Modulation of IL-1β and TGF-β1 Gene Expression in Stress-Induced Depression Rat Supplemented with Malaysian Acacia Honey
Abstract
1. Introduction
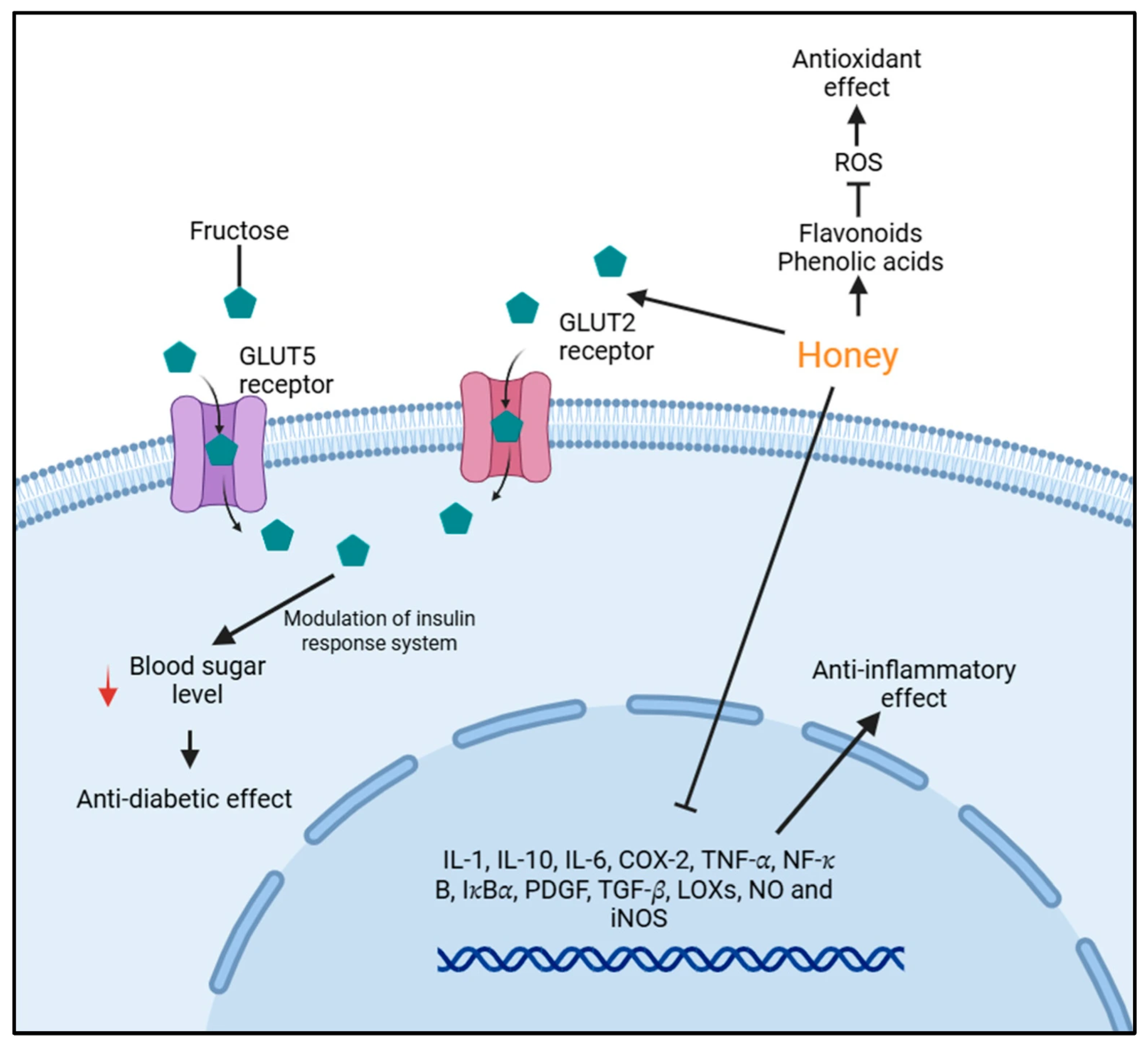
2. Methodology
2.1. Chemicals
2.2. Honey Collection
2.3. Housing and Husbandry of Rats
2.4. Stress-Depression Model
2.5. Hemolysis Assay
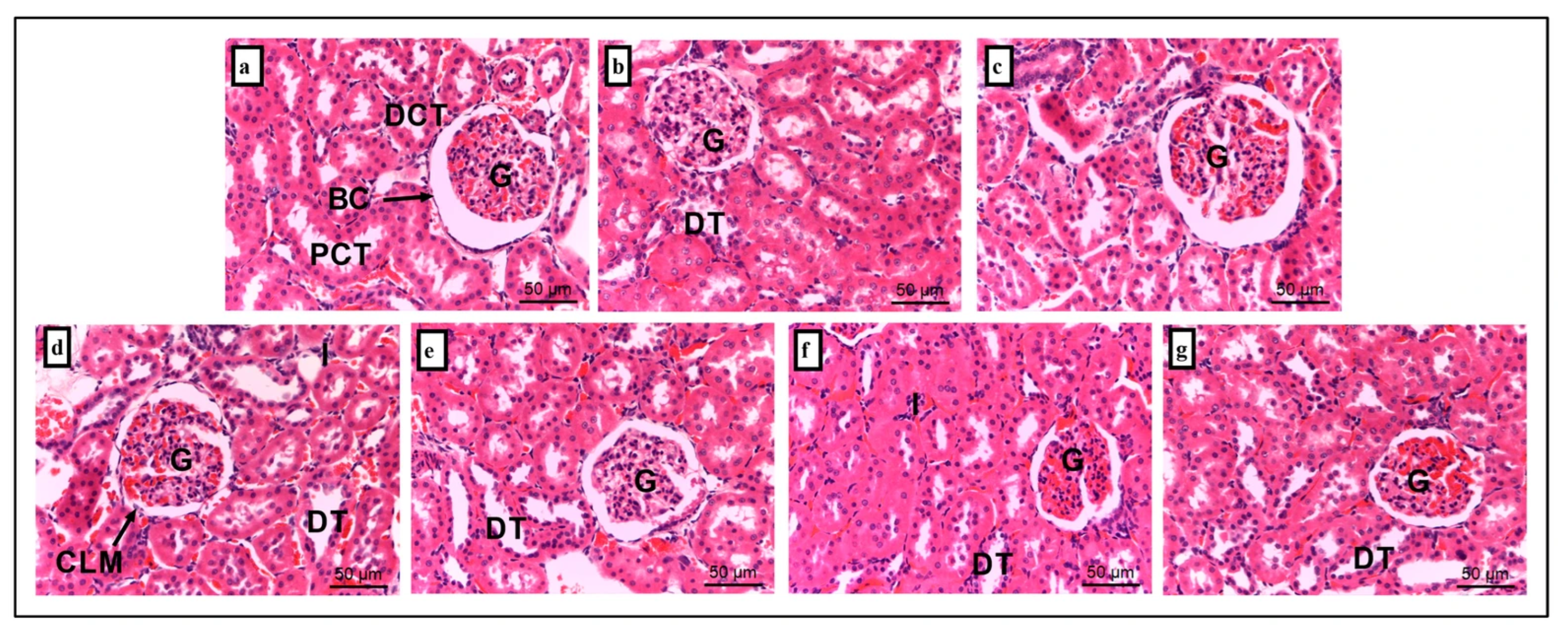
2.6. Histopathology
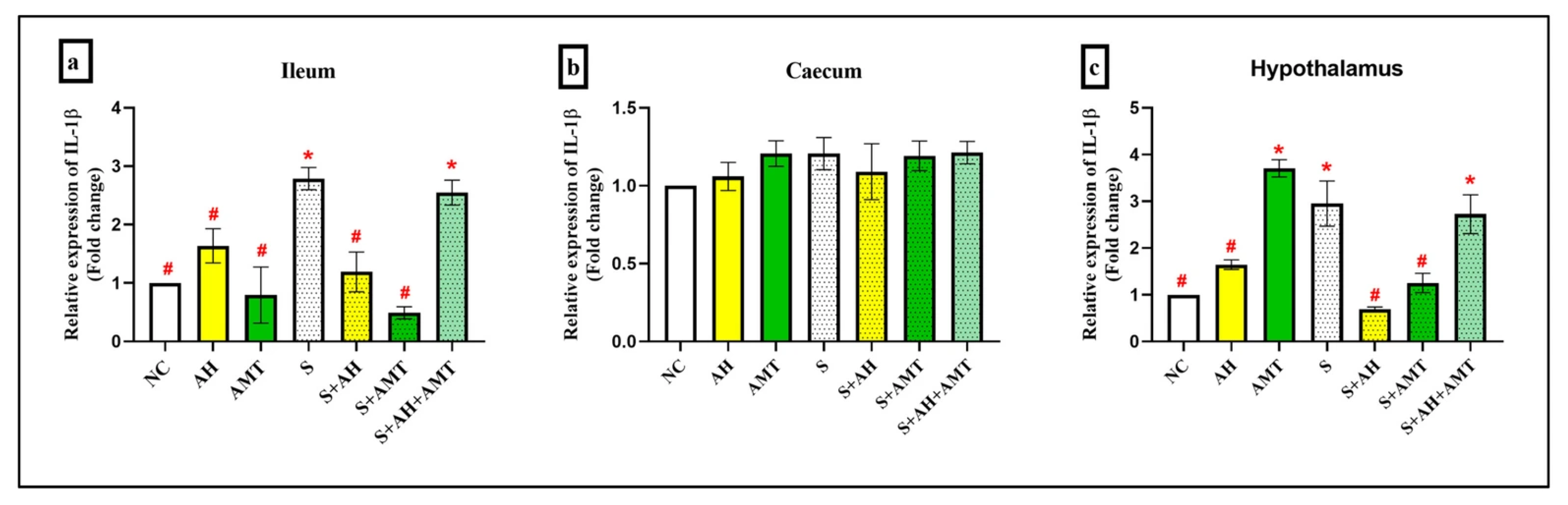
2.7. Quantitative Real-Time PCR
2.8. Statistical Analysis
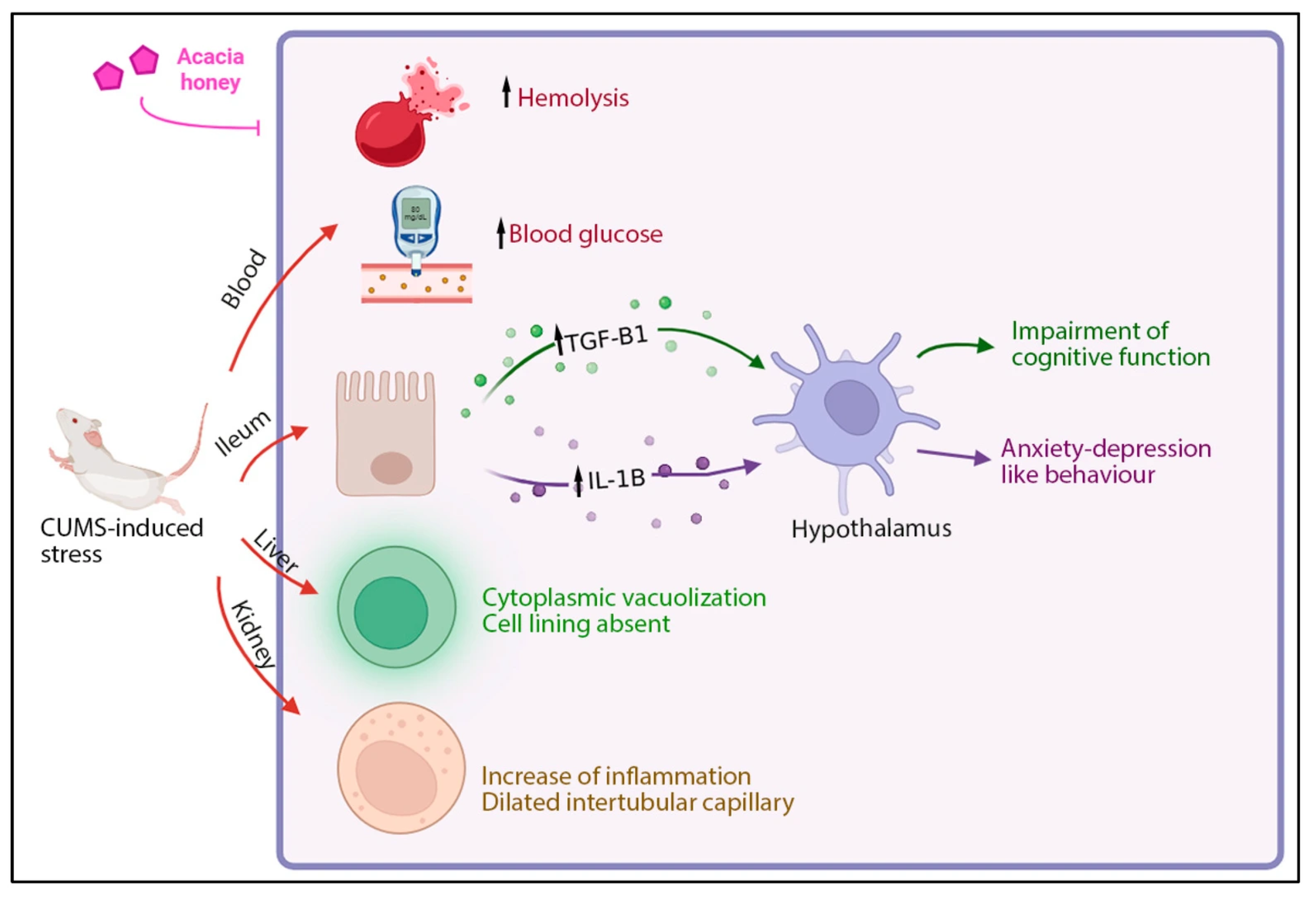
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AH | Malaysian Acacia honey |
| ALT | Alanine aminotransferase |
| AMT | Amitriptyline |
| ANOVA | Analysis of variance |
| AST | Aspartate transaminase |
| BDNF | Brain-derived neurotrophic factor |
| BC | Bowman’s capsule |
| c-FOS | Celular-FOS |
| CLM | Cell lining missing |
| COX-2 | Cyclooxygenase-2 |
| CPV | Cytoplasmic vacuolation |
| Ct | Threshold cycle |
| CUMS | Chronic unpredictable mild stress |
| CV | Central vein |
| DCT | Distal convoluted tubules |
| DNA | Deoxyribonucleic acid |
| DT | Dilated intertubular capillaries |
| ES | Erythrocyte suspension |
| G | Glomerulus |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GGT | Gamma-glutamyltransferase |
| GLUT2 receptor | Glucose Transporter Type 2 receptor |
| GLUT5 receptor | Glucose Transporter Type 5 receptor |
| h | Hour |
| H2O2 | Hydrogen peroxide |
| HPA | Hypothalamic–pituitary–adrenal |
| H&E | Hematoxylin-eosin |
| iNOS | Inducible Nitric Oxide Synthase |
| I | Inflammation |
| IL-1 | Interleukin-1 |
| IL-1α | Interleukin-1 alpha |
| IL-1β | Interleukin-1 beta |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IFN-γ | Interferon gamma |
| KC | Kupffer cell |
| LPS | Lipopolysaccharide |
| LOXs | Lipoxygenases |
| min | minute |
| mRNA | Messenger ribonucleic acid |
| MDD | major depressive disorder |
| NC | Normal control |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric Oxide |
| IκBα | Inhibitor of kappa B alpha |
| PBS | Phosphate-buffered saline |
| PCT | Proximal convoluted tubules |
| PDGF | Platelet-Derived Growth Factor |
| RNA | Ribonucleic acid |
| RO | Reverse osmosis water |
| ROS | Reactive oxygen species |
| S | Stress-induced group |
| SEM | Standard error of mean |
| Sn | Sinusoidal |
| SD | Sprague-Dawley |
| SPT | Sucrose preference test |
| TGF-β | Transforming growth factor beta |
| TGF-β1 | Transforming growth factor beta 1 gene |
| TNF-α | Tumor necrosis factor-alpha |
References
