Occurrence, Homologue Profiles and Risk Assessment of Short- and Medium-Chain Chlorinated Paraffins in Edible Vegetable Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of SCCPs and MCCPs in Edible Vegetable Oils
2.1.1. Materials and Reagents
2.1.2. Instruments
2.1.3. Extraction Protocol
2.1.4. Instrumental Analysis
2.1.5. Quality Assurance and Quality Control (QA/QC)
2.2. Dietary Consumption Data of Vegetable Oils in China
2.3. Chlorinated Paraffin Exposure Assessment
2.3.1. Probabilistic Assessment Method
2.3.2. Risk Characterization
3. Results
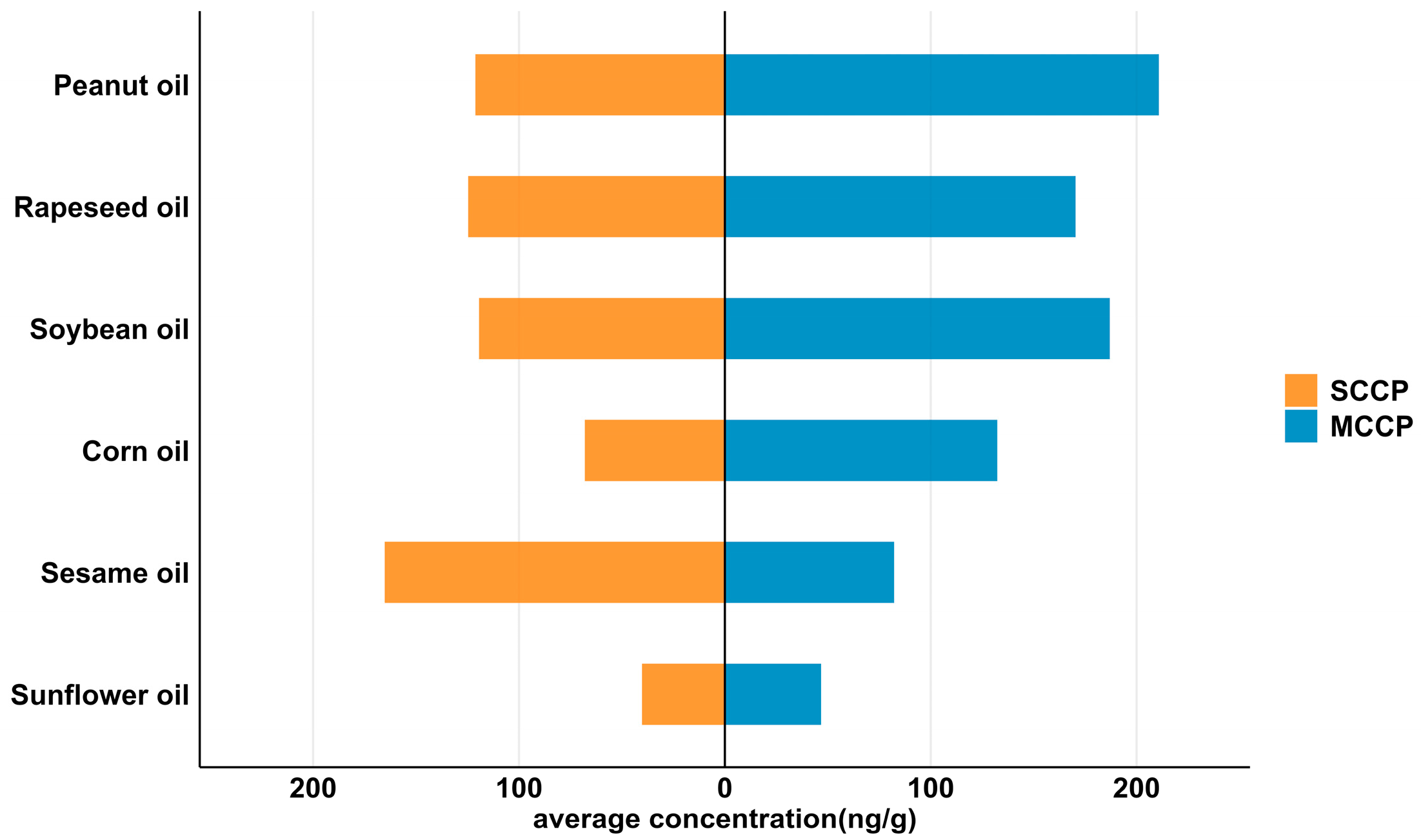
3.1. Concentrations of SCCPs and MCCPs in Edible Vegetable Oils
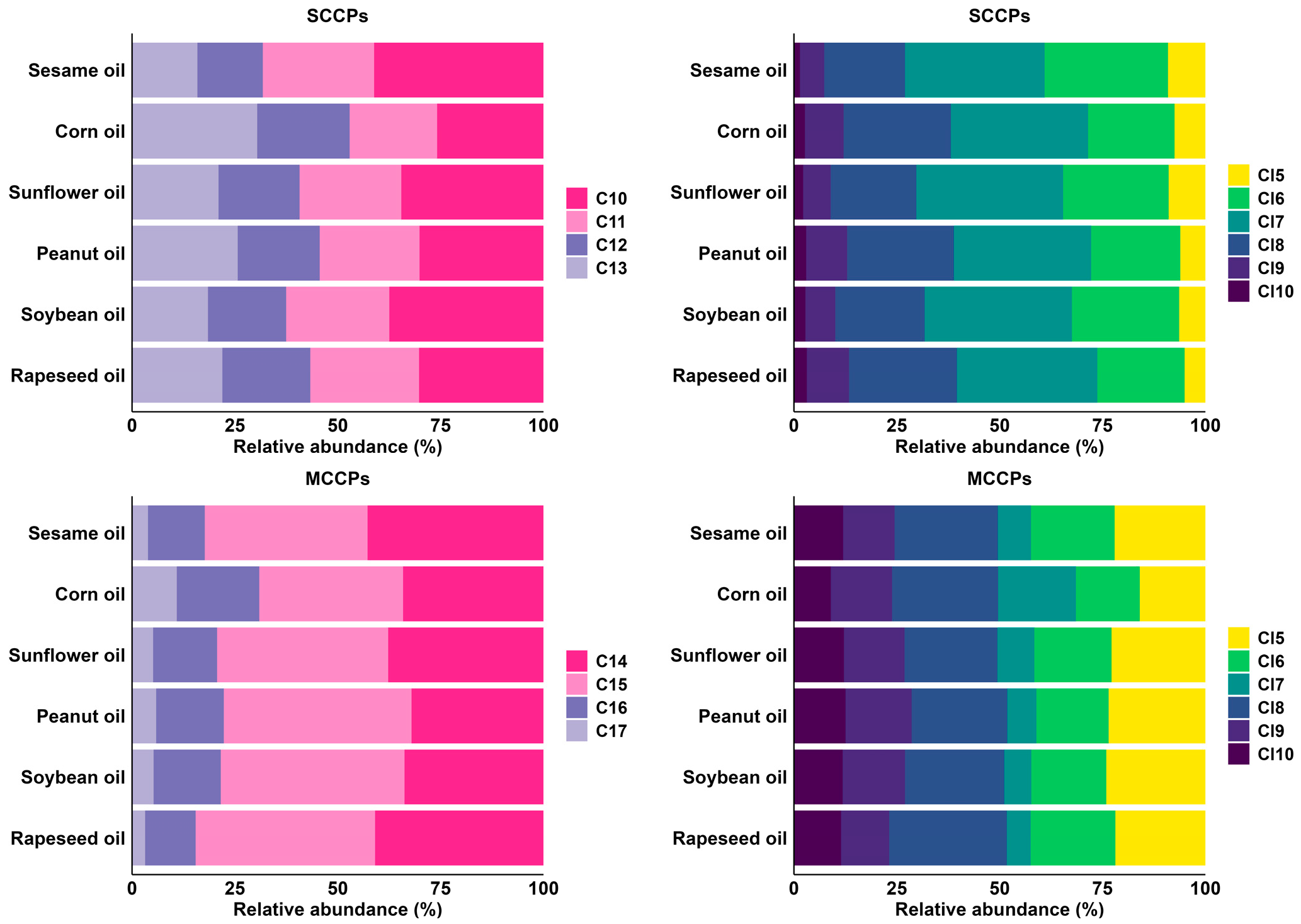
3.2. Congener Group Profiles of SCCPs and MCCPs in Different Vegetable Oils
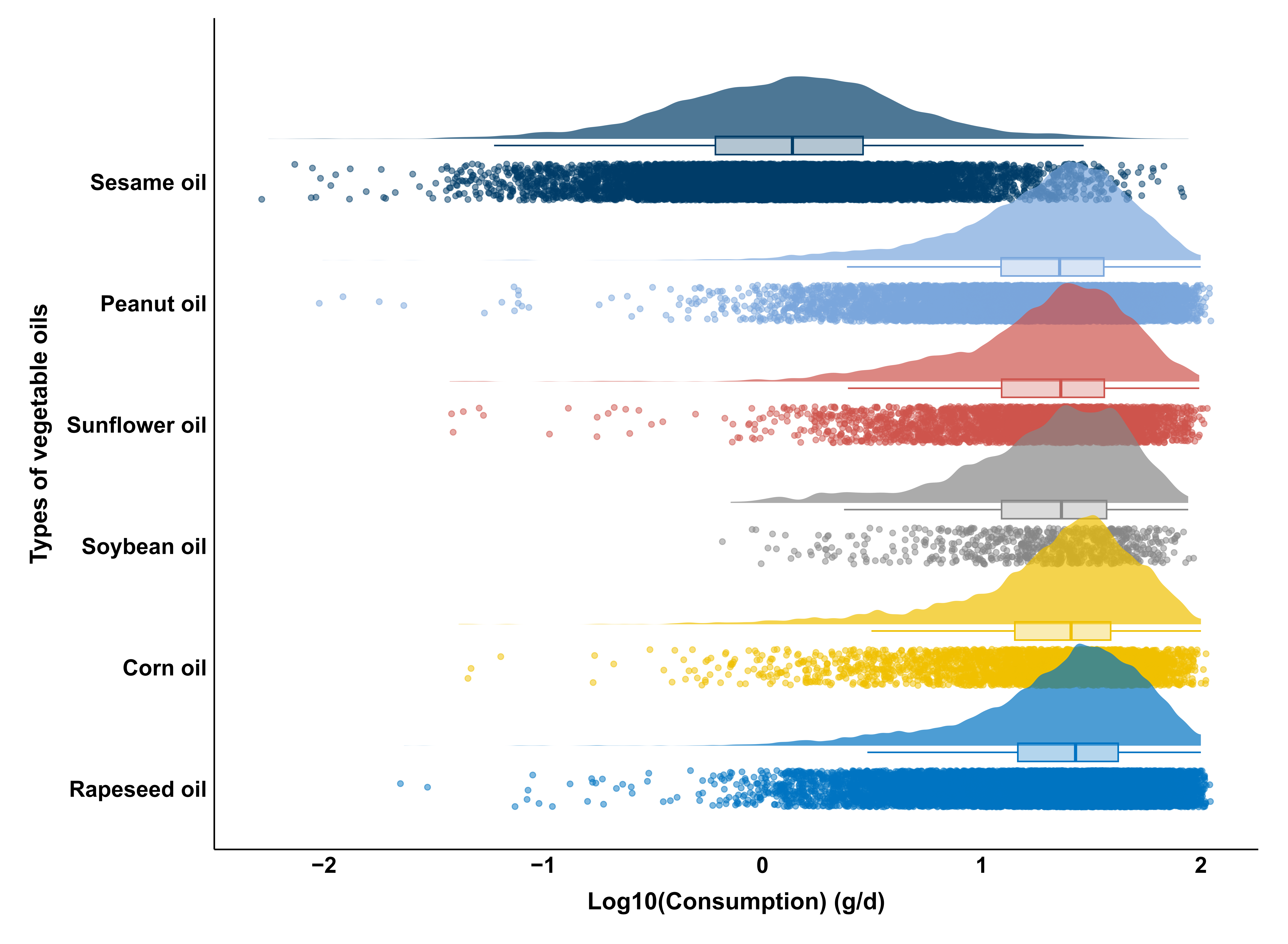
3.3. Vegetable Oil Consumption
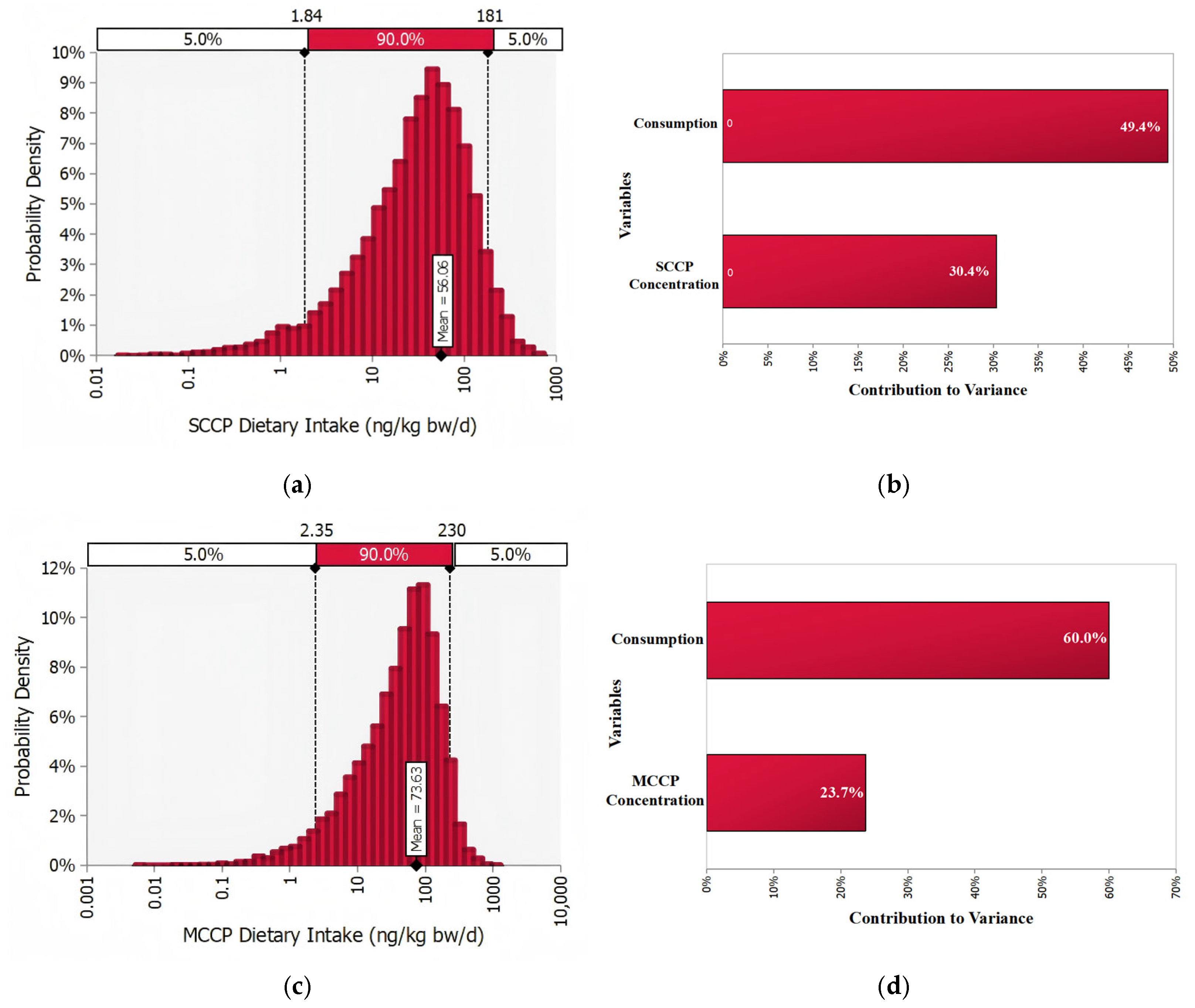
