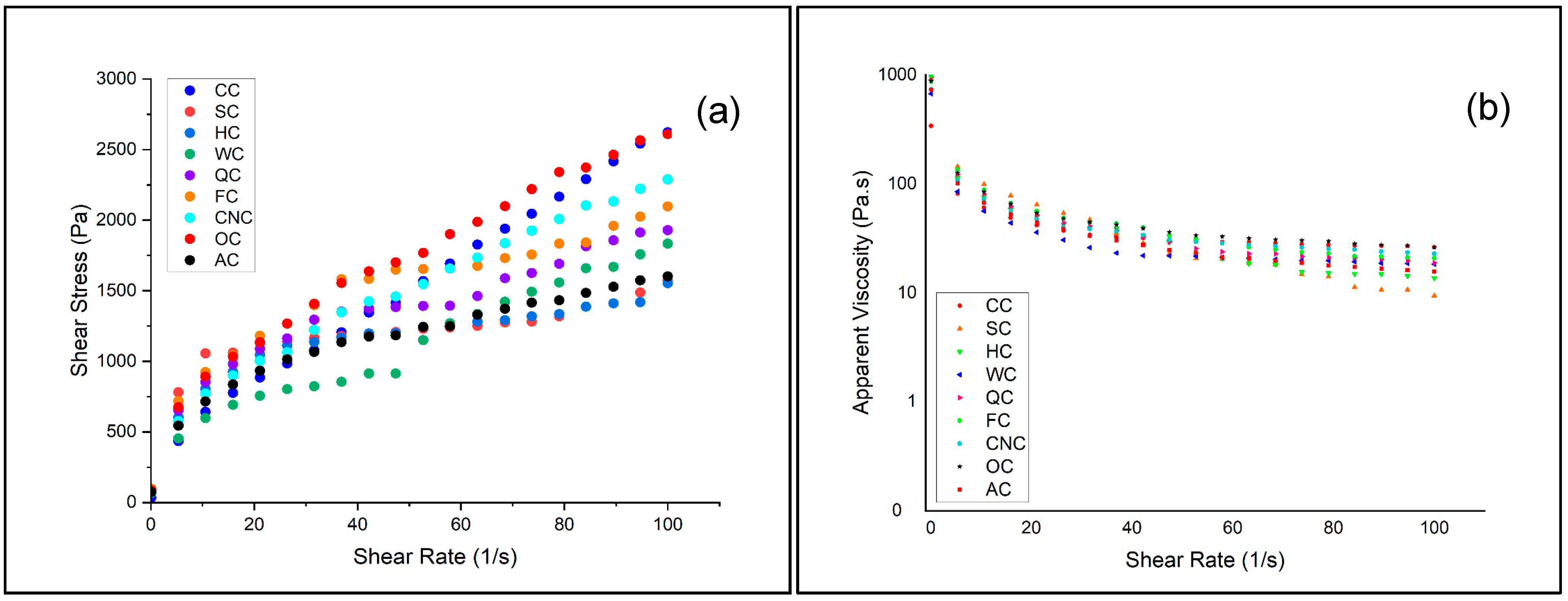
3.1. Rheological Characterization of Muffin Batters
The rheological characteristics of muffin batters were analyzed using a controlled-stress rheometer to evaluate how different milk alternatives influenced flow behavior and viscoelastic properties (
Figure 1).
The viscosity of muffin batter is a critical determinant of product quality. Inappropriate viscosity may negatively influence aeration and texture during baking. Excessively high viscosity restricts gas bubble expansion, leading to dense and compact structures, while overly low viscosity causes structural collapse and insufficient crumb development. Therefore, a well-balanced viscosity is essential to ensure proper aeration, optimal volume, and stable texture in muffins [
47]. The flow behavior of aquafaba-based muffin batters prepared with different milk types was investigated using steady-shear testing, and the results are presented in
Figure 1a,b.
All samples exhibited non-Newtonian, shear-thinning behavior, characterized by a decrease in apparent viscosity with increasing shear rate. This pseudoplastic flow is typical for cake and muffin batters, where weak intermolecular bonds are disrupted under shear forces. However, clear differences were observed depending on the type of milk used. Batters prepared with coconut milk (CNC), oat milk (OC), and flaxseed milk (FC) demonstrated higher shear stress and viscosity values, indicating stronger internal cohesion and higher resistance to deformation. This effect may be attributed to the elevated lipid and soluble fiber fractions in these plant-based milks, which increase water-binding capacity and contribute to a more viscous batter matrix [
48,
49,
50]. In contrast, almond milk (AC) and hazelnut milk (HC) resulted in lower shear stress and viscosity across the tested range, reflecting weaker structural networks and more fluid-like consistency [
51,
52]. Quinoa milk (QC) and walnut milk (WC) showed intermediate values, suggesting that their protein and mineral components provided partial reinforcement of batter structure (Table 4). The control batter prepared with cow’s milk (CC) exhibited intermediate behavior, positioned between the high-viscosity and low-viscosity formulations.
These observations are consistent with previous reports showing that batters enriched with hydrocolloids or fiber-rich plant ingredients exhibit higher viscosity and improved stability, while formulations with lower solids or weaker emulsifying capacity tend to produce more fluid systems [
53,
54,
55]. The shear-thinning behavior observed across all formulations is technologically advantageous, as it facilitates air incorporation during mixing and supports gas retention during baking. However, the magnitude of viscosity strongly depends on milk composition, highlighting the role of plant-based milk type in determining structural reinforcement and flow behavior of aquafaba-based muffin batters. The results demonstrate that milk alternatives with higher fiber or lipid fractions (coconut, oat, flaxseed) enhance batter consistency, while nut-based milks (almond, hazelnut) yield weaker networks. This indicates that the choice of milk significantly influences the rheological performance of aquafaba-based muffin formulations, with direct implications for aeration, expansion, and final product texture.
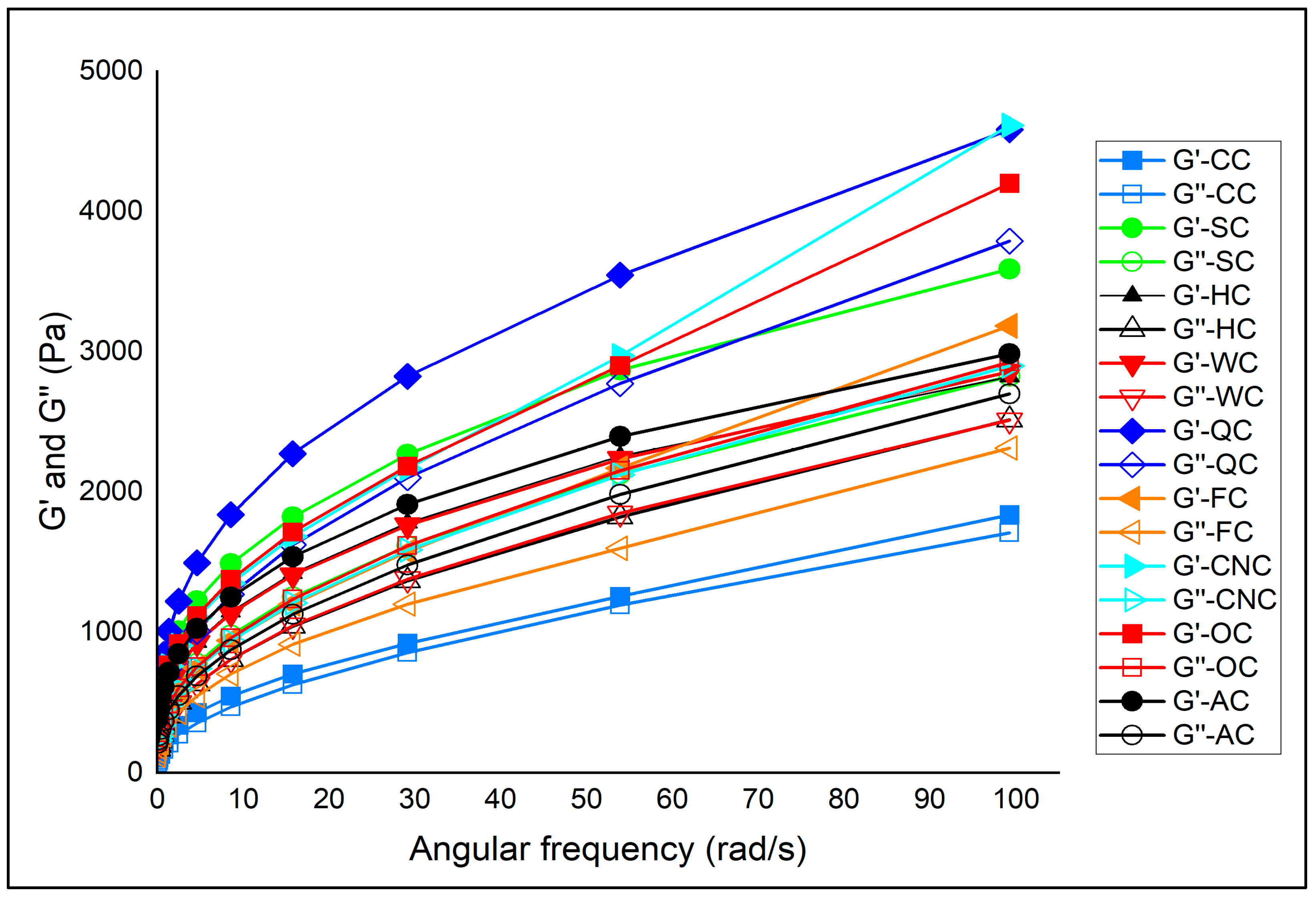
The viscoelastic behavior of muffin batters prepared with aquafaba and different milk alternatives was investigated by frequency sweep tests, and the results are shown in
Figure 2 and
Table 2. Frequency sweep measurements are crucial for understanding batter performance, as they provide information on the balance between elastic (
G′) and viscous (
G″) moduli. A predominance of
G′ over
G″ indicates solid-like behavior and a more stable network, which is desirable for gas retention and structure setting during baking. Conversely, higher
G″ or tan
δ values point to weaker elastic networks and greater viscous character, which may compromise batter stability and product texture [
56].
