Physicochemical Analysis and Digestive Enzymes Inhibition of a Selected Malaysian Apis cerana Honey
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physicochemical Analysis
2.2.1. Moisture
2.2.2. Baume (Density)
2.2.3. Brix Value
2.2.4. Sugar Profiling
2.2.5. pH and Free Acidity
2.2.6. Ash
2.2.7. Electrical Conductivity
- K = cell constant (cm−1)
- G = electrical conductance (mS), as obtained using a conductivity cell
- 11.691 = electrical conductivity of fresh distilled water (mS/cm) and 0.1 M potassium chloride solution, total value means at 20 °C
- SH = honey’s electrical conductivity (mS/cm)
- K = cell constant (cm−1), calculated in Equation (1)
- G = conductance (mS)
2.2.8. Colour Analysis
2.2.9. Choline
2.3. Enzyme Analysis
2.3.1. Inhibition of the Pancreatic Lipase Activity
2.3.2. Inhibition of the α-Amylase Activity
2.3.3. Inhibition of the α-Glucosidase Activity
2.3.4. Different Treatments of Honey on α-Glucosidase Activity
2.4. Statistical Analyses
2.5. Flowchart of the Study
3. Results
3.1. Physicochemical Analysis
3.2. Enzyme Analysis
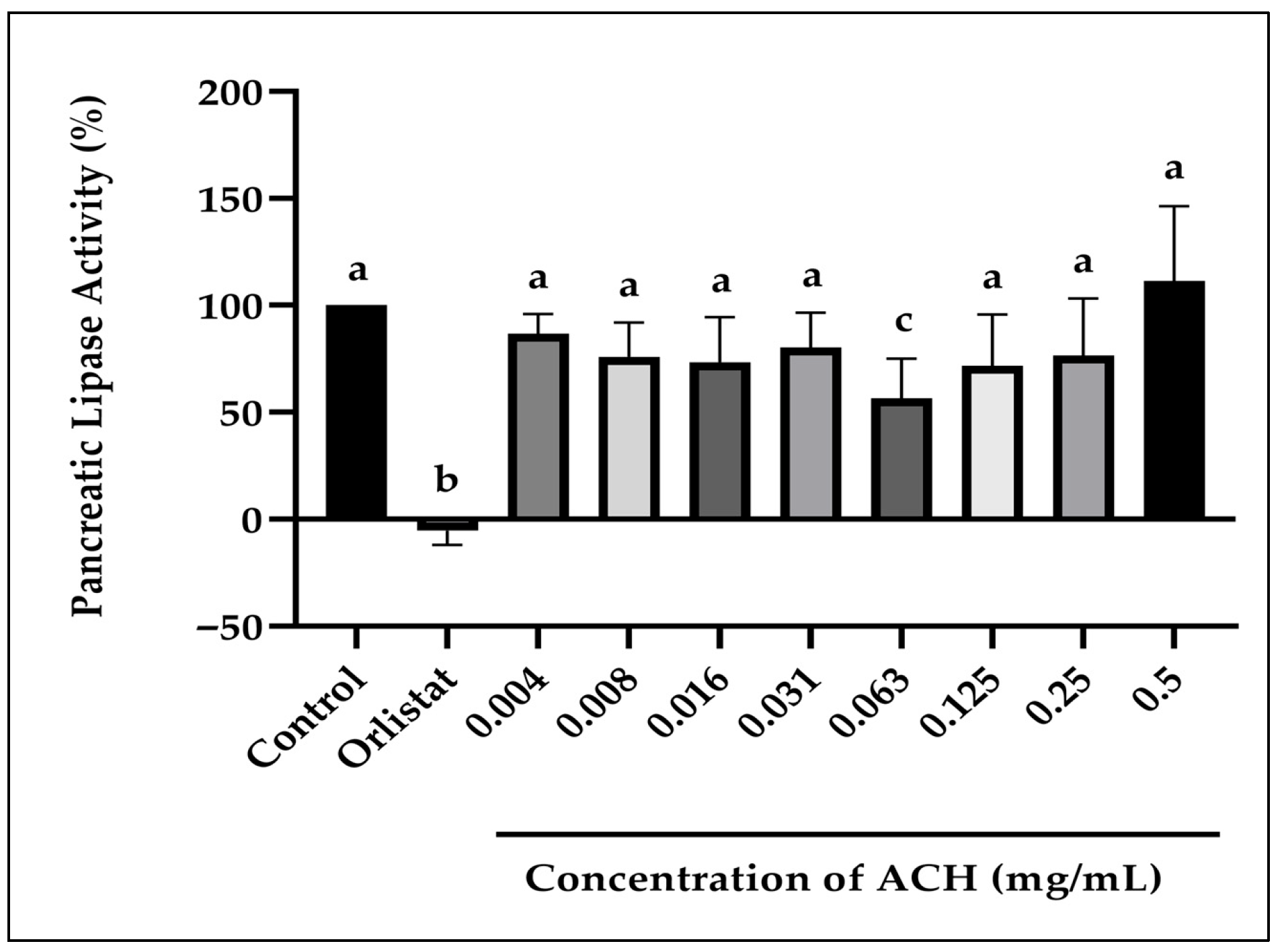
3.2.1. Pancreatic Lipase Activity
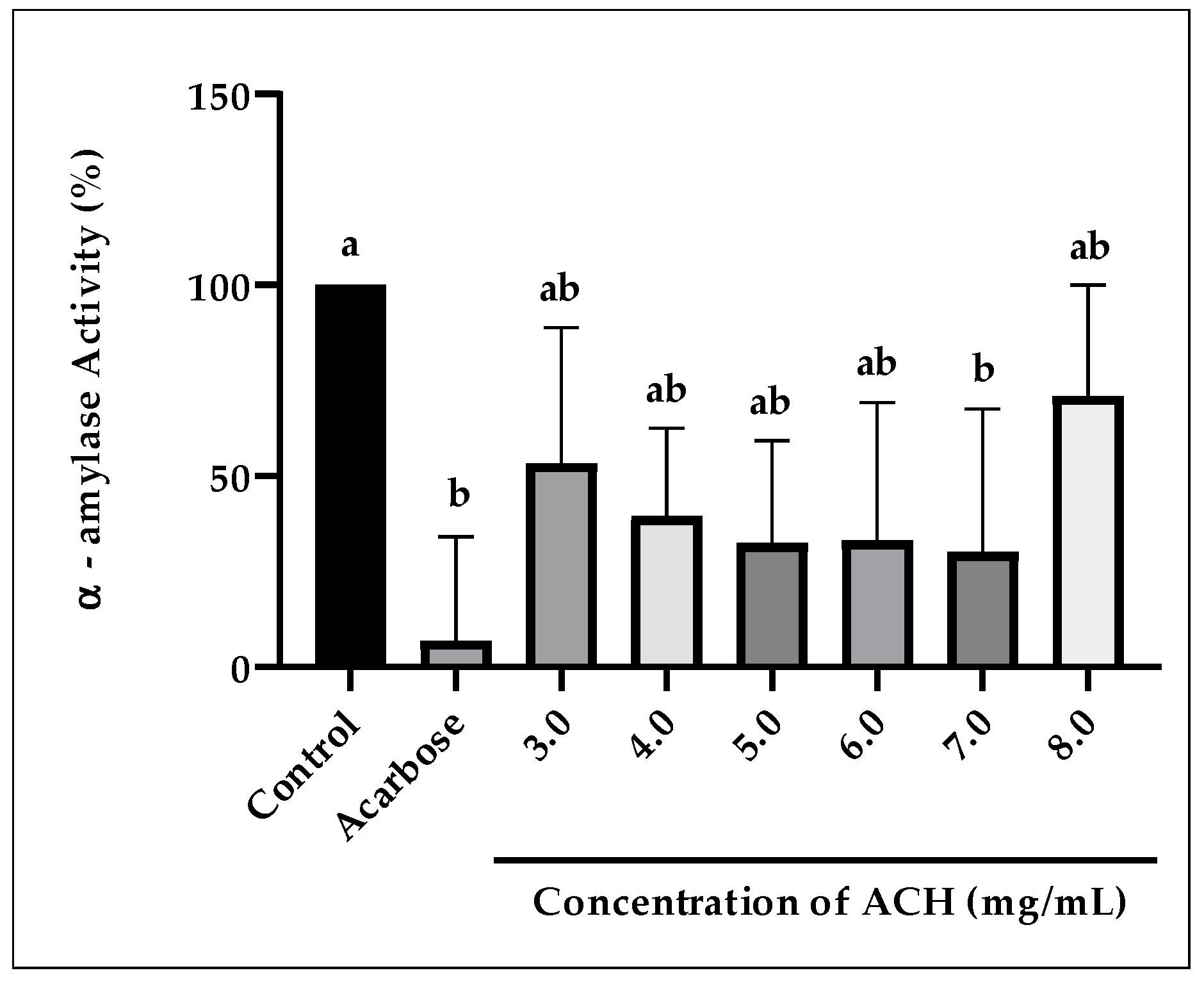
3.2.2. α-Amylase Activity
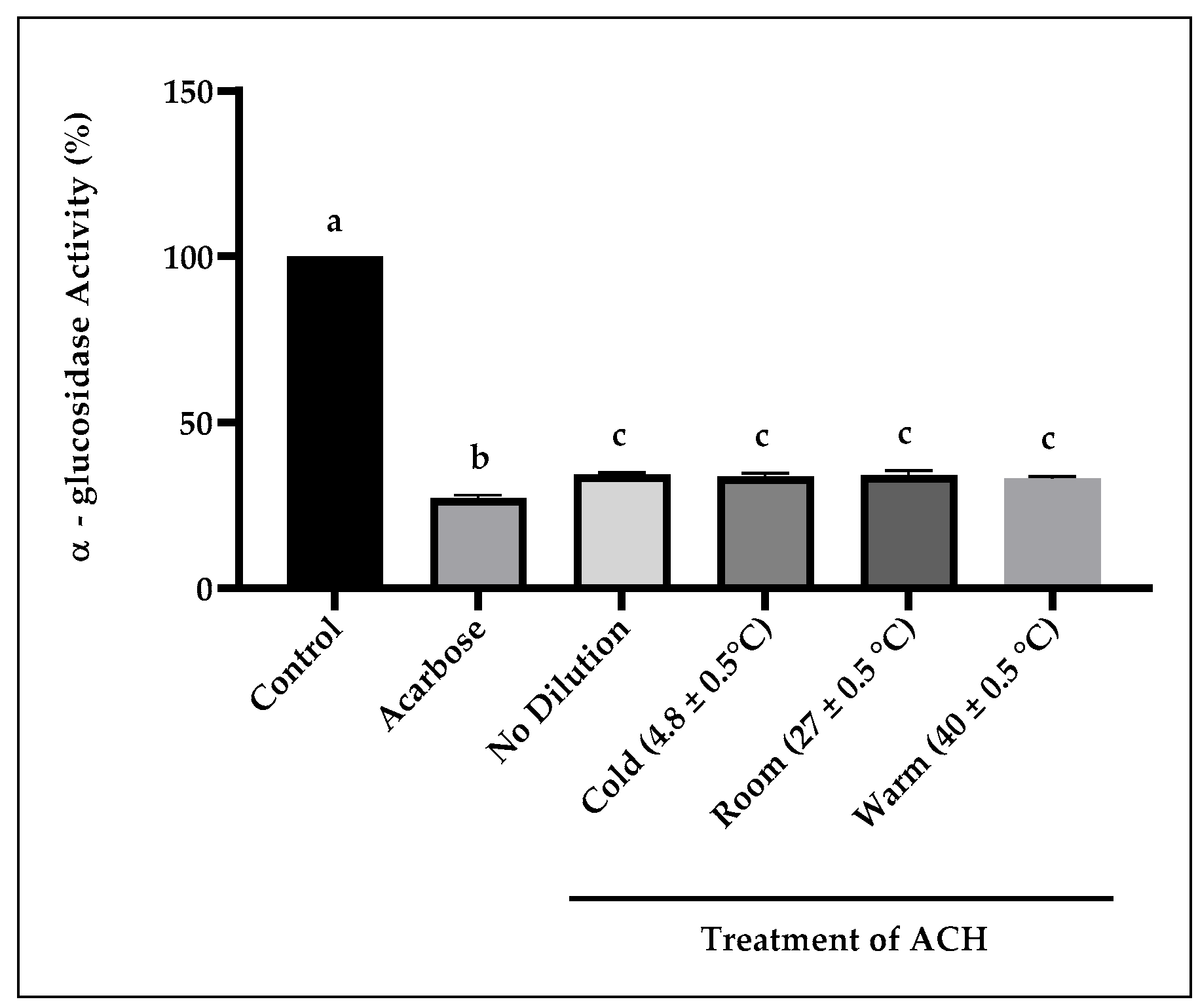
3.2.3. α-Glucosidase Activity
4. Discussion
4.1. Physicochemical Analysis
4.2. Enzyme Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACH | Apis cerana honey |
| IMR | Institute for Medical Research |
| NIH | National Institutes of Health |
| HPLC | High-performance Liquid Chromatography |
| IHC | International Honey Commission |
| USDA | United States Department of Agriculture |
| ICS | Ion Chromatography System |
| SPB | Sorenson’s Phosphate Buffer |
| PNPG | 4-nitrophenyl β-D-glucopyranoside |
| MFA | Malaysian Food Act 1983 |
| CA | Codex Alimentarius 2009 |
| F | Fructose |
| G | Glucose |
| F/G | Fructose and Glucose ratio |
References
- World Obesity. World Obesity Atlas 2025. Available online: https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_Atlas_2025_rev1.pdf (accessed on 1 May 2025).