3.4. Probability Assessment of Exposure to SCCPs and MCCPs in Vegetable Oils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brits, M.; de Boer, J.; Rohwer, E.R.; De Vos, J.; Weiss, J.M.; Brandsma, S.H. Short-, medium-, and long-chain chlorinated paraffins in South African indoor dust and cat hair. Chemosphere 2020, 238, 124643. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Wang, Z.; Bogdal, C.; Scheringer, M.; Hungerbühler, K. Global production, use, and emission volumes of short-chain chlorinated paraffins-A minimum scenario. Sci. Total Environ. 2016, 573, 1132–1146. [Google Scholar] [CrossRef]
- van Mourik, L.M.; Gaus, C.; Leonards, P.E.G.; de Boer, J. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 2016, 155, 415–428. [Google Scholar] [CrossRef]
- Liu, Y.; Han, X.; Zhao, N.; Fang, X.; Zhang, S.; Li, S.; Jiang, W.; Ding, L. The association of liver function biomarkers with internal exposure of short- and medium-chain chlorinated paraffins in residents from Jinan, China. Environ. Pollut. 2021, 268 Pt A, 115762. [Google Scholar] [CrossRef]
- Simond, A.E.; Houde, M.; Lesage, V.; Michaud, R.; Verreault, J. Metabolomic profiles of the endangered St. Lawrence Estuary beluga population and associations with organohalogen contaminants. Sci. Total Environ. 2020, 717, 137204. [Google Scholar] [CrossRef]
- Gao, W.; Cao, D.; Wang, Y.; Wu, J.; Wang, Y.; Wang, Y.; Jiang, G. External Exposure to Short- and Medium-Chain Chlorinated Paraffins for the General Population in Beijing, China. Environ. Sci. Technol. 2018, 52, 32–39. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Environment Canada. Chlorinated Paraffins. In Follow-Up Report on a PSL 1 Assessment for Which Data Were Insufficient to Conclude whether the Substances Were “Toxic” to the Environment and to the Human Health; Canadian Environmental Protection Agency: Ottawa, ON, Canada, 2008. [Google Scholar]
- Cao, Y.; Harada, K.H.; Liu, W.; Yan, J.; Zhao, C.; Niisoe, T.; Adachi, A.; Fujii, Y.; Nouda, C.; Takasuga, T.; et al. Short-chain chlorinated paraffins in cooking oil and related products