Gold Nanoparticles as a Possible Tool to Untangle Some Structural Features of the Gluten Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thiol Labeling
2.3. SDS-PAGE
2.4. Trypsin Hydrolysis of AuNP-Bound Proteins
2.5. High-Resolution LC-MS/MS Analysis
2.6. LC-MS/MS Data Acquisition
2.7. Bioinformatic Analysis
2.8. Statistical Analysis
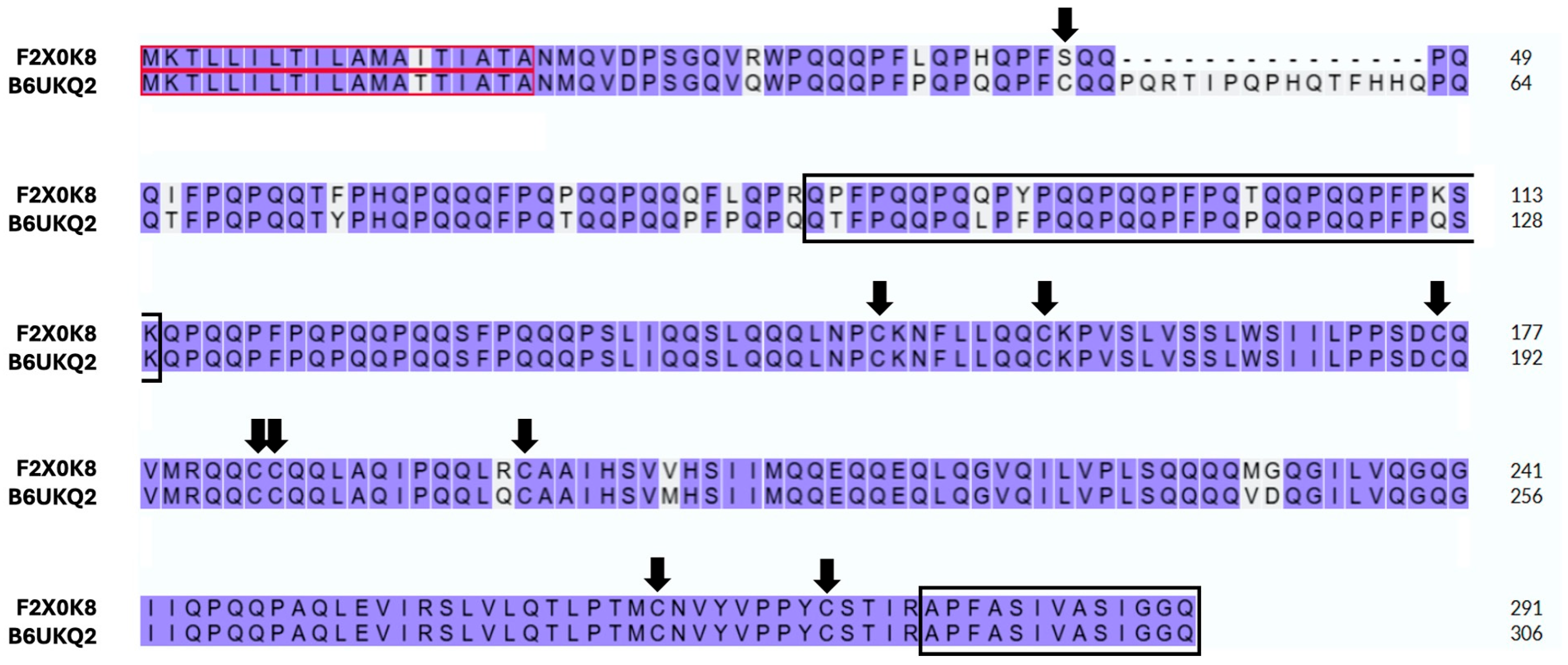
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-ME | 2-mercaptoethanol |
| ACN | Acetonitrile |
| AGC | Automatic gain control |
| AmBic | Ammonium bicarbonate |
| AuNP | Thiol-reactive gold nanoparticles |
| db | Dry basis |
| DTNB | Ellman’s reagent, 5,5′-dithiobis-2-nitrobenzoic acid |
| DTT | DL-Dithiothreitol |
| FA | Formic acid |
| FWHM | Full width at half maximum |
| HGL | High molecular weight gliadins |
| HMW | High molecular weight |
| IAF | 5-iodoacetamido fluorescein |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| LMW | Low molecular weight |
| NP | Nanoparticles |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TRIDC | Triticum turgidum subsp. dicoccoides |
| TRITD | Triticum turgidum subsp. durum |
| TRITI | Triticum dicoccon var. timopheevii |
| TRIUA | Triticum Urartu |
| UHPLC | Ultra-high-performance liquid chromatography |
| WHEAT | Triticum aestivum |
References
- Shewry, P. What is gluten—Why is it special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, E.; Fontaine, J.; Grua-Priol, J.; Assaf, A.; Thouand, G.; Le-Bail, A. Study of structural changes of gluten proteins during bread dough mixing by Raman spectroscopy. Food Chem. 2021, 358, 129916. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chemistry of wheat gluten proteins: Qualitative composition. Cereal Chem. 2023, 100, 23–35. [Google Scholar] [CrossRef]
- Belton, P.S. On the elasticity of wheat gluten. J. Cereal Sci. 1999, 29, 103–107. [Google Scholar] [CrossRef]
- Singh, H.; MacRitchie, F. Application of polymer science to properties of gluten. J. Cereal Sci. 2001, 33, 231–243. [Google Scholar] [CrossRef]
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Phil. Trans. R. Soc. B Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef]