- Joseph, J.J.; Golden, S.H. Cortisol Dysregulation: The Bidirectional Link between Stress, Depression, and Type 2 Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef]
- Maydych, V. The Interplay between Stress, Inflammation, and Emotional Attention: Relevance for Depression. Front. Neurosci. 2019, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Atagana, O.S.; Asagba, S.O. Protective Effects of Honey against Cadmium-Induced Alteration of Some Biochemical Parameters in Rats. Toxicol. Environ. Chem. 2014, 96, 1557–1563. [Google Scholar] [CrossRef]
- Liu, Z.; Lei, M.; Bai, Y. Chronic Stress Mediates Inflammatory Cytokines Alterations and Its Role in Tumorigenesis. J. Inflamm. Res. 2025, 18, 1067–1090. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Galante, J.; Vainre, M.; Stochl, J.; Dufour, G.; Jones, P.B. Immune Dysregulation among Students Exposed to Exam Stress and Its Mitigation by Mindfulness Training: Findings from an Exploratory Randomised Trial. Sci. Rep. 2020, 10, 5812. [Google Scholar] [CrossRef]
- Holzer, P.; Reichmann, F.; Farzi, A. Neuropeptide Y, Peptide YY and Pancreatic Polypeptide in the Gut-Brain Axis. Neuropeptides 2012, 46, 261–274. [Google Scholar] [CrossRef]
- Moraczewski, J.; Awosika, A.; Aedma, K. Tricyclic Antidepressants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Fructose Might Contribute to the Hypoglycemic Effect of Honey. Molecules 2012, 17, 1900–1915. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Effects of Daily Consumption of Honey Solution on Hematological Indices and Blood Levels of Minerals and Enzymes in Normal Individuals. J. Med. Food 2003, 6, 135–140. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey: A Novel Antioxidant. Molecules 2012, 17, 4400–4423. [Google Scholar] [CrossRef] [PubMed]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori Honey Is Packed Full of Antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Dar, S.A.; Ahmed, M.M.M.; Aly, M.M.; Vlainić, J. Antioxidant Capacity and Therapeutic Applications of Honey: Health Benefits, Antimicrobial Activity and Food Processing Roles. Antioxidants 2025, 14, 959. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.S.; Abdah, M.A.; Ramasamy, K.; Hasan, M.H.; Hamimi, I.A.; Zolkapli, E. Physicochemical Analysis and Sugar Profiling of Acacia Honey. Malays. J. Microsc. 2018, 14, 157–164. [Google Scholar]
- Samat, S. Physicochemical Profile of Selected Malaysian Honey and The Effect of Pineapple Honey on Obesity In Vitro and In Vivo Model. Ph.D. Thesis, Universiti Teknologi Mara, Shah Alam, Selangor, 2021. [Google Scholar]