Across all formulations, both
G′ and
G″ increased with frequency, confirming the weak gel-like and shear-dependent nature of muffin batters. Importantly,
G′ values were consistently higher than
G″ in all samples, indicating that elastic contributions dominated over viscous losses, which is characteristic of stable bakery batters [
57].
Among the samples, coconut milk (CNC) exhibited the highest
K′ value (1838.30 Pa.s
n) together with a relatively high
K″, indicating that CNC batters possessed stronger elastic resistance and enhanced structural reinforcement compared to other formulations [
58]. Quinoa (QC) and oat milk (OC) batters also showed high
K′ values (1671.60 and 1706.20 Pa.s
n, respectively), reflecting solid-like networks and good stability. By contrast, almond (AC) and hazelnut (HC) batters had lower
K′ values (1235.10 and 1048.20 Pa.s
n, respectively). The control sample with cow’s milk (CC) displayed the lowest
K′ (598.32 Pa.s
n), highlighting the reinforcing role of certain plant-based milks in aquafaba systems [
59].
The loss factor (tan
δ) provided further insights. Lower tan
δ values (closer to elastic dominance) were observed in SC (0.609), OC (0.631), and CNC (0.636), indicating stronger elastic contributions and better stability. Conversely, higher tan
δ values in FC (0.692) and CC (0.778) indicated a relatively greater viscous character, which may lead to reduced gas retention and less stable crumb structure [
60]. Almond and hazelnut batters showed intermediate tan
δ values (0.624 and 0.643, respectively), suggesting moderate viscoelastic balance but weaker networks compared to CNC or OC.
These findings align with previous studies indicating that the presence of fiber- or hydrocolloid-rich constituents in plant matrices tends to increase
G′ and decrease tan
δ, thus improving batter stability and baking performance. The observed differences among formulations may therefore be partially attributed to the compositional variation in the plant-based milks used (e.g., solids, protein, and fiber contents) [
55,
60,
61].
Frequency sweep analysis demonstrated that milk type significantly influenced the viscoelastic properties of aquafaba-based muffin batters. Coconut, oat, and quinoa milks promoted stronger elastic networks and lower tan δ values, enhancing batter stability and potentially improving gas retention during baking. Almond and hazelnut milks, however, contributed to weaker structures, indicating reduced elastic reinforcement. These rheological trends are expected to directly influence the textural attributes and volume development of the final baked products.
3.2. Physical Properties of Muffin Samples
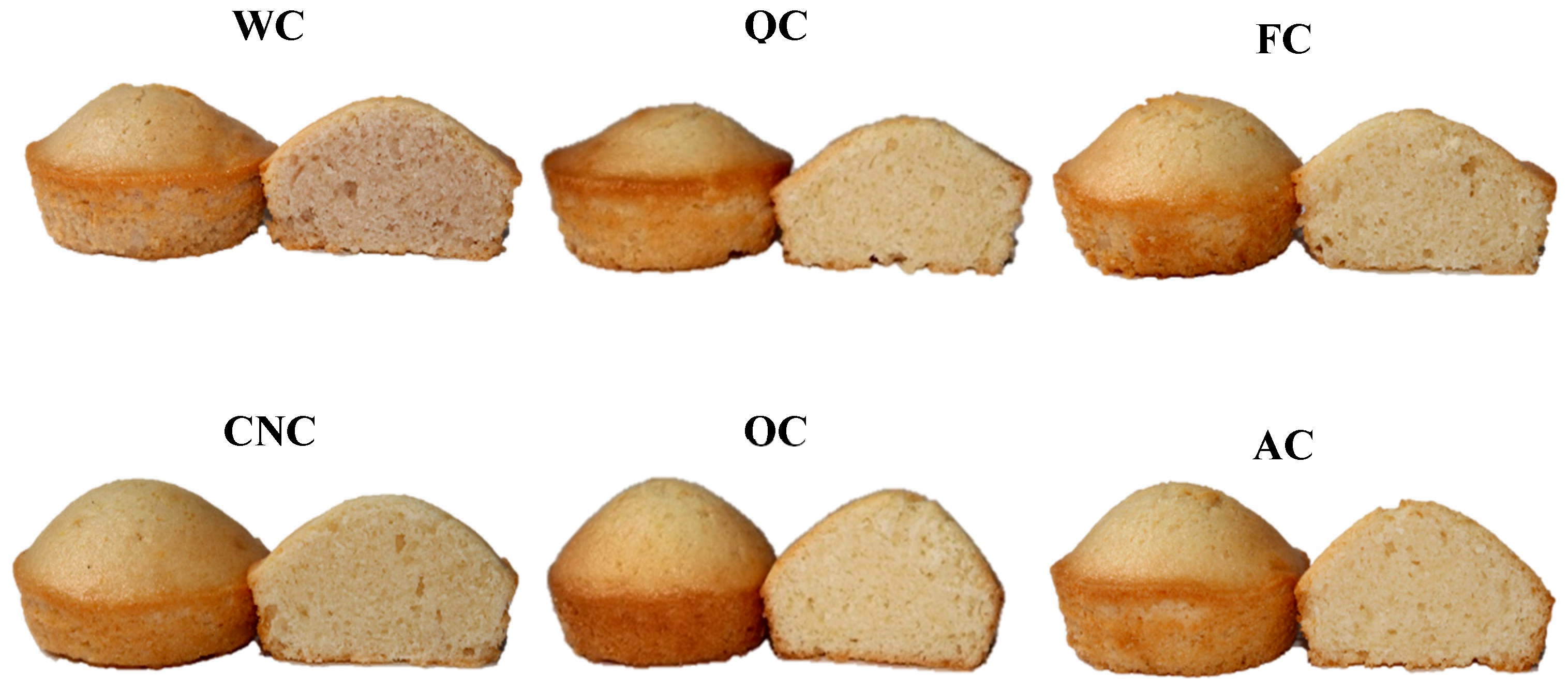
The physical attributes and external appearance of the muffin samples are summarized in
Table 3 and illustrated in
Figure 3. Following baking, the percentage of baking loss varied between 5.63% in the AC sample and 6.91% in the OC sample. Although these variations were not statistically significant (
p > 0.05), the slightly lower values observed in AC and CNC muffins suggest improved moisture retention during baking. In contrast, the higher loss recorded for the OC muffins may be associated with the presence of soluble fiber and β-glucans, which can modify water distribution and evaporation dynamics in the batter matrix.
Table 3 and
Figure 3 summarize the muffins’ physical characteristics and visual appearance, respectively. After baking, baking loss ranged from 5.63% in the AC muffin to 6.91% in the OC muffin. Although the differences were not statistically significant (
p > 0.05), slightly lower losses in AC and CNC muffins may indicate better water retention capacity, whereas OC exhibited higher loss, which may be related to its fiber and β-glucan content influencing water distribution during baking [
62].
