Gelatin-Based Films Containing Extracts of Prickly Pear (Opuntia guerrana): Characterization and Evaluation of Bioactive Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Physicochemical Analysis of PP and FP
2.2. Extracts Obtained from PP and FP
2.3. Antioxidant Activity of PP and FP
2.4. Edible Films Elaboration
2.5. Gelatin Edible Film Characterization
2.6. Mechanical Properties
2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Biodegradability
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analysis of PP and FP
3.2. Antioxidant Activity of PP and FP from Opuntia guerrana
3.3. Gelatin Films Physicochemical Properties
Fourier Transform Infrared Spectroscopy
3.4. Gelatin-Based Films Optical Properties
3.5. Gelatin Films Barrier Properties
3.6. Thermal Properties of Gelatin Films
3.7. Mechanical Properties of Gelatin Films
3.8. Functional Properties of Gelatin Films
3.9. Biodegradability of Gelatin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PP | Powder peel |
| FP | Fresh pulp |
| GF | Gelatin film |
| GFP | Gelatin film + fresh pulp |
| GPP | Gelatin film + powder peel |
| GM | Gelatin film + mix (PP and FP) |
References
- Toniciolli Rigueto, C.V.; Rosseto, M.; Alessandretti, I.; de Oliveira, R.; Wohlmuth, D.A.R.; Ferreira Menezes, J.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin films from wastes: A review of production, characterization, and application trends in food preservation and agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel Materials in the Preparation of Edible Films and Coatings—A Review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Tyuftin, A.A.; Kerry, J.P. Gelatin films: Study review of barrier properties and implications for future studies employing biopolymer films. Food Packag. Shelf Life 2021, 29, 100688. [Google Scholar] [CrossRef]
- Manzur-Valdespino, S.; Arias-Rico, J.; Ramírez-Moreno, E.; Sánchez-Mata, M.d.C.; Jaramillo-Morales, O.A.; Angel-García, J.; Zafra-Rojas, Q.Y.; Barrera-Gálvez, R.; Cruz-Cansino, N.d.S. Applications and Pharmacological Properties of Cactus Pear (Opuntia spp.) Peel: A Review. Life 2022, 12, 1903. [Google Scholar] [CrossRef]
- Reyes-Agüero, J.A.; Aguirre-Rivera, J.R.; Hernández, H.M.J.A. Systematic notes and a detailed description of Opuntia ficus-indica (L.) Mill.(Cactaceae). Agrociencia 2005, 39, 395–408. [Google Scholar]
- Rodrigues, C.; Paula, C.D.d.; Lahbouki, S.; Meddich, A.; Outzourhit, A.; Rashad, M.; Pari, L.; Coelhoso, I.; Fernando, A.L.; Souza, V.G.L. Opuntia spp.: An Overview of the Bioactive Profile and Food Applications of This Versatile Crop Adapted to Arid Lands. Foods 2023, 12, 1465. [Google Scholar] [CrossRef]