from China. Chemosphere 2015, 138, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choo, G.; Ekpe, O.D.; Kim, J.; Oh, J.E. Short-chain chlorinated paraffins in various foods from Republic of Korea: Levels, congener patterns, and human dietary exposure. Environ. Pollut. 2020, 263 Pt B, 114520. [Google Scholar] [CrossRef]
- Cui, L.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Qiao, L.; Xu, C.; Wang, K.; Huang, D. Short- and Medium-Chain Chlorinated Paraffins in Foods from the Sixth Chinese Total Diet Study: Occurrences and Estimates of Dietary Intakes in South China. J. Agric. Food Chem. 2020, 68, 9043–9051. [Google Scholar] [CrossRef]
- Krätschmer, K.; Schächtele, A.; Vetter, W. Short- and medium-chain chlorinated paraffin exposure in South Germany: A total diet, meal and market basket study. Environ. Pollut. 2021, 272, 116019. [Google Scholar] [CrossRef]
- Nilsson, M.L.; Waldebäck, M.; Liljegren, G.; Kulin, H.; Markides, K.E. Pressurized-fluid extraction (PFE) of chlorinated paraffins from the biodegradable fraction of source-separated household waste. Fresenius J. Anal. Chem. 2001, 370, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, L.; Zhang, Y.; Cao, X.; Qiao, L.; Huang, D.; Liu, Y.; Ai, Q.; Zheng, M. A review on human exposure to chlorinated paraffins. Environ. Chem. 2023, 42, 2139–2152. [Google Scholar]
- Huang, J.W.; Bai, Y.Y.; Zeeshan, M.; Liu, R.Q.; Dong, G.H. Effects of exposure to chlorinated paraffins on human health: A scoping review. Sci. Total Environ. 2023, 886, 163953. [Google Scholar] [CrossRef]
- Gao, W.; Bai, L.; Ke, R.; Cui, Y.; Yang, C.; Wang, Y.; Jiang, G. Distributions and Congener Group Profiles of Short-Chain and Medium-Chain Chlorinated Paraffins in Cooking Oils in Chinese Markets. J. Agric. Food Chem. 2020, 68, 7601–7608. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on Persistent Organic Pollutants (Recast) (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg/2019/1021/oj (accessed on 10 October 2025).