- Veraverbeke, W.S.; Delcour, J.A. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. Crit. Rev. Food Sci. Nutr. 2002, 42, 179–208. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Gobaa, S.; Bancel, E.; Branlard, G.; Kleijer, G.; Stamp, P. Proteomic analysis of wheat recombinant inbred lines: Variations in prolamin and dough rheology. J. Cereal Sci. 2008, 47, 610–619. [Google Scholar] [CrossRef]
- Shewry, P.R.; Belton, P.S. What do we really understand about wheat gluten structure and functionality? J. Cereal Sci. 2024, 117, 103895. [Google Scholar] [CrossRef]
- Bonomi, F.; D’Egidio, M.G.; Iametti, S.; Marengo, M.; Marti, A.; Pagani, M.A.; Ragg, E.M. Structure-quality relationship in commercial pasta: A molecular glimpse. Food Chem. 2012, 135, 348–355. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effects of Physical and Chemical Factors on the Structure of Gluten, Gliadins and Glutenins as Studied with Spectroscopic Methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, F.; Mora, G.; Pagani, M.A.; Iametti, S. Probing structural features of water-insoluble proteins by front-face fluorescence. Anal. Biochem. 2004, 329, 104–111. [Google Scholar] [CrossRef]
- Quayson, E.T.; Marti, A.; Morris, C.F.; Marengo, M.; Bonomi, F.; Seetharaman, K.; Iametti, S. Structural consequences of the interaction of puroindolines with gluten proteins. Food Chem. 2018, 253, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K. Gluten Proteins. In ICC Handbook of 21st Century Cereal Science and Technology; Shewry, P.R., Koksel, H., Taylor, J.R.N., Eds.; Elsevier Academic Press: London, UK, 2023; pp. 71–78. [Google Scholar]
- Zang, P.; Gao, Y.; Chen, P.; Lv, C.; Zhao, G. Recent Advances in the Study of Wheat Protein and Other Food Components Affecting the Gluten Network and the Properties of Noodles. Foods 2022, 11, 3824. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, F.; Ferranti, P.; Mamone, G. Wheat Flour: Chemistry and Biochemistry. In Bakery Products Science and Technology: Second Edition; Wiley: Hoboken, NJ, USA, 2014; pp. 55–74. [Google Scholar] [CrossRef]
- Caramanico, R.; Barbiroli, A.; Marengo, M.; Fessas, D.; Bonomi, F.; Lucisano, M.; Pagani, M.A.; Iametti, S.; Marti, A. Interplay between starch and proteins in waxy wheat. J. Cereal Sci. 2017, 75, 198–204. [Google Scholar] [CrossRef]
