Optimization of β-Carotene Enrichment of Coconut Oil from Canistel (Pouteria campechiana L.) Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Experimental Design for Optimization of β-Carotene Enrichment
2.2.1. Process of β-Carotene Enrichment
2.2.2. Process of Heating to Determine the Retention of β-Carotene in Enriched Coconut Oil
2.3. Sample Preparation for β-Carotene Determination
2.3.1. Quantification of β-Carotene by UV-Vis Spectrophotometry
2.3.2. Determination of Extraction Efficiency
2.4. Chemical Composition Analysis of Oils
2.4.1. Saponification Value (SV)
- B—Volume in mL of standard HCL required for blank test;
- S—Volume in mL of standard HCL required for the sample test;
- N—Normality of the standard HCL;
- W—Weight of oil (g).
2.4.2. Acid Value (AV)
2.4.3. Peroxide Value (PV)
2.4.4. Iodine Value (IV)
2.4.5. Color Variation
2.5. Phytochemical Analysis of Oils
2.5.1. Preparation of Methanolic Extracts
2.5.2. Total Phenolic Content (TPC)
2.5.3. Total Flavonoid Content (TFC)
2.5.4. DPPH Radical Scavenging Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. β-Carotene Content in Canistel
3.2. Model Fitting and Statistical Validation
3.3. Effect of Extraction Conditions on β-Carotene Enrichment
3.4. Physicochemical Properties of Enriched Coconut Oil
3.5. Color Characteristics of Enriched Oil
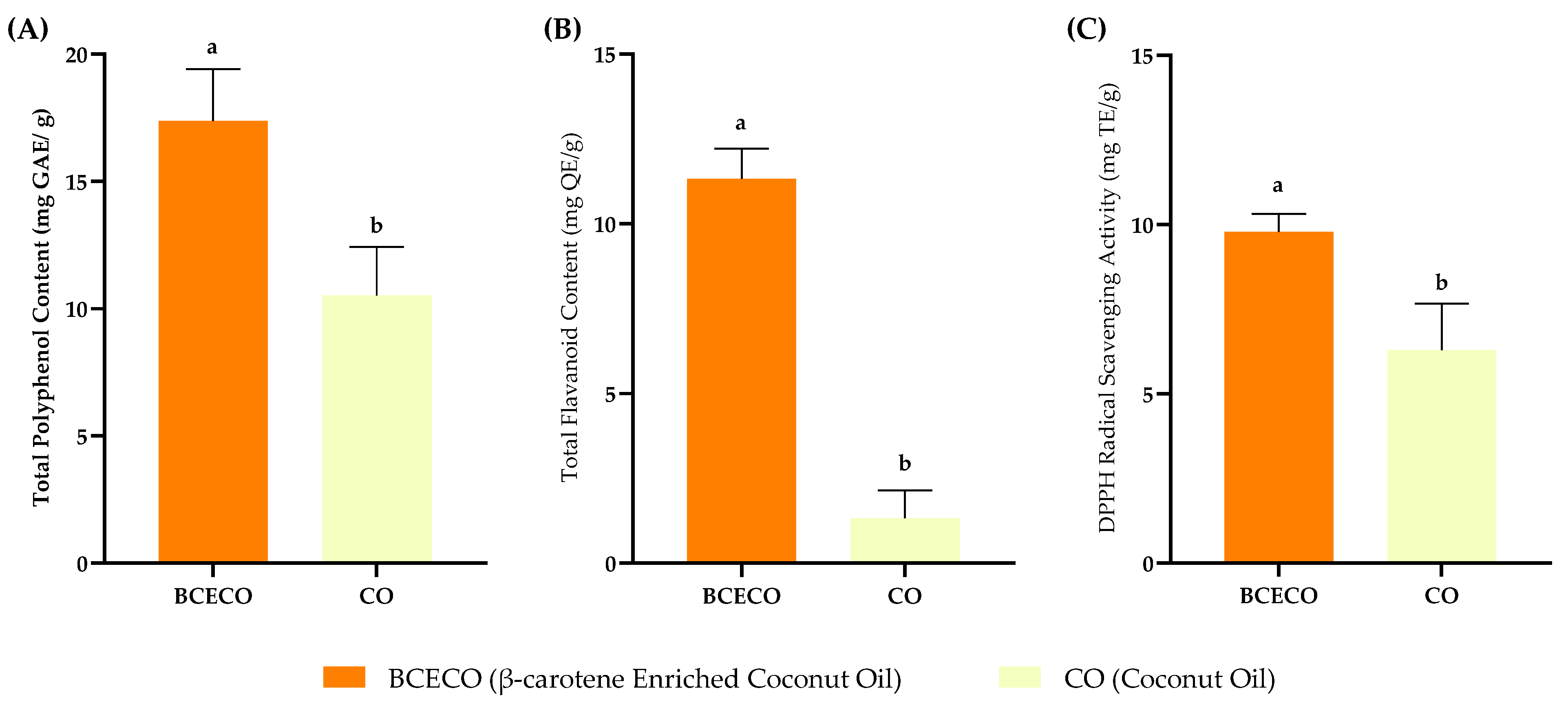
3.6. Functional Properties of Enriched Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| AV | Acid Value |
| β-Carotene | Beta-Carotene |
| CCD | Central Composite Design |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GAE | Gallic Acid Equivalent |
| IV | Iodine Value |
| L*, a*, b* | CIE Color Space Coordinates (Lightness, Red-Green, Yellow-Blue) |
| PV | Peroxide Value |
| QE | Quercetin Equivalent |
| R2 | Coefficient of Determination |
| RSM | Response Surface Methodology |
| SV | Saponification Value |
| TFC | Total Flavonoid Content |
| TPC | Total Phenolic Content |
| TE | Trolox Equivalent |
| UV–Vis | Ultraviolet–Visible Spectrophotometry |
References
- Kumar, R.; Oruna-Concha, M.J.; Niranjan, K.; Vimaleswaran, K.S. A Review on Vitamin A Deficiency and Depleted Immunity in South Asia: From Deficiency to Resilience. Nutrition 2024, 124, 112452. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Vitamin A deficiency. Nutrition Landscape Information System (NLiS). 2023. Available online: https://www.who.int/data/nutrition/nlis/info/vitamin-a-deficiency (accessed on 18 August 2025).
- Zheng, X.; Yang, Y.; Al-Babili, S. Exploring the Diversity and Regulation of Apocarotenoid Metabolic Pathways in Plants. Front. Plant Sci. 2021, 12, 787049. [Google Scholar] [CrossRef]
- Arshad, Z.; Shahid, S.; Hasnain, A.; Yaseen, E.; Rahimi, M. Functional Foods Enriched with Bioactive Compounds: Therapeutic Potential and Technological Innovations. Food Sci. Nutr. 2025, 13, e71024. [Google Scholar] [CrossRef]
