Chicken Liver from Broilers Fed Wheat Germ Expeller: A Source of Minerals and Energy in the Human Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Proximate Composition Analysis of Feed and Livers
2.4. Analysis of Mineral Content in Feed and Livers
2.5. Statistical Analysis
3. Results and Discussion
3.1. Growth Performance
3.2. Weight and Proximate Composition of Livers
3.3. Macroelements
3.4. Microelements
3.5. Risk Assessment Calculation
3.6. Principal Components Analysis
3.7. Broiler Liver and Nutrient Reference Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOAC | Association of Official Analysis Chemists |
| BW | Body Weight |
| CT | Control Treatment |
| EBP | Edible By-Products |
| EDI | Estimated Daily Intake |
| EX | Experimental diet |
| FAO | Food and Agriculture Organization of the United Nations |
| FAAS | Flame Atomic Absorption Spectrometry |
| FWGE | Fermented Wheat Germ Extract |
| HQ | Hazard Quotient |
| NRV | NRV |
| PCA | Principal Component Analysis |
| RfD | Reference Dose |
| THQ | Total Hazard Quotient |
| USDA | United States Department of Agriculture |
| WEG | Wheat Germ Expeller |
| WHO | World Health Organization |
| WWB | Wet Weight Basis |
References
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian Liver: The Forgotten Organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, F.A. The Availability, the Price, the Tradition, the Religion, the Income, the Social, the Development and the Economic Influences on the Meat Consumption. Biomed. J. Sci. Tech. Res. 2024, 55, 47203–47212. [Google Scholar] [CrossRef]
- Statistics Poland. Statistical Yearbook of the Republic of Poland; Statistics Poland: Warsaw, Poland, 2024.
- Qu, Z.; Tang, J.; Sablani, S.S.; Ross, C.F.; Sankaran, S.; Shah, D.H. Quality Changes in Chicken Livers during Cooking. Poult. Sci. 2021, 100, 101316. [Google Scholar] [CrossRef] [PubMed]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Gujska, E. Effect of Different Cooking Methods on Folate Content in Chicken Liver. Foods 2020, 9, 1431. [Google Scholar] [CrossRef]
- Latoch, A.; Stasiak, D.M.; Siczek, P. Edible Offal as a Valuable Source of Nutrients in the Diet—A Review. Nutrients 2024, 16, 1609. [Google Scholar] [CrossRef]
- Alao, B.; Falowo, A.; Chulayo, A.; Muchenje, V. The Potential of Animal By-Products in Food Systems: Production, Prospects and Challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- European Food Safety Authority. EFSA Strategy 2027: Science, Safe Food, Sustainability: Updated Edition; Publications Office: Luxembourg, 2025. [Google Scholar]
- United Nations The Sustainable Development Goals Report 2022; Social and United Nations Department of Economic: New York, NY, USA, 2022; ISBN 978-92-1-047887-8.
- Bearth, A.; Khunnutchanart, K.; Gasser, O.; Hasler, N. The Whole Beast: Consumers’ Perceptions of and Willingness-to-Eat Animal by-Products. Food Qual. Prefer. 2021, 89, 104144. [Google Scholar] [CrossRef]
- Henchion, M.; McCarthy, M. Facilitators and Barriers for Foods Containing Meat Coproducts. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 237–250. ISBN 978-0-12-814874-7. [Google Scholar]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A Review on the Use of Agro-Industrial CO-Products in Animals’ Diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Sugiharto, S.; Nuengjamnong, C. An Update Review on the Use of Agro-Industrial Byproducts on Carcass and Meat Quality of Broiler Chickens. Discov. Food 2025, 5, 203. [Google Scholar] [CrossRef]