- Sun, H.; Pouya, S.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and type 2 Diabetes: Two diseases with a need for combined treatment strategies—EASO can lead the way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef]
- Ruban, A.; Stoenchev, K.; Ashrafian, H.; Teare, J. Current treatments for obesity. Clin. Med. 2019, 19, 205–212. [Google Scholar] [CrossRef]
- Sibony, R.W.; Segev, O.; Dor, S.; Raz, I. Drug Therapies for Diabetes. Int. J. Mol. Sci. 2023, 24, 17147. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, S. Pancreatic lipase inhibitors: The road voyaged and successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Jin, Z.; Svensson, B. Food-derived non-phenolic α-amylase and α-glucosidase inhibitors for controlling starch digestion rate and guiding diabetes-friendly recipes. LWT 2022, 153, 112455. [Google Scholar] [CrossRef]
- Multum, C. Acarbose. Available online: https://www.drugs.com/mtm/acarbose.html (accessed on 6 June 2024).
- Ranneh, Y.; Md Akim, A.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Abu Bakar, M.F. Honey and its nutritional and anti-inflammatory Value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Junaidin, J.; Abdurachman, A.; Sudiana, K. Comparison of antioxidant and antidiabetic properties of meliponine honey from different stingless bee species and origins: A scoping review. Pediatr. Endocrinol. Diabetes Metab. 2025, 31, 59–67. [Google Scholar] [CrossRef]
- Zaldivar-Ortega, A.K.; Morfin, N.; Angeles-Hernandez, J.C.; Gonzalez-Montiel, L.; Vicente-Flores, M.; Aguirre-Alvarez, G.; Cenobio-Galindo, A.d.J. Functional characterization of Scaptotrigona mexicana Honey: Physicochemical properties, antioxidant capacity, and α-amylase inhibition for food process applications. Processes 2025, 13, 2788. [Google Scholar] [CrossRef]
- Zulkifli, M.F.; Mohd Radzi, M.N.F.; Saludes, J.P.; Dalisay, D.S.; Wan Ismail, W.I. Potential of natural honey in controlling obesity and its related complications. J. Evid.-Based Integr. Med. 2022, 27, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Dar, S.A.; Ahmed, M.M.M.; Aly, M.M.; Vlainić, J. Antioxidant capacity and therapeutic applications of honey: Health benefits, antimicrobial activity and food processing roles. Antioxidants 2025, 14, 959. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- Usman, A.N.; Fendi, F.; Ahmad, M.; Budiaman, B.; Sartini, S.; Nulandari, Z.; Agustin, D.I.; Munjiyah, N. A review of different honey from Indonesia and Malaysia. BIO Web Conf. 2024, 96, 01024. [Google Scholar] [CrossRef]
- Katuwal, D.R.; Pokhrel, A.; Khanal, D. Comparative study of Apis cerana and Apis mellifera. J. Agric. For. Res. 2023, 2, 41–48. [Google Scholar]
- Zhang, Y.Z.; Wang, S.; Chen, Y.F.; Wu, Y.Q.; Tian, J.; Si, J.J.; Zhang, C.P.; Zheng, H.Q.; Hu, F.L. Authentication of Apis cerana honey and Apis mellifera honey based on Major Royal Jelly Protein 2 Gene. Molecules 2019, 24, 289. [Google Scholar] [CrossRef] [PubMed]
- Krishnasree, V.; Mary Ukkuru, P. In vitro antidiabetic activity and glycemic index of bee honeys. Indian J. Tradit. Knowl. 2017, 16, 134–140. [Google Scholar]
- FAO/WHO. Standard for Honey CXS 12-1981, Adopted in 1981, Revised in 1987, 2001, Amended in 2019, 2022, Codex Alimentarius. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B12-1981%252FCXS_012e.pdf (accessed on 8 June 2024).
- Bogdanov, S. Harmonised Methods of the International Honey Commission. In Bee Product Science; International Honey Commission: Rome, Italy, 2009; Volume 5, pp. 1–62. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 10 June 2024).