- Jamwal, N.; Jasrotia, R.; Badyal, N.; Hajam, Y.A.; Langer, S. Honey: An Antidiabetic and Hypoglycemic Agent to Reverse Diabetes Induced Complications BT—Honey in Food Science and Physiology; Kumar, R., Hajam, Y.A., Bala Dhull, S., Giri, A., Eds.; Springer Nature: Singapore, 2024; pp. 369–388. ISBN 978-981-97-3565-5. [Google Scholar]
- Sukri, N.M.; Ooi, F.K.; Chee, K.C.; Sirajudeen, K.N.S. Effect of Acacia Honey Drink Consumed Pre and During Exercise on Glucose Metabolism, Total Antioxidant Status, and Running Performance in the Heat. IIUM Med. J. Malaysia 2022, 21, 85–95. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Ismail, M.S.; Mohamed, M.; Ooi, F.K. Blood Glucose Metabolism, Serum, and Urine Osmolality in Response to Sodium-Enriched Acacia Honey Drink Consumption during Rehydration after Exercise in Hot and Humid Environment. J. Sustain. Sci. Manag. 2020, 15, 72–83. [Google Scholar] [CrossRef]
- Schell, K.R.; Fernandes, K.E.; Shanahan, E.; Wilson, I.; Blair, S.E.; Carter, D.A.; Cokcetin, N.N. The Potential of Honey as a Prebiotic Food to Re-Engineer the Gut Microbiome Toward a Healthy State. Front. Nutr. 2022, 9, 957932. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef]
- Harro, J. Animal Models of Depression: Pros and Cons. Cell Tissue Res. 2019, 377, 5–20. [Google Scholar] [CrossRef]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic Unpredictable Mild Stress for Modeling Depression in Rodents: Meta-Analysis of Model Reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef]
- van Zyl, P.J.; Dimatelis, J.J.; Russell, V.A. Behavioural and Biochemical Changes in Maternally Separated Sprague–Dawley Rats Exposed to Restraint Stress. Metab. Brain Dis. 2016, 31, 121–133. [Google Scholar] [CrossRef]
- Abidin, Q.H.Z.; Ismail, W.I.W.; Ismail, N.E.; Eshak, Z. Preliminary Study on Malaysian Honey as Anti- Stress Agent. Thai J. Pharm. Sci. 2017, 41, 133–136. [Google Scholar]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Lei, H.; Wang, L.; Xue, L.; Wang, X.; Zhu, X. Optimized animal model to mimic the reality of stress-induced depression in the clinic. BMC Phychiatry 2017, 17, 171. [Google Scholar] [CrossRef]
- Okoko, T.; Ere, D. Reduction of Hydrogen Peroxide—Induced Erythrocyte Damage by Carica Papaya Leaf Extract. Asian Pac. J. Trop. Biomed. 2012, 2, 449–453. [Google Scholar] [CrossRef]