- Emide, D.; Magni, C.; Saitta, F.; Cardone, G.; Botticella, E.; Fessas, D.; Iametti, S.; Lafiandra, D.; Sestili, F.; Marti, A.; et al. Molecular insights into the role of amylose/amylopectin ratio on gluten protein organization. Food Chem. 2023, 404, 134675. [Google Scholar] [CrossRef]
- Magni, C.; Emide, D.; Iametti, S.; Barbiroli, A. A Thiolomic Approach to Map Free Thiol Distribution in Food Proteins. In Proteomics Applied to Foods. Methods and Protocols in Food Science; Ferranti, P., Ed.; Humana: New York, NY, USA, 2024; pp. 73–85. [Google Scholar] [CrossRef]
- Marengo, M.; Mamone, G.; Ferranti, P.; Polito, L.; Iametti, S.; Bonomi, F. Topological features of the intermolecular contacts in gluten-forming proteins: Exploring a novel methodological approach based on gold nanoparticles. Food Res. Int. 2019, 119, 492–498. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Balasubramanian, S.K.; Yang, L.; Yung, L.Y.L.; Ong, C.N.; Ong, W.Y.; Yu, L.E. Characterization, Purification, and Stability of Gold Nanoparticles. Biomaterials 2010, 31, 9023–9030. [Google Scholar] [CrossRef]
- International Association for Cereal Science and Technology (ICC). Method No. 158: Gluten Index Method for Assessing Gluten Strength in Durum Wheat (Triticum durum); ICC: Vienna, Austria, 1995. [Google Scholar]
- Compostella, F.; Pitirollo, O.; Silvestri, A.; Polito, L. Glyco-gold nanoparticles: Synthesis and applications. Beilstein J. Org. Chem. 2017, 13, 1008–1021. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction Coefficient of Gold Nanoparticles with Different Sizes and Different Capping Ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Iametti, S.; Marengo, M.; Miriani, M.; Pagani, M.A.; Marti, A.; Bonomi, F. Integrating the information from proteomic approaches: A “thiolomics” approach to assess the role of thiols in protein-based networks. Food Res. Int. 2013, 54, 980–987. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Jimenez-Ruiz, A.; Perez-Tejeda, P.; Grueso, E.; Castillo, P.M.; Prado-Gotor, R. Non-functionalized gold nanoparticles: Synthetic routes and synthesis condition dependence. Chem. Eur. J. 2015, 21, 9596–9609. [Google Scholar] [CrossRef]