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Nielsen, S.S. Introduction to the Chemical Analysis of Foods; Jones and Bartlett Publishers, Inc.: Boston, MA, USA, 1994. [Google Scholar]
- United States Department of Agriculture. United States Standards for Grades of Extracted Honey. 1985. Available online: https://www.ams.usda.gov/sites/default/files/media/Extracted_Honey_Standard%5B1%5D.pdf (accessed on 10 June 2024).
- Chater, P.I.; Wilcox, M.D.; Cherry, P.; Herford, A.; Mustar, S.; Wheater, H.; Brownlee, I.; Seal, C.; Pearson, J. Inhibitory activity of extracts of Hebridean brown seaweeds on lipase activity. J. Appl. Phycol. 2016, 28, 1303–1313. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mustar, S.; Khalid, N.M.; Abd Rashed, A.; Mohd Noh, M.F.; Wilcox, M.D.; Chater, P.I.; Brownlee, I.A.; Pearson, J.P. Inhibitory activities of three Malaysian edible seaweeds on lipase and α-amylase. J. Appl. Phycol. 2013, 25, 1405–1412. [Google Scholar] [CrossRef]
- Apostolidis, E.; Karayannakidis, P.D.; Kwon, Y.I.; Lee, C.M.; Seeram, N.P. Seasonal variation of phenolic antioxidant-mediated α-glucosidase inhibition of Ascophyllum nodosum. Plant Foods Hum. Nutr. 2011, 66, 313–319. [Google Scholar] [CrossRef]
- Legal Research Board. Part VIII, Standards and Particular Labelling Requirements for Food (Cereal, Cereal Product, Starch and Bread), Sweetening Substance: Honey. In Food Act 1983 (Act 281) & Regulations (As at 15th August 2013); International Law Book Services: Petaling Jaya, Malaysia, 2013; pp. 126–127. [Google Scholar]
- Sajid, M.; Yamin, M.; Asad, F.; Yaqub, S.; Ahmad, S.; Ali Muhammad Samee Mubarik, M.; Ahmad, B.; Ahmad, W.; Qamer, S. Comparative study of physio-chemical analysis of fresh and branded honeys from Pakistan. Saudi J. Biol. Sci. 2020, 27, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Tischer Seraglio, S.K.; Schulz, M.; Brugnerotto, P.; Silva, B.; Valdemiro Gonzaga, L.; Fett, R.; Oliveira Costa, A.C. Quality, composition and health-protective properties of citrus honey: A review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef] [PubMed]
- Adgaba, N.; Al-Ghamdi, A.; Sharma, D.; Tadess, Y.; Alghanem, S.M.; Ali Khan, K.; Ansari, M.J.; Mohamed, G.K.A. Physico-chemical, antioxidant and anti-microbial properties of some Ethiopian mono-floral honeys. Saudi J. Biol. Sci. 2020, 27, 2366–2372. [Google Scholar] [CrossRef]
- Mongi, R.J. Influence of botanical origin and geographical zones on physicochemical properties, mineral contents and consumer acceptance of honey in Tanzania. Food Chem. Adv. 2024, 4, 100731. [Google Scholar] [CrossRef]
- Santos Filipe, M.; Kowalczyk, T.; Kukula-Koch, W.; Wieczfinska, J.; Bangay, G.; Diaz-Lanza, A.M.; Cardoso, R.V.C.; Mandim, F.; Falcão, S.I.; Vilas-Boas, M.; et al. Evaluating the quality, physicochemical properties, and biological activities of Centauri® honey from Turkey. Food Biosci. 2024, 62, 105028. [Google Scholar] [CrossRef]
- Data Support Company. Balances. How Does a Moisture Analyzer Work? 2023. Available online: https://www.dscbalances.com/blogs/articles/how-does-a-moisture-analyzer-work?srsltid=AfmBOorkHSy_xEHThRRj1dhTMJ8Ib2HBhs12tKG7QI (accessed on 30 October 2025).