- Abidin, Q.H.Z.; Rujhan, N.H.M.; Wan Ismail, W.I.; Ismail, N.E.; Eshak, Z. Potential Used of Tualang and Acacia Honey in Ameliorating Stress-Depression Disorder: A Preliminary Study. In Proceedings of the Seoul International Congress of Endocrinology and Metabolism, Seoul, Republic of Korea, 4–6 November 2015; Korea Endocrine Society: Seoul, Republic of Korea, 2015; p. 309. [Google Scholar]
- Abidin, Q.H.Z. Anti Stress-Depression Effects of Acacia Honey on Chronic Unpredictable Mild Stress Rats. Ph.D. Thesis, Universiti Teknologi Mara, Shah Alam, Selangor, 2017. [Google Scholar]
- Markov, D.D. Sucrose Preference Test as a Measure of Anhedonic Behavior in a Chronic Unpredictable Mild Stress Model of Depression: Outstanding Issues. Brain Sci. 2022, 12, 1287. [Google Scholar] [CrossRef]
- He, L.W.; Zeng, L.; Tian, N.; Li, Y.; He, T.; Tan, D.M.; Zhang, Q.; Tan, Y. Optimization of Food Deprivation and Sucrose Preference Test in SD Rat Model Undergoing Chronic Unpredictable Mild Stress. Anim. Model. Exp. Med. 2020, 3, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Fan, R.; Li, T.; Yang, Z.; Hu, E.; Yu, Z.; Tian, J.; Luo, W.; Zhang, C. Metabolomics Analysis of the Prefrontal Cortex in a Rat Chronic Unpredictable Mild Stress Model of Depression. Front. Psychiatry 2022, 13, 815211. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.J.; Yang, Y.H.C.; Cruz, A.M.; Beall, C.; Ellacott, K.L.J. Brain-Body Control of Glucose Homeostasis—Insights From Model Organisms. Front. Endocrinol. 2021, 12, 662769. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Azizi-soleiman, F. Honey and Glycemic Control: A Systematic Review. PharmaNutrition 2020, 11, 100180. [Google Scholar] [CrossRef]
- Ayoub, S.; Javed, M.; Sattar, K.; Ullah, H.; Hajjar, W.; Alasiri, S. Honey and Diabetes Mellitus: Obstacles and Challenges—Road to Be Repaired. Saudi J. Biol. Sci. 2017, 24, 1030–1033. [Google Scholar] [CrossRef]
- Mushtaq, S.; Imtiyaz, Z.; Wali, A.F.; Khan, A.; Rashid, S.M.; Amin, I.; Ali, A.; Rehman, M.U.; Arafah, A. Honey: A Powerful Natural Antioxidant and Its Possible Mechanism of Action BT—Therapeutic Applications of Honey and Its Phytochemicals; Rehman, M.U., Majid, S., Eds.; Springer: Singapore, 2020; Volume 1, pp. 11–29. ISBN 978-981-15-6799-5. [Google Scholar]
