Wet-Spinning Technology for Plant-Based Meat Alternative: Influence of Protein Composition on Physicochemical and Textural Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Maaterials
2.2. Method of Preparation
2.3. Preparation of Wet-Spun Meat Alternatives
2.3.1. Zeta Potential
2.3.2. Solution Analysis
2.3.3. pH
2.3.4. Color
2.3.5. Cooking Loss
- CL = Cooking Loss
- W1 = Weight of the uncooked samples (g)
- W2 = Weight of the cooked sample (g)
2.3.6. Moisture Content
2.3.7. Texture Profile Analysis (TPA)
2.3.8. Warner–Bratzler Shear Force
2.3.9. Sensory Analysis
2.3.10. Statistical Analysis
3. Results and Discussion
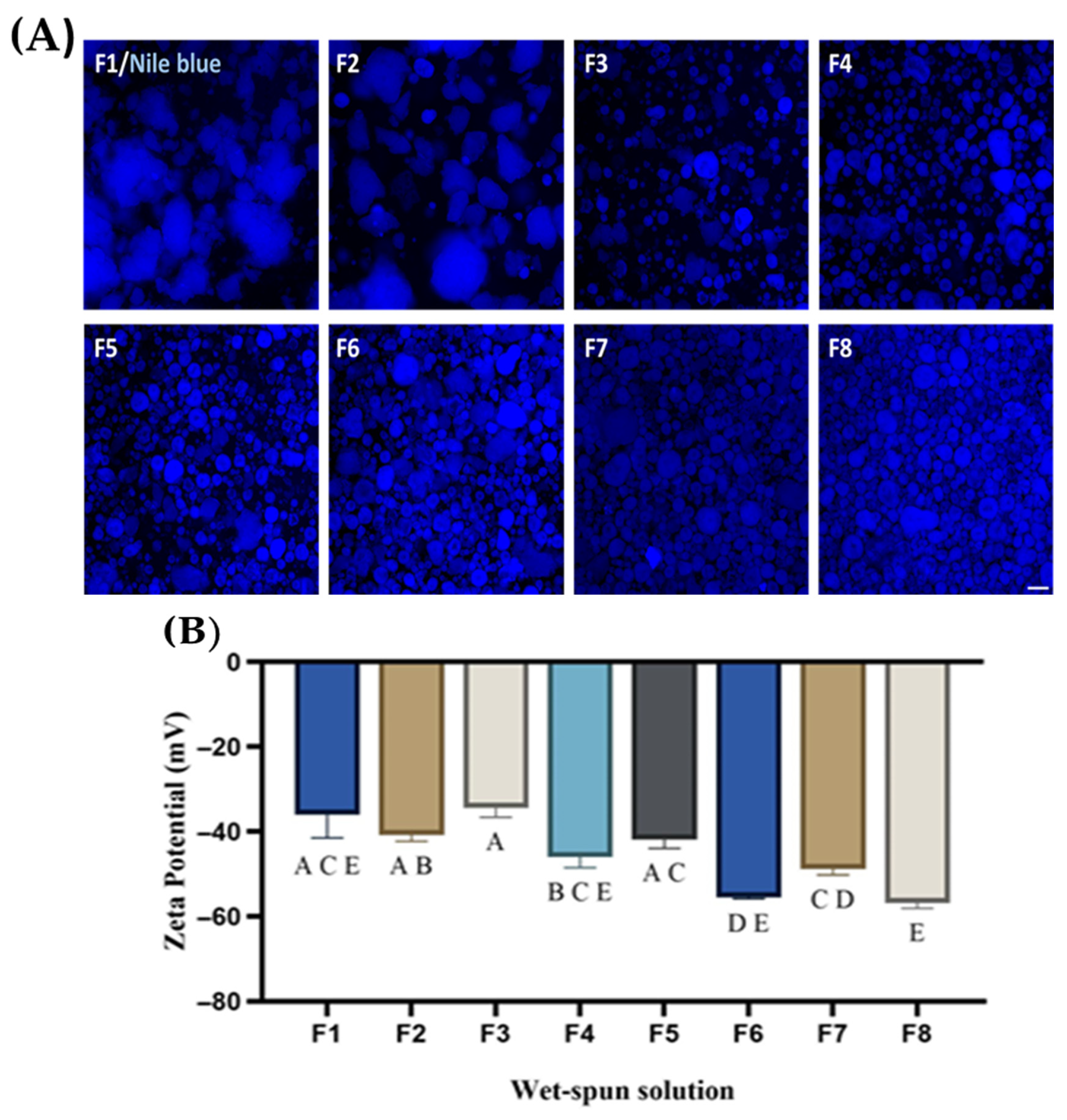
3.1. Zeta Potential and Phase Behavior
3.2. pH and Color
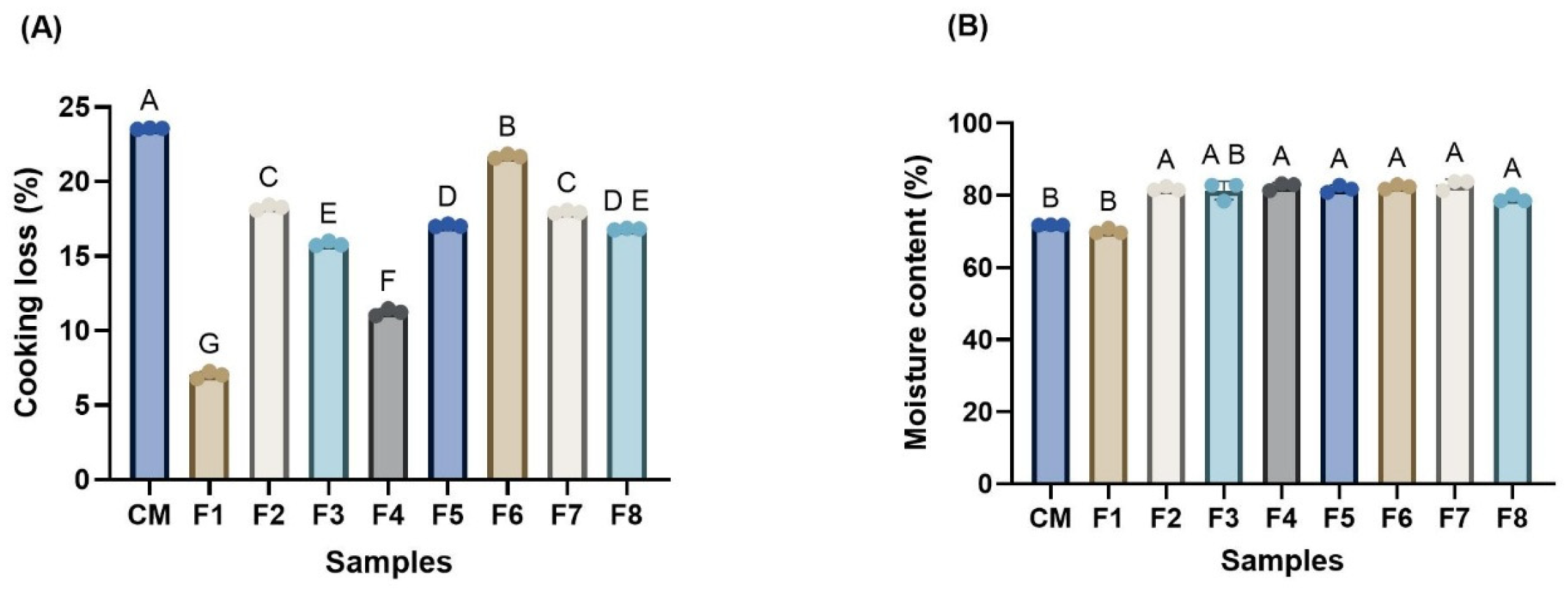
3.3. Cooking Loss and Moisture Content
3.4. Comparative Analysis of WBSF and TPA
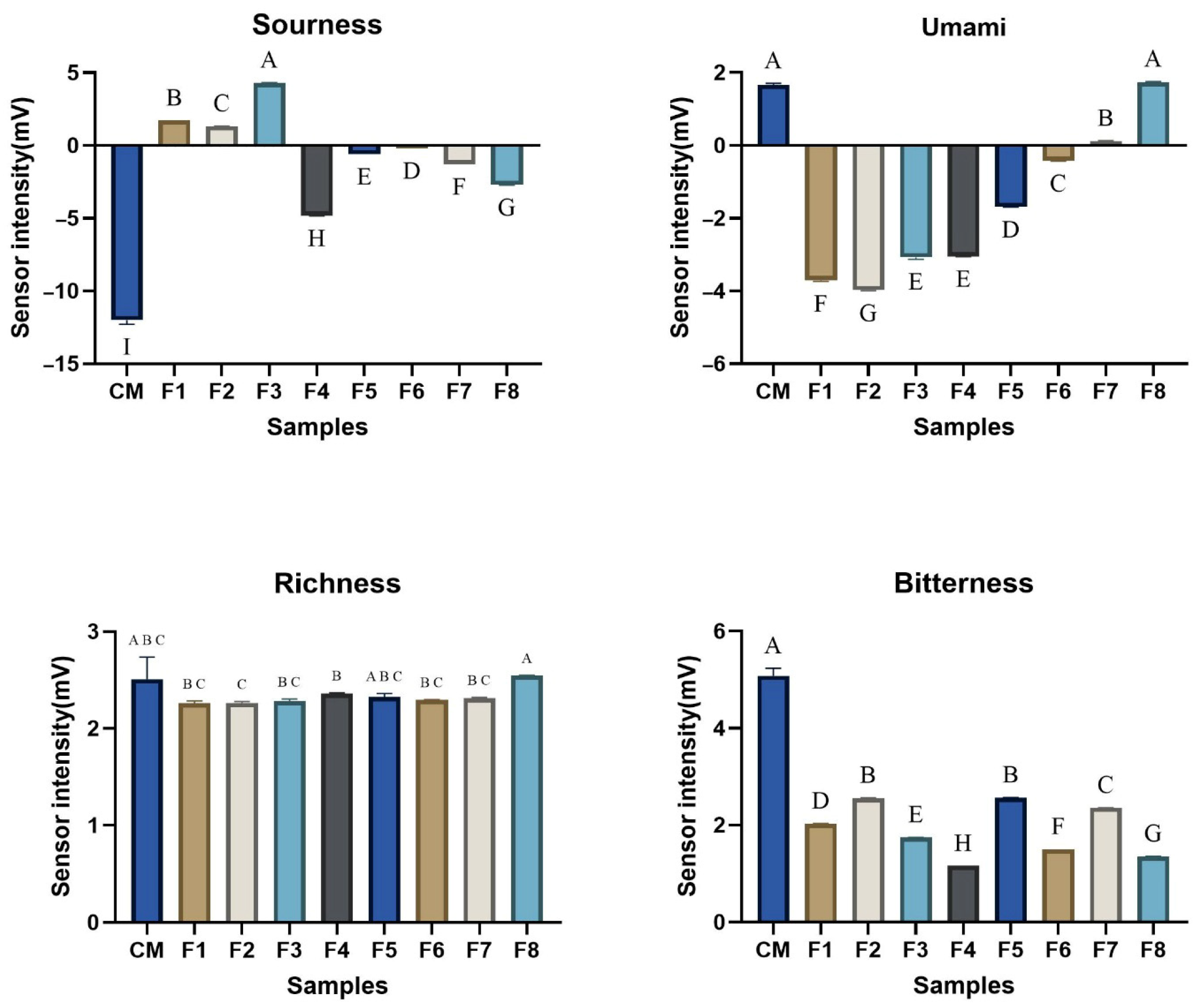
3.5. Comparative Analysis of Taste Characteristics
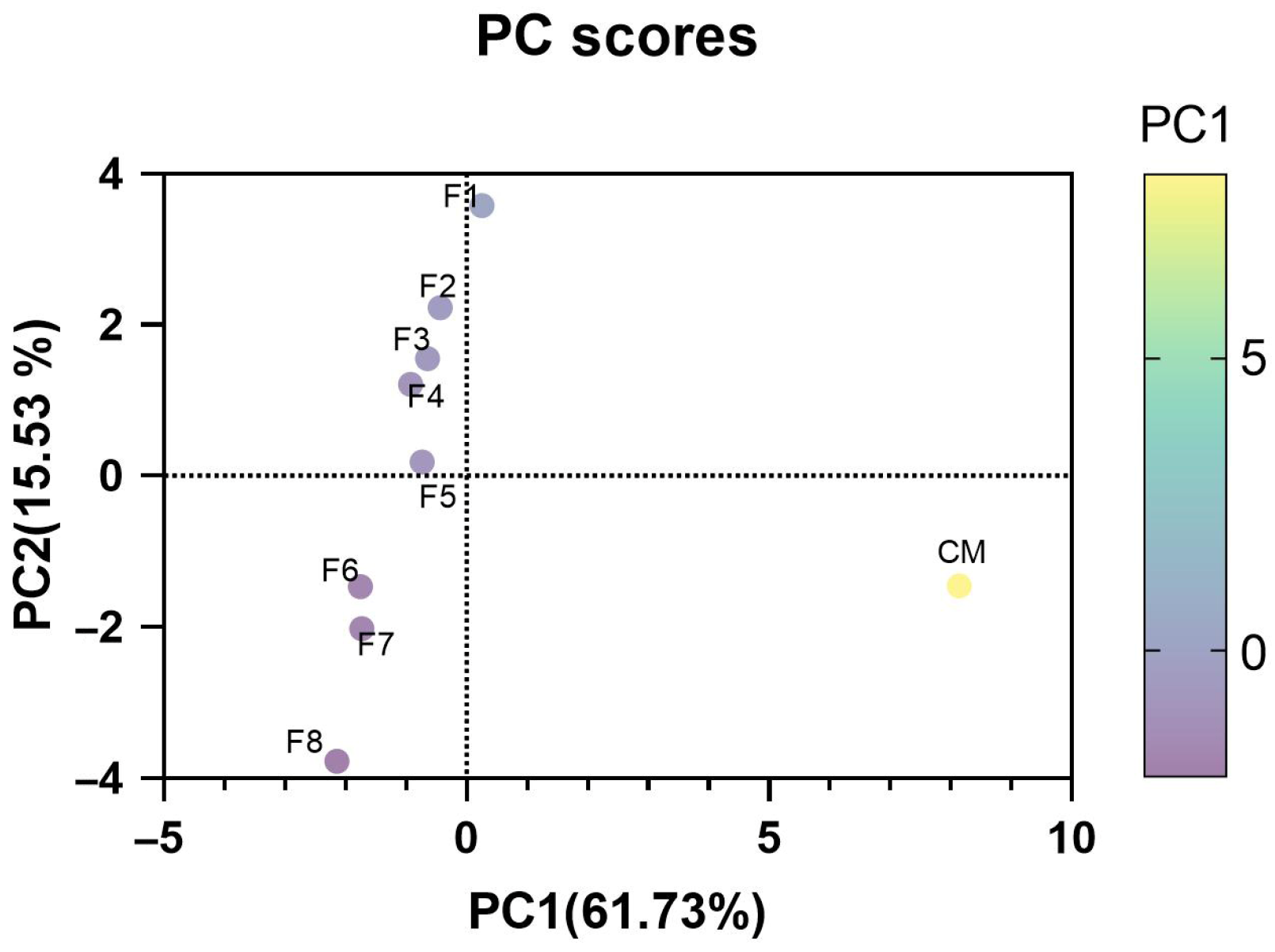
3.6. Principal Component Analysis for Quality Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Zhao, Y.; Jin, X.; Zhu, X.; Fang, Y. Material perspective on the structural design of artificial meat. Adv. Sustain. Syst. 2021, 5, 2100017. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Gill, M.; Wijk, M.v.; Herrero, M.; Ramankutty, N. Livestock policy for sustainable development. Nat. Food 2020, 1, 160–165. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Plant-based meat analogues. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–126. [Google Scholar]
- Kumari, S.; Alam, A.N.; Hossain, M.J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Sensory evaluation of plant-based meat: Bridging the gap with animal meat, challenges and future prospects. Foods 2023, 13, 108. [Google Scholar] [CrossRef]
- Wei, Z.; Wei, K.; Liu, J.; Zhou, Y. The relationship between agricultural and animal husbandry economic development and carbon emissions in Henan Province, the analysis of factors affecting carbon emissions, and carbon emissions prediction. Mar. Pollut. Bull. 2023, 193, 115134. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Kumari, S.; Kim, C.-J.; Lee, E.-Y.; Alam, A.N.; Chung, Y.-S.; Hwang, Y.-H.; Joo, S.-T. Effect of adding cultured meat tissue on physicochemical and taste characteristics of hybrid cultured meat manufactured using wet-spinning. Food Sci. Anim. Resour. 2024, 44, 1440. [Google Scholar] [CrossRef] [PubMed]