- European Union (EU). Farm to Fork Strategy—For a Fair, Healthy and Environmentally-Friendly FoodSystem; European Union (EU): Brusel, Belgium, 2020; pp. 1–23. [Google Scholar]
- Liu, W.R.; Zeng, D.; She, L.; Su, W.X.; He, D.C.; Wu, G.Y.; Ma, X.R.; Jiang, S.; Jiang, C.H.; Ying, G.G. Comparisons of Pollution Characteristics, Emission Situations, and Mass Loads for Heavy Metals in the Manures of Different Livestock and Poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef]
- Volpato, J.A.; Ribeiro, L.B.; Torezan, G.B.; Da Silva, I.C.; Martins, I.D.O.; Genova, J.L.; De Oliveira, N.T.E.; Carvalho, S.T.; Carvalho, P.L.D.O.; Vasconcellos, R.S. Characterization of the Variations in the Industrial Processing and Nutritional Variables of Poultry By-Product Meal. Poult. Sci. 2022, 101, 101926. [Google Scholar] [CrossRef]
- Hill, G.; Sayadi, A.; Gendreau, J.D.; Tobar, Z.; Liu, Y.; Pitesky, M.E.; Simmons, C.W. Assessment of the Variation in Nutritional Composition and Safety of Dried Recovered Food from United States Households and Prospects for Use in Chicken Feed. Front. Sustain. Food Syst. 2023, 7, 1180249. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 68/2013 of 16 January 2013 on the Catalogue of Feed Materials. Off. J. Eur. Union 2013, 29, 1–64. [Google Scholar]
- Ge, Y.; Sun, A.; Ni, Y.; Cai, T. Study and Development of a Defatted Wheat Germ Nutritive Noodle. Eur. Food Res. Techno. 2001, 212, 344–348. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Mohdaly, A.A.A.; Elneairy, N.A.A. Wheat Germ: An Overview on Nutritional Value, Antioxidant Potential and Antibacterial Characteristics. Food Nutr. Sci. 2015, 6, 265–277. [Google Scholar] [CrossRef]
- Zou, Q.; Meng, W.; Li, C.; Wang, T.; Li, D. Feeding Broilers with Wheat Germ, Hops and Grape Seed Extract Mixture Improves Growth Performance. Front. Physiol. 2023, 14, 1144997. [Google Scholar] [CrossRef]
- Goluch, Z.; Okruszek, A.; Sierżant, K.; Wierzbicka-Rucińska, A. The Influence of Wheat Germ Expeller on Performance and Selected Parameters of Carbohydrate, Lipid, and Protein Metabolism in Blood Serum for Broilers. Agriculture 2023, 13, 753. [Google Scholar] [CrossRef]
- Elghafar, R.A.; Abaza, M.; Ellakany, H.F.; Abd El-Hady, A.M.; El-Sabrout, K. The Effect of Fermented Wheat Germ Extract on Broiler Chicks’ Growth Performance, Immunological Status, and Carcass Characteristics. Ann. Anim. Sci. 2024, 24, 1323–1331. [Google Scholar] [CrossRef]
- Goluch, Z.; Słupczyńska, M.; Okruszek, A.; Haraf, G.; Wereńska, M.; Wołoszyn, J. The Energy and Nutritional Value of Meat of Broiler Chickens Fed with Various Addition of Wheat Germ Expeller. Animals 2023, 13, 499. [Google Scholar] [CrossRef]
- Wang, F.; Glenn, A.J.; Tessier, A.J.; Mei, Z.; Haslam, D.E.; Guasch-Ferré, M.; Tobias, D.K.; Eliassen, A.H.; Manson, J.E.; Clish, C.; et al. Integration of Epidemiological and Blood Biomarker Analysis Links Haem Iron Intake to Increased Type 2 Diabetes Risk. Nat. Metab. 2024, 6, 1807–1818. [Google Scholar] [CrossRef]