- Iwaki, S.; Aono, S.; Hayakawa, K.; Fu, B.X.; Otobe, C. Changes in Protein Non-Covalent Bonds and Aggregate Size during Dough Formation. Foods 2020, 9, 1643. [Google Scholar] [CrossRef]
- Kuktaite, R.; Larsson, H.; Johansson, E. Variation in Protein Composition of Wheat Flour and Its Relationship to Dough Mixing Behavior. J. Cereal Sci. 2004, 40, 31–39. [Google Scholar] [CrossRef]
- Delcour, J.A.; Joye, I.J.; Pareyt, B.; Wilderjans, E.; Brijs, K.; Lagrain, B. Wheat Gluten Functionality as a Quality Determinant in Cereal-Based Food Products. Annu. Rev. Food Sci. Technol. 2012, 3, 469–492. [Google Scholar] [CrossRef]
- Schmid, M.; Wieser, H.; Koehler, P. Isolation and characterization of High-Molecular-Weight (HMW) gliadins from wheat flour. Cereal Chem. 2016, 93, 536–542. [Google Scholar] [CrossRef]
- Schmid, M.; Wieser, H.; Koehler, P. Disulphide structure of high-molecular-weight (HMW-) gliadins as affected by terminators. J. Cereal Sci. 2017, 78, 66–74. [Google Scholar] [CrossRef]
- Ferrante, P.; Masci, S.; D’Ovidio, R.; Lafiandra, D.; Volpi, C.; Mattei, B. A Proteomic approach to verify in vivo expression of a novel γ-gliadin containing an extra cysteine residue. Proteomics 2006, 6, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, P.; Gianibelli, M.C.; Larroque, O.; Lafiandra, D.; Masci, S. Considerations about the effect of incorporation of two rare LMW-GS in durum wheat in comparison to bread wheat doughs. Options Mediterr. 2008, 81, 119–122. [Google Scholar]
- Lutz, E.; Wieser, H.; Koehler, P. Identification of disulfide bonds in wheat gluten proteins by means of Mass Spectrometry/Electron Transfer Dissociation. J. Agric. Food Chem. 2012, 60, 3708–3716. [Google Scholar] [CrossRef]
- Iwaki, S.; Hayakawa, K.; Fu, B.-X.; Otobe, C. Changes in Hydrophobic Interactions among Gluten Proteins during Dough Formation. Processes 2021, 9, 1244. [Google Scholar] [CrossRef]
- Aussenac, T.; Carceller, J.L.; Kleiber, D. Changes in SDS Solubility of Gluten Polymers during Dough Mixing and Resting. Cereal Chem. 2001, 78, 39–45. [Google Scholar] [CrossRef]
- Peng, P.; Wang, X.; Zou, X.; Zhang, X.; Hu, X. Dynamic Behaviors of Protein and Starch and Interactions Associated with Glutenin Composition in Wheat Dough Matrices during Sequential Thermo-Mechanical Treatments. Food Res. Int. 2022, 154, 110986. [Google Scholar] [CrossRef]