- Estrella-Osuna, D.E.; Ruiz-Cruz, S.; Rodríguez-Félix, F.; Figueroa-Enríquez, C.E.; González-Ríos, H.; Fernández-Quiroz, D.; Márquez-Ríos, E.; Tapia-Hernández, J.A.; Pérez-Álvarez, J.Á.; Suárez-Jiménez, G.M. Rheological Properties and Antioxidant Activity of Gelatin-Based Edible Coating Incorporating Tomato (Solanum lycopersicum L.) Extract. Gels 2024, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Coelhoso, I. Polysaccharide Films/Membranes for Food and Industrial Applications. Polysaccharides 2025, 6, 48. [Google Scholar] [CrossRef]
- Figueroa-Enriquez, C.E.; Rodríguez-Félix, F.; Ruiz-Cruz, S.; Castro-Enriquez, D.D.; Gonzalez-Rios, H.; Perez-Alvarez, J.Á.; Madera-Santana, T.J.; Burruel-Ibarra, S.E.; Tapia-Hernández, J.A.; Estrella-Osuna, D.E. Edible Coating of Sodium Alginate With Gelatin Nanoparticles and Pitaya Extract (Stenocereus thurberi): Physicochemical and Antioxidant Properties. J. Food Qual. 2025, 2025, 5756522. [Google Scholar] [CrossRef]
- Weldearegay, S.G.; Gaddala, B.; Fentie, E.G.; Sundramurthy, V.P.; Priya, L.S. Valorization of waste prickly pear peels: Optimization for pectin extraction, characterization, and development of edible film. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Amor, N.B.; Lo Vecchio, G.; Nava, V.; Rando, R.; Ben Mansour, H.; Lo Turco, V. Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef]
- AOAC. Oficial Methodos of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Pinedo-Espinoza, J.M.; Aguirre-Mancilla, C.L.; Jiménez-Alvarado, R.; Fuente, G.I.d.l.; Ramírez-Pimentel, J.G.; Hernández-Fuentes, A.D. Bioactive compounds and antioxidant activity evolution during the ripening process of 12 Opuntia spp. fruit accessions. Emir. J. Food Agric. 2017, 29, 138–148. [Google Scholar] [CrossRef]
- Espino-Manzano, S.O.; León-López, A.; Aguirre-Álvarez, G.; González-Lemus, U.; Prince, L.; Campos-Montiel, R.G. Application of Nanoemulsions (W/O) of Extract of Opuntia oligacantha C.F. Först and Orange Oil in Gelatine Films. Molecules 2020, 25, 3487. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- González-Aguayo, E.; Campos-Montiel, R.; Pinedo-Espinoza, J.; Aguirre-Álvarez, G.; Hernández-Fuentes, A.D. Estabilidad del color en extractos de diferentes genotipos de tunas rojas (Opuntia spp.). Rev. Científica Biológico Agropecu. Tuxpan 2014, 2, 728–735. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sood, A.; Saini, C.S. Utilization of peel of white pomelo for the development of pectin based biodegradable composite films blended with casein and egg albumen. Food Chem. Adv. 2022, 1, 100054. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.d.J.; Pimentel-González, D.J.; Del Razo-Rodríguez, O.E.; Medina-Pérez, G.; Carrillo-Inungaray, M.L.; Reyes-Munguía, A.; Campos-Montiel, R.G. Antioxidant and antibacterial activities of a starch film with bioextracts microencapsulated from cactus fruits (Opuntia oligacantha). Food Sci. Biotechnol. 2019, 28, 1553–1561. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Jamilah, B.; Nur Hanani, Z.A. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Fauzan, H.R.; Ningrum, A.; Supriyadi, S. Evaluation of a Fish Gelatin-Based Edible Film Incorporated with Ficus carica L. Leaf Extract as Active Packaging. Gels 2023, 9, 918. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Susmitha, A.; Sasikumar, K.; Rajan, D.; Padmakumar M, A.; Nampoothiri, K.M. Development and characterization of corn starch-gelatin based edible films incorporated with mango and pineapple for active packaging. Food Biosci. 2021, 41, 100977. [Google Scholar] [CrossRef]
- Mora-Palma, R.M.; Martinez-Munoz, P.E.; Contreras-Padilla, M.; Feregrino-Perez, A.; Rodriguez-Garcia, M.E. Evaluation of water diffusion, water vapor permeability coefficients, physicochemical and antimicrobial properties of thin films of nopal mucilage, orange essential oil, and orange pectin. J. Food Eng. 2024, 366, 111865. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmed, A.R.; Mohamed, H.I.; Al-Otaibi, H.H.; Ramadan, K.M.A.; Elkatry, H.O. Utilization of Prickly Pear Peels Flour as a Natural Source of Minerals, Dietary Fiber and Antioxidants: Effect on Cakes Production. Agronomy 2023, 13, 439. [Google Scholar] [CrossRef]
- Nouraddini, M.; Esmaiili, M.; Mohtarami, F. Development and characterization of edible films based on eggplant flour and corn starch. Int. J. Biol. Macromol. 2018, 120, 1639–1645. [Google Scholar] [CrossRef]
- Bourhia, M.; Elmahdaoui, H.; Moussa, S.I.; Ullah, R.; Bari, A. Potential Natural Dyes Food from the Powder of Prickly Pear Fruit Peels (Opuntia spp.) Growing in the Mediterranean Basin under Climate Stress. BioMed Res. Int. 2020, 2020, 7579430. [Google Scholar] [CrossRef]
- Ettalibi, F.; Elmahdaoui, H.; Amzil, J.; Gadhi, C.; Harrak, H. Drying impact on physicochemical and biochemical criteria of prickly pear fruit peels of three varieties of Opuntia spp. Mater. Today Proc. 2020, 27, 3243–3248. [Google Scholar] [CrossRef]
- Alzaeem, I.; Ebrahim, K. Evaluation of Quality of Storage of Prickly Pear (Opuntia Ficus Indica (L.)) Using Two Packaging Methods. Online J. Anim. Feed. Res. 2023, 5, 19–28. [Google Scholar]