- Shahinfar, H.; Jayedi, A.; Shab-Bidar, S. Dietary Iron Intake and the Risk of Type 2 Diabetes: A Systematic Review and Dose–Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2022, 61, 2279–2296. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Huang, Y.; Zhang, T.; Sun, Q.; Zhang, Y.; Zhang, P.; Wang, G.; Zhang, J.; Wu, J. Associations of Dietary Total, Heme, Non-Heme Iron Intake with Diabetes, CVD, and All-Cause Mortality in Men and Women with Diabetes. Heliyon 2024, 10, e38758. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Guan, L.; Ren, Y.; Zhao, Y.; Liu, D.; Zhang, D.; Liu, L.; Liu, F.; Chen, X.; Cheng, C.; et al. Dietary Iron Intake and Risk of Death Due to Cardiovascular Diseases: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Asia Pac. J. Clin. Nutr. 2020, 29, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gianattasio, K.Z.; Bennett, E.E.; Stewart, J.D.; Xu, X.; Park, E.S.; Smith, R.L.; Ying, Q.; Whitsel, E.A.; Power, M.C. The Associations of Dietary Copper With Cognitive Outcomes. Am. J. Epidemiol. 2022, 191, 1202–1211. [Google Scholar] [CrossRef]
- Miao, Q.; Zhang, J.; Yun, Y.; Wu, W.; Luo, C. Association between Copper Intake and Essential Hypertension: Dual Evidence from Mendelian Randomization Analysis and the NHANES Database. Front. Nutr. 2024, 11, 1454669. [Google Scholar] [CrossRef]
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch--Ernst, K.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific Opinion on the Tolerable Upper Intake Level for Manganese. EFSA J. 2023, 21, e8413. [Google Scholar] [CrossRef]
- Kamaly, H.F.; Sharkawy, A.A. Health Risk Assessment of Metals in Chicken Meat and Liver in Egypt. Environ. Monit. Assess. 2023, 195, 802. [Google Scholar] [CrossRef]
- Ali, H.S.; Almashhadany, D.A.; Khalid, H.S. Determination of Heavy Metals and Selenium Content in Chicken Liver at Erbil City, Iraq. Ital. J. Food Saf. 2020, 9, 8659. [Google Scholar] [CrossRef]
- Hossain, E.; Nesha, M.; Chowdhury, M.A.Z.; Rahman, S.H. Human Health Risk Assessment of Edible Body Parts of Chicken through Heavy Metals and Trace Elements Quantitative Analysis. PLoS ONE 2023, 18, e0279043. [Google Scholar] [CrossRef]
- Srikanthithasan, K.; Gariglio, M.; Diaz Vicuna, E.; Fiorilla, E.; Miniscalco, B.; Zambotto, V.; Cappone, E.E.; Stoppani, N.; Soglia, D.; Raspa, F.; et al. Dietary Processed Former Foodstuffs for Broilers: Impacts on Growth Performance, Digestibility, Hematobiochemical Profiles and Liver Gene Abundance. J. Anim. Sci. Biotechnol. 2024, 15, 122. [Google Scholar] [CrossRef]
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Seong, P.N.; Cho, S.H.; Park, K.M.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Ba, H.V. Characterization of Chicken By-Products by Mean of Proximate and Nutritional Compositions. Korean J. Food Sci. Anim. Resour. 2015, 35, 179–188. [Google Scholar] [CrossRef]
- Alshamy, Z.; Richardson, K.C.; Harash, G.; Hünigen, H.; Röhe, I.; Hafez, H.M.; Plendl, J.; Al Masri, S. Structure and Age-Dependent Growth of the Chicken Liver Together with Liver Fat Quantification: A Comparison between a Dual-Purpose and a Broiler Chicken Line. PLoS ONE 2019, 14, e0226903. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 9831:2004; Animal Feeding Stuffs, Animal Products, and Faeces or Urine—Determination of Gross Calorific Value—Bomb Calorimeter Method (ISO 9831:1998). ISO: Geneva, Switzerland, 1998.