- Abedi, E.; Pourmohammadi, K. Chemical Modifications and Their Effects on Gluten Protein: An Extensive Review. Food Chem. 2021, 343, 128398. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef] [PubMed]
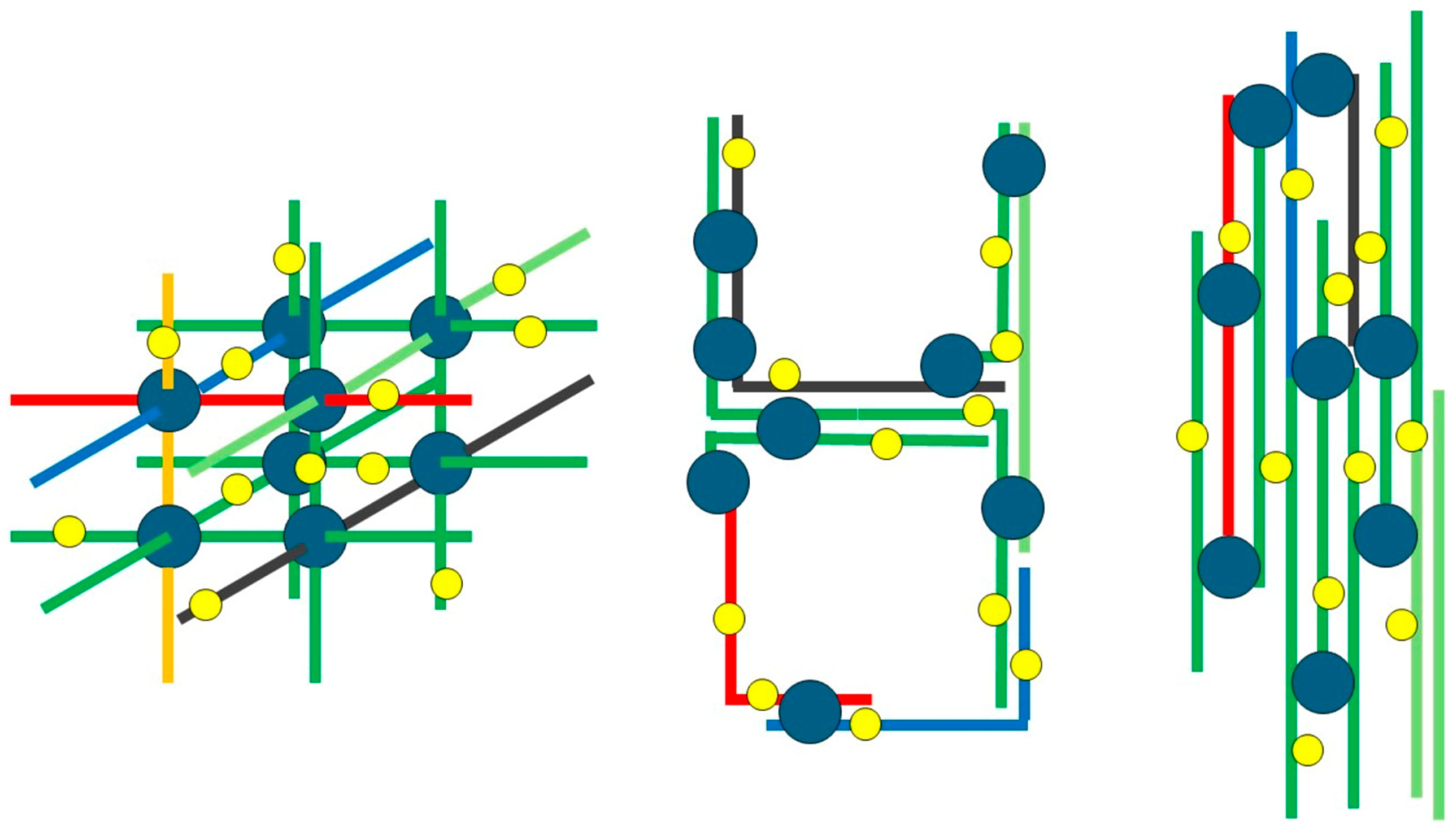
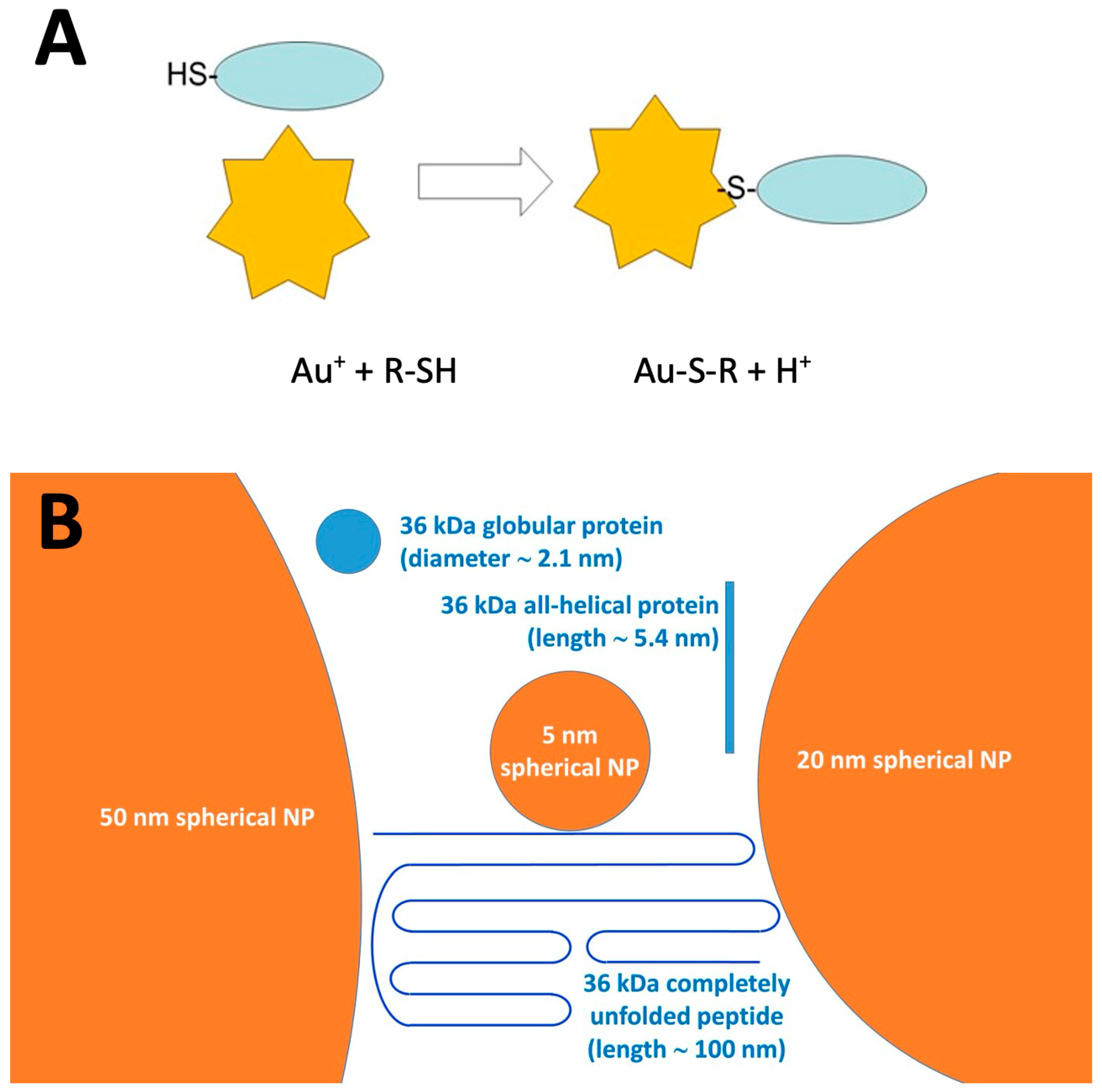
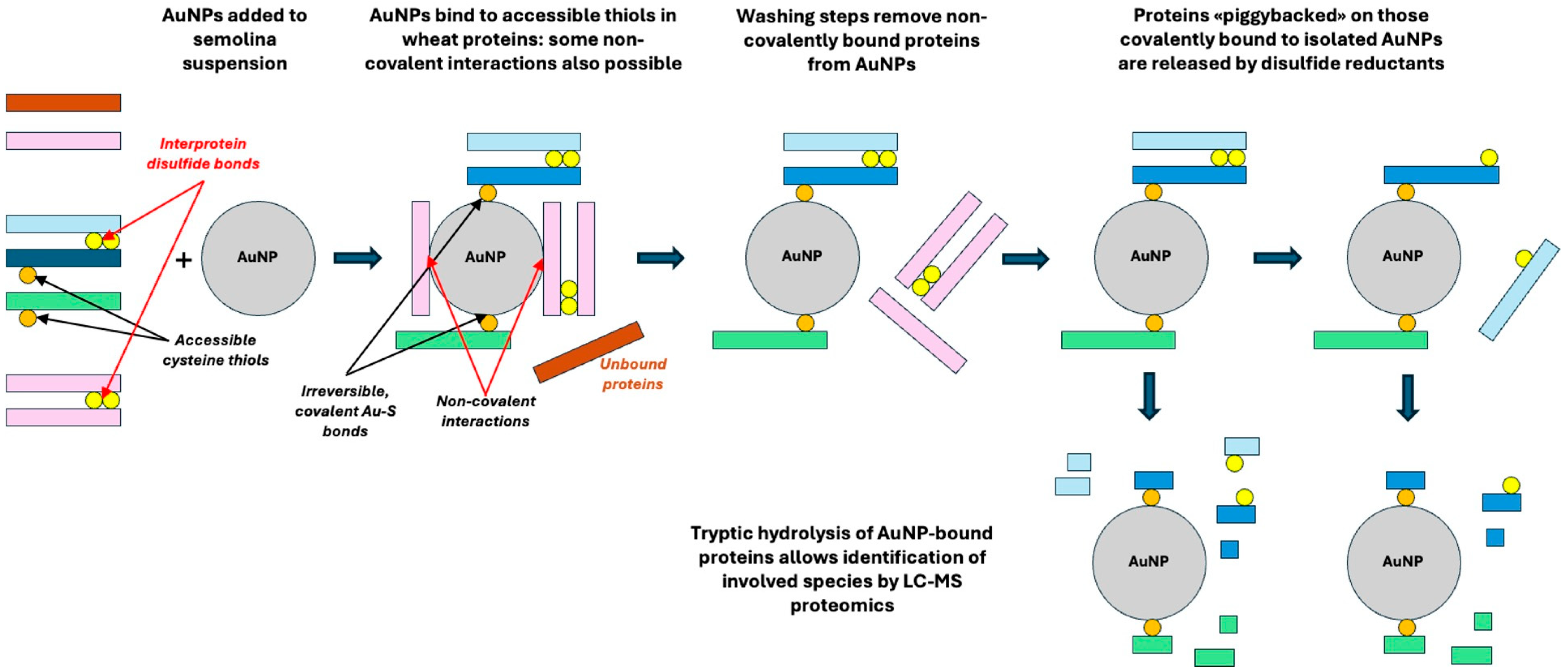
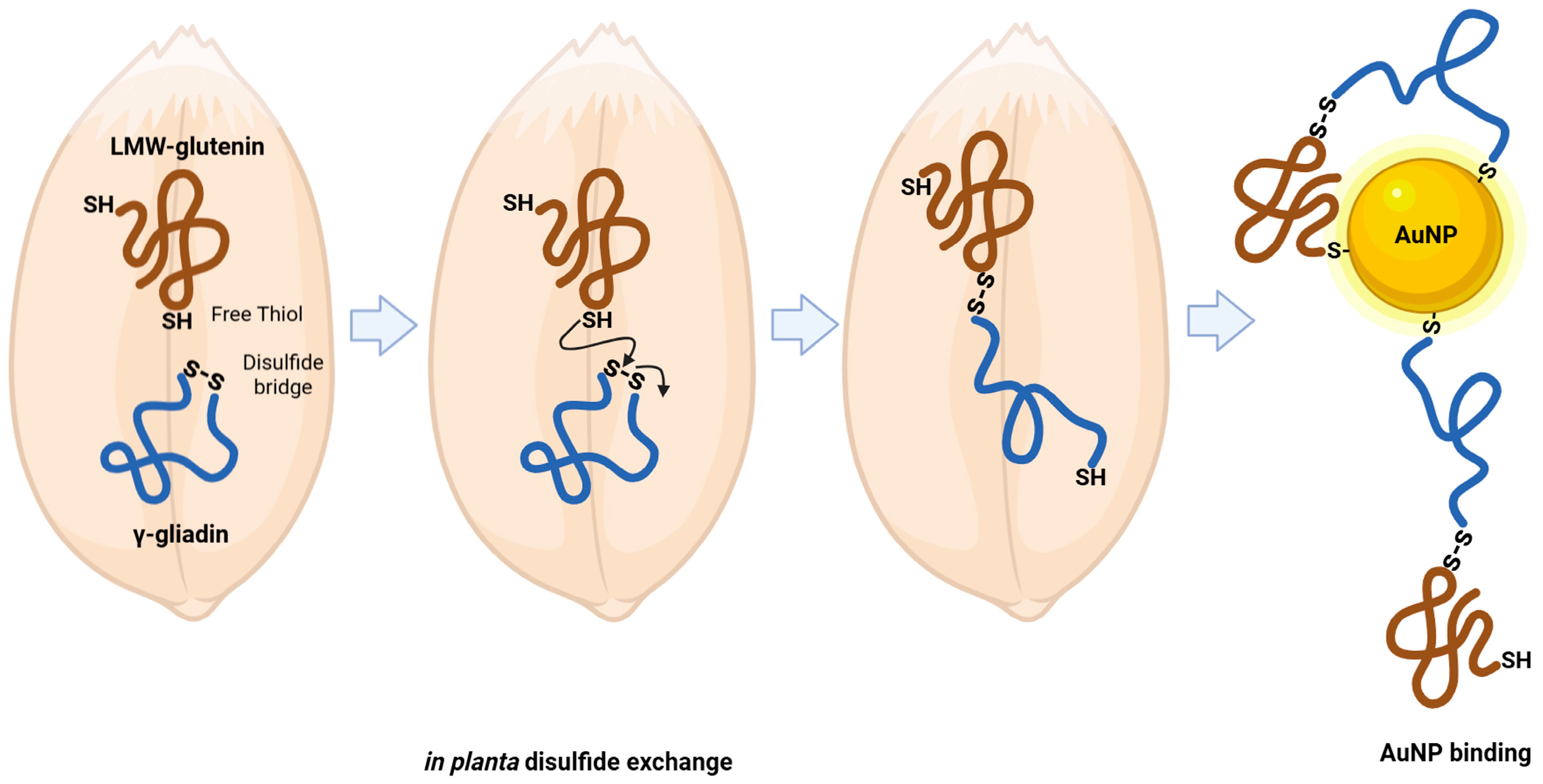
| Protein Family | Individual Proteins | Free Cysteine Thiols | Thiols Involved in Disulfide Bonds | ||||
|---|---|---|---|---|---|---|---|
| N- Terminus | Repeat Region | C- Terminus | N- Terminus | Repeat Region | C- Terminus | ||
| Gliadins | Alpha and beta | none | none | none | none | none | 6 * |
| Gamma | none | none | none | none | none | 8 * | |
| Omega | none | none | none | none | none | none | |
| HMW Glutenins | x-type | none | none | none | 3 ** | none | 1 ** |
| Dx5 | none | none | none | 3 ** | 1 ** | 1 ** | |
| y-type | none | none | none | 5 ** | 1 ** | 1 ** | |
| LMW Glutenins | s- and m-type | 1 | none | 1 | none | none | 6 * |
| i-type | none | none | 2 | none | none | 6 ** | |
| Buffer Only | Buffer + 1% SDS | ||||||
|---|---|---|---|---|---|---|---|
| UniProt ID | Mass | Origin | Protein Name | UniProt ID | Mass | Origin | Protein Name |
| Prolamin family | |||||||
| A7LHB5 | 33,068.8 | WHEAT | Alpha-gliadin | ||||
| M7ZZV2 | 23,557.9 | TRIUA | Alpha/beta-gliadin MM1 | ||||
| H6VLP7 | 32,301.8 | WHEAT | Alpha-gliadin | ||||
| F2X0K8 | 33,083.4 | WHEAT | Gamma-gliadin | F2X0K8 | 33,083.4 | WHEAT | Gamma-gliadin |
| A0A2U8JD23 | 41,892.9 | WHEAT | Gamma-gliadin B4 | ||||
| R9XWC4 | 37,468.1 | WHEAT | Gamma-gliadin | ||||
| A7LHB4 | 33,014.7 | WHEAT | Alpha-gliadin | ||||
| P04726 | 33,941.6 | WHEAT | Alpha/beta-gliadin clone PW1215 | ||||
| A0A023WHQ9 | 32,897.4 | WHEAT | Alpha-gliadin | ||||
| R9XSV1 | 32,807.4 | WHEAT | Alpha-gliadin | ||||
| R9XU90 | 36,002.7 | WHEAT | Alpha-gliadin | ||||
| R9XUS6 | 40,793.6 | WHEAT | Gamma-gliadin | ||||
| Q0GK30 | 32,803.5 | TRITI | Prolamin | Q0GK30 | 32,803.5 | TRITI | Prolamin |
| R4JB53 | 37,803.1 | WHEAT | Low-molecular-weight glutenin subunit | ||||