- Hossain, M.S.; Wazed, M.A.; Das Shuvo, S.; Sultana, Z.; Akhter Preya, M.S.; Khanom, H.; Asha, S.; Kamal, M.M.; Mondal, B.K.; Ahmad, T. Fortified and Functional Foods: Trends, Innovations, and Their Public Health Impact for Future Nutrient Enrichment. J. Agric. Food Res. 2025, 23, 102275. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, X.; Wang, Z.; Zhang, W.; Jiang, Z. Structural Characteristics and Stability Analysis of Coconut Oil Body and Its Application for Loading β-Carotene. Food Chem. 2024, 446, 138818. [Google Scholar] [CrossRef]
- Evangelista-Lozano, S.; Robles-Jímarez, H.R.; Pérez-Barcena, J.F.; Agama-Acevedo, E.; Briones-Martínez, R.; Cruz-Castillo, J.G. Fruit Characterization of Pouteria Campechiana ([Kunth] Baehni) in Three Different Stages of Maturity. Fruits 2021, 76, 116–122. [Google Scholar] [CrossRef]
- Do, T.V.T.; Suhartini, W.; Phan, C.U.; Zhang, Z.; Goksen, G.; Lorenzo, J.M. Nutritional Value, Phytochemistry, Health Benefits, and Potential Food Applications of Pouteria Campechiana (Kunth) Baehni: A Comprehensive Review. J. Funct. Foods 2023, 103, 105481. [Google Scholar] [CrossRef]
- Jiménez-Parra, J.E.; Ortiz-García, M.M.; Chavez-Pesqueira, M.; Andueza-Noh, R.H.; Potter, D.; Arias, R.S.; Martínez-Castillo, J. Diversity and Genetic Structure of Kanisté (Pouteria Campechiana), an Underutilized Tropical Fruit Tree Native to the Yucatán Peninsula, Mexico. J. Agric. Food Res. 2025, 23, 102271. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.D.A.; Senarathne, S.M.A.C.U.; Wijesinghe, W.A.J.P. Extraction and Characterization of Natural Food Colorants from Canistel Fruit (Pouteria Campechiana): A Comprehensive Evaluation of Physicochemical Properties. Adv. Technol. 2025, 5, 32–44. [Google Scholar] [CrossRef]
- Saraiva, B.R.; Licci, N.M.; Anjo, F.A.; Vital, A.C.P.; da Silva, J.B.; Bruschi, M.L.; Matumoto-Pintro, P.T. Effects of Inulin and Canistel Addition in the Physical Characteristics of Fat-Reduced Processed Cheese. Res. Soc. Dev. 2021, 9, e4289119917. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A Comprehensive Review on Carotenoids in Foods and Feeds: Status Quo, Applications, Patents, and Research Needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Sahoo, J.; Chatli, M.K. A Simple UV-Vis Spectrophotometric Method for Determination of β-Carotene Content in Raw Carrot, Sweet Potato and Supplemented Chicken Meat Nuggets. LWT Food Sci. Technol. 2011, 44, 1809–1813. [Google Scholar] [CrossRef]
- Hagos, M.; Redi-Abshiro, M.; Chandravanshi, B.S.; Yaya, E.E. Development of Analytical Methods for Determination of β-Carotene in Pumpkin (Cucurbita Maxima) Flesh, Peel, and Seed Powder Samples. Int. J. Anal. Chem. 2022, 2022, 9363692. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- ISO 660:2009; Animal and Vegetable Fats and Oils; Determination of Acid Value and Acidity. International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- Tubino, M.; Aricetti, J.A. A Green Potentiometric Method for the Determination of the Iodine Number of Biodiesel. Fuel 2013, 103, 1158–1163. [Google Scholar] [CrossRef]