- Aryee, A.N.; Boye, J.I. Comparative study of the effects of processing on the nutritional, physicochemical and functional properties of lentil. J. Food Process. Preserv. 2017, 41, e12824. [Google Scholar] [CrossRef]
- Richter, J.K.; Montero, M.L.; Ikuse, M.; Wagner, C.E.; Ross, C.F.; Saunders, S.R.; Ganjyal, G.M. The interaction between wheat and pea protein influences the final chemical and sensory characteristics of extruded high moisture meat analogs. J. Food Sci. 2024, 89, 104–120. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of ingredients and additives in plant-based meat analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. The science of plant—based foods: Constructing next—generation meat, fish, milk, and egg analogs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4049–4100. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Future Foods: How Modern Science is Transforming the Way We Eat; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Zhang, M.; Mittal, G. Measuring tenderness of meat products by Warner Bratzler shear press. J. Food Process. Preserv. 1993, 17, 351–367. [Google Scholar] [CrossRef]
- Damez, J.-L.; Clerjon, S. Meat quality assessment using biophysical methods related to meat structure. Meat Sci. 2008, 80, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Sensini, A.; Zucchelli, A.; Carloni, R.; Focarete, M.L. Hierarchical fibrous structures for muscle—inspired soft—actuators: A review. Appl. Mater. Today 2020, 20, 100772. [Google Scholar] [CrossRef]
- Offer, G.; Knight, P.; Jeacocke, R.; Almond, R.; Cousins, T.; Elsey, J.; Parsons, N.; Sharp, A.; Starr, R.; Purslow, P. The structural basis of the water-holding, appearance and toughness of meat and meat products. Food Struct. 1989, 8, 17. [Google Scholar]
- Yuliarti, O.; Kovis, T.J.K.; Yi, N.J. Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 2021, 288, 110138. [Google Scholar] [CrossRef]