- Valero-Galván, J.; González-Fernández, R.; Sigala-Hernández, A.; Núñez-Gastélum, J.A.; Ruiz-May, E.; Rodrigo-García, J.; Larqué-Saavedra, A.; Martínez-Ruiz, N.d.R. Sensory attributes, physicochemical and antioxidant characteristics, and protein profile of wild prickly pear fruits (O. macrocentra Engelm., O. phaeacantha Engelm., and O. engelmannii Salm-Dyck ex Engelmann.) and commercial prickly pear fruits (O. ficus-indica (L.) Mill.). Food Res. Int. 2021, 140, 109909. [Google Scholar] [CrossRef]
- Todaro, M.; Alabiso, M.; Di Grigoli, A.; Scatassa, M.L.; Cardamone, C.; Mancuso, I.; Mazza, F.; Bonanno, A. Prickly Pear By-Product in the Feeding of Livestock Ruminants: Preliminary Investigation. Animals 2020, 10, 949. [Google Scholar] [CrossRef]
- Abou-Zaid, F.O.; Ahmed, F.; Zedan, A.E.-H.I. Using of Prickly Pear (Opuntia Spp.) Fruit Juice and Peels in Cookies Production. Alex. Sci. Exch. J. 2022, 43, 239–248. [Google Scholar] [CrossRef]
- Scarano, P.; Tartaglia, M.; Zuzolo, D.; Prigioniero, A.; Guarino, C.; Sciarrillo, R. Recovery and Valorization of Bioactive and Functional Compounds from the Discarded of Opuntia ficus-indica (L.) Mill. Fruit Peel. Agronomy 2022, 12, 388. [Google Scholar] [CrossRef]
- Bellumori, M.; Innocenti, M.; Andrenelli, L.; Melani, F.; Cecchi, L.; Pandino, G.; Mauromicale, G.; La Malfa, S.; Mulinacci, N. Composition of discarded Sicilian fruits of Opuntia ficus indica L.: Phenolic content, mineral profile and antioxidant activity in peel, seeds and whole fruit. Food Chem. 2023, 428, 136756. [Google Scholar] [CrossRef]
- Yahia, E.M.; Mondragon-Jacobo, C. Nutritional components and anti-oxidant capacity of ten cultivars and lines of cactus pear fruit (Opuntia spp.). Food Res. Int. 2011, 44, 2311–2318. [Google Scholar] [CrossRef]
- Bousbia, N.; Mazari, A.; Lamoudi, L.; Akretche-kelfat, S.; Chibane, N.; Dif, M.E. Evaluation of the phytochemical composition and the antioxidant activity of cactus pear flowers and fruit derivatives. Agrobiologia 2022, 12, 3235–3243. [Google Scholar]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A Systematic Review of Its Phytochemicals and Pharmacological Activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Pérez-Marroquín, X.A.; Aguirre-Cruz, G.; Campos-Lozada, G.; Callejas-Quijada, G.; León-López, A.; Campos-Montiel, R.G.; García-Hernández, L.; Méndez-Albores, A.; Vázquez-Durán, A.; Aguirre-Álvarez, G. Green Synthesis of Silver Nanoparticles for Preparation of Gelatin Films with Antimicrobial Activity. Polymers 2022, 14, 3453. [Google Scholar] [CrossRef]