VI, a key indicator of muffin structural quality, was highest in the control sample (119.00 mm), consistent with the structural contributions of egg proteins. Plant-based milk alternatives are often reported to exhibit weaker gelation, water holding, and viscoelastic properties compared with cow’s milk, which could explain their tendency toward lower VI values [
63]. Among plant-based variants, OC (102.33 mm), AC (100.67 mm), and HC (101.33 mm) exhibited satisfactory leavening performance, whereas WC had the lowest VI (91.00 mm). Although no formal threshold has been established for VI values in bakery products, measurements above 100 mm are generally regarded as indicative of favorable structural attributes. Similar findings have been reported in other plant-based systems, where aquafaba–milk combinations achieved VI values above 100 mm [
15,
21], while lentil protein–based muffins were able to maintain comparable volume indices despite complete substitution of egg and milk proteins [
64]. These results highlight that, although plant-based proteins and flours often reduce structural expansion, optimized formulations can still yield VI values within the desirable range for good muffin quality. Statistical analysis confirmed that the VI values differed significantly among samples (
p < 0.05), supporting the visual distinctions in muffin height (
Figure 3).
SI, was determined from the height difference between the two lateral edges of the muffins, expressed as an absolute value. SI values ranged between 7.00 and 10.33 mm. The absence of negative readings indicates that none of the samples underwent structural collapse during baking [
65]. According to Dadalı and Elmacı [
66], cakes generally exhibit positive SI values, reflecting a higher central region compared to the edges as a consequence of gas expansion and structural stabilization during baking. Nevertheless, when SI values become excessively high, they may indicate surface irregularities, often arising from uneven batter distribution or thermal gradients within the oven. In the present study, the SC sample displayed the lowest SI value (7.33 mm), corresponding to the most uniform surface profile. By contrast, the AC and FC samples showed elevated SI values (≥10 mm), reflecting greater surface unevenness and irregularity. These variations were not statistically significant (
p > 0.05), indicating that the type of plant-based milk substitute did not exert a measurable effect on surface symmetry.
UI, defined as the mean absolute difference between central and peripheral heights, serves as an indicator of lateral symmetry and surface regularity in muffins. Values approaching zero are desirable, as they reflect an even rise and a well-balanced surface profile. In this study, UI values ranged from −1.00 to 1.00 mm, with SC and FC samples exhibiting values of 0.00 mm. These findings are consistent with previous reports confirming that very low UI values correspond to uniform muffin morphology [
66]. Statistical evaluation showed no significant variation among the formulations (
p > 0.05), indicating that milk substitution had no discernible influence on muffin surface symmetry.
Muffin batters with higher storage modulus (
G′) values tended to form firmer and more elastic networks, which in turn limited air incorporation and reduced the final muffin expansion. For instance, the coconut milk sample (CNC), which exhibited the highest
K′ value (1838.30 Pa.s
n), also showed relatively high hardness and a moderate VI, supporting the idea that excessive batter rigidity restricts bubble growth during baking. In contrast, almond (AC) and hazelnut (HC) batters, with comparatively low
K′ values (1235.10 and 1048.20 Pa.s
n, respectively), displayed weaker structures that permitted greater fluidity but limited the development of a stable muffin height. The control sample (CC), characterized by the lowest
K′ (598.32 Pa.s
n) and the highest tan
δ (0.778), showed reduced elastic dominance. This indicates that cow’s milk proteins, when combined with aquafaba, were not able to form a viscoelastic network as strong as those observed in certain plant-based milk formulations. Previous studies have shown that aquafaba alone can establish elastic gel-like structures, while the presence of fiber- or hydrocolloid-rich plant ingredients (such as β-glucans or seed mucilages) further enhances
G′ and reduces tan
δ, thereby improving batter stability and baking performance [
49,
62,
63]. These findings support the present observation that coconut, oat, and quinoa milks reinforced aquafaba-based batters more effectively than cow’s milk, highlighting the importance of milk composition in determining rheological behavior and final muffin quality.
The influence of different milk alternatives on muffin texture was investigated, and the corresponding results are summarized in
Table 3. The data indicate that the type of milk used had a measurable effect on the textural attributes of the final product. The highest hardness values were recorded in SC (1616.01 gf) and HC (1584.69 gf), whereas the lowest were observed in WC (976.59 gf) and FC (1015.00 gf). Most plant-based milks increased hardness compared to CC, likely due to differences in protein composition and water distribution within the crumb [
22]. Springiness measurements revealed that CC (0.91) exhibited the highest elasticity, while FC (0.84) and WC (0.86) showed reduced values. consistent with previous reports showing that egg or milk proteins provide superior elasticity compared to plant-based substitutes [
56,
60]. The highest cohesiveness was also found in CC (0.71), whereas SC, HC, FC, CNC, and AC samples exhibited lower cohesiveness (0.58–0.60). These results reveal that plant-based milk alternatives were less effective in maintaining internal structural binding. Regarding chewiness, CC (828.54), SC (823.56) and HC (832.87) showed the highest values, whereas FC (513.33) and WC (534.95) had the lowest. Resilience was highest in CC (0.33) and lowest in SC (0.25), HC (0.26) and AC (0.26), indicating that cow’s milk contributed to superior structural recovery compared to plant-based milks. In conclusion, the results indicate that milk alternatives substantially altered the textural characteristics of muffins, underlining the importance of optimizing formulations to achieve the desired texture profile.
Color is a critical indicator of muffin quality, largely governed by Maillard browning and caramelization during baking. The crust color arises mainly from reactions between reducing sugars and amino compounds, while the crumb color depends on the specific composition and pigmentation of the ingredients [
14,
28]. In this study, color parameters were measured using the CIELAB system for both crust and crumb portions, and the corresponding results are summarized in
Table 3.
Crust brightness (
L*) ranged from 58.98 (CC) to 69.12 (AC). The highest crust lightness was observed in AC, followed by CNC (67.46) and WC (65.12). In contrast, CC (58.98) showed the lowest value, which can be attributed to the pigments naturally present in egg yolk [
67]. Similar increases in brightness have been reported in cakes formulated with plant-based proteins or fibers that enhance water binding and surface reflectance [
21,
68]. Crust
a* values, representing redness, were highest in CC (12.48), whereas all plant-based variants showed significantly lower
a* values. This reduction indicates that the absence of egg-derived pigments limited reddish hues [
68]. Crust
b* values followed a similar trend, with CC exhibiting the highest yellowness (30.99), while WC (24.57) and QC (24.49) displayed markedly lower values. Since internal muffin temperature does not exceed 100 °C, Maillard reactions are restricted, and observed color differences can be primarily attributed to raw ingredients [
65]. The yellowish tone of the CC sample (
Figure 3) can be attributed to the carotenoids present in egg yolk [
69].
Crumb lightness (
L*) was highest in CC (75.03) and lowest in WC (64.69), producing a noticeably darker crumb. Across all samples, the crumb appeared more reddish or yellowish than the crust. Notably, WC was the only sample with a positive crumb
a* (1.22), indicating a distinct reddish tone, whereas CC and the other plant-based muffins displayed negative
a* values. Crumb
b* values were highest in CC (24.33) and lowest in WC (12.11). These differences may be attributed to arise from compositional factors in walnut milk, such as phenolic compounds and fiber, which can participate in Maillard or oxidation reactions and thereby contribute to a darker internal color [
70].
Beyond the basic
L*,
a*, and
b* parameters, Chroma (
C*), hue angle (
h°), and total color difference (
ΔE) were also computed to provide a more comprehensive assessment of visual appearance. The control muffins (CC) exhibited the most intense coloration, with
C* values of 33.49 for the crust and 24.43 for the crumb, whereas the walnut-based samples (WC) showed the lowest saturation (25.03 and 12.17, respectively). Hue angle evaluation indicated a gradual transition in crust color from orange-yellow in CC (67.88°) to brighter yellow tones in the flaxseed (FC, 81.01°) and almond (AC, 83.19°) muffins. In contrast, the crumb of CC showed a hue of 275.35°, corresponding to the blue-green region of the CIELAB color space—a result of negative
a* combined with dominant positive
b* coordinates. The largest total color deviations (
ΔE) for the crust were observed in AC (18.20) and CNC (15.85), whereas WC demonstrated the greatest difference in crumb color (16.72). These outcomes clearly demonstrate that incorporating plant-based milks into muffin formulations leads to visually perceptible color modifications relative to the control sample [
22].
3.4. Chemical Composition of Muffin Samples
The chemical composition of muffins is a decisive factor for both nutritional quality and technological functionality. Moisture content affects textural softness and shelf-life stability, while protein and fat levels determine structural integrity and flavor development during baking. Ash and mineral contents provide insights into the micronutrient contribution of different formulations, whereas dietary fiber and total carbohydrate fractions are directly linked with health-promoting properties and caloric density [
74].