- Lam, A.; Can Karaca, A.; Tyler, R.; Nickerson, M. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Schreuders, F.K.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Grabowska, K.J.; Tekidou, S.; Boom, R.M.; van der Goot, A.-J. Shear structuring as a new method to make anisotropic structures from soy–gluten blends. Food Res. Int. 2014, 64, 743–751. [Google Scholar] [CrossRef]
- Abdullah, N.S.B. Physicochemical and Sensory Properties of Imitation Chicken Nuggets Produced from Chickpea Flour anf Textured Vegetable Protein. Master’s Thesis, Universiti Sains Islam Malaysia, Nilai, Malaysia, 2016. [Google Scholar]
- Kumari, S.; Kim, S.-H.; Kim, C.-J.; Chung, Y.S.; Hwang, Y.-H.; Joo, S.-T. Development and comparative evaluation of imitated fiber from different protein sources using wet-spinning. Food Sci. Anim. Resour. 2024, 44, 1156. [Google Scholar] [CrossRef]
- Ling, Y.; Liu, Y.; Yin, R.; West, A. An eco-friendly droplet-wet spinning technology for producing high-quality hemp/cotton blend yarn. J. Clean. Prod. 2024, 475, 143689. [Google Scholar] [CrossRef]
- Ozipek, B.; Karakas, H. Wet spinning of synthetic polymer fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 174–186. [Google Scholar]
- Pereira, C.; Pinto, T.V.; Santos, R.M.; Correia, N. Sustainable and Naturally Derived Wet Spun Fibers: A Systematic Literature Review. Fibers 2024, 12, 75. [Google Scholar] [CrossRef]
- Var, C.; Palamutcu, S. Diverse Approaches in Wet-Spun Alginate Filament Production from the Textile Industry Perspective: From Process Optimization to Composite Filament Production. Polymers 2024, 16, 1817. [Google Scholar] [CrossRef] [PubMed]
- Rekola, S.-M.; Kårlund, A.; Mikkonen, S.; Kolehmainen, M.; Pomponio, L.; Sozer, N. Structure, texture and protein digestibility of high moisture extruded meat alternatives enriched with cereal brans. Appl. Food Res. 2023, 3, 100262. [Google Scholar] [CrossRef]
- Smetana, S.; Larki, N.A.; Pernutz, C.; Franke, K.; Bindrich, U.; Toepfl, S.; Heinz, V. Structure design of insect-based meat analogs with high-moisture extrusion. J. Food Eng. 2018, 229, 83–85. [Google Scholar] [CrossRef]