- Nikhat, S.; Fazil, M. History, phytochemistry, experimental pharmacology and clinical uses of honey: A comprehensive review with special reference to Unani medicine. J. Ethnopharmacol. 2022, 282, 114614. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Singh, S. Honey moisture reduction and its quality. J. Food Sci. 2018, 55, 3861–3871. [Google Scholar] [CrossRef]
- Ananias, K.R.; Machado de Melo, A.A.; José de Moura, C. Analysis of moisture content, acidity and contamination by yeast and molds in Apis mellifera L. honey from central Brazil. Braz. J. Microbiol. 2013, 44, 679–683. [Google Scholar] [CrossRef]
- Castillo Martinez, T.; García Osorio, C.; García Muñiz, J.G.; Aguilar Ávila, J.; Ramírez Valverde, R. Sugars and °brix honey from Apis mellifera, Melipona beecheii and commercial honey from a local market in Mexico. Vet. Mex. OA 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Oroian, M. Measurement, prediction and correlation of density, viscosity, surface tension and ultrasonic velocity of different honey types at different temperatures. J. Food Eng. 2013, 119, 167–172. [Google Scholar] [CrossRef]
- Vosoghi, M.; Yousefi, S.; Honarvar, M. Physicochemical and sensory properties of honey powder from different climatic regions. Appl. Food Res. 2025, 5, 100843. [Google Scholar] [CrossRef]
- Albu, A.; Radu-Rusu, C.G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality assessment of raw honey issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- Tigistu, T.; Worku, Z.; Mohammed, A. Evaluation of the physicochemical properties of honey produced in Doyogena and Kachabira Districts of Kembata Tambaro zone, Southern Ethiopia. Heliyon 2021, 7, e06803. [Google Scholar] [CrossRef]
- Smetanska, I.; Alharthi, S.S.; Selim, K.A. Physicochemical, antioxidant capacity and color analysis of six honeys from different origin. J. King Saud Univ. Sci. 2021, 33, 101447. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Missaoui, R.; Donsí, F.; Ferrari, G.; Hamdi, S. Physicochemical, rheological, and thermal properties of six types of honey from various floral origins in Tunisia. Int. J. Food Prop. 2015, 18, 2624–2637. [Google Scholar] [CrossRef]
- Cabrera, M.; Santander, E. Physicochemical and sensory analysis of honeys from eastern Formosa province (Argentina) and its relationship with their botanical origin. Food Chem. Adv. 2022, 1, 100026. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Gomes, V.V.; Bandeira, A.M.P.; Cordovil, K.P.S.; Bandeira Filho, J.d.R.; Braghini, F.; Biluca, F.C.; Gonzaga, L.V.; Fett, R.; da Costa, K.S.; de Azevedo, M.M.R.; et al. Physicochemical characterization and antioxidant activity of honey samples of Apis mellifera and different species of Meliponinae subfamily from the Brazilian eastern Amazon region. Food Sci. Technol. 2022, 42, e114921. [Google Scholar] [CrossRef]
- Raweh, H.S.A.; Hadj Ahmed, A.Y.B.; Iqbal, J.; Alqarni, A.S. Monitoring and evaluation of free acidity levels in Talh honey originated from Talh tree Acacia gerrardii Benth. J. King Saud Univ. Sci. 2022, 34, 101678. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Owayss, A.A.; Mahmoud, A.A.; Hannan, M.A. Mineral content and physical properties of local and imported honeys in Saudi Arabia. J. Saudi Chem. Soc. 2014, 18, 618–625. [Google Scholar] [CrossRef]
- Al-Doghairi, M.A.; Al-Rehiayani, S.; Ibrahim, G.H.; Osman, K.A. Physicochemical and antimicrobial properties of natural honeys produced in Al-Qassim Region, Saudi Arabia. J. King Abdulaziz Univ. Meteorol. Env. Arid Land Agric. Sci. 2007, 18, 3–18. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Vazquez, M. Differences between honeydew and blossom honeys: A review. Trends Food Sci. Technol. 2017, 59, 79–87. [Google Scholar] [CrossRef]