According to the results presented in
Table 4, the control muffin (CC) containing cow’s milk showed the highest protein (8.51 g/100 g) and fat (22.21 g/100 g) contents, which translated into the highest calculated energy value (448.90 kcal/100 g). In contrast, all plant-based formulations exhibited reduced protein levels, ranging from 5.40 to 5.92 g/100 g, with concomitant decreases in fat. Among them, AC and QC muffins displayed the lowest fat contents (19.92 and 19.61 g/100 g, respectively), which led to significantly lower energy values compared to the control (
p < 0.05). SC and HC muffins stood out with higher dietary fiber (1.02 and 0.99 g/100 g, respectively), highlighting the fiber-enriching potential of these plant-based milks.
Table 4.
Chemical composition (g/100 g) and mineral composition (mg/1000 g) of muffin samples.
Table 4.
Chemical composition (g/100 g) and mineral composition (mg/1000 g) of muffin samples.
| | Moisture | Ash | Protein | Fat | TDF | Total Carbohydrate | Energy (kcal/100 g) |
|---|
| CC | 14.45 ± 0.00 c | 0.73 ± 0.03 bc | 8.51 ± 0.07 a | 22.21 ± 0.02 a | 0.70 ± 0.01 bc | 53.45 ± 0.07 e | 448.90 ± 0.00 a |
| SC | 14.91 ± 0.08 b | 0.82 ± 0.02 b | 5.75 ± 0.07 bc | 20.36 ± 0.04 c | 1.02 ± 0.02 a | 57.20 ± 0.14 d | 436.90 ± 0.00 f |
| HC | 14.45 ± 0.03 c | 0.73 ± 0.03 bc | 5.42 ± 0.04 d | 20.36 ± 0.05 c | 0.99 ± 0.01 a | 58.10 ± 0.00 bc | 439.10 ± 0.28 d |
| WC | 14.86 ± 0.07 b | 0.72 ± 0.02 c | 5.92 ± 0.09 b | 20.82 ± 0.06 b | 0.49 ± 0.01 e | 57.20 ± 0.00 d | 440.70 ± 0.14 c |
| QC | 16.18 ± 0.06 a | 0.97 ± 0.03 a | 5.46 ± 0.00 d | 19.61 ± 0.01 e | 0.64 ± 0.01 cd | 57.10 ± 0.00 d | 428.20 ± 0.14 g |
| FC | 14.62 ± 0.02 c | 0.82 ± 0.02 a | 5.40 ± 0.00 d | 20.23 ± 0.03 c | 0.60 ± 0.02 d | 58.25 ± 0.07 b | 437.85 ± 0.35 e |
| CNC | 14.82 ± 0.02 b | 0.80 ± 0.01 bc | 5.68 ± 0.04 c | 20.06 ± 0.02 d | 0.73 ± 0.00 b | 57.95 ± 0.07 c | 436.35 ± 0.07 f |
| OC | 14.20 ± 0.00 d | 0.93 ± 0.02 a | 5.44 ± 0.00 d | 20.76 ± 0.02 b | 0.60 ± 0.02 d | 58.05 ± 0.07 bc | 442.10 ± 0.00 b |
| AC | 13.72 ± 0.05 e | 0.76 ± 0.01 bc | 5.66 ± 0.01 c | 19.92 ± 0.04 d | 0.99 ± 0.01 a | 58.95 ± 0.07 a | 439.75 ± 0.07 d |
| | P | Na | Mg | K | Ca |
| CC | 2188.36 ± 103.85 a | 2201.69 ± 121.29 a | 138.99 ± 9.98 b | 1014.14 ± 25.31 d | 301.99 ± 13.85 a |
| SC | 1917.80 ± 22.01 b | 2015.84 ± 36.11 ab | 174.31 ± 1.80 a | 1331.41 ± 5.28 a | 235.61 ± 3.92 bc |
| HC | 1809.51 ± 53.03 bc | 1936.65 ± 58.66 bc | 171.40 ± 4.73 a | 1163.73 ± 33.21 bc | 252.08 ± 2.63 b |
| WC | 1669.66 ± 39.61 c | 1802.11 ± 51.70 c | 168.99 ± 4.29 a | 1142.05 ± 39.47 bc | 155.31 ± 0.79 d |
| QC | 1765.32 ± 21.23 bc | 1896.96 ± 27.27 bc | 171.22 ± 0.98 a | 1153.24 ± 14.43 bc | 146.15 ± 3.06 d |
| FC | 1698.42 ± 13.60 c | 1776.845 ± 11.96 c | 169.43 ± 0.96 a | 1212.24 ± 5.84 b | 165.94 ± 1.05 d |
| CNC | 1815.14 ± 8.13 bc | 2030.32 ± 17.317 ab | 168.96 ± 3.83 a | 1141.18 ± 11.53 bc | 230.29 ± 1.03 c |
| OC | 1648.82 ± 17.88 c | 1769.86 ± 18.17 c | 163.69 ± 1.56 a | 1120.66 ± 4.24 c | 228.54 ± 5.07 c |
| AC | 1721.91 ± 31.98 c | 1862.19 ± 33.94 bc | 161.80 ± 5.03 a | 1169.46 ± 15.75 bc | 244.99 ± 0.67 bc |
The mineral profile also varied markedly among samples. Control muffins were richer in calcium (301.99 mg/100 g) compared with the plant-based counterparts, which ranged from 146.15 mg/100 g in QC to 252.08 mg/100 g in HC. On the other hand, SC muffin contained the highest potassium content (1331.41 mg/100 g), nearly 30% higher than the control. CC, SC and CNC muffins showed high sodium concentrations (>2000 mg/100 g), exceeding the WHO recommended daily maximum sodium intake of 2000 mg [
75], whereas the other formulations remained between 1770 and 1937 mg/100 g. Magnesium content was consistently higher in plant-based samples (161.8–174.3 mg/100 g) than in the control (138.99 mg/100 g), pointing to their potential in enhancing micronutrient diversity.
The compositional shifts observed here reflect well-known patterns in dairy substitution. Cow’s milk typically contributes higher protein and calcium levels, while plant-based milks reduce energy density and fat content but increase fiber and magnesium [
76,
77]. Soy- and nut-based systems are particularly associated with improved fiber fractions in bakery applications [
78], whereas reduced calcium in quinoa and walnut milks mirrors earlier findings on mineral dilution in gluten-free formulations [
77]. Elevated sodium in SC and CNC muffins (>2000 mg/100 g) aligns with reports that some commercial plant milks contain added salts for stabilization [
79]. Such differences highlight the dual challenge and opportunity of reformulation: achieving nutritional adequacy while maintaining functional properties.
3.5. Fatty Acid Composition
The fatty acid composition of bakery products plays a decisive role in both nutritional quality and technological functionality. Saturated fatty acids (SFA) are generally associated with structural stability but excessive intake is linked with cardiovascular diseases, while unsaturated fatty acids (UFA), including monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA), are considered beneficial for lipid metabolism and inflammation regulation [
80]. In muffins, the choice of milk replacer can alter the lipid profile by modulating the proportion of these fatty acid groups.
The results given in
Table 5 reveal that palmitic acid (C16:0) and stearic acid (C18:0) were the dominant SFAs across all samples. CC muffin showed significantly higher SFA content (13.40%) compared to plant-based formulations, which ranged between 11.44 and 11.98%. This demonstrates that substitution with alternative milks successfully reduced the proportion of SFAs, thus potentially improving the nutritional lipid quality. Among plant-based milks, FC and QC muffins exhibited slightly elevated SFA values relative to other vegan samples. Although both flaxseed and quinoa are generally recognized for their high PUFA content, flaxseed also contains appreciable amounts of palmitic and stearic acids [
81], while quinoa oil includes approximately 20% palmitic acid alongside oleic and linoleic acids [
82]. These intrinsic profiles likely explain the modest increase in SFA observed in FC and QC formulations.
Oleic acid (C18:1) was the predominant MUFA, with values consistently around 35% in all formulations, showing no significant differences (
p > 0.05). This uniformity indicates that the replacement of cow’s milk with plant-based alternatives did not markedly affect MUFA content, which is favorable given the well-known role of oleic acid in supporting cardiovascular health [
83]. Linoleic acid (C18:2) represented the main PUFA, ranging from 50.33% in CC to 53.21% in WC. Muffins formulated with walnut, soy, and oat milk exhibited the highest PUFA levels, in agreement with the intrinsic fatty acid profiles of these plant sources, which have been reported to contain higher proportions of unsaturated fatty acids compared to dairy counterparts [
84]. Conversely, flaxseed and coconut milk muffins showed comparatively lower PUFA proportions, despite flaxseed being widely recognized as a rich source of α-linolenic acid (C18:3) [
85]. Notably, WC and FC muffins recorded the highest α-linolenic acid contents (0.341% and 0.309%, respectively), confirming their potential as functional formulations enriched in omega-3 fatty acids [
86].