- Kim, T. Texturization of Pulse Proteins: Peas, Lentils, and Faba Beans. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2018. [Google Scholar]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Koksel, F.; Masatcioglu, M.T. Physical properties of puffed yellow pea snacks produced by nitrogen gas assisted extrusion cooking. Lwt 2018, 93, 592–598. [Google Scholar] [CrossRef]
- Beck, S.M.; Knoerzer, K.; Foerster, M.; Mayo, S.; Philipp, C.; Arcot, J. Low moisture extrusion of pea protein and pea fibre fortified rice starch blends. J. Food Eng. 2018, 231, 61–71. [Google Scholar] [CrossRef]
- Masatcioglu, M.T.; Koksel, F. Functional and thermal properties of yellow pea and red lentil extrudates produced by nitrogen gas injection assisted extrusion cooking. J. Sci. Food Agric. 2019, 99, 6796–6805. [Google Scholar] [CrossRef]
- Sengar, A.S.; Botinestean, C.; O’Connor, A.; Tiwari, B.K.; Tiwari, U.; Pathania, S. Developing freeze-structured meat alternatives using pea and faba proteins: Evaluating their partial and complete substitution in beef patties. Food Struct. 2025, 45, 100451. [Google Scholar] [CrossRef]
- Venkatachalam, C.D.; Rajan, D.; Arunachalam, S. Optimization of Legume Based Meat Alternative Using Freeze Structuring and Evaluation of its Quality Indices. Suranaree J. Sci. Technol. 2024, 31, 020029. [Google Scholar] [CrossRef]
- Choi, H.W.; Jeon, H.; Lee, J.-Y.; Choi, Y.J.; Hahn, J. Mechanical Stretching Technology for Plant-Based Meat Analogs with Enhanced Texture Utilizing Wheat Gluten and Pea Protein Isolate. LWT 2025, 218, 117479. [Google Scholar] [CrossRef]
- Zhao, Y.-R.; Peng, N.; Li, Y.-Q.; Liang, Y.; Guo, Z.-W.; Wang, C.-Y.; Wang, Z.-Y.; Wang, C.; Ren, X. Physicochemical properties, structural characteristics and protein digestibility of pea protein-wheat gluten composited meat analogues prepared via high-moisture extrusion. Food Hydrocoll. 2024, 156, 110283. [Google Scholar] [CrossRef]
- Cui, B.; Liang, H.; Li, J.; Zhou, B.; Chen, W.; Liu, J.; Li, B. Development and characterization of edible plant-based fibers using a wet-spinning technique. Food Hydrocoll. 2022, 133, 107965. [Google Scholar] [CrossRef]