- Abano, E.E.; Sam-Amoah, L.K.; Bart-Plange, A. Variation in Ultrasonic Frequency and Time as Pre-Treatments to Air-Drying of Carrot. J. Agric. Eng. 2013, 43, e23. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djekic, I.; Miocinovic, J.; Djordjevic, V.; Lorenzo, J.M.; Barba, F.J.; Mörlein, D.; Tomasevic, I. What Is the Color of Milk and Dairy Products and How Is It Measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- Molole, G.J.; Gure, A.; Abdissa, N. Determination of Total Phenolic Content and Antioxidant Activity of Commiphora Mollis (Oliv.) Engl. Resin. BMC Chem. 2022, 16, 48. [Google Scholar] [CrossRef]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total Phenolic, Flavonoid Contents, and Antioxidant Activities of Fruit, Seed, and Bark Extracts of Zanthoxylum Armatum DC. Sci. World J. 2020, 2020, 8780704. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- de Lanerolle, M.S.; Buddhika Priyadarshani, A.M.; Sumithraarachchi, D.B.; Jansz, E.R. The Carotenoids of Pouteria Campechiana (Sinhala: Ratalawulu). J. Natl. Sci. Found. Sri Lanka 2008, 36, 95–98. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid Extraction Methods: A Review of Recent Developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Demiray, E.; Tulek, Y. Degradation Kinetics of β-Carotene in Carrot Slices during Convective Drying. Int. J. Food Prop. 2017, 20, 151–156. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on Natural Food Pigments—A Mini-Review on Carotenoids, Anthocyanins, and Betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Norshazila, S.; Koy, C.N.; Rashidi, O.; Ho, L.H.; Azrina, I.; Nurul Zaizuliana, R.A.; Zarinah, Z. The Effect of Time, Temperature and Solid to Solvent Ratio on Pumpkin Carotenoids Extracted Using Food Grade Solvents. Sains Malays. 2017, 46, 231–237. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal Degradation Kinetics of Lutein, β-Carotene and β-Cryptoxanthin in Virgin Olive Oils. J. Food Compos. Anal. 2011, 24, 811–820. [Google Scholar] [CrossRef]
- Portillo-López, R.; Morales-Contreras, B.E.; Lozano-Guzmán, E.; Basilio-Heredia, J.; Muy-Rangel, M.D.; Ochoa-Martínez, L.A.; Rosas-Flores, W.; Morales-Castro, J. Vegetable Oils as Green Solvents for Carotenoid Extraction from Pumpkin (Cucurbita Argyrosperma Huber) Byproducts: Optimization of Extraction Parameters. J. Food Sci. 2021, 86, 3122–3136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Shivhare, U.S.; Sandhu, K.S. Thermal Degradation Kinetics of Carotenoids and Visual Color of Papaya Puree. J. Food Sci. 2002, 67, 2692–2695. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, V. RSM and ANN Approach for Optimization of Ultrasonic Assisted Extraction of Pumpkin Seed Oil and Their Quality Assessment. Food Chem. Adv. 2023, 3, 100552. [Google Scholar] [CrossRef]