- Latimer, G.W., Jr. Official Methods of Analysis of AOAC International, 20th ed.; Association of Official Analysis Chemists International: Rockville, MD, USA, 2016; ISBN 978-0-935584-87-5. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International., 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005; ISBN 0-935584-54-4. [Google Scholar]
- Buryakov, N.P.; Zagarin, A.Y.; Fathala, M.M.; Aleshin, D.E. The Role of Supplementing a Complex Phytobiotic Feed Additive Containing (Castanea Sativa Mill) Extract in Combination with Calcium Butyrate, Zinc–Methionine and Essential Oils on Growth Indicators, Blood Profile and Carcass Quality of Broiler Chickens. Vet. Sci. 2023, 10, 212. [Google Scholar] [CrossRef]
- Zoraunye, A.; Chikumba, N.; Munengwa, A.; Mugova, C.; Chikwanda, D. Response of ROSS 308 Broiler Chickens (Gallus Domesticus) to Dietary Supplementation with Inorganic Copper. Greener J. Agric. Sci. 2023, 13, 15–21. [Google Scholar] [CrossRef]
- Kokoszyński, D.; Bernacki, Z.; Saleh, M.; Stęczny, K.; Binkowska, M. Body Conformation and Internal Organs Characteristics of Different Commercial Broiler Lines. Rev. Bras. Cienc. Avic. 2017, 19, 47–52. [Google Scholar] [CrossRef]
- Henry, S.; Darwish, S.; Saleh, A.; Khalifa, A. Carcass Characteristics and Nutritional Composition of Some Edible Chicken By-Products. Egypt. J. Food Sci. 2019, 47, 81–90. [Google Scholar] [CrossRef]
- Daryoush Shakouri, M.; Malekzadeh, M. Responses of Broiler Chickens to the Nutrient Recommendations of NRC (1994) and the Ross Broiler Management Manual. Rev. Colomb. Cienc. Pecu. 2016, 29, 91–98. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 177–197. ISBN 978-0-470-65071-4. [Google Scholar]
- Karomy, A.; Habib, H.N.; Kasim, S. Influence of Different Levels of Crude Protein and Metabolizable Energy on Production Performance of Ross Broiler. JBAH 2019, 9, 20–24. [Google Scholar] [CrossRef]
- Tables of Composition and Nutritional Value of Food; PZWL Wydawnictwo Lekarskie: Warsaw, Poland, 2020; ISBN 978-83-200-6258-8.
- U.S. Department of Agriculture FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 July 2025).
- Ministry of Agriculture and Rural and Development of the Slovak Republic the Slovak Food Composition Database (SFCDB). Available online: http://www.pbd-online.sk/en (accessed on 10 October 2025).
- National Institute of Nutrition and Seafood Research-NIFES Seafood Data Norwegian Food Composition Table. Available online: https://www.matvaretabellen.no/en/ (accessed on 10 October 2025).
- Technical University of Denmark The Danish Food Composition Database. Available online: https://frida.fooddata.dk/?lang=en (accessed on 10 October 2025).
- Public Health England McCance and Widdowson’s The Composition of Foods Integrated Dataset 2021. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 10 October 2025).
- Bird, R.; Eskin, M. The Emerging Role of Phosphorus in Human Health. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 96, pp. 27–88. ISBN 978-0-12-820648-5. [Google Scholar]
- Bernal, A.; Zafra, M.A.; Simón, M.J.; Mahía, J. Sodium Homeostasis, a Balance Necessary for Life. Nutrients 2023, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Karásek, F.; Štenclová, H.; Šťastník, O.; Mrkvicová, E.; Pavlata, L.; Nedomová, Š.; Zeman, L. The Effect of Calcium and Magnesium Supplementation on Performance and Bone Strength of Broiler Chickens. Potravinárstvo 2017, 11, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Majewska, D.; Szczerbińska, D.; Ligocki, M.; Bucław, M.; Sammel, A.; Tarasewicz, Z.; Romaniszyn, K.; Majewski, J. Comparison of the Mineral and Fatty Acid Profiles of Ostrich, Turkey and Broiler Chicken Livers. Brit. Poult. Sci. 2016, 57, 193–200. [Google Scholar] [CrossRef]