- de Wit, M.; du Toit, A.; Osthoff, G.; Hugo, A. Cactus pear antioxidants: A comparison between fruit pulp, fruit peel, fruit seeds and cladodes of eight different cactus pear cultivars (Opuntia ficus-indica and Opuntia robusta). J. Food Meas. Charact. 2019, 13, 2347–2356. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Effect of date fruit waste extract as an antioxidant additive on the properties of active gelatin films. Food Chem. 2021, 355, 129631. [Google Scholar] [CrossRef]
- Fatima, S.; Mir, M.I.; Khan, M.R.; Sayyed, R.Z.; Mehnaz, S.; Abbas, S.; Sadiq, M.B.; Masih, R. The Optimization of Gelatin Extraction from Chicken Feet and the Development of Gelatin Based Active Packaging for the Shelf-Life Extension of Fresh Grapes. Sustainability 2022, 14, 7881. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Monitoring the freshness of fish fillets by colorimetric gelatin composite film incorporated with curcumin extract. Biocatal. Agric. Biotechnol. 2023, 50, 102722. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Song, L. Characterization of antioxidant and antibacterial gelatin films incorporated with Ginkgo biloba extract. RSC Adv. 2019, 9, 27449–27454. [Google Scholar] [CrossRef] [PubMed]
- Iahnke, A.O.e.S.; Costa, T.M.H.; de Oliveira Rios, A.; Flôres, S.H. Antioxidant films based on gelatin capsules and minimally processed beet root (Beta vulgaris L. var. Conditiva) residues. J. Appl. Polym. Sci. 2016, 133, 4309. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Pérez, S.; Hernández-Carranza, P.; Ávila-Sosa, R. Physical and Antioxidant Characterization of Edible Films Added with Red Prickly Pear (Opuntia ficus-indica L.) cv. San Martín Peel and/or Its Aqueous Extracts. Food Bioprocess Technol. 2018, 11, 368–379. [Google Scholar] [CrossRef]
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.S.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia Ficus Indica Edible Parts: A Food and Nutritional Security Perspective. Food Rev. Int. 2022, 38, 930–952. [Google Scholar] [CrossRef]
- López-Palestina, C.U.; Aguirre-Mancilla, C.L.; Raya-Pérez, J.C.; Ramirez-Pimentel, J.G.; Vargas-Torres, A.; Hernández-Fuentes, A.D. Physicochemical and antioxidant properties of gelatin-based films containing oily tomato extract (Solanum lycopersicum L.). CyTA-J. Food 2019, 17, 142–150. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Jawad, M.; Shah, Y.A.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. A Comparative Study of the Properties of Gelatin (Porcine and Bovine)-Based Edible Films Loaded with Spearmint Essential Oil. Biomimetics 2023, 8, 172. [Google Scholar] [CrossRef]