Reviews emphasize that bovine milk fat is naturally enriched in palmitic and stearic acids, which play a role in gas retention and structural stabilization of baked goods. However, plant-based substitutes introduce higher proportions of oleic and linoleic acids, which enhance nutritional quality and provide additional health benefits [
13]. Thus, the observed increase in UFA content, particularly in walnut- and flaxseed-based muffins, indicates a clear advantage from both technological and nutritional perspectives. UFAs accounted for 86–88% of the lipid fraction in all formulations, with the highest TUFA values recorded in plant-based muffins (>88%). This shift towards a more favorable unsaturated-to-saturated fatty acid ratio underscores the potential of plant-based milks to improve the health profile of muffins without compromising structural integrity. These findings confirm that strategic use of milk alternatives can deliver bakery products with both functional performance and enhanced nutritional value.
3.7. Total Amino Acid (AA) Composition
The amino acid composition of muffins, presented in
Table 8, revealed distinct variations among the control and plant-based formulations. Beyond their structural role in proteins, amino acids participate in a wide range of physiological processes including energy metabolism, immune response, neurotransmission, and tissue repair [
97].
Acidic amino acids were dominant in WC, QC, and FC muffins. WC (1.895 g/100 g) and QC (1.850 g/100 g) showed glutamic acid levels nearly fourfold higher than CC (0.476 g/100 g), while QC also contained the highest aspartic acid (0.224 g/100 g vs. 0.065 g/100 g in CC). These increments indicate that certain plant-based alternatives, particularly walnut and quinoa, enhanced the abundance of flavor-active amino acids, consistent with their reported contribution to umami perception [
98,
99].
Among the basic amino acids, arginine was most abundant. WC (1.928 g/100 g) recorded nearly double the CC level (1.007 g/100 g), followed by QC (1.780 g/100 g) and FC (1.398 g/100 g). This highlights the arginine-rich nature of nut- and seed-derived proteins, which is nutritionally relevant since arginine is involved in nitric oxide synthesis and immune regulation [
100]. Lysine content varied substantially among samples. While CC contained 0.114 g/100 g, levels dropped markedly in SC (0.010 g/100 g) and HC (0.006 g/100 g), supporting the well-known limitation of lysine in many plant proteins [
101]. However, certain formulations such as WC (0.246 g/100 g) and QC (0.228 g/100 g) showed elevated lysine contents, indicating that specific plant sources may partially compensate for this deficiency.
Neutral amino acids also showed strong variability. WC and QC demonstrated the highest values for several essential neutral amino acids, including leucine (0.602 and 0.589 g/100 g, respectively), nearly doubling CC (0.330 g/100 g). FC also showed an elevated leucine level (0.427 g/100 g). By contrast, SC and HC contained very low leucine (0.051 and 0.031 g/100 g). Similar patterns were observed for valine and isoleucine, which peaked in WC and QC but were minimal in soy- and hazelnut-based formulations. These branched-chain amino acids (BCAAs) are fundamental to protein synthesis and energy metabolism, and their variability has direct implications for the nutritional quality of vegan muffins [
102]. Methionine also displayed a similar trend: while WC and QC maintained levels comparable to CC, SC and HC recorded substantial reductions (0.015 and 0.013 g/100 g, respectively, vs. 0.088 g/100 g in CC). This underscores methionine as another limiting amino acid in soy and nut-based milk alternatives. A distinctive feature was the presence of hydroxyproline in WC (0.225 g/100 g), QC (0.200 g/100 g), and OC (0.078 g/100 g), whereas it was undetectable in CC. Hydroxyproline is generally associated with collagen; its detection in vegan samples may derive from hydrolyzed plant proteins or collagen-like analogues, potentially influencing textural attributes of the muffins [
103].
Substituting cow’s milk with plant-based alternatives reshaped the amino acid spectrum of muffins. While CC retained higher methionine, certain formulations such as WC and QC reached lysine levels comparable to or exceeding the control. Moreover, WC, QC, and FC exhibited elevated glutamic acid, arginine, and leucine. These findings underscore both the nutritional limitations and the functional advantages of plant-based milks, emphasizing that amino acid balance in vegan muffins is determined by the specific substitute employed.
3.8. Shelf-Life Evaluation of Muffin Samples
During the 30-day storage period, significant differences were observed among the muffin formulations with respect to physicochemical, textural, color, and microbiological properties (
Supplementary Tables S1 and S2).
Moisture content showed a biphasic trend. Between day 0 and day 15, moisture levels increased in all samples, due to equilibration and redistribution of water within the matrix. The highest values were recorded in HC and QC (19.94% and 19.87%, respectively), whereas CC and AC remained comparatively lower (17.02% and 18.28%). By day 30, however, moisture declined sharply across all formulations, most prominently in SC (9.17%) and CC (10.07%), while FC retained the highest level (15.71%). This reduction indicates progressive dehydration during storage, a typical phenomenon in bakery systems. The relatively stable hydration in FC may be linked to flaxseed mucilage, which provides water retention capacity [
104]. Ash content exhibited moderate variations. On day 30, OC and AC recorded the highest values (1.18% and 1.11%), possibly reflecting concentration effects due to moisture loss and localized redistribution of minerals. In contrast, SC showed the lowest ash value (0.57%). Fat content also declined over time, consistent with lipid oxidation and hydrolysis. The decrease was especially marked in CC (22.21% to 16.66%), whereas WC and SC maintained higher levels (21.84% and 21.73% at day 30). These results reflect the susceptibility of unsaturated fatty acids to oxidation under ambient storage [
105].
Textural attributes could be assessed up to day 15, but the same parameters were not applicable at day 30, so the values were not measurable. Hardness increased substantially, particularly in CNC (from 1416.21 gf to 4153.08 gf) and AC (1404.69 gf to 4116.30 gf). A parallel rise in chewiness was observed in AC (748.21 to 1236.76), while springiness and resilience values decreased, indicating structural weakening. Cohesiveness also declined consistently reflecting starch retrogradation and protein–starch interactions that limit crumb elasticity [
15].
Color parameters were markedly affected by storage. Crust
L* values declined initially, most clearly in CC (58.98 to 54.13), whereas QC maintained higher brightness. Crust a* values remained consistently higher in CC (12.48–14.10), reflecting stronger Maillard browning promoted by egg proteins and lactose [
101]. Crust
b* values were highest and increased in CC (30.99 to 33.28), but remained lower in WC (24.57 to 23.60). For crumb color,
L* decreased notably in OC (70.62 to 62.33),
a* values rose in WC (1.22 to 2.78), and
b* values declined slightly across most samples. These changes highlight the combined effects of Maillard reactions and plant-derived pigments on color stability during storage.
Aerobic plate counts (APC) varied between 1.5 × 102 CFU/g (OC, day 0) and 9.2 × 103 CFU/g (SC, day 15), remaining below levels typically linked to spoilage. All samples also met the food safety requirements for ready-to-eat bakery products defined by EC No. 2073/2005, as E. coli, coliforms, Staphylococcus aureus, yeasts, molds, and Salmonella spp. were not detected. These results confirm that hygienic conditions were maintained during production and that microbial safety was preserved throughout storage. Importantly, the use of different plant-based milks did not compromise microbiological quality, and all muffin formulations remained stable and safe for 30 days under ambient conditions.
All muffin formulations maintained acceptable quality and microbial stability during 30 days of storage. The main challenges were moisture loss and texture hardening, most pronounced in SC and CC. By contrast, flaxseed- and nut-based variants (FC, AC, CNC) held water better and retained their structure more effectively. Importantly, every formulation remained microbiologically safe under ambient conditions, showing that a wide range of plant-based milks can be used to produce bakery products with an extended shelf life.