- Marina, A.M.; Che Man, Y.B.; Nazimah, S.A.H.; Amin, I. Chemical Properties of Virgin Coconut Oil. JAOCS J. Am. Oil Chem. Soc. 2009, 86, 301–307. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids—A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, L.; Peng, F.; Ma, Y.; Deng, Z.; Li, H. Natural Antioxidants Enhance the Oxidation Stability of Blended Oils Enriched in Unsaturated Fatty Acids. J. Sci. Food Agric. 2024, 104, 2907–2916. [Google Scholar] [CrossRef]
- Yildiz, A.Y.; Öztekin, S.; Anaya, K. Effects of Plant-Derived Antioxidants to the Oxidative Stability of Edible Oils under Thermal and Storage Conditions: Benefits, Challenges and Sustainable Solutions. Food Chem. 2025, 479, 143752. [Google Scholar] [CrossRef]
- Yang, J.H.; Tran, T.T.T.; Le, V.V.M. Effects of Natural Antioxidants on the Palm Olein Quality during the Heating and Frying. J. Food Meas. Charact. 2020, 14, 2713–2720. [Google Scholar] [CrossRef]
- Pandurangaiah, S.; Sadashiva, A.T.; Shivashankar, K.S.; Sudhakar Rao, D.V.; Ravishankar, K.V. Carotenoid Content in Cherry Tomatoes Correlated to the Color Space Values L*, a*, b*: A Non-Destructive Method of Estimation. J. Hortic. Sci. 2020, 15, 27–34. [Google Scholar] [CrossRef]
- Manasa, V.; Vaishnav, S.R.; Tumaney, A.W. Physicochemical Characterization and Nutraceutical Compounds of the Selected Spice Fixed Oils. J. Food Sci. Technol. 2021, 58, 3094–3105. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and PH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Bhimjiyani, V.H.; Borugadda, V.B.; Naik, S.; Dalai, A.K. Enrichment of Flaxseed (Linum usitatissimum) Oil with Carotenoids of Sea Buckthorn Pomace via Ultrasound-Assisted Extraction Technique: Enrichment of Flaxseed Oil with Sea Buckthorn. Curr. Res. Food Sci. 2021, 4, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Marinaccio, L.; Zengin, G.; Bender, O.; Dogan, R.; Atalay, A.; Masci, D.; Flamminii, F.; Stefanucci, A.; Mollica, A. Lycopene Enriched Extra Virgin Olive Oil: Biological Activities and Assessment of Security Profile on Cells. Food Biosci. 2024, 60, 104466. [Google Scholar] [CrossRef]



| Independent Variable | Symbol | Levels | ||||
|---|---|---|---|---|---|---|
| −α (−1.141) | −1 | 0 | 1 | α (1.141) | ||
| Temperature (℃) | X1 | −23.79 | 30 | 45 | 60 | 66.21 |
| Oil percentage (% oil: solid) | X2 | −39.64 | 50 | 75 | 100 | 110.36 |
| Run | Independent Variable | Response Variable | Extraction Efficiency (%) | |
|---|---|---|---|---|
| Factor 1 Heating Temperature (°C) | Factor 2 Oil Percentage (%) | β-Carotene Content (µg/mL) | ||
| 1 | 30 | 50 | 2.5136 ± 0.29 | 10.52 ± 0.38 |
| 2 | 45 | 110.35 | 3.3711 ± 0.25 | 31.14 ± 1.09 |
| 3 | 60 | 50 | 0.6981 ± 0.03 | 2.92 ± 1.88 |
| 4 | 45 | 39.64 | 3.2908 ± 0.31 | 10.92 ± 0.62 |
| 5 | 60 | 100 | 0.9236 ± 0.10 | 7.73 ± 1.05 |
| 6 | 30 | 100 | 3.8222 ± 0.52 | 31.99 ± 1.76 |