- Choi, S.H.; Nam, M.S. Classification of honeydew and blossom honeys by principal component analysis of physicochemical parameters. Korean J. Agric. Sci. 2020, 47, 67–81. [Google Scholar] [CrossRef]
- Sipos, L.; Végh, R.; Bodor, Z.; Zinia Zaukuu, J.L.; Hitka, G.; Bázár, G.; Kovacs, Z. Classification of bee pollen and prediction of sensory and colorimetric attributes: A sensometric fusion approach by e-Nose, e-Tongue and NIR. Sensors 2020, 20, 6768. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Domínguez, R.; Reyes-Carrillo, J.L.; de la Cruz-Larios, L.; González-Eguiarte, D.R. Bee honey color variation throughout the year in Huejotitán, Jalisco, México. Sustain. Agri Food Environ. Res. 2018, 6, 3. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Kontakos, S.; Drouza, C.; Papastephanou, C. Characterization and botanical differentiation of monofloral and multifloral honeys produced in Cyprus, Greece, and Egypt using physicochemical parameter analysis and mineral content in conjunction with Supervised Statistical Techniques. J. Anal. Methods Chem. 2018, 2018, 7698251. [Google Scholar] [CrossRef]
- Šedík, P.; Hudecová, M.; Predanócyová, K. Exploring consumers’ preferences and attitudes to honey: Generation approach in Slovakia. Foods 2023, 12, 1941. [Google Scholar] [CrossRef]
- Rane, M.; Kulkarni, S.; Saabir, F. Determination of color, phenolic acid, flavonoid and antioxidant capacity of honey samples from Western Ghats of Maharashtra. J. Chem. Health Risks 2024, 14, 1869–1873. [Google Scholar]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef]
- Burns, B.C.; Belani, J.D.; Wittorf, H.N.; Brailoiu, E.; Brailoiu, G.C. Choline—An essential nutrient with health benefits and a signaling molecule. Int. J. Mol. Sci. 2025, 26, 7159. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.Y.; Bhagwat, S.A.; Williams, J.R.; Howe, J.C.; Holden, J.M. USDA Database for the Choline Content of Common Foods; Nutrient Data Laboratory: Beltsville, MD, USA, 2008; pp. 1–37. Available online: https://data.nal.usda.gov/system/files/Choln02.pdf (accessed on 10 June 2024).
- Xue, X.; Ding, Y.; Gong, P.; Lu, L.; Wang, J.; Zhang, Y.; Wu, C.; Su, S.; Wei, L.; Liu, Y.; et al. Determination of choline and acetylcholine in honey by Ultra Performance Liquid Chromatography—Tandem Mass Spectrometry. FENXI CESHI XUEBAO (J. Instrum. Anal.) 2022, 41, 341–347. [Google Scholar]
- Holmbäck, U.; Forslund, A.; Grudén, S.; Alderborn, G.; Söderhäll, A.; Hellström, P.M.; Lennernäs, H. Effects of a novel combination of orlistat and acarbose on tolerability, appetite, and glucose metabolism in persons with obesity. Obes. Sci. Pract. 2020, 6, 313–323. [Google Scholar] [CrossRef]
- Pan, G.; Lu, Y.; Wei, Z.; Li, Y.; Li, L.; Pan, X. A review on the in vitro and in vivo screening of α-glucosidase inhibitors. Heliyon 2024, 10, e37467. [Google Scholar] [CrossRef]
- Hanefeld, M.; Schaper, F. The role of alpha-glucosidase inhibitors (acarbose). In Pharmacotherapy of Diabetes: New Developments; Mogensen, C.E., Ed.; Springer: Boston, MA, USA, 2007; pp. 143–152. [Google Scholar] [CrossRef]
- Fratianni, F.; Ombra, M.N.; d’Acierno, A.; Caputo, L.; Amato, G.; De Feo, V.; Coppola, R.; Nazzaro, F. Polyphenols content and in vitro α-glycosidase activity of different Italian monofloral honeys, and their effect on selected pathogenic and probiotic bacteria. Microorganisms 2021, 9, 1694. [Google Scholar] [CrossRef]
- Gercek, Y.C.; Dagsuyu, E.; Basturk, F.N.; Kırkıncı, S.; Yıldırım, N.; Kıskanc, G.; Ozmener, B.; Unlu, Y.S.; Kalkan, S.N.; Bozta, K.; et al. Enzyme inhibitory, physicochemical, and phytochemical properties and botanical sources of honey, bee pollen, bee bread, and propolis obtained from the same apiary. Antioxidants 2024, 13, 1483. [Google Scholar] [CrossRef]