- Pikor, D.; Hurła, M.; Słowikowski, B.; Szymanowicz, O.; Poszwa, J.; Banaszek, N.; Drelichowska, A.; Jagodziński, P.P.; Kozubski, W.; Dorszewska, J. Calcium Ions in the Physiology and Pathology of the Central Nervous System. Int. J. Mol. Sci. 2024, 25, 13133. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and Pathophysiology of Potassium Homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Magnesium (Mg2+) Deficiency, Not Well-Recognized Non-Infectious Pandemic: Origin and Consequence of Chronic Inflammatory and Oxidative Stress-Associated Diseases. Cell Physiol. Biochem. 2022, 57, 1–23. [CrossRef]
- Shastak, Y.; Rodehutscord, M. A Review of the Role of Magnesium in Poultry Nutrition. World’s Poult. Sci. J. 2015, 71, 125–138. [Google Scholar] [CrossRef]
- Hefnawy, A.; Khaiat, H. The Importance of Copper and the Effects of Its Deficiency and Toxicity in Animal Health. Int. J. Livest. Res. 2015, 5, 1–20. [Google Scholar] [CrossRef]
- Barua, S.; Ciannella, S.; Tijani, L.; Gomez-Pastora, J. Iron in Blood Cells: Function, Relation to Disease, and Potential for Magnetic Separation. Biotech. Bioeng. 2023, 120, 1707–1724. [Google Scholar] [CrossRef]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic Crossroads of Iron and Copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef]
- Suttle, N. (Ed.) Mineral Nutrition of Livestock, 4th ed.; CABI: Wallingford, UK, 2010; ISBN 978-1-84593-473-6. [Google Scholar]
- Da Cruz Ferreira Júnior, H.; Da Silva, D.L.; De Carvalho, B.R.; De Oliveira, H.C.; Cunha Lima Muniz, J.; Junior Alves, W.; Eugene Pettigrew, J.; Eliza Facione Guimarães, S.; Da Silva Viana, G.; Hannas, M.I. Broiler Responses to Copper Levels and Sources: Growth, Tissue Mineral Content, Antioxidant Status and mRNA Expression of Genes Involved in Lipid and Protein Metabolism. BMC Vet. Res. 2022, 18, 223. [Google Scholar]
- Hu, Y.; Wang, C.; Wu, W.; Qu, Y.; Zhang, W.; Li, D.; Zhu, L.; Gao, F.; Wu, B.; Zhang, L.; et al. Organic Zinc with Moderate Chelation Strength Enhances Zinc Absorption in the Small Intestine and Expression of Related Transporters in the Duodenum of Broilers. Front. Physiol. 2022, 13, 952941. [Google Scholar] [CrossRef] [PubMed]
- Maria, D.D.B.; Vieira, S.L.; Horn, R.M.; Marchi, M.L.A.; Favero, A. Phytase Improves Zinc Utilization by Broiler Chickens. Animals 2024, 14, 3423. [Google Scholar] [CrossRef] [PubMed]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of Zinc in Health and Disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Byrne, L.; Murphy, R.A. Relative Bioavailability of Trace Minerals in Production Animal Nutrition: A Review. Animals 2022, 12, 1981. [Google Scholar] [CrossRef]
- Blasi, F.; Trovarelli, V.; Mangiapelo, L.; Ianni, F.; Cossignani, L. Grape Pomace for Feed Enrichment to Improve the Quality of Animal-Based Foods. Foods 2024, 13, 3541. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, S.; Zhao, W.; Wang, S.; Sun, C.; Chen, B.; Zhu, Y. Effects of Dried Blueberry Pomace and Pineapple Pomace on Growth Performance and Meat Quality of Broiler Chickens. Animals 2023, 13, 2198. [Google Scholar] [CrossRef]
- Gungor, E.; Altop, A.; Erener, G. Effect of Raw and Fermented Grape Pomace on the Growth Performance, Antioxidant Status, Intestinal Morphology, and Selected Bacterial Species in Broiler Chicks. Animals 2021, 11, 364. [Google Scholar] [CrossRef]
- Tóth, T.; Horváth, R.É.; Dóka, O.; Kovács, M.; Fébel, H. The Effects of Mineral Supplementation in Rapeseed Cake Diet on Thyroid Function and Meat Quality in Broiler Chickens. Agriculture 2024, 14, 2333. [Google Scholar] [CrossRef]
- Goluch, Z.; Czernecki, T.; Haraf, G.; Okruszek, A.; Wereńska, M. Impact of Various Types of Heat Processing on the Content of Selected Trace Elements of Goose Breast Meat. Appl. Sci. 2025, 15, 6795. [Google Scholar] [CrossRef]
- CASRN 7439-96-5; US EPA-IRIS Manganese. U.S. Environmental Protection Agency: Washington, DC, USA, 1995.