- Martinez-Armenta, C.; Camacho-Rea, M.C.; Martínez-Nava, G.A.; Espinosa-Velázquez, R.; Pineda, C.; Gomez-Quiroz, L.E.; López-Reyes, A. Therapeutic Potential of Bioactive Compounds in Honey for Treating Osteoarthritis. Front. Pharmacol. 2021, 12, 642836. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.; Ai, Y.; Li, L.; Zhao, S.; Liu, Z.; Peng, Q.; Deng, S.; Huang, Y.; Mo, Y.; et al. Amitriptyline Reduces Sepsis-Induced Brain Damage Through TrkA Signaling Pathway. J. Mol. Neurosci. 2020, 70, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Milenina, L.S.; Krutetskaya, Z.I.; Antonov, V.G.; Krutetskaya, N.I. Tricyclic Antidepressant Amitriptyline Suppresses Ca2+ Responses in Rat Peritoneal Macrophages. Cell Tissue Biol. 2024, 18, 439–450. [Google Scholar] [CrossRef]
- Turpin, C.; Catan, A.; Guerin-Dubourg, A.; Debussche, X.; Bravo, S.B.; Álvarez, E.; van Den Elsen, J.; Meilhac, O.; Rondeau, P.; Bourdon, E. Enhanced Oxidative Stress and Damage in Glycated Erythrocytes. PLoS ONE 2020, 15, e0235335. [Google Scholar] [CrossRef] [PubMed]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.; Piatti, E. Honey Flavonoids as Protection Agents against Oxidative Damage to Human Red Blood Cells. Food Chem. 2007, 104, 1635–1640. [Google Scholar] [CrossRef]
- Vallelian, F.; Buehler, P.W.; Schaer, D.J. Hemolysis, Free Hemoglobin Toxicity, and Scavenger Protein Therapeutics. Blood 2022, 140, 1837–1844. [Google Scholar] [CrossRef]
- Pelusi, S.; Valenti, L.; Fargion, S. Oxidative Stress and Hepatic Iron Overload BT—Studies on Hepatic Disorders; Albano, E., Parola, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 345–356. ISBN 978-3-319-15539-5. [Google Scholar]
- Greenhalgh, S.N.; Thompson, A.I.; Henderson, N.C.; Iredale, J.P. Oxidative Stress and Liver Inflammation BT—Studies on Hepatic Disorders; Albano, E., Parola, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 123–147. ISBN 978-3-319-15539-5. [Google Scholar]
- Wang, X.; Ling, W.; Zhu, Y.; Ji, C.; An, X.; Qi, Y.; Li, S.; Zhang, C.; Tong, R.; Jiang, D.; et al. Spermidine Alleviates Copper-Induced Oxidative Stress, Inflammation and Cuproptosis in the Liver. FASEB J. 2025, 39, e70453. [Google Scholar] [CrossRef]
- Yaman, T.; Yener, Z.; Celik, I. Histopathological and Biochemical Investigations of Protective Role of Honey in Rats with Experimental Aflatoxicosis. BMC Complement. Altern. Med. 2016, 16, 232. [Google Scholar] [CrossRef]
- Van Avondt, K.; Nur, E.; Zeerleder, S. Mechanisms of Haemolysis-Induced Kidney Injury. Nat. Rev. Nephrol. 2019, 15, 671–692. [Google Scholar] [CrossRef]
- Xie, T.; Yao, L.; Li, X. Advance in Iron Metabolism, Oxidative Stress and Cellular Dysfunction in Experimental and Human Kidney Diseases. Antioxidants 2024, 13, 659. [Google Scholar] [CrossRef]