- Zulhilmi Cheng, M.Z.S.; Zawawi, N.; Ooi, D.J.; Chan, K.W.; Ismail, N.; Ishak, N.A.; Mohd Esa, N. In vitro investigation of antioxidant and antidiabetic properties of phenolic-rich extract from stingless bee honey (Heterotrigona itama). Malays. J. Med. Health Sci. 2023, 19, 141–150. [Google Scholar] [CrossRef]
- Setiawan, R.D.; Melia, S.; Juliyarsi, I.; Rusdimansyah. Investigation of Stingless Bee Honey from West Sumatra as an Antihyperglycemic Food. Prev. Nutr. Food Sci. 2024, 29, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Peláez-Acero, A.; Garrido-Islas, D.B.; Campos-Montiel, R.G.; González-Montiel, L.; Medina-Pérez, G.; Luna-Rodríguez, L.; González-Lemus, U.; de Jesús Cenobio-Galindo, A. The application of ultrasound in honey: Antioxidant activity, inhibitory effect on α-amylase and α-glucosidase, and in vitro digestibility assessment. Molecules 2022, 27, 5825. [Google Scholar] [CrossRef]
- Moein, M.; Moein, S.; Farmani, F.; Rozbehan, S.; Sabahi, Z. Examination the antioxidant potentials and antidiabetic properties of phenolic extracts of some Iranian honeys. J. Nephropharmacol. 2022, 11, e6. [Google Scholar] [CrossRef]
- Guo, J.; Ding, Q.; Zhang, Z.; Zhang, Y.; He, J.; Yang, Z.; Zhou, P.; Gong, X. Evaluation of the antioxidant activities and phenolic profile of Shennongjia Apis cerana honey through a comparison with Apis mellifera honey in China. Molecules 2023, 28, 3270. [Google Scholar] [CrossRef] [PubMed]

| Analysis | Result (ACH) | Malaysian Food Act 1983 (Act 281) & Regulations (as of 15 August 2013) [28] | Codex Alimentarius 2009 (CXS 12-1981) [20] |
|---|---|---|---|
| Moisture (Refractometer) | 22.7 ± 0.1% | <20% | <20% |
| <23% (Hea) | |||
| Moisture (Air-oven drying) | 24.4 ± 0.2% | NA | NA |
| Moisture (Moisture analyser) | 8.6 ± 0.5% | NA | NA |
| Baume (°Be′) | 40.1 ± 0.0 °Be′ | NA | NA |
| Brix concentration (Brix%) | 75.5 ± 0.1% | NA | NA |
| Fructose (F) | 29.75 ± 0.27 g/100 g | NA | NA |
| Glucose (G) | 28.76 ± 0.23 g/100 g | NA | NA |
| Sum of F and G | 58.81 ± 0.50 g/100 g | ≥60% | ≥45 g/100 g (H, H/B) |
| ≥60 g/100 g (B) | |||
| F/G ratio | 1.03 | NA | NA |
| Sucrose | 8.44 ± 0.06 g/100 g | <10% | <10 g/100 g (refer to content) |
| <5 g/100 g (B) | |||
| <15 g/100 g (L, Bor) | |||
| Maltose | 0.59 ± 0.00 g/100 g | NA | NA |
| Lactose | ND | NA | NA |
| pH | 4.13 ± 0.01 | NA | |
| Free acidity | 93.7 ± 0.6 meq/1000 g | <40 meq/1000 g | <50 meq/1000 g |
| Ash | 2.8 ± 0.5 g/100 g | <1% | <1.0 g/100 g |
| Electrical conductivity | 1.66 ± 0.00 mS/cm | NA | ≥0.8 mS/cm (H, C, H/C) |
| <0.8 mS/cm (B) | |||
| Colour analysis | 150.0 ± 0.0 mm Pfund (Dark Amber) | NA | NA |
| Choline | ND | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustar, S.; Ibrahim, N.; Pauzi, N.A.; Abd Rashed, A.; Md Noh, M.F. Physicochemical Analysis and Digestive Enzymes Inhibition of a Selected Malaysian Apis cerana Honey. Foods 2025, 14, 3958. https://doi.org/10.3390/foods14223958
Mustar S, Ibrahim N, Pauzi NA, Abd Rashed A, Md Noh MF. Physicochemical Analysis and Digestive Enzymes Inhibition of a Selected Malaysian Apis cerana Honey. Foods. 2025; 14(22):3958. https://doi.org/10.3390/foods14223958
Chicago/Turabian StyleMustar, Suraiami, Nurliayana Ibrahim, Noor Athirah Pauzi, Aswir Abd Rashed, and Mohd Fairulnizal Md Noh. 2025. "Physicochemical Analysis and Digestive Enzymes Inhibition of a Selected Malaysian Apis cerana Honey" Foods 14, no. 22: 3958. https://doi.org/10.3390/foods14223958
APA StyleMustar, S., Ibrahim, N., Pauzi, N. A., Abd Rashed, A., & Md Noh, M. F. (2025). Physicochemical Analysis and Digestive Enzymes Inhibition of a Selected Malaysian Apis cerana Honey. Foods, 14(22), 3958. https://doi.org/10.3390/foods14223958