- Nagamine, S.; Akagi, M.; Nakagawa, K.; Kobayashi, T. Effect of coagulant concentration on soy protein-based fiber for meat substitute by wet spinning combined with ionic cross-linking of alginate. J. Chem. Eng. Jpn. 2023, 56, 2256377. [Google Scholar] [CrossRef]
- Chuang, R.; Naidu, A.; Galipon, J. Enhancing Meat Analog Texture Using Wet—Spun Fibroin Protein Fibers: A Novel Approach to Mimic Whole—Muscle Meat. J. Texture Stud. 2025, 56, e70001. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Liu, H.; Chen, Q.; Liu, Q.; Kong, B. Soy protein isolate-sodium alginate colloidal particles for improving the stability of high internal phase Pickering emulsions: Effects of mass ratios. Food Chem. X 2024, 21, 101094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Yang, C.; Guan, Y.; Liu, N.; Chen, Y.; Wang, K.; Huang, S.; Liu, Y.; Zhu, Q. Effect of ionic type of celluloses attached to pea protein on high internal phase Pickering emulsions properties. Food Chem. X 2025, 28, 102542. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Low-temperature and long-time heating regimes on non-volatile compound and taste traits of beef assessed by the electronic tongue system. Food Chem. 2020, 320, 126656. [Google Scholar] [CrossRef]
- Berlanga-Reyes, C.M.; Carvajal-Millan, E.; Hicks, K.B.; Yadav, M.P.; Rascón-Chu, A.; Lizardi-Mendoza, J.; Toledo-Guillén, A.R.; Islas-Rubio, A.R. Protein/arabinoxylans gels: Effect of mass ratio on the rheological, microstructural and diffusional characteristics. Int. J. Mol. Sci. 2014, 15, 19106–19118. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Li, W.; Martin, G.J.; Ashokkumar, M. An investigation into the mechanism of alkaline extraction-isoelectric point precipitation (AE-IEP) of high-thiol plant proteins. Appl. Sci. 2023, 13, 6469. [Google Scholar] [CrossRef]
- Matalanis, A.; Jones, O.G.; McClements, D.J. Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocoll. 2011, 25, 1865–1880. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, D.; Guo, Y.; Sun, L. Applications of mixed polysaccharide-protein systems in fabricating multi-structures of binary food gels—A review. Trends Food Sci. Technol. 2021, 109, 197–210. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, M.-H.; Bi, X.; Du, J. Physicochemical properties, structural characteristics and in vitro digestion of brown rice–pea protein isolate blend treated by microbial transglutaminase. Food Hydrocoll. 2023, 141, 108673. [Google Scholar] [CrossRef]