| Q42451 | 84,637.6 | WHEAT | Glu-B1-1b HMW glutenin subunit | ||||
| A0A1D8V7C6 | 61,970.4 | WHEAT | High molecular weight glutenin subunit | ||||
| A0A142ESP2 | 66,181 | TRIDC | High molecular weight glutenin subunit 1Ay protein | ||||
| P08453 | 37,122.6 | WHEAT | Gamma-gliadin | ||||
| Q9M4L9 | 31,490.7 | WHEAT | Alpha-gliadin | ||||
| LMW-Glutenin family | |||||||
| A2IBV5 | 40,435.1 | WHEAT | Glutenin subunit | A2IBV5 | 40,435.1 | WHEAT | Glutenin subunit |
| B2BZC7 | 32,721.7 | WHEAT | LMW-m glutenin subunit 0154A5-M | ||||
| Q9FEQ2 | 44,567.1 | TRITD | Low molecular weight glutenin subunit (Fragment) | ||||
| A0A0S2GJR1 | 39,893.7 | WHEAT | Low-molecular-weight glutenin subunit | ||||
| Q0GQX1 | 33,978.8 | WHEAT | Low molecular weight glutenin subunit (Fragment) | ||||
| O49958 | 39,791.5 | TRITD | Low molecular weight glutenin subunit (Fragment) | ||||
| HMW-Glutenin family | |||||||
| A0A2L1K3K6 | 73,890.6 | TRITD | High molecular weight glutenin (Fragment) | A0A2L1K3K6 | 73,890.6 | TRITD | High molecular weight glutenin (Fragment) |
| A0A2P1E384 | 66,122.9 | TRIDC | High molecular weight glutenin subunit 1Ay | ||||
| Q8RVX0 | 86,038.3 | TRITD | High molecular weight glutenin subunit 1Bx20 (Fragment) | ||||
| A0A0K0KDM6 | 85,285.7 | WHEAT | High molecular weight glutenin subunit 1Dy3 | ||||
| A0A142BX75 | 10,150.8 | TRITD | High-molecular-weight glutenin subunit (Fragment) | ||||
| Q41553 | 88,476.2 | WHEAT | HMW glutenin subunit Ax2 | Q41553 | 88,476.2 | WHEAT | HMW glutenin subunit Ax2 |
| A3RF26 | 32,042.5 | WHEAT | Truncated high molecular weight glutenin subunit 1By9 | ||||
| A0A1D8V7C6 | 61,970.4 | WHEAT | High molecular weight glutenin subunit | ||||
| Q03872 | 89,819.5 | WHEAT | High molecular weight glutenin subunit 1Ax1 | ||||
| Q18MZ6 | 80,069.8 | WHEAT | High-molecular-weight glutenin subunit Bx17 | ||||
| Q0Q5D8 | 77,315.6 | WHEAT | High-molecular-weight glutenin By8 | ||||
| UniProt ID | Peptide 1 | Peptide 2 | Protein Name |
|---|---|---|---|
| A0A2L1K3K6 | C(+Disulfide)RPVAVSQVVR | Q(Gln->pyro-Glu)LQC(+Disulfide)ER | High molecular weight glutenin (Fragment) |
| A0A2L1K3K6 | C(+Disulfide)RPVAVSQVVR | QLQC(+Disulfide)ER | High molecular weight glutenin (Fragment) |