- U.S. Department of Health and Human Services. Toxicological Profile for Zinc; Agency for Toxic Substances and Disease Registry (US): Atlanta, GA, USA, 2005.
- Taylor, A.A.; Tsuji, J.S.; McArdle, M.E.; Adams, W.J.; Goodfellow, W.L. Recommended Reference Values for Risk Assessment of Oral Exposure to Copper. Risk Anal. 2023, 43, 211–218. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency. Provisional Peer Reviewed Toxicity Values for Iron and Compounds (CASRN 7439-89-6) Derivation of Subchronic and Chronic Oral RfDs; Office of Research and Development, National Center for Environmental Assessment, Superfund Health Risk Technical Support Center: Cincinnati, OH, USA, 2006.
- Lewis, J. Codex Nutrient Reference Values; FAO: Rome, Italy, 2019; ISBN 978-92-5-131957-4. [Google Scholar]
- European Parlament REGULATION (EU) No 1169/2011 of the European Parrlament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32011R1169 (accessed on 10 November 2025).
- Calvo, M.S.; Dunford, E.K.; Uribarri, J. Industrial Use of Phosphate Food Additives: A Mechanism Linking Ultra-Processed Food Intake to Cardiorenal Disease Risk? Nutrients 2023, 15, 3510. [Google Scholar] [CrossRef]
- Campos, N.D.S.; Alvarenga, F.B.M.; Sabarense, C.M.; Oliveira, M.A.L.D.; Timm, J.G.; Vieira, M.A.; Sousa, R.A.D. Evaluation of the Influence of Different Cooking Pot Types on the Metallic Elements Content in Edible Chicken Tissues by MIP OES. Braz. J. Food Technol. 2020, 23, e2019308. [Google Scholar] [CrossRef]
- Akaichi, F.; Glenk, K.; Revoredo-Giha, C. Bundling Food Labels: What Role Could the Labels “Organic,” “Local” and “Low Fat” Play in Fostering the Demand for Animal-friendly Meat. Agribusiness 2022, 38, 349–370. [Google Scholar] [CrossRef]
- Akaichi, F.; Glenk, K.; Revoredo-Giha, C. Could Animal Welfare Claims and Nutritional Information Boost the Demand for Organic Meat? Evidence from Non-Hypothetical Experimental Auctions. J. Clean. Prod. 2019, 207, 961–970. [Google Scholar] [CrossRef]