- List of Key Controlled New Pollutants (2023 Edition). Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk02/202212/W020221230613338823204.pdf (accessed on 10 October 2025).
- European Union. Council Decision (EU) 2025/868 of 23 April 2025 on the Position to be Taken on Behalf of the European Union at the Twelfth Meeting of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants as Regards the Requests for Extension of Specific Exemptions and the Proposals For amendment of Annex A to that Convention. Available online: http://data.europa.eu/eli/dec/2025/868/oj (accessed on 10 October 2025).
- Xia, D.; Gao, L.; Zheng, M.; Li, J.; Zhang, L.; Wu, Y.; Tian, Q.; Huang, H.; Qiao, L. Human Exposure to Short- and Medium-Chain Chlorinated Paraffins via Mothers’ Milk in Chinese Urban Population. Environ. Sci. Technol. 2017, 51, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, T.; Pan, F.; Li, J.; Mao, W. Status of cooking oil consumption among Chinese adults aged 18–59 years during 2017–2020. Chin. J. Food Hyg. 2023, 35, 909–914. [Google Scholar] [CrossRef]
- WHO. FAO Principles and Methods for the Risk Assessment of Chemicals in Food; WHO Press: Geneva, Switzerland, 2009; ISBN 978-92-4-157240-8. [Google Scholar]
- EFSA Scientific Committee (SC). Scientific opinion on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 282, 1–31. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L. Risk assessment of chlorinated paraffins in feed and food. EFSA J. 2020, 18, e05991. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, X.; Zhou, Y.; Wu, Y.; Cao, Y.; Yang, J.; Zhang, J. Biomonitoring, exposure routes and risk assessment of chlorinated paraffins in humans: A mini-review. Environ. Sci. Process Impacts 2023, 25, 1588–1603. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gong, Y.; Luo, Y.; Cao, R.; Yang, J.; Cheng, L.; Gao, Y.; Zhang, H.; Chen, J.; Geng, N. Toxic effects and toxicological mechanisms of chlorinated paraffins: A review for insight into species sensitivity and toxicity difference. Environ. Int. 2023, 178, 108020. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, J.; Xue, Z.; Jin, X.; Jin, Y.; Fu, Z. The environmental distribution and toxicity of short-chain chlorinated paraffins and underlying mechanisms: Implications for further toxicological investigation. Sci. Total Environ. 2019, 695, 133834. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, J.; He, Q.; Wang, B.; Xu, X.; Zhao, X.; Gao, M.; Yan, B. Short-chain chlorinated paraffins induce liver injury in mice through mitochondrial disorders and disruption of cholesterol-bile acid pathway. Environ. Pollut. 2025, 364 Pt 1, 125323. [Google Scholar] [CrossRef]
- Wang, R.; He, J.; Gao, L.; Xu, C.; Qiao, L.; Cui, L. Characterization of short- and medium-chain chlorinated paraffins in butter in Beijing. Environ. Chem. 2018, 37, 2473–2480. [Google Scholar]
- Shen, Y.; Krätschmer, K.; Bovee, T.; Louisse, J.; van Leeuwen, S.P. Chlorinated paraffins (CPs) in vegetable oils from the Dutch market and the effects of the refining process on their levels. Food Control 2023, 153, 109889. [Google Scholar] [CrossRef]
- Tomasko, J.; Stupak, M.; Hajslova, J.; Pulkrabova, J. Application of the GC-HRMS based method for monitoring of short- and medium-chain chlorinated paraffins in vegetable oils and fish. Food Chem. 2021, 355, 129640. [Google Scholar] [CrossRef]
- Harada, K.H.; Takasuga, T.; Hitomi, T.; Wang, P.; Matsukami, H.; Koizumi, A. Dietary exposure to short-chain chlorinated paraffins has increased in Beijing, China. Environ. Sci. Technol. 2011, 45, 7019–7027. [Google Scholar] [CrossRef]
- Catalogue of Priority Control Chemicals (1st Batch). Available online: https://www.mee.gov.cn/gkml/hbb/bgg/201712/t20171229_428832.htm (accessed on 10 October 2025).