| A0A2P1E384 | C(+Disulfide)RPVALSQVAR | QLQC(+Disulfide)ER | High molecular weight glutenin subunit 1Ay |
| Q9FEQ2 | VFLQQQC(+Disulfide)IPVAMQR | SQMLQQSIC(+Disulfide)HVMQR | Low molecular weight glutenin subunit (Fragment) |
| A0A0S2GJR1 | TLPTMC(+Disulfide)SVNVPVYGTTTIVPFGVGTR | VFLQQQ(Deamidated)C(+Disulfide)SPVAMPQSLAR | Low-molecular-weight glutenin subunit |
| NP-Bound Gluten Proteins After EtOH Wash | NP-Bound Gluten Proteins After EtOH/DTT Wash | ||||||
|---|---|---|---|---|---|---|---|
| UniProt ID | Mass | Origin | Protein Name | UniProt ID | Mass | Origin | Protein Name |
| Prolamin family | |||||||
| A7LHB5 | 33,068.8 | WHEAT | Alpha-gliadin | ||||
| M7ZZV2 | 23,557.9 | TRIUA | Alpha/beta-gliadin MM1 | ||||
| H6VLP7 | 32,301.8 | WHEAT | Alpha-gliadin | ||||
| F2X0K8 | 33,083.4 | WHEAT | Gamma-gliadin | F2X0K8 | 33,083.4 | WHEAT | Gamma-gliadin |
| A0A2U8JD23 | 41,892.9 | WHEAT | Gamma-gliadin B4 | ||||
| R9XWC4 | 37,468.1 | WHEAT | Gamma-gliadin | ||||
| A7LHB4 | 33,014.7 | WHEAT | Alpha-gliadin | ||||
| P04726 | 33,941.6 | WHEAT | Alpha/beta-gliadin clone PW1215 | ||||
| A0A023WHQ9 | 32,897.4 | WHEAT | Alpha-gliadin | ||||
| R9XSV1 | 32,807.4 | WHEAT | Alpha-gliadin | ||||
| R9XU90 | 36,002.7 | WHEAT | Alpha-gliadin | ||||
| R9XUS6 | 40,793.6 | WHEAT | Gamma-gliadin | ||||
| Q0GK30 | 32,803.5 | TRITI | Prolamin | ||||
| LMW-Glutenin family | |||||||
| P10385 | 41,020.4 | WHEAT | Glutenin, low molecular weight subunit | ||||
| O49958 | 39,791.5 | TRITD | Low molecular weight glutenin subunit (Fragment) | ||||
| R4JB53 | 37,803.1 | WHEAT | Low-molecular-weight glutenin subunit | ||||
| A2IBV5 | 40,435.1 | WHEAT | Glutenin subunit | A2IBV5 | 40,435.1 | WHEAT | Glutenin subunit |
| B2BZC7 | 32,721.7 | WHEAT | LMW-m glutenin subunit 0154A5-M | ||||
| Q9FEQ2 | 44,567.1 | TRITD | Low molecular weight glutenin subunit (Fragment) | ||||
| A0A0S2GJR1 | 39,893.7 | WHEAT | Low-molecular-weight glutenin subunit | ||||
| HMW-Glutenin family | |||||||
| Q03872 | 89,819.5 | WHEAT | High molecular weight glutenin subunit 1Ax1 | ||||
| Q03871 | 75,701.9 | WHEAT | HMW glutenin subunit 1By9 | ||||
| Q0Q5D8 | 77,315.6 | WHEAT | High-molecular-weight glutenin By8 | ||||