| Trait | CT | EX5 | EX10 | EX15 | SEM | p Value |
|---|---|---|---|---|---|---|
| Liver weight (g) | 50.9 | 49.3 | 45.8 | 43.3 | 1.05 | 0.060 |
| Normalized liver mass (%) (g/100 g of BW) | 2.03 | 2.07 | 2.02 | 1.92 | 0.03 | 0.437 |
| Gross energy (MJ/100 g WWB) | 0.87 a | 0.85 | 0.85 | 0.80 b | 0.090 | 0.028 |
| Moisture (% WWB) | 68.4 | 68.4 | 68.9 | 70.3 | 0.354 | 0.193 |
| Crude fat (% WWB) | 5.86 | 5.66 | 5.00 | 4.09 | 0.266 | 0.051 |
| Crude protein (% WWB) | 20.2 | 19.5 | 20.4 | 20.5 | 0.196 | 0.291 |
| Crude ash (% WWB) | 1.46 | 1.40 | 1.45 | 1.47 | 0.019 | 0.596 |
| Minerals | CT | EX5 | EX10 | EX15 | SEM | p Value |
|---|---|---|---|---|---|---|
| P | 394.0 | 344.7 | 413.0 | 419.4 | 12.3 | 0.262 |
| Na | 139.2 A | 123.9 A | 101.3 B | 93.5 B | 4.42 | 0.001 |
| Ca | 19.4 a | 24.2 a | 12.3 | 11.1 b | 1.67 | 0.010 |
| K | 218.6 | 187.0 | 210.5 | 204.2 | 5.22 | 0.205 |
| Mg | 29.4 b | 35.9 a | 30.7 | 30.4 | 0.79 | 0.013 |
| Minerals | CT | EX5 | EX10 | EX15 | SEM | p Value |
|---|---|---|---|---|---|---|
| Cu | 0.81 | 0.76 | 0.73 | 0.86 | 0.020 | 0.114 |
| Fe | 21.3 | 21.7 | 25.4 | 26.6 | 0.981 | 0.218 |
| Zn | 2.58 | 2.58 | 2.72 | 3.01 | 0.080 | 0.139 |
| Mn | 0.46 | 0.43 | 0.40 | 0.39 | 0.010 | 0.087 |
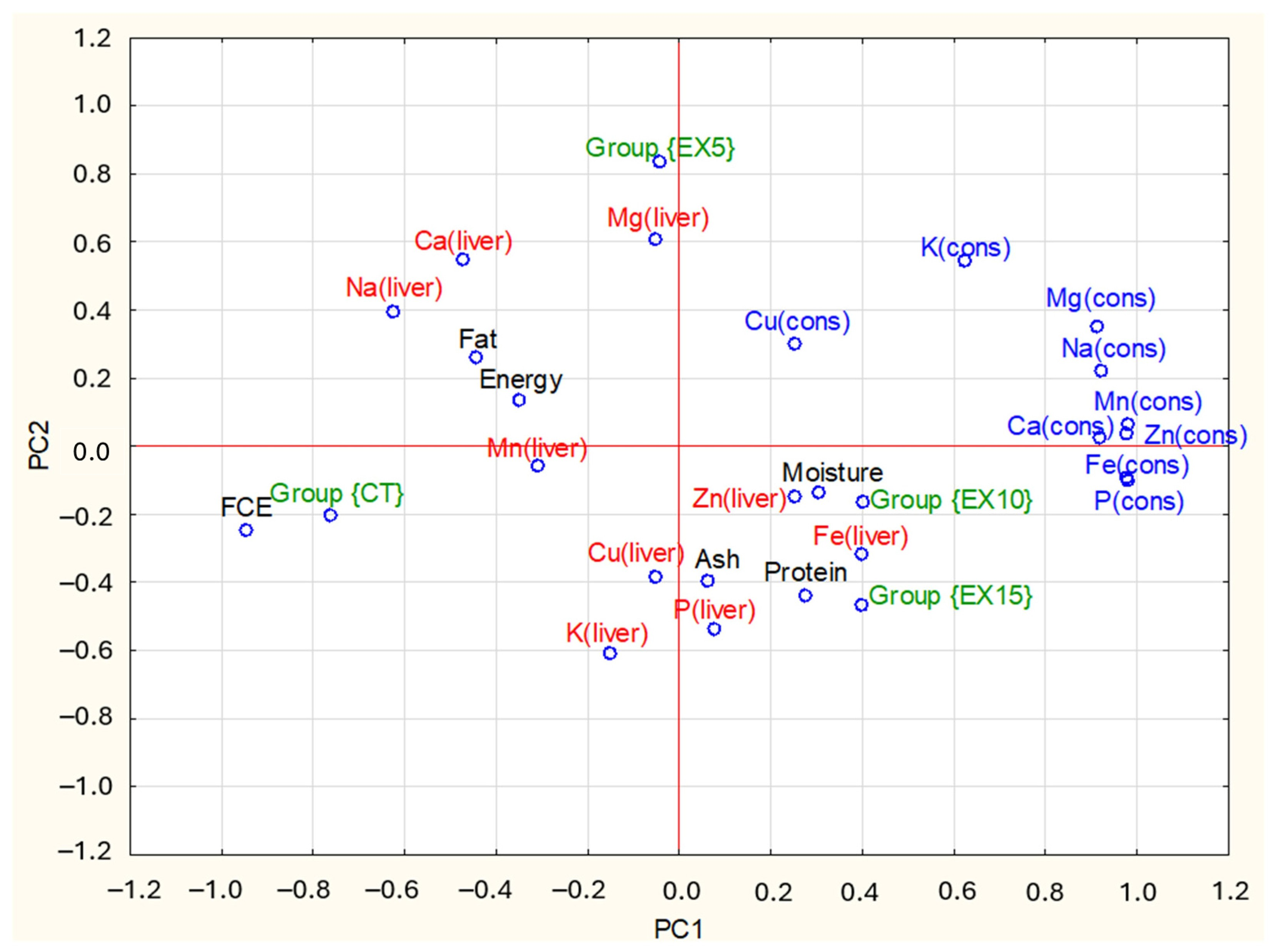
| Items | PC1 1 | PC2 1 | |
|---|---|---|---|
| Mineral contents of livers: | P (liver) | 0.08 | −0.54 |
| Na (liver) | −0.62 | 0.40 | |
| Ca (liver) | −0.47 | 0.55 | |
| K (liver) | −0.15 | −0.61 | |
| Mg (liver) | −0.05 | 0.61 | |
| Fe (liver) | 0.40 | −0.32 | |
| Zn (liver) | 0.25 | −0.15 | |
| Cu (liver) | −0.05 | −0.39 | |
| Mn (liver) | −0.31 | −0.06 | |
| Chemical composition of livers: | Moisture | 0.31 | −0.14 |
| Protein | 0.28 | −0.44 | |
| Fat | −0.44 | 0.26 | |
| Ash | 0.06 | −0.40 | |