- Abdel-moneim, W.M.; Ghafeer, H.H. The Potential Protective Effect of Natural Honey against Cadmium-Induced Hepatotoxicity and Nephrotoxicity. Mansoura J. Forensic Med. Clin. Toxicol. 2007, XV, 75–98. [Google Scholar] [CrossRef]
- Yaacob, W.M.H.W.; Long, I.; Zakaria, R.; Othman, Z. Tualang Honey and Its Methanolic Fraction Protect against LPS-Induced Neuroinflammation and Amyloid Deposition in Male Rats. Asian J. Med. Biomed. 2018, 59. [Google Scholar]
- Bird, L. IL-22 Protects against Stress-Induced Anxiety. Nat. Rev. Immunol. 2025, 25, 75. [Google Scholar] [CrossRef]
- Uchakin, P.N.; Tobin, B.; Cubbage, M.; Marshall, G.J.; Sams, C. Immune Responsiveness Following Academic Stress in First-Year Medical Students. J. Interf. Cytokine Res. Off. J. Int. Soc. Interf. Cytokine Res. 2001, 21, 687–694. [Google Scholar] [CrossRef]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B Increases Intestinal Tight Junction Permeability by Up-Regulation of MIR200C-3p, Which Degrades Occludin MRNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef]
- Ma, Y.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M. Cyclooxygenase-2-Related Signaling in the Hypothalamus Plays Differential Roles in Response to Various Acute Stresses. Brain Res. 2013, 1508, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Farzi, A.; Hassan, A.M.; Zenz, G.; Jacan, A.; Reichmann, F. Visceral Inflammation and Immune Activation Stress the Brain. Front. Immunol. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Li, C.; Yao, J.; Yang, C.; Yu, S.; Yang, Z.; Wang, L.; Li, S.; He, N. Gut Microbiota-Derived Short Chain Fatty Acids Act as Mediators of the Gut-Liver-Brain Axis. Metab. Brain Dis. 2025, 40, 122. [Google Scholar] [CrossRef] [PubMed]
- Farhana, Z.; Alhewairini, S.; Mahamood, M. The Gut–Brain Axis, Cognition and Honey. In Therapeutic Applications of Honey and Its Phytochemicals; Springer: Singapore, 2020; pp. 331–343. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zhou, H.; Jin, L.; Liu, C.; Yang, M.; Zhao, X.; Ding, W.; Xie, W.; Kong, H. GLP-1R Activation Attenuates the Progression of Pulmonary Fibrosis via Disrupting NLRP3 Inflammasome/PFKFB3-Driven Glycolysis Interaction and Histone Lactylation. J. Transl. Med. 2024, 22, 954. [Google Scholar] [CrossRef]
- Kolb, M.; Margetts, P.J.; Anthony, D.C.; Pitossi, F.; Gauldie, J. Transient Expression of IL-1β Induces Acute Lung Injury and Chronic Repair Leading to Pulmonary Fibrosis. J. Clin. Investig. 2001, 107, 1529–1536. [Google Scholar] [CrossRef]