- Helmick, H.; Tonner, T.; Hauersperger, D.; Ettestad, S.; Hartanto, C.; Okos, M.; Liceaga, A.; Bhunia, A.K.; Kokini, J.L. Physicochemical characterization of changes in pea protein as the result of cold extrusion. Food Chem. 2023, 423, 136240. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, E.; Erkol, M. The effect of moisture content on colour characteristics of walnuts. Int. J. Food Eng. 2009, 5. [Google Scholar] [CrossRef]
- Hutchings, J.B. Chemistry of food colour. In Food Colour and Appearance; Springer: Berlin/Heidelberg, Germany, 1994; pp. 367–469. [Google Scholar]
- Matiacevich, S.B.; Buera, M.P. A critical evaluation of fluorescence as a potential marker for the Maillard reaction. Food Chem. 2006, 95, 423–430. [Google Scholar] [CrossRef]
- Larsen, R.A. Hydration as a factor in bread flour quality. Cereal Chem 1964, 41, 1964. [Google Scholar]
- Rasper, V.; DeMan, J.M. Measurement of Hydration Capacity of Wheat Flour Starck Mixtures. Cereal Chem. 1980, 57, 27–31. [Google Scholar]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The current situation of pea protein and its application in the food industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Grondin, M.; Hamel, F.; Averill-Bates, D.A.; Sarhan, F. Wheat proteins enhance stability and function of adhesion molecules in cryopreserved hepatocytes. Cell Transplant. 2009, 18, 79–88. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, H.; Zhou, Q.; Li, W.; Gao, J.; Liao, X.; Yu, Z.; Zheng, M.; Zhou, Y.; Sui, X. Influence mechanism of wheat bran cellulose and cellulose nanocrystals on the storage stability of soy protein isolate films: Conformation modification and molecular interaction perspective. Food Hydrocoll. 2023, 139, 108475. [Google Scholar] [CrossRef]