| Energy | −0.35 | 0.13 | |
| Mineral consumption with feed: | P (cons) | 0.98 | −0.10 |
| Na (cons) | 0.92 | 0.22 | |
| Ca (cons) | 0.92 | 0.02 | |
| K (cons) | 0.63 | 0.54 | |
| Mg (cons) | 0.91 | 0.35 | |
| Fe (cons) | 0.98 | −0.10 | |
| Zn (cons) | 0.98 | 0.04 | |
| Cu (cons) | 0.25 | 0.30 | |
| Mn (cons) | 0.98 | 0.06 | |
| FCE 2 | −0.95 | −0.25 | |
| Groups of chickens 3: | Group {CT} | −0.76 | −0.20 |
| Group {EX5} | −0.04 | 0.83 | |
| Group {EX10} | 0.40 | −0.16 | |
| Group {EX15} | 0.40 | −0.47 |
| Mineral | NRVs 1 | CT | EX5 | EX10 | EX15 |
|---|---|---|---|---|---|
| P | 700 | 53.3 | 49.21 | 59.0 | 59.9 |
| Ca | 1000 | 1.94 | 2.42 | 1.21 | 1.11 |
| Mg | 310 | 9.5 | 11.6 | 9.9 | 9.8 |
| Fe | 14 | 152.1 | 155.0 | 181.4 | 190.0 |
| Zn | 11 | 23.5 | 23.5 | 24.7 | 27.4 |
| Cu | 0.9 | 90.0 | 84.4 | 81.1 | 95.6 |
| Mn | 3 | 15.3 | 14.3 | 13.3 | 13.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goluch, Z.; Król, B.; Haraf, G.; Okruszek, A.; Sierżant, K. Chicken Liver from Broilers Fed Wheat Germ Expeller: A Source of Minerals and Energy in the Human Diet. Foods 2025, 14, 3962. https://doi.org/10.3390/foods14223962
Goluch Z, Król B, Haraf G, Okruszek A, Sierżant K. Chicken Liver from Broilers Fed Wheat Germ Expeller: A Source of Minerals and Energy in the Human Diet. Foods. 2025; 14(22):3962. https://doi.org/10.3390/foods14223962
Chicago/Turabian StyleGoluch, Zuzanna, Barbara Król, Gabriela Haraf, Andrzej Okruszek, and Kamil Sierżant. 2025. "Chicken Liver from Broilers Fed Wheat Germ Expeller: A Source of Minerals and Energy in the Human Diet" Foods 14, no. 22: 3962. https://doi.org/10.3390/foods14223962
APA StyleGoluch, Z., Król, B., Haraf, G., Okruszek, A., & Sierżant, K. (2025). Chicken Liver from Broilers Fed Wheat Germ Expeller: A Source of Minerals and Energy in the Human Diet. Foods, 14(22), 3962. https://doi.org/10.3390/foods14223962