- Wang, C.; Gao, W.; Liang, Y.; Wang, Y.; Jiang, G. Concentrations and congener profiles of chlorinated paraffins in domestic polymeric products in China. Environ. Pollut. 2018, 238, 326–335. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, S.; Cao, J.; Tian, J.; Zhou, W.; Gao, H.; Dong, S. Chlorinated paraffins in takeout food and its packaging in Beijing, China and dietary exposure risk. Environ. Res. 2024, 252 Pt 1, 118768. [Google Scholar] [CrossRef]
- Tang, S.; Gao, F.; Huang, Z.; Liu, P.; Huang, J. Edible vegetable oil consumption cognition of residents in China and its analysis based on survey data from five provinces. China Oils Fats 2023, 48, 1–5. (in Chinese). [Google Scholar] [CrossRef]
- Li, H.; Gao, S.; Yang, M.; Zhang, F.; Cao, L.; Xie, H.; Chen, X.; Cai, Z. Dietary exposure and risk assessment of short-chain chlorinated paraffins in supermarket fresh products in Jinan, China. Chemosphere 2020, 244, 125393. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Cao, D.; Lv, K.; Wu, J.; Wang, Y.; Wang, C.; Wang, Y.; Jiang, G. Elimination of short-chain chlorinated paraffins in diet after Chinese traditional cooking-a cooking case study. Environ. Int. 2019, 122, 340–345. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.J.; Limonier, F.; Poma, G.; Bombeke, J.; Winand, R.; Vanneste, K.; Andjelkovic, M.; Van Hoeck, E.; Joly, L.; Covaci, A. Concentrations and distribution of chlorinated paraffins in Belgian foods. Environ. Pollut. 2021, 291, 118236. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Wu, N.; Gao, L.; Zhang, L.; Zhou, T.; Cao, P.; Chen, J.; Zhou, P. Occurrence, Homologue Profiles and Risk Assessment of Short- and Medium-Chain Chlorinated Paraffins in Edible Vegetable Oils. Foods 2025, 14, 3988. https://doi.org/10.3390/foods14233988
Lu Y, Wu N, Gao L, Zhang L, Zhou T, Cao P, Chen J, Zhou P. Occurrence, Homologue Profiles and Risk Assessment of Short- and Medium-Chain Chlorinated Paraffins in Edible Vegetable Oils. Foods. 2025; 14(23):3988. https://doi.org/10.3390/foods14233988
Chicago/Turabian StyleLu, Yu, Nan Wu, Lirong Gao, Lei Zhang, Tingting Zhou, Pei Cao, Jinyao Chen, and Pingping Zhou. 2025. "Occurrence, Homologue Profiles and Risk Assessment of Short- and Medium-Chain Chlorinated Paraffins in Edible Vegetable Oils" Foods 14, no. 23: 3988. https://doi.org/10.3390/foods14233988
APA StyleLu, Y., Wu, N., Gao, L., Zhang, L., Zhou, T., Cao, P., Chen, J., & Zhou, P. (2025). Occurrence, Homologue Profiles and Risk Assessment of Short- and Medium-Chain Chlorinated Paraffins in Edible Vegetable Oils. Foods, 14(23), 3988. https://doi.org/10.3390/foods14233988