- Day, L.; Augustin, M.; Batey, I.; Wrigley, C. Wheat-gluten uses and industry needs. Trends Food Sci. Technol. 2006, 17, 82–90. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Beyrer, M. Impact of native pea proteins on the gelation properties of pea protein isolates. Food Struct. 2023, 37, 100340. [Google Scholar] [CrossRef]
- Li-Chan, E.; Lacroix, I. Properties of proteins in food systems: An introduction. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–25. [Google Scholar]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Shimoni, Y.; Galili, G. Intramolecular disulfide bonds between conserved cysteines in wheat gliadins control their deposition into protein bodies. J. Biol. Chem. 1996, 271, 18869–18874. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Chen, B.; Rao, J. Pea protein isolate–high methoxyl pectin soluble complexes for improving pea protein functionality: Effect of pH, biopolymer ratio and concentrations. Food Hydrocoll. 2018, 80, 245–253. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ninomiya, K. Umami and food palatability. J. Nutr. 2000, 130, 921S–926S. [Google Scholar] [CrossRef] [PubMed]
- Woychik, J.; Boundy, J.A.; Dimler, R. Wheat gluten proteins, amino acid composition of proteins in wheat gluten. J. Agric. Food Chem. 1961, 9, 307–310. [Google Scholar] [CrossRef]
- Ruusunen, M.; Puolanne, E. Reducing sodium intake from meat products. Meat Sci. 2005, 70, 531–541. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Ma, X.; Yu, M.; Liu, Z.; Deng, D.; Cui, Y.; Tian, Z.; Wang, G. Effect of amino acids and their derivatives on meat quality of finishing pigs. J. Food Sci. Technol. 2020, 57, 404–412. [Google Scholar] [CrossRef] [PubMed]

| Wheat Protein (%) | Pea Protein Isolate (%) | Sodium Alginate (%) | |
|---|---|---|---|
| F1 | 16 | 0 | 2 |
| F2 | 14 | 4 | 2 |
| F3 | 12 | 6 | 2 |
| F4 | 10 | 8 | 2 |
| F5 | 8 | 10 | 2 |
| F6 | 6 | 12 | 2 |
| F7 | 4 | 14 | 2 |
| F8 | 0 | 16 | 2 |
| CM | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | SEM | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 5.62 G | 5.49 G | 5.91 F | 5.98 E | 6.05 D | 6.07 D | 6.14 C | 6.20 B | 6.26 A | 0.007 | <0.001 |
| Color | |||||||||||
| CIE L* | 53.79 I | 62.88 H | 64.96 G | 66.26 F | 68.50 E | 70.45 D | 71.45 C | 72.46 B | 73.90 A | 0.049 | <0.001 |