3.10. Chemometric Analysis
3.10.1. Correlation Coefficient
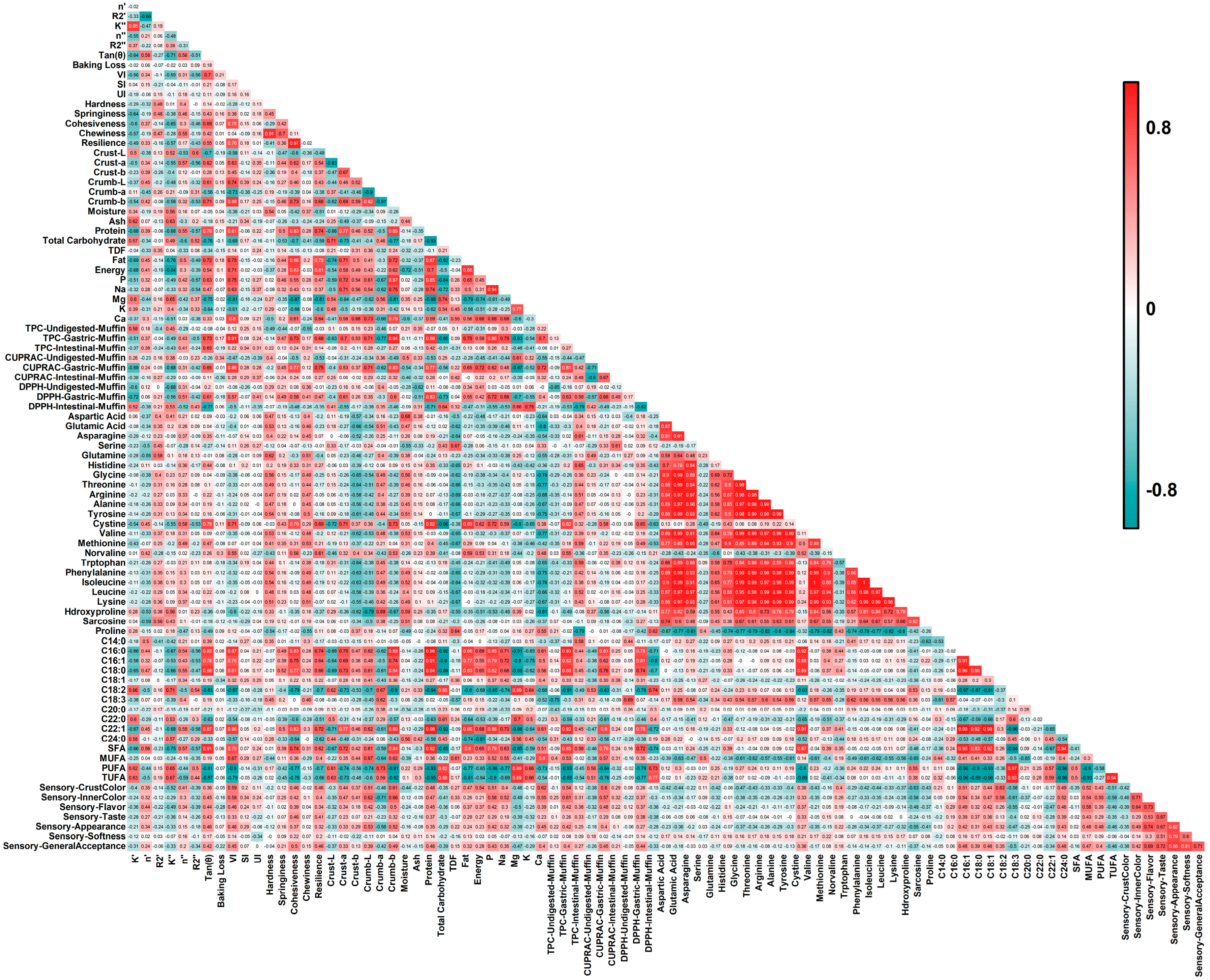
The Pearson correlation heatmap (
Figure 6) provided an integrative overview of interrelationships among rheological model parameters, physicochemical attributes, compositional variables, and functional indices of aquafaba-based vegan muffins. Several strong positive and negative correlations were observed, reflecting how rheological behavior, moisture dynamics, and antioxidant potential collectively define product quality.
A strong positive correlation between the storage and loss consistency indices (K′–K″, R2 = 0.85) indicated that samples with higher elastic consistency also exhibited greater viscous response, reflecting coherent viscoelastic network formation in batters stabilized by plant-based hydrocolloids [
107]. Conversely, K′ correlated negatively with DPPH values in undigested and gastric phases but positively in the intestinal phase (−0.71 ≤ R
2 ≤ 0.77), suggesting that matrix elasticity influenced the release of antioxidant compounds during digestion.
Hardness and chewiness were highly correlated (R2 = 0.91), as were resilience and cohesiveness (R2 = 0.97), confirming that nutrient-rich formulations supported stronger internal bonding and improved structural recovery. Cohesiveness also correlated positively with protein and fat contents (R2 ≈ 0.85), highlighting the reinforcing role of macronutrients in texture development. Similarly, the volume index showed positive associations with protein and TPC in the gastric phase (R2 = 0.81–0.91), linking increased volume with enhanced phenolic release and color intensity (crumb b*, R2 = 0.88).
Strong intercorrelations among amino acids such as aspartic acid, glutamic acid, and glycine (R
2 = 0.87–0.90) reflected their shared origin in legume- and seed-derived proteins. Proline, in contrast, exhibited a pronounced negative correlation with these amino acids (R
2 ≈ –0.80), suggesting that its cyclic structure limited participation in hydrogen-bonded protein networks [
108]. The antioxidant assays also displayed tight relationships: TPC and CUPRAC values across digestive phases were strongly aligned (R
2 ≥ 0.90), demonstrating coherent redox behavior of polyphenol-rich systems [
109].
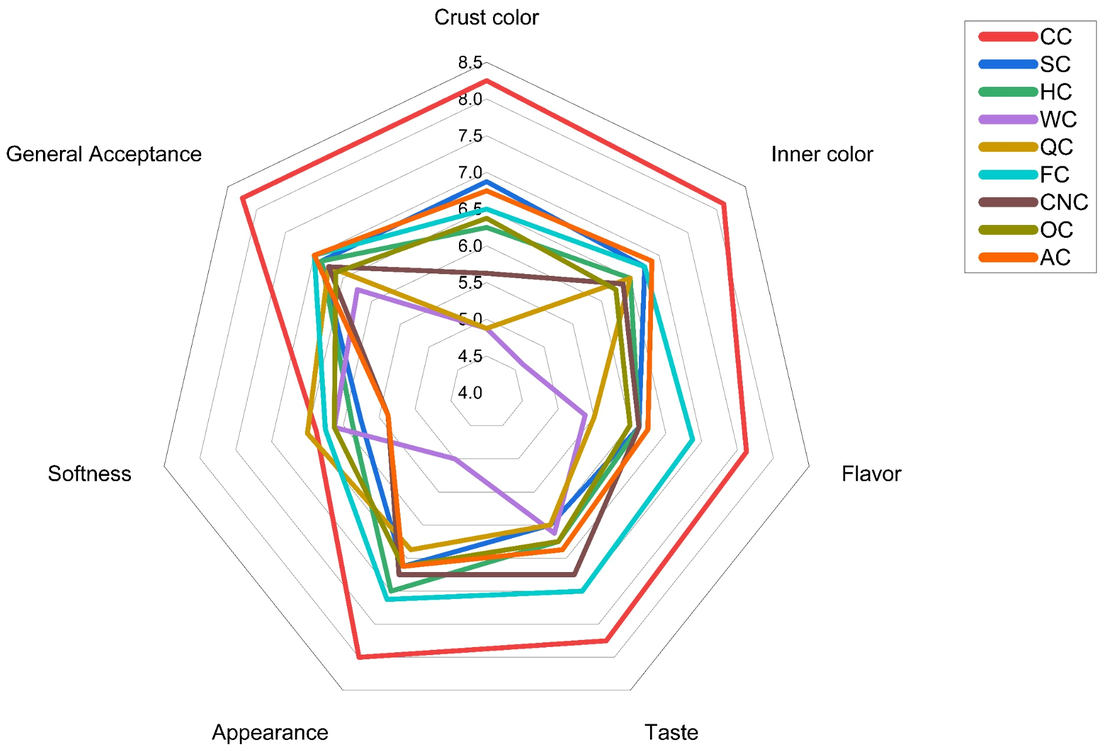
In addition to the compositional and rheological relationships, strong associations were observed among the sensory attributes themselves, as well as between instrumental and perceptual measures. General acceptability was closely linked with softness, appearance, and taste (R2 = 0.72–0.86), confirming that consumer preference was primarily driven by mouthfeel and visual quality. Instrumental cohesiveness and resilience also correlated positively with sensorial general acceptability (R2 ≈ 0.45–0.50), indicating that structurally stable matrices were perceived as softer and more pleasant. Positive correlations between crumb L* and b* values and sensory color scores (R2 = 0.51–0.66) indicate that lighter and more yellowish crumb tones were perceived as visually appealing, while the negative association between crumb a* and sensory inner color (R2 = –0.71) suggests that excessive reddish hues were less favored. Conversely, the weak negative relationship between crust L* and crust color perception (R2 ≈ −0.30) implies that a slightly darker crust was preferred for an optimal baked appearance
The results indicate that rheological strength, compositional density, and antioxidant capacity are the primary factors shaping the physical and functional characteristics of plant-based muffins, forming the statistical basis for the PCA and HCA results discussed in the following sections.