- Troncone, E.; Marafini, I.; Stolfi, C.; Monteleone, G. Transforming Growth Factor-Β1/Smad7 in Intestinal Immunity, Inflammation, and Cancer. Front. Immunol. 2018, 9, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Mashima, H.; Sakai, T.; Matsuhashi, T.; Jin, M.; Ohnishi, H. Functional Roles of TGF-Β1 in Intestinal Epithelial Cells through Smad-Dependent and Non-Smad Pathways. Dig. Dis. Sci. 2013, 58, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Kim, S.H.; Kim, E.H. The Molecular Mechanism of Transforming Growth Factor-β Signaling for Intestinal Fibrosis: A Mini-Review. Front. Pharmacol. 2019, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-Beta Signal Transduction: Biology, Function and Therapy for Diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Rodari, M.M.; Cerf-Bensussan, N.; Parlato, M. Dysregulation of the Immune Response in TGF-β Signalopathies. Front. Immunol. 2022, 13, 1066375. [Google Scholar] [CrossRef]
- Kajumba, M.M.; Kakooza-Mwesige, A.; Nakasujja, N.; Koltai, D.; Canli, T. Treatment-Resistant Depression: Molecular Mechanisms and Management. Mol. Biomed. 2024, 5, 43. [Google Scholar] [CrossRef]
- Cao, B.B.; Zhang, X.X.; Du, C.Y.; Liu, Z.; Qiu, Y.H.; Peng, Y.P. TGF-Β1 Provides Neuroprotection via Inhibition of Microglial Activation in 3-Acetylpyridine-Induced Cerebellar Ataxia Model Rats. Front. Neurosci. 2020, 14, 187. [Google Scholar] [CrossRef]
- Xie, J.; Xiong, S.; Li, Y.; Xia, B.; Li, M.; Zhang, Z.; Shi, Z.; Peng, Q.; Li, C.; Lin, L.; et al. Phenolic Acids from Medicinal and Edible Homologous Plants: A Potential Anti-Inflammatory Agent for Inflammatory Diseases. Front. Immunol. 2024, 15, 1345002. [Google Scholar] [CrossRef]
- Bang, E.; Tobery, A.; Montgomery, K.S.; Fincher, A.S.; Earnest, D.J.; Murchison, D.A.; Griffith, W.H. Amitriptyline Decreases GABAergic Transmission in Basal Forebrain Neurons Using an Optogenetic Model of Aging. Front. Aging Neurosci. 2021, 13, 673155. [Google Scholar] [CrossRef]
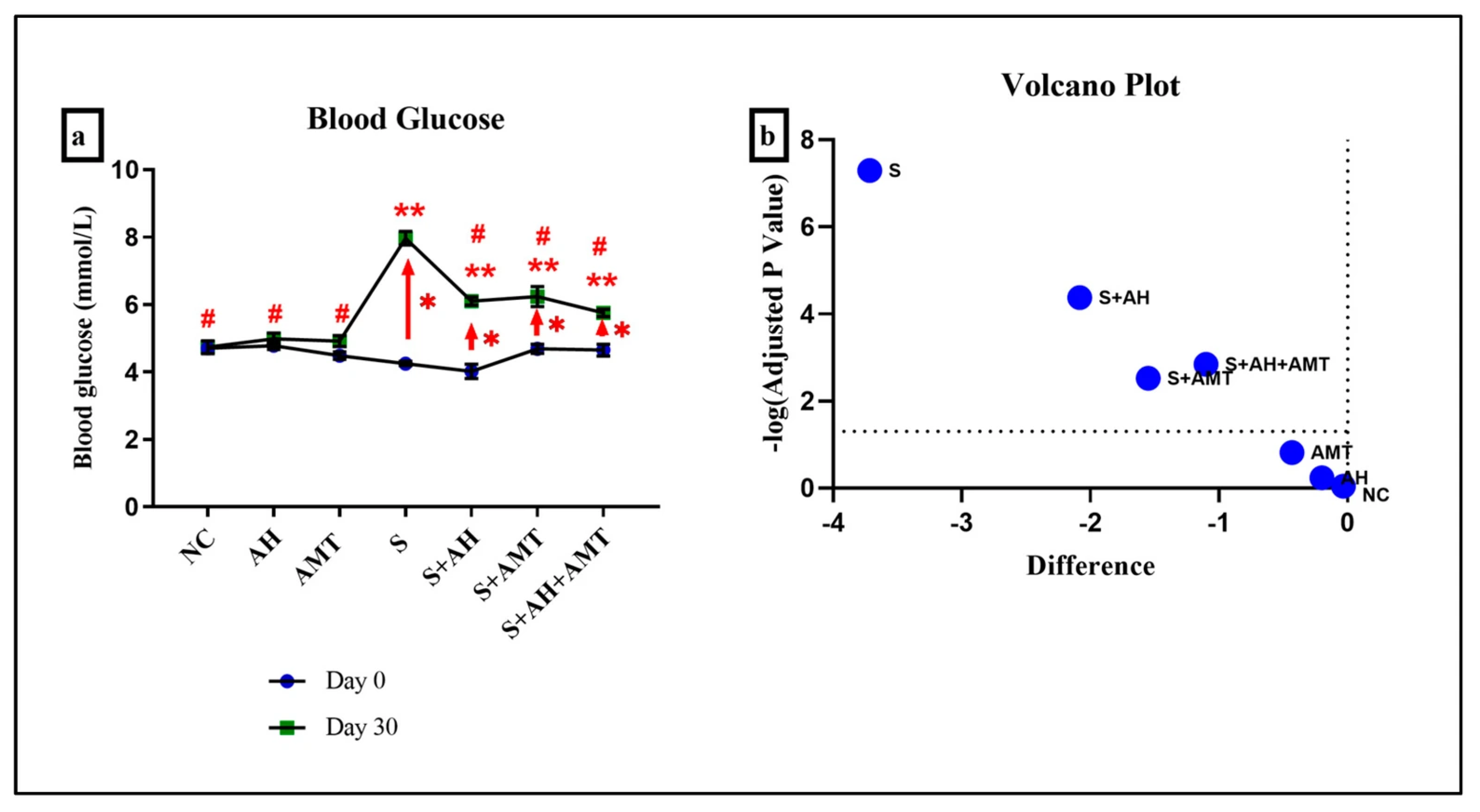
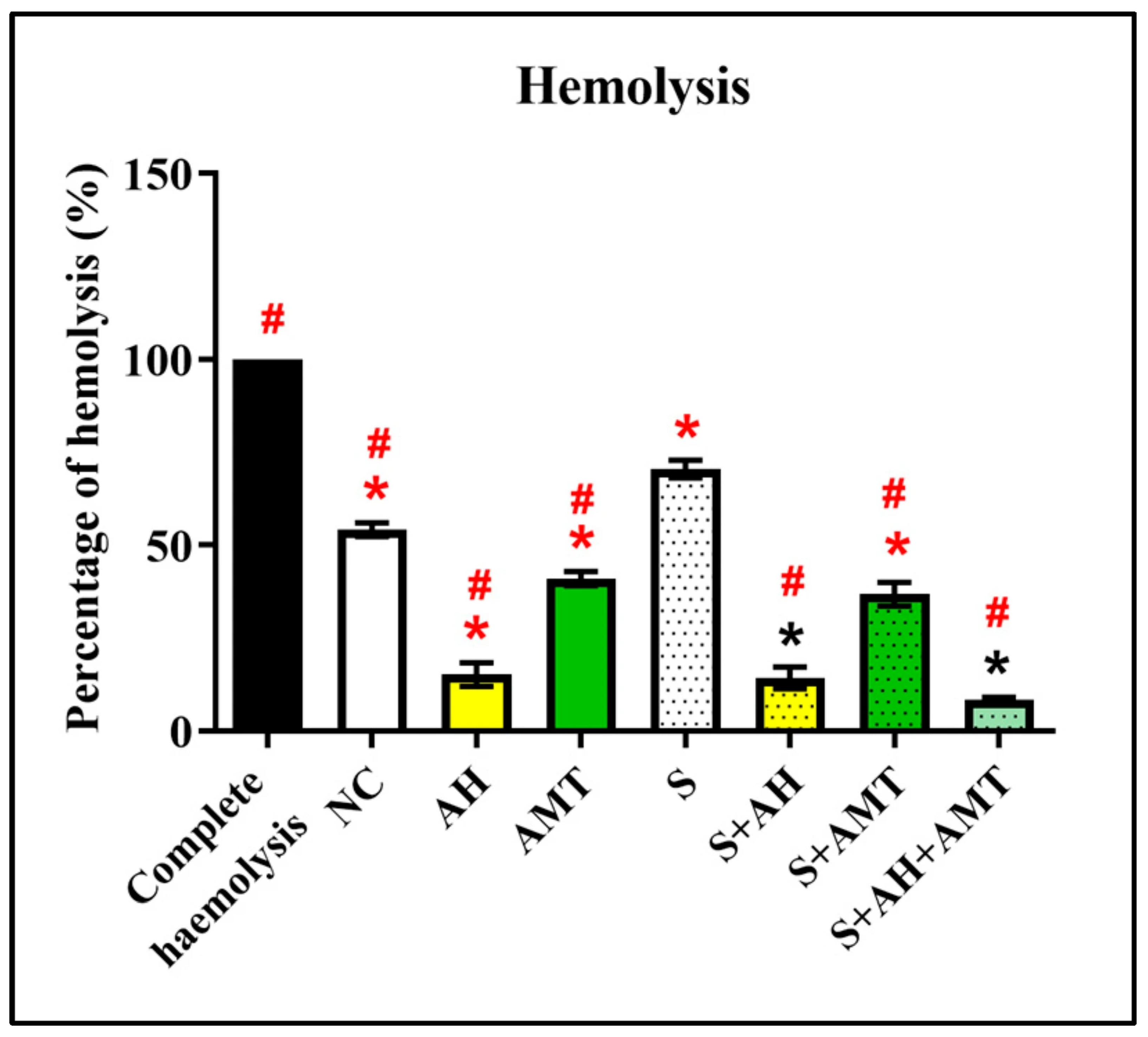
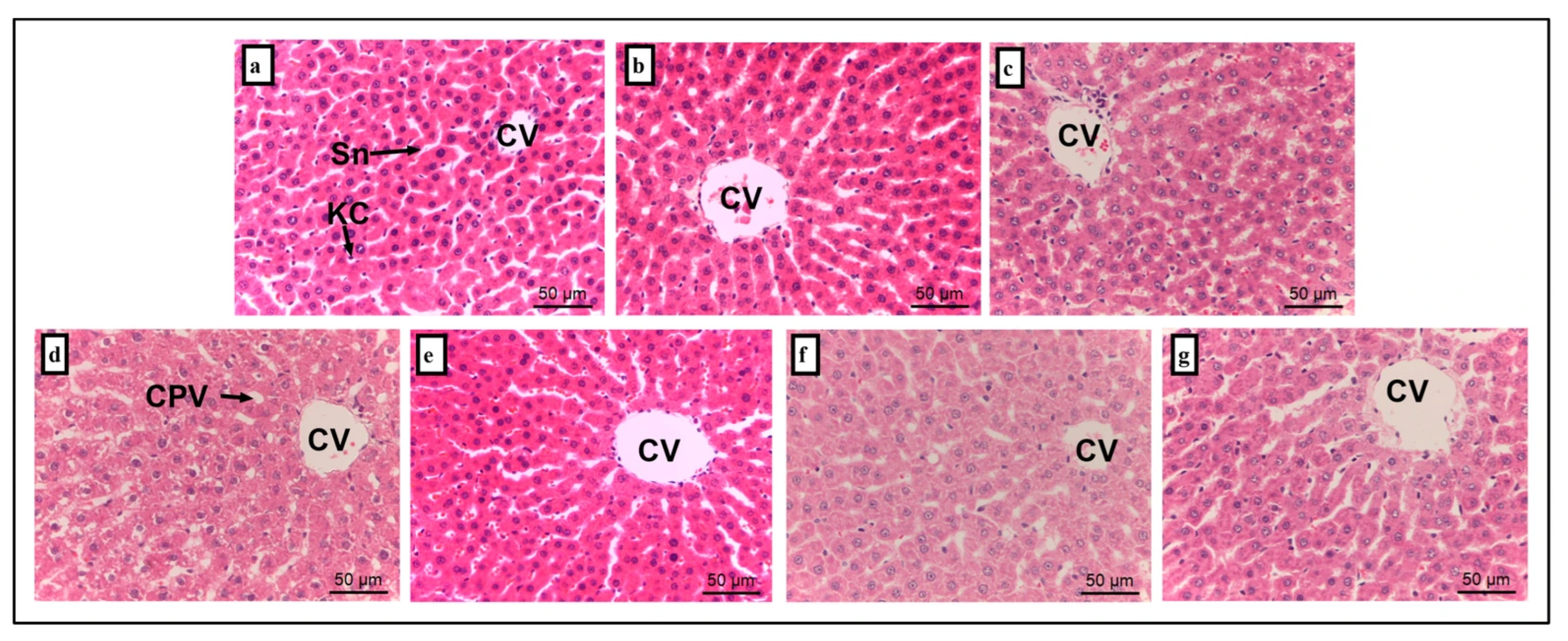

| No | Stressor * | Procedure |
|---|---|---|
| 1 | Overnight illumination | Illumination of cages containing rats for 24 h |
| 2 | Cage tilting | The cage is tilted for 24 h |
| 3 | Forced swimming | The rats were forced to swim for 5 min |
| 4 | White noise | The rats were introduced to intermittent 60 dB white noise stress for 2 h |
| 5 | Damp bedding | The bedding was damped for 24 h |
| 6 | Restricted movement | The rats will live in a closed compartment system for 2 h |