- Aguirre-Alvarez, G.; Pimentel-González, D.J.; Campos-Montiel, R.G.; Foster, T.; Hill, S.E. The effect of drying temperature on mechanical properties of pig skin gelatin films El efecto de la temperatura de secado sobre las propiedades mecánicas de películas de gelatina de cerdo. CyTA-J. Food 2011, 9, 243–249. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Biodegradable Films Based on Gelatin and Papaya Peel Microparticles with Antioxidant Properties. Food Bioprocess Technol. 2018, 11, 536–550. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant potential of eugenol and ginger essential oils with gelatin/chitosan films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Villasante, J.; Martin-Lujano, A.; Almajano, M.P. Characterization and Application of Gelatin Films with Pecan Walnut and Shell Extract (Carya illinoiensis). Polymers 2020, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
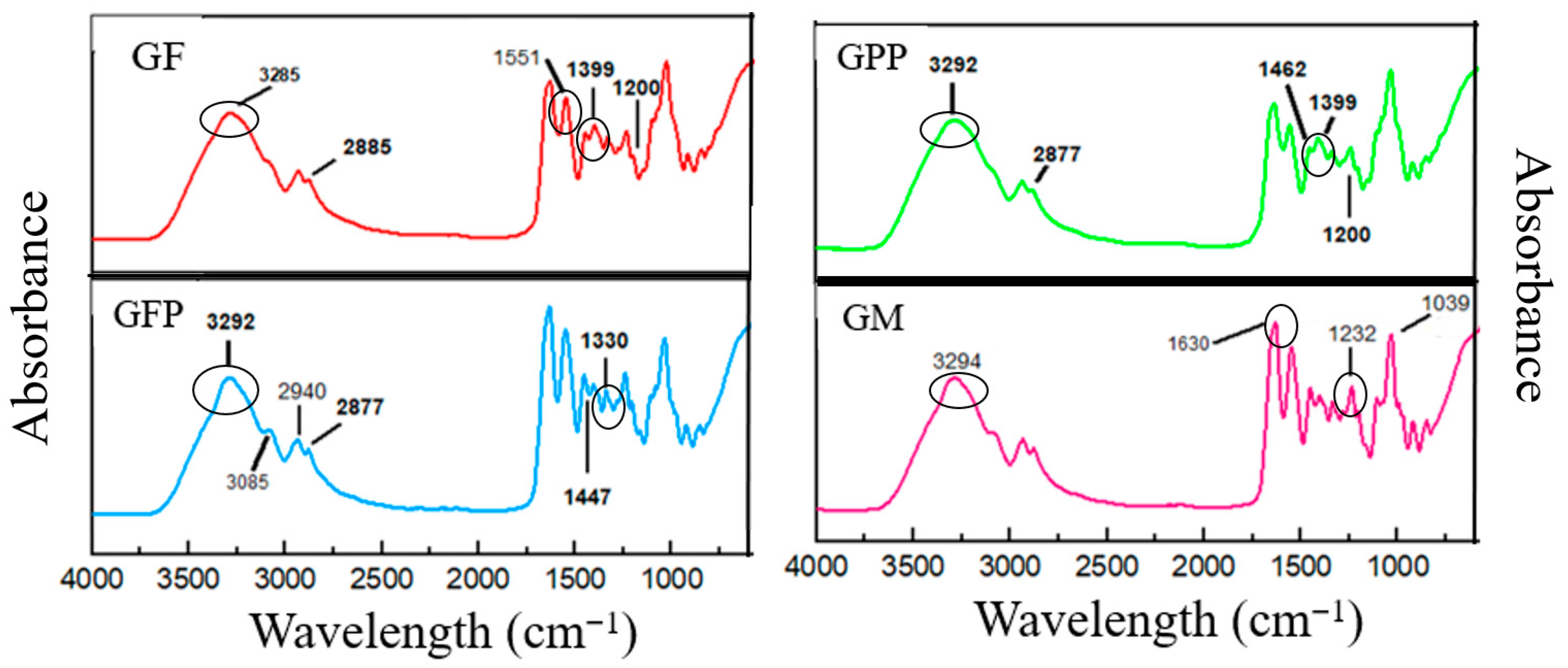
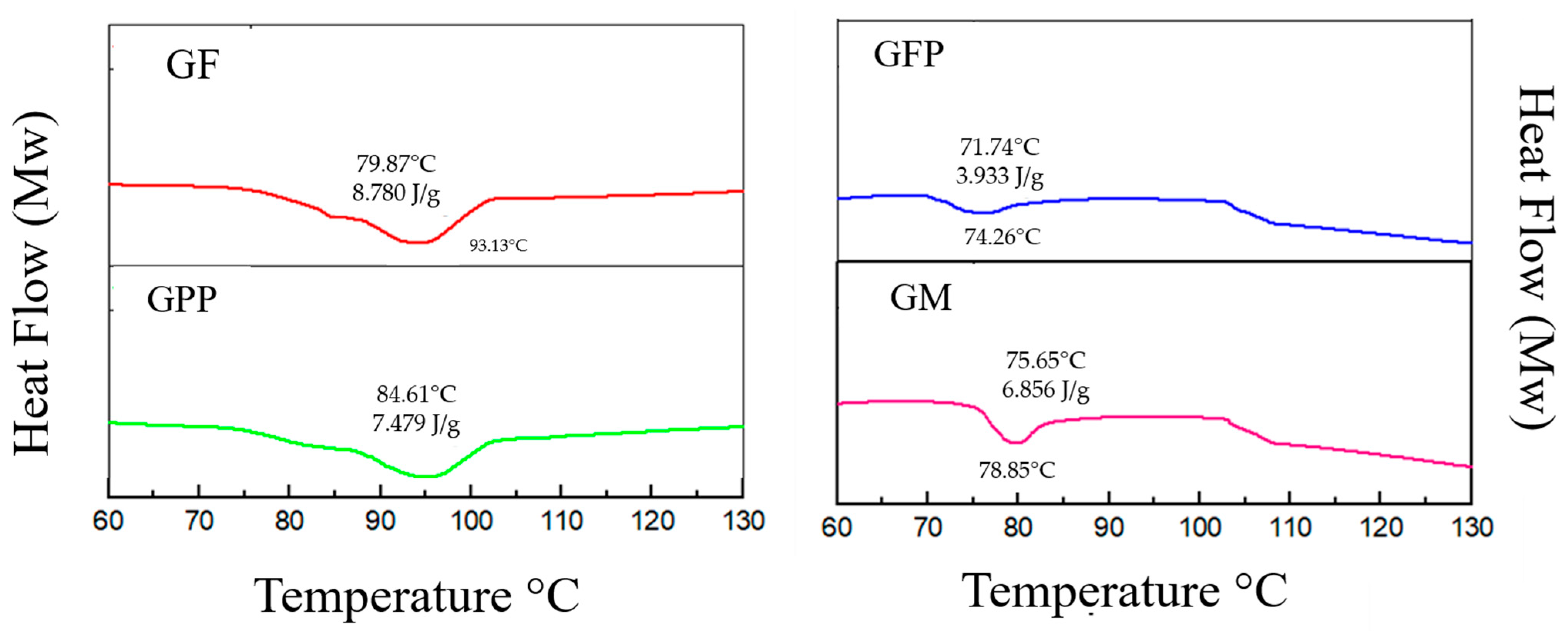

| Formulations | Gelatin (w/w) | Solvent (v/v) | Glycerol (w/v) | PP (w/v) | FP (w/v) |
|---|---|---|---|---|---|
| GF | 2% | 180 mL | 1% | ------- | ------- |
| GFP | 2% | 180 mL | 1% | ------- | 2% |
| GPP | 2% | 180 mL | 1% | 2% | ------- |
| GM | 2% | 180 mL | 1% | 2% | 1% |
| Parameter | PP (%w/w) | FP (%w/w) |
|---|---|---|
| Humidity | 3.66 ± 0.57 | 79.63 ± 0.52 |
| Protein | 4.41 ± 0.06 | 0.440 ± 0.01 |
| Fat | 2.50 ± 0.73 | 0.251 ± 0.02 |
| Ash | 11.96 ± 0.15 | 0.793 ± 0.00 |
| Fiber | 9.41 ± 1.53 | 0.827 ± 0.02 |
| Carbohydrates | 49.2 | 97.68 |
| Sample | Phenols (mg EAG/mL/g) | Flavonoids (mg EQ/g) | Betalains (mg/g) | DPPH Inhibition (%) | ABTS Inhibition (%) |
|---|---|---|---|---|---|
| PP | 3611.94 ± 22.39 | 906.67 ± 8.10 | 128.53 ± 0.34 | 72.82 ± 2.86 | 98.19 ± 0.21 |
| FP | 1810.95 ± 15.02 | 566.96 ± 27.21 | 139.05 ± 1.96 | 38.10 ± 1.50 | 67.42 ± 0.35 |
| Formulation | L | a* | b* | Opacity | |
|---|---|---|---|---|---|
| GF | 91.08 ± 1.03 a | −0.31 ± 0. 14 a | −1.74 ± 0.23 b | 0.98 ± 0.06 a |  |
| GFP | 67.59 ± 2.16 b | 33.53 ± 2.34 b | −15.39 ± 1.03 a | 1.06 ± 0.00 a | |
| GPP | 46.26 ± 1.14 c | 39.53 ± 2.28 c | 10.73 ± 1.31 d | 2.31 ± 0.01 b | |
| GM | 43.52 ± 1.80 d | 41.45 ± 1.63 d | 8.82 ± 1.46 c | 2.64 ± 0.09 c |
| Formulation | WVP (g/m s Pa) | Humidity (%w/w) | Solubility (%w/w) | Swelling Degree (%w/w) |
|---|---|---|---|---|
| GF | 5.81 × 10−10 ± 2.27 × 10−8 b | 31.82 ± 1.76 a | 92.65 ± 1.63 a | 237.19 ± 18.05 a |