3.10.2. Principal Component Analysis
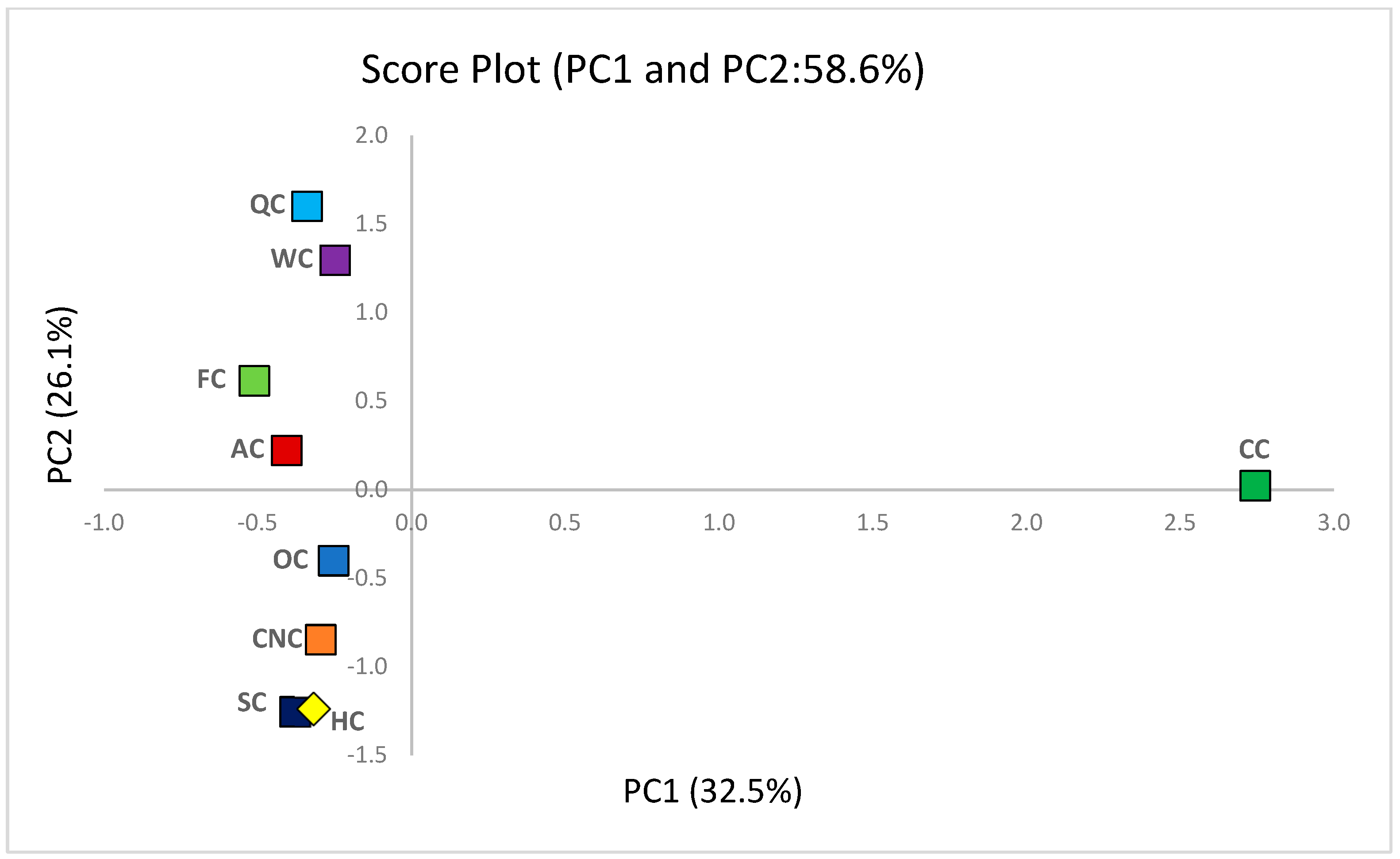
PCA was performed to reduce the multidimensional dataset and to identify the principal factors governing the physical, compositional, and functional variations among aquafaba-based vegan muffins (
Supplementary Table S3). The first two principal components (PC1 and PC2) explained 58.6% of the total variance (
Figure 7 and
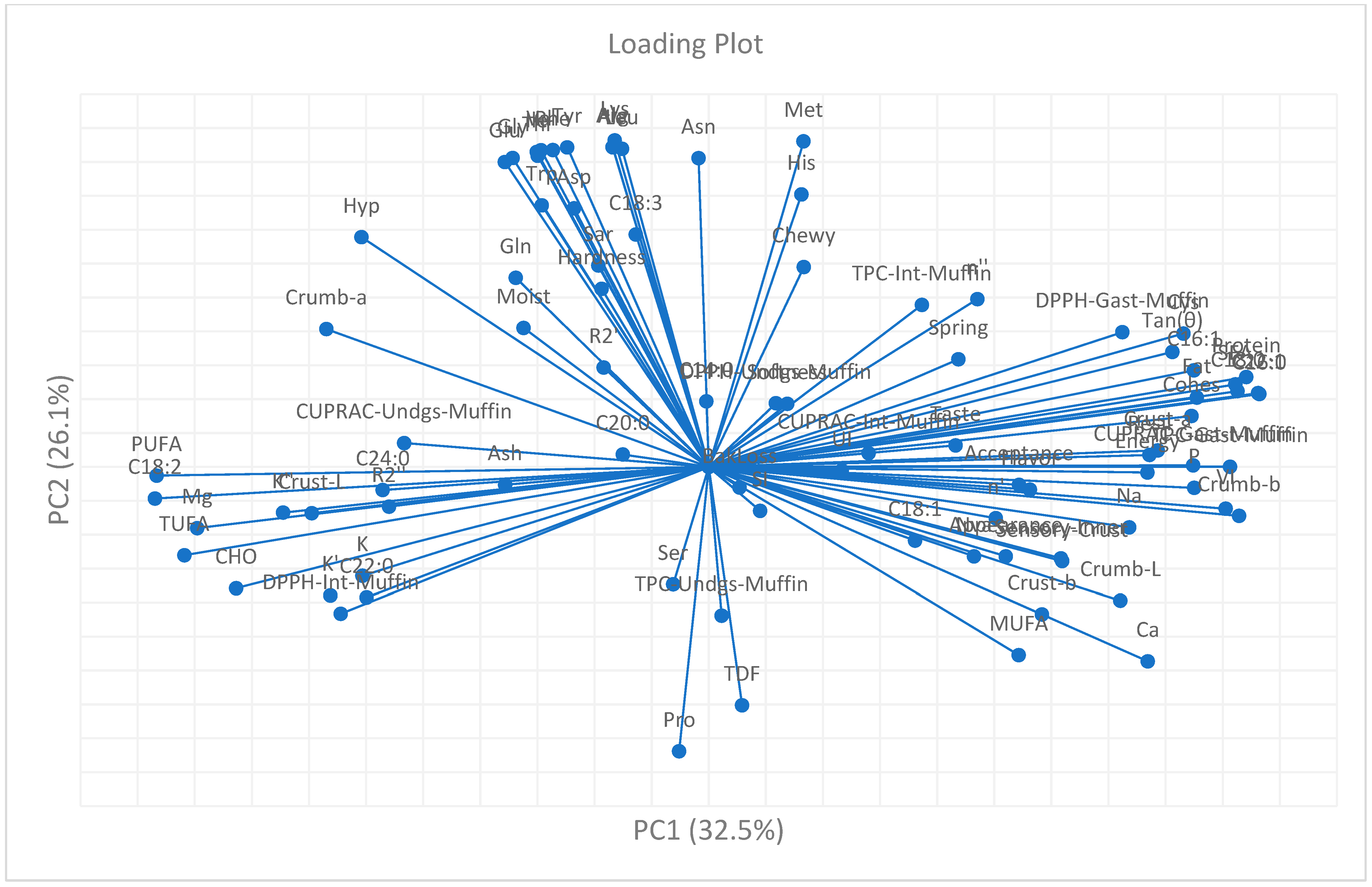
Figure 8), indicating an adequate dimensional reduction to visualize the dominant patterns within the dataset. PC1 (32.5% of the variance) was primarily defined by rheological and compositional attributes, whereas PC2 (26.1%) reflected variations in antioxidant response and amino acid composition.