| A0A1D8V7C6 | 61,970.4 | WHEAT | High molecular weight glutenin subunit | ||||
| A0A142ESP2 | 66,181 | TRIDC | High molecular weight glutenin subunit 1Ay protein | A0A142ESP2 | 66,181 | TRIDC | High molecular weight glutenin subunit 1Ay protein |
| Q42451 | 84,637.6 | WHEAT | Glu-B1-1b HMW glutenin subunit | ||||
| A0A2L1K3K6 | 73,890.6 | TRITD | High molecular weight glutenin (Fragment) | ||||
| A0A2P1E384 | 66,122.9 | TRIDC | High molecular weight glutenin subunit 1Ay | ||||
| Q8RVX0 | 86,038.3 | TRITD | High molecular weight glutenin subunit 1Bx20 (Fragment) | Q8RVX0 | 86,038.3 | TRITD | High molecular weight glutenin subunit 1Bx20 (Fragment) |
| A0A0K0KDM6 | 85,285.7 | WHEAT | High molecular weight glutenin subunit 1Dy3 | ||||
| A0A142BX75 | 10,150.8 | TRITD | High-molecular-weight glutenin subunit (Fragment) | ||||
| Q41553 | 88,476.2 | WHEAT | HMW glutenin subunit Ax2 | ||||
| A3RF26 | 32,042.5 | WHEAT | Truncated high molecular weight glutenin subunit 1By9 | A3RF26 | 32,042.5 | WHEAT | Truncated high molecular weight glutenin subunit 1By9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emide, D.; D’Auria, G.; Iametti, S.; Barbiroli, A.; Marengo, M.; Mamone, G.; Ferranti, P.; Bonomi, F. Gold Nanoparticles as a Possible Tool to Untangle Some Structural Features of the Gluten Network. Foods 2025, 14, 3985. https://doi.org/10.3390/foods14233985
Emide D, D’Auria G, Iametti S, Barbiroli A, Marengo M, Mamone G, Ferranti P, Bonomi F. Gold Nanoparticles as a Possible Tool to Untangle Some Structural Features of the Gluten Network. Foods. 2025; 14(23):3985. https://doi.org/10.3390/foods14233985
Chicago/Turabian StyleEmide, Davide, Giovanni D’Auria, Stefania Iametti, Alberto Barbiroli, Mauro Marengo, Gianfranco Mamone, Pasquale Ferranti, and Francesco Bonomi. 2025. "Gold Nanoparticles as a Possible Tool to Untangle Some Structural Features of the Gluten Network" Foods 14, no. 23: 3985. https://doi.org/10.3390/foods14233985
APA StyleEmide, D., D’Auria, G., Iametti, S., Barbiroli, A., Marengo, M., Mamone, G., Ferranti, P., & Bonomi, F. (2025). Gold Nanoparticles as a Possible Tool to Untangle Some Structural Features of the Gluten Network. Foods, 14(23), 3985. https://doi.org/10.3390/foods14233985