| 7 | 45 | 75 | 3.2126 ± 0.52 | 20.17 ± 0.17 |
| 8 | 45 | 75 | 3.7887 ± 0.41 | 23.78 ± 0.27 |
| 9 | 23.79 | 75 | 2.5360 ± 0.23 | 15.92 ± 0.11 |
| 10 | 66.21 | 75 | 0.0326 ± 0.00 | 0.20 ± 0.03 |
| 11 | 45 | 75 | 2.5538 ± 0.64 | 16.03 ± 0.59 |
| 12 | 45 | 75 | 2.5293 ± 0.29 | 15.88 ± 0.14 |
| 13 | 45 | 75 | 2.2546 ± 0.51 | 14.15 ± 0.53 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14.93 | 5 | 2.99 | 9.57 | <0.0049 a |
| A-Temperature | 8.52 | 1 | 8.52 | 27.31 | <0.0012 a |
| B-Oil percentage | 2.40 | 1 | 0.3393 | 7.69 | <0.0370 a |
| AB | 1.75 | 1 | 0.2933 | 5.61 | <0.0410 a |
| A2 | 5.28 | 1 | 5.28 | 5.23 | <0.0245 a |
| B2 | 0.63 | 1 | 0.1607 | 2.02 | <0.0410 a |
| Residual | 2.18 | 7 | 0.3119 | ||
| Lack of Fit | 0.6270 | 3 | 0.2090 | 0.5372 | 0.6815 b |
| Pure Error | 1.56 | 4 | 0.3890 | ||
| Cor Total | 17.11 | 12 |
| Dependent Variable | Experimented Value | Predicted Value |
|---|---|---|
| β-carotene content | 2.215 (µg/mL) | 2.22 (µg/mL) |
| Test Parameter | Type of Oil | |
|---|---|---|
| β-Carotene-Enriched Coconut Oil | Coconut Oil | |
| Saponification value (mg KOH/g) | 248.12 ± 1.2 a | 251.23 ± 2.3 a |
| Peroxide value (meqO2/kg) | 0.05 ± 0.01 b | 0.09 ± 0.01 a |
| Acid value (mg KOH/g) | 0.10 ± 0.01 a | 0.17 ± 0.02 a |
| Iodine Value (I2 g/100 g) | 6.88 ± 0.12 b | 7.91 ± 0.10 a |
| Color Parameter | β-Carotene-Enriched Coconut Oil | Coconut Oil |
|---|---|---|
| L* | 30.09 ± 0.51 a | 30.12 ± 0.68 a |
| a* | 5.31 ± 0.32 b | 6.29 ± 0.71 a |
| b* | 8.67 ± 0.33 a | 1.75 ± 0.17 b |
| Hue angle (h°) | 58.51 ± 1.82 a | 15.55 ± 2.20 b |
| Yellowness index | 41.16 ± 1.72 a | 8.30 ± 0.83 b |
| Redness/yellowness ratio | 0.61 ± 0.04 b | 3.59 ± 0.54 a |
| Chroma (C*) | 10.17 ± 0.33 a | 6.53 ± 0.69 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jans, H.M.; Wijerathna, M.C.; Fernando, G.S.N.; Hafiz, M.S. Optimization of β-Carotene Enrichment of Coconut Oil from Canistel (Pouteria campechiana L.) Using Response Surface Methodology. Foods 2025, 14, 3947. https://doi.org/10.3390/foods14223947
Jans HM, Wijerathna MC, Fernando GSN, Hafiz MS. Optimization of β-Carotene Enrichment of Coconut Oil from Canistel (Pouteria campechiana L.) Using Response Surface Methodology. Foods. 2025; 14(22):3947. https://doi.org/10.3390/foods14223947
Chicago/Turabian StyleJans, Harshaka Maduwantha, Madushi Chathurika Wijerathna, Ganwarige Sumali Nivanthi Fernando, and Maryam S. Hafiz. 2025. "Optimization of β-Carotene Enrichment of Coconut Oil from Canistel (Pouteria campechiana L.) Using Response Surface Methodology" Foods 14, no. 22: 3947. https://doi.org/10.3390/foods14223947
APA StyleJans, H. M., Wijerathna, M. C., Fernando, G. S. N., & Hafiz, M. S. (2025). Optimization of β-Carotene Enrichment of Coconut Oil from Canistel (Pouteria campechiana L.) Using Response Surface Methodology. Foods, 14(22), 3947. https://doi.org/10.3390/foods14223947