| CIE a* | 7.17 A | 1.295 E | 0.55 F | 1.51 D | 1.49 D | 1.59 C | 1.24 E | 0.57 F | 2.28 B | 0.008 | <0.001 |
| CIE b* | 2.08 F | 16.89 B | 14.25 E | 16.05 D | 16.22 C,D | 16.41 C | 15.94 D | 17.11 B | 19.29 A | 0.008 | <0.001 |
| CM | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | SEM | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WBSF (kg/cm2) | 1.22 D | 4.71 A | 4.62 A | 4.57 A | 4.51 A | 4.45 B | 4.40 B | 4.34 B,C | 4.26 C | 0.007 | <0.0001 |
| Hardness (N) | 25.88 B,C | 26.53 B | 25.00 D | 23.03 E | 22.00 F | 23.53 E | 25.03 C,D | 27.02 B | 32.20 A | 1.990 | <0.0001 |
| Springiness | 0.86 F | 0.87 F | 0.87 F | 0.88 E | 0.88 E | 0.89 D | 0.91 C | 0.92 B | 0.93 A | 0.049 | <0.0001 |
| Gumminess (N) | 3.63 H | 6.50 G | 6.97 F,G | 7.80 E,F | 8.20 E | 9.00 D | 10.50 C | 12.00 B | 13.27 A | 0.068 | <0.0001 |
| Chewiness (N) | 4.79 H | 6.20 G | 6.80 F | 7.20 E,F | 7.50 E | 8.10 D | 8.91 C | 9.50 B | 11.73 A | 0.008 | <0.0001 |
| Cohesiveness | 0.26 C,D,E,F | 0.25 F | 0.25 F | 0.26 E | 0.26 E | 0.27 D | 0.28 C | 0.29 B | 0.30 A | 0.008 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Kim, S.-H.; Kim, C.-J.; Hwang, Y.-H.; Joo, S.-T. Wet-Spinning Technology for Plant-Based Meat Alternative: Influence of Protein Composition on Physicochemical and Textural Properties. Foods 2025, 14, 3913. https://doi.org/10.3390/foods14223913
Kumari S, Kim S-H, Kim C-J, Hwang Y-H, Joo S-T. Wet-Spinning Technology for Plant-Based Meat Alternative: Influence of Protein Composition on Physicochemical and Textural Properties. Foods. 2025; 14(22):3913. https://doi.org/10.3390/foods14223913
Chicago/Turabian StyleKumari, Swati, So-Hee Kim, Chan-Jin Kim, Young-Hwa Hwang, and Seon-Tea Joo. 2025. "Wet-Spinning Technology for Plant-Based Meat Alternative: Influence of Protein Composition on Physicochemical and Textural Properties" Foods 14, no. 22: 3913. https://doi.org/10.3390/foods14223913
APA StyleKumari, S., Kim, S.-H., Kim, C.-J., Hwang, Y.-H., & Joo, S.-T. (2025). Wet-Spinning Technology for Plant-Based Meat Alternative: Influence of Protein Composition on Physicochemical and Textural Properties. Foods, 14(22), 3913. https://doi.org/10.3390/foods14223913







