Google Scholar] [CrossRef]
- Yang, W.; Tang, J.; Ou, Q.; Yan, X.; Liu, L.; Liu, Y. Recyclable Ag-Deposited TiO2 SERS Substrate for Ultrasensitive Malachite Green Detection. ACS Omega 2021, 6, 27271–27278. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, C.; Qiao, Y.; Wang, P.; Wang, J.; Shi, J.; Liu, B.; Wang, Z.; Hou, E.; Chang, L.; et al. A novel three-dimensional porous Ag/TiO2 hybrid aerogels with high dense hot spot as effective SERS substrate for ultrasensitive detection. Spectrochim. Acta A 2024, 322, 124849. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, S.; Jiang, S.; Xu, J. Improved SERS Performance on Ag-Coated Amorphous TiO2 Random Nanocavities by the Enhanced Light–Matter Coupling Effect. ACS Sustain. Chem. Eng. 2024, 12, 3234–3242. [Google Scholar] [CrossRef]
- Yang, L.; Sang, Q.; Du, J.; Yang, M.; Li, X.; Shen, Y.; Han, X.; Jiang, X.; Zhao, B. A Ag synchronously deposited and doped TiO2 hybrid as an ultrasensitive SERS substrate: A multifunctional platform for SERS detection and photocatalytic degradation. Phys. Chem. Chem. Phys. 2018, 20, 15149–15157. [Google Scholar] [CrossRef]
- Yang, J.; Song, G.; Zhou, L.; Wang, X.; You, L.; Li, J. Highly sensitively detecting tetramethylthiuram disulfide based on synergistic contribution of metal and semiconductor in stable Ag/TiO2 core-shell SERS substrates. Appl. Surf. Sci. 2021, 539, 147744. [Google Scholar] [CrossRef]
- Huang, S.; Wu, C.; Wang, Y.; Yang, X.; Yuan, R.; Chai, Y. Ag/TiO2 nanocomposites as a novel SERS substrate for construction of sensitive biosensor. Sensor. Actuat. B-Chem. 2021, 339, 129843. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Yu, H.; Liu, Y.; Gao, J.; Yang, L.; Zhang, M.; He, G.; Sun, Z. Effect of TiO2 arrays on surface enhanced Raman scattering (SERS) performance for Ag/TiO2 substrates. Nanotechnology 2021, 32, 075708. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, H.; Chen, X.; Wang, X.; Wei, H.; Guo, Z. Pulsed laser deposited Ag nanoparticles on nickel hydroxide nanosheet arrays for highly sensitive surface-enhanced Raman scattering spectroscopy. Appl. Surf. Sci. 2014, 316, 66–71. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, H.; Zhao, J.; Yi, H.; Wang, X. Pulsed laser deposition of Ag nanoparticles on titanium hydroxide/oxide nanobelt arrays for highly sensitive surface-enhanced Raman spectroscopy. Appl. Surf. Sci. 2015, 347, 499–504. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Jing, Y.; Wang, R.; Chen, L.; Zhang, J.; Sui, S.; Wang, X. Rapid detection of artificial food colorants with surface enhanced Raman spectroscopy: Engineering a novel gold-induced silver triangle nanosheet. J. Alloys Compd. 2024, 1006, 176246. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, X.; Xiao, T.; Zhang, L.; Lv, P.; Zhao, J. Preparation and properties of MnO2–TiO2 nanotube array composite electrodes using titanium foam as the current collector. Int. J. Hydrogen Energy 2018, 43, 8859–8867. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, L.; Jia, Y.; Chang, Y.; Tan, X.; Yang, L.; Chen, H. Design of carbon loaded porous TiO2 foams by the hydrothermal-assisted annealing carbonization of fruit residue for solar-driven water evaporation. Sol. Energy Mater. Sol. Cells 2019, 202, 110116. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, D.; Luo, S.; Jiang, L.; Wang, Q.; Chen, L.; Wei, G.-F.; Wang, X. The engineered interfacial Pd–O–Ti sites on the TiO2 nanobelts to accelerate water dissociation for the alkaline hydrogen evolution. Electrochim. Acta 2024, 507, 145198. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR Surface Characterization of TiO2 Particles Used in Polymer Encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar]