Positive loadings of protein (0.94), fat (0.86), saturated fatty acids (0.92), and volume index (0.91) on PC1 demonstrated that nutrient-rich and structurally coherent batters exhibited higher viscoelastic strength and greater expansion during baking. These variables were closely aligned with cohesiveness (0.85), resilience (0.77), and crumb
b* (0.93), indicating that color development and textural uniformity were directly related to macronutrient content and matrix organization. In contrast, negative loadings of PUFA (−0.97), TUFA (−0.92), and total carbohydrate (−0.83) denoted an opposite compositional trend associated with unsaturated lipid enrichment and carbohydrate dominance, which are typically linked with softer, less elastic matrices [
110,
111].
PC2 exhibited strong positive loadings for a cluster of amino acids, including arginine (0.94), alanine (0.94), tyrosine (0.94), valine (0.94), and methionine (0.96), reflecting the influence of proteinogenic components on the nutritional profile of legume- and seed-based formulations. Negative loading of proline (−0.84) on the same axis confirmed its antagonistic behavior due to its cyclic imino structure, which limits participation in hydrogen-bonded protein networks. In parallel, antioxidant indices measured in the gastric phase of the milk fraction—including total phenolic content (TPC: 0.76), CUPRAC (0.73), and DPPH (0.73)—showed co-alignment along PC2, highlighting phase-dependent clustering of polyphenolic responses during simulated digestion.
The PCA score plot revealed clear sample differentiation. The control muffin (CC) was distinctly separated along PC1 due to its higher protein, fat, and SFA loadings, reflecting its strong structural and compositional integrity. Plant-based milk formulations formed subgroups on the negative side of PC1: quinoa (QC) and walnut (WC) muffins positioned higher along PC2, indicating enhanced amino acid and antioxidant characteristics [
112,
113], while soy (SC) and hazelnut (HC) muffins clustered lower, corresponding to higher unsaturated lipid content and reduced viscoelastic strength [
114].
PCA confirmed the multivariate relationships suggested by the correlation analysis. The integrated clustering of rheological, compositional, and antioxidant parameters demonstrates that the structural stability, amino acid complexity, and redox potential jointly determine the functionality and nutritional quality of aquafaba-based vegan muffins.
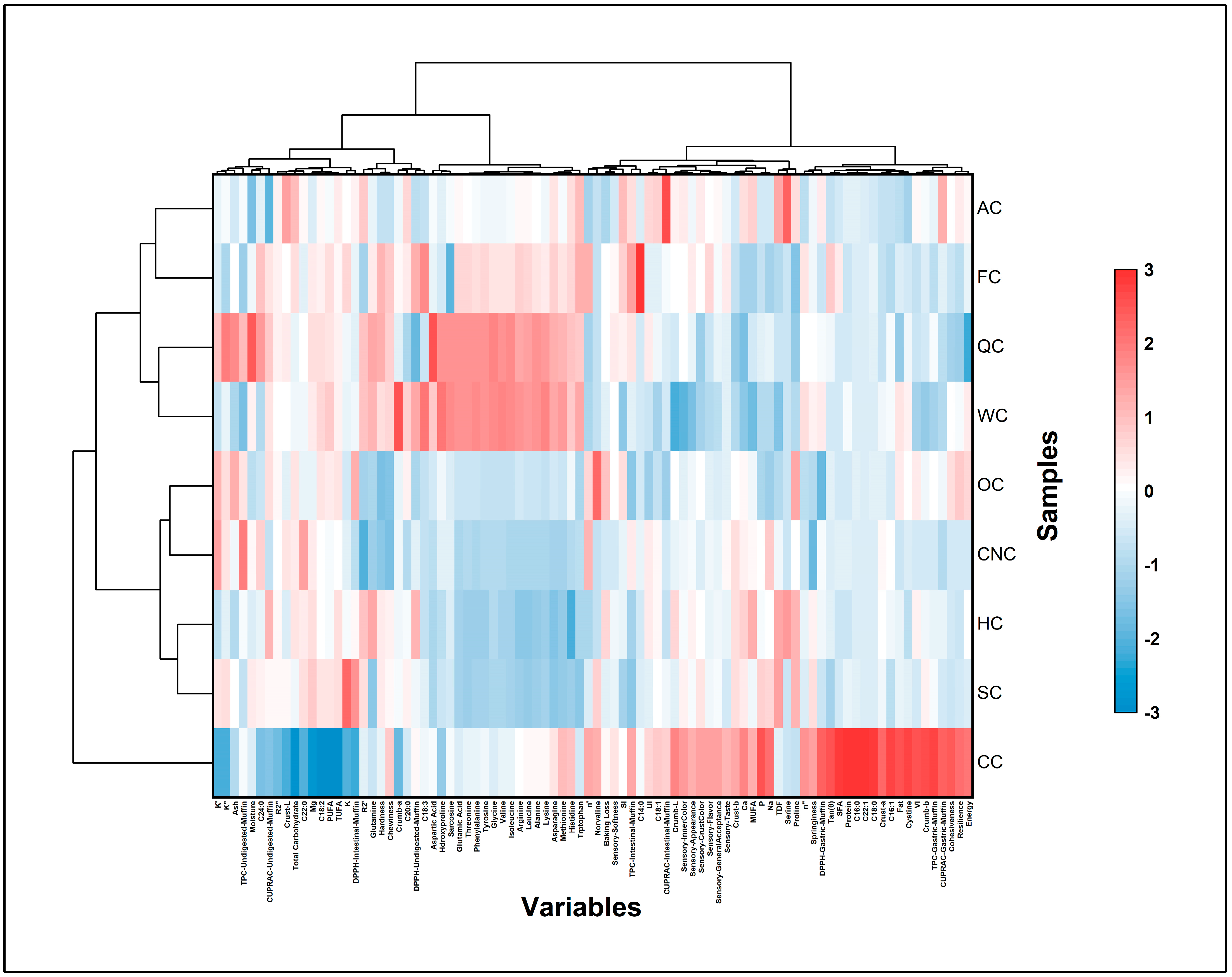
3.10.3. Hierarchical Cluster Analysis
HCA was performed to evaluate the relationships among muffin formulations and to identify groups with comparable physicochemical and functional characteristics. Clustering was conducted based on Ward’s method with Euclidean distance as the similarity metric. The resulting dendrogram (
Figure 9) illustrated the overall relational pattern among samples. This multivariate approach complemented the PCA findings, providing an enhanced interpretation of formulation proximity and differentiation within the dataset [
115].
Cluster I was composed of CC, SC, HC, WC, and QC samples, all of which exhibited comparable patterns associated with elevated levels of unsaturated lipids, moderate antioxidant capacity, and limited viscoelastic strength. The alignment of soy and hazelnut muffins within this cluster indicates that lipid-dominant matrices improved moisture retention but weakened gas-cell stability, producing softer and less cohesive textures [
114]. Similarly, walnut- and quinoa-based formulations displayed parallel behavior, as their polyunsaturated lipid profiles favored nutritional enhancement but restricted elastic recovery within the baked matrix. These observations suggest that formulations dominated by plant-derived oils formed a coherent group defined by moderate structural integrity and reduced rheological strength, consistent with previous reports on nut- and seed-based bakery systems [
57,
68].
Cluster II included FC, CNC, OC, and AC muffins, which shared higher levels of protein and dietary fiber, as well as stronger textural resilience and enhanced antioxidant bioaccessibility. The proximity of FC and CNC samples reflected the contribution of mucilage- and hydrocolloid-rich compounds that supported a cohesive and elastic crumb network [
43,
116]. OC and AC muffins were also positioned closely, characterized by improved viscoelastic balance and elevated phenolic content—an effect likely arising from synergistic starch–protein–fiber interactions within their matrices. These results highlight the capacity of hydrocolloid- and fiber-dominant systems to reinforce both mechanical and biofunctional quality in vegan bakery products [
60,
68].
Together, the PCA and HCA outcomes demonstrate that the type of plant-based milk exerts a decisive influence on the multidimensional behavior of aquafaba-based muffins. Fiber- and hydrocolloid-rich milks (Cluster II) promoted structural cohesion and functional enhancement, whereas lipid-oriented formulations (Cluster I) were associated with softer, less elastic textures. This integrated interpretation underscores the relevance of multivariate modeling for identifying formulation-specific relationships in plant-based bakery systems.