| 7 | Rest | No stressor was given to the rats |
| Primer Name | Primer Sequence (5′ to 3′) |
|---|---|
| F_GAPDH | AGTGCCAGCCTCGTCTCATA |
| R_GAPDH | GATGGTGATGGGTTTCCCGT |
| F_Interleukin 1 beta | GACTTCACCATGGAACCCGT |
| R_Interleukin 1 beta | GGAGACTGCCCATTCTCGAC |
| F_TGFβ1 | CGTCAGACATTCGGGAAGCA |
| R_TGFβ1 | TGCCGTACAACTCCAGTGAC |
| Group | Liver | Kidney | ||
|---|---|---|---|---|
| Cytoplasmic Vacuolation | Cell Lining Absence | Dilated Intertubular Capillaries | Inflammation | |
| NC | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| AH | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.20 ± 0.20 | 0.20 ± 0.20 |
| AMT | 1.20 ± 0.37 * | 0.20 ± 0.20 | 0.60 ± 0.25 | 0.60 ± 0.40 |
| S | 1.40 ± 0.25 * | 1.20 ± 0.37 * | 1.40 ± 0.51 * | 2.20 ± 0.20 * |
| S + AH | 0.40± 0.25 # | 0.20 ± 0.20 # | 0.60 ± 0.25 | 0.60 ± 0.25 # |
| S + AMT | 1.20 ± 0.20 * | 0.20 ± 0.20 # | 0.60 ± 0.40 | 0.60 ± 0.25 # |
| S + AH + AMT | 0.00 ± 0.00 # | 0.00 ± 0.00 # | 0.80 ± 0.37 | 0.40 ± 0.25 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.S.; Muhammad, H.; Nik Zainuddin, N.A.S.; Md Nasir, N.L.; Abd Rahman, M.R.A.; Siu, L.M.; Md Akim, A.; Ramasamy, K.; Hazizul Hasan, M.; Eshak, Z. Modulation of IL-1β and TGF-β1 Gene Expression in Stress-Induced Depression Rat Supplemented with Malaysian Acacia Honey. Foods 2025, 14, 3895. https://doi.org/10.3390/foods14223895
Mohamed AS, Muhammad H, Nik Zainuddin NAS, Md Nasir NL, Abd Rahman MRA, Siu LM, Md Akim A, Ramasamy K, Hazizul Hasan M, Eshak Z. Modulation of IL-1β and TGF-β1 Gene Expression in Stress-Induced Depression Rat Supplemented with Malaysian Acacia Honey. Foods. 2025; 14(22):3895. https://doi.org/10.3390/foods14223895
Chicago/Turabian StyleMohamed, Anis Syamimi, Hussin Muhammad, Nik Aina Syazana Nik Zainuddin, Nur Liana Md Nasir, Mohd Rahimi Ashraf Abd Rahman, Lau Mei Siu, Abdah Md Akim, Kalavathy Ramasamy, Mizaton Hazizul Hasan, and Zolkapli Eshak. 2025. "Modulation of IL-1β and TGF-β1 Gene Expression in Stress-Induced Depression Rat Supplemented with Malaysian Acacia Honey" Foods 14, no. 22: 3895. https://doi.org/10.3390/foods14223895
APA StyleMohamed, A. S., Muhammad, H., Nik Zainuddin, N. A. S., Md Nasir, N. L., Abd Rahman, M. R. A., Siu, L. M., Md Akim, A., Ramasamy, K., Hazizul Hasan, M., & Eshak, Z. (2025). Modulation of IL-1β and TGF-β1 Gene Expression in Stress-Induced Depression Rat Supplemented with Malaysian Acacia Honey. Foods, 14(22), 3895. https://doi.org/10.3390/foods14223895