- Wang, Q.; Qiu, L.; Tan, X.; Liu, Z.; Gao, S.; Wang, R. Amorphous TiO2 granular nanodisks on porous Ti foam for highly effective solar cells and photocatalysts. J. Taiwan Inst. Chem. Eng. 2019, 102, 85–91. [Google Scholar] [CrossRef]
- Han, D.; Guo, B.; Li, Y.; Feng, W.; Liu, K.; Wu, T.; Wan, Y.; Wang, L.; Gao, M.; Liu, Y.; et al. Simultaneous photocatalytic degradation and SERS detection of tetracycline with self-sustainable and recyclable ternary PI/TiO2/Ag flexible microfibers. Microsyst. Nanoeng. 2024, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Dong, J.; Ren, Y.; Zhou, W.; Han, Q.; Zhang, C.; Ren, K.; Wang, Y.; Gao, W.; Qi, J. Fabrication of plasmonic Au-Ag alloy nanostars for ultrasensitive SERS detection. Spectrochim. Acta A 2025, 339, 126208. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ozaki, Y.; Xu, Z.; Zhao, B. Effect of TiO2 on Altering Direction of Interfacial Charge Transfer in a TiO2-Ag-MPY-FePc System by SERS. Angew. Chem. Int. Ed. 2019, 58, 8172–8176. [Google Scholar] [CrossRef]
- Polyanskiy, M.N. Refractiveindex.info database of optical constants. Sci. Data 2024, 11, 94. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Farrokhpour, H.; Ghandehari, M. Theoretical Spectroscopic Study on the Au, Ag, Au/Ag, and Ag/Au Nanosurfaces and Their Cytosine/Nanosurface Complexes: UV, IR, and Charge-Transfer SERS Spectra. J. Phys. Chem. C 2019, 123, 16345–16358. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.; Dai, Z.; Liu, J.; Yang, S.; Zhou, L.; Xiao, X.; Jiang, C.; Roy, V.A.L. Low-Cost, Disposable, Flexible and Highly Reproducible Screen Printed SERS Substrates for the Detection of Various Chemicals. Sci. Rep. 2015, 5, 10208. [Google Scholar] [PubMed]
- Yao, Y.; Wang, W.; Tian, K.; Ingram, W.M.; Cheng, J.; Qu, L.; Li, H.; Han, C. Highly reproducible and sensitive silver nanorod array for the rapid detection of Allura Red in candy. Spectrochim. Acta A 2018, 195, 165–171. [Google Scholar]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal--amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Jing, Y.; Zhang, D.; Wang, R.; Chen, L.; Zhang, J.; Sui, S.; Wang, X. Highly Sensitive SERS Detection of Food Colorants via Charge Transfer of Metal and Semiconductor in Ag/TiO2/Ti Foam. Foods 2025, 14, 3998. https://doi.org/10.3390/foods14233998
Wang Q, Jing Y, Zhang D, Wang R, Chen L, Zhang J, Sui S, Wang X. Highly Sensitive SERS Detection of Food Colorants via Charge Transfer of Metal and Semiconductor in Ag/TiO2/Ti Foam. Foods. 2025; 14(23):3998. https://doi.org/10.3390/foods14233998
Chicago/Turabian StyleWang, Qunlong, Yuting Jing, De Zhang, Ruijing Wang, Linlin Chen, Jianghua Zhang, Shaofeng Sui, and Xuefeng Wang. 2025. "Highly Sensitive SERS Detection of Food Colorants via Charge Transfer of Metal and Semiconductor in Ag/TiO2/Ti Foam" Foods 14, no. 23: 3998. https://doi.org/10.3390/foods14233998
APA StyleWang, Q., Jing, Y., Zhang, D., Wang, R., Chen, L., Zhang, J., Sui, S., & Wang, X. (2025). Highly Sensitive SERS Detection of Food Colorants via Charge Transfer of Metal and Semiconductor in Ag/TiO2/Ti Foam. Foods, 14(23), 3998. https://doi.org/10.3390/foods14233998