| GFP | 6.15 × 10−10 ± 1.28 × 10−8 b | 36.71 ± 1.31 a | 92.34 ± 0.12 a | 309.40 ± 59.07 a |
| GPP | 7.84 × 10−10 ± 1.05 × 10−9 ab | 36.44 ± 1.82 b | 91.23 ± 1.62 b | 238.93 ± 12.40 b |
| GM | 6.74 × 10−10 ± 5.1 × 10−9 b | 36.00 ± 0.67 b | 89.69 ± 0.60 a | 325.23 ± 17.17 c |
| Formulation | Thickness (mm) | TS (MPa) | EAB % | YM (MPa) |
|---|---|---|---|---|
| GF | 0.098 ± 0.01 c | 222.57 ± 6.12 a | 33.50 ± 1.41 b | 0.328 ± 0.06 a |
| GFP | 0.121 ± 0.01 b | 163.20 ± 3.14 b | 42.20 ± 1.38 a | 0.157 ± 0.01 b |
| GPP | 0.278 ± 0.01 a | 57.32 ± 3.82 c | 18.33 ± 0.10 c | 0.155 ± 0.06 b |
| GM | 0.275 ± 0.01 a | 60.02 ± 2.28 c | 43.10 ± 1.25 a | 0.054 ± 0.02 c |
| Formulation | Fenols (mg EAG/g) | Flavonoids (mg EQ/g) | Betalain (mg/g) | ABTS Inhibition % | DPPH Inhibition % |
|---|---|---|---|---|---|
| GF | ------------ | ------------ | ----------- | 42.31 ± 0.82 d | 12.30 ± 1.57 c |
| GFP | 625.87 ± 3.76 c | 175.29 ± 5.88 c | 20.30 ± 0.34 c | 65.28 ± 0.14 c | 15.42 ± 2.61 b |
| GPP | 1304.98 ± 23.14 a | 1289.02 ± 12.51 a | 119.89 ± 1.46 a | 98.24 ± 0.08 b | 38.50 ± 2.11 a |
| GM | 1144 ± 31.34 b | 1159.61 ± 21.33 b | 98.68 ± 0.64 b | 98.00 ± 0.14 a | 42.72 ± 1.56 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-López, A.; Flores-Gutiérrez, E.V.; Cenobio-Galindo, A.d.J.; Islas-Moreno, A.; Aguirre-Álvarez, G.; Carreño-Márquez, I.J.A. Gelatin-Based Films Containing Extracts of Prickly Pear (Opuntia guerrana): Characterization and Evaluation of Bioactive Properties. Foods 2025, 14, 3911. https://doi.org/10.3390/foods14223911
León-López A, Flores-Gutiérrez EV, Cenobio-Galindo AdJ, Islas-Moreno A, Aguirre-Álvarez G, Carreño-Márquez IJA. Gelatin-Based Films Containing Extracts of Prickly Pear (Opuntia guerrana): Characterization and Evaluation of Bioactive Properties. Foods. 2025; 14(22):3911. https://doi.org/10.3390/foods14223911
Chicago/Turabian StyleLeón-López, Arely, Elvia Verónica Flores-Gutiérrez, Antonio de Jesús Cenobio-Galindo, Asael Islas-Moreno, Gabriel Aguirre-Álvarez, and Iván Jalil Antón Carreño-Márquez. 2025. "Gelatin-Based Films Containing Extracts of Prickly Pear (Opuntia guerrana): Characterization and Evaluation of Bioactive Properties" Foods 14, no. 22: 3911. https://doi.org/10.3390/foods14223911
APA StyleLeón-López, A., Flores-Gutiérrez, E. V., Cenobio-Galindo, A. d. J., Islas-Moreno, A., Aguirre-Álvarez, G., & Carreño-Márquez, I. J. A. (2025). Gelatin-Based Films Containing Extracts of Prickly Pear (Opuntia guerrana): Characterization and Evaluation of Bioactive Properties. Foods, 14(22), 3911. https://doi.org/10.3390/foods14223911







