Synergistic Preservation of Fresh Pork: Coupling Electrostatic Field and Packaging During Controlled Freezing-Point Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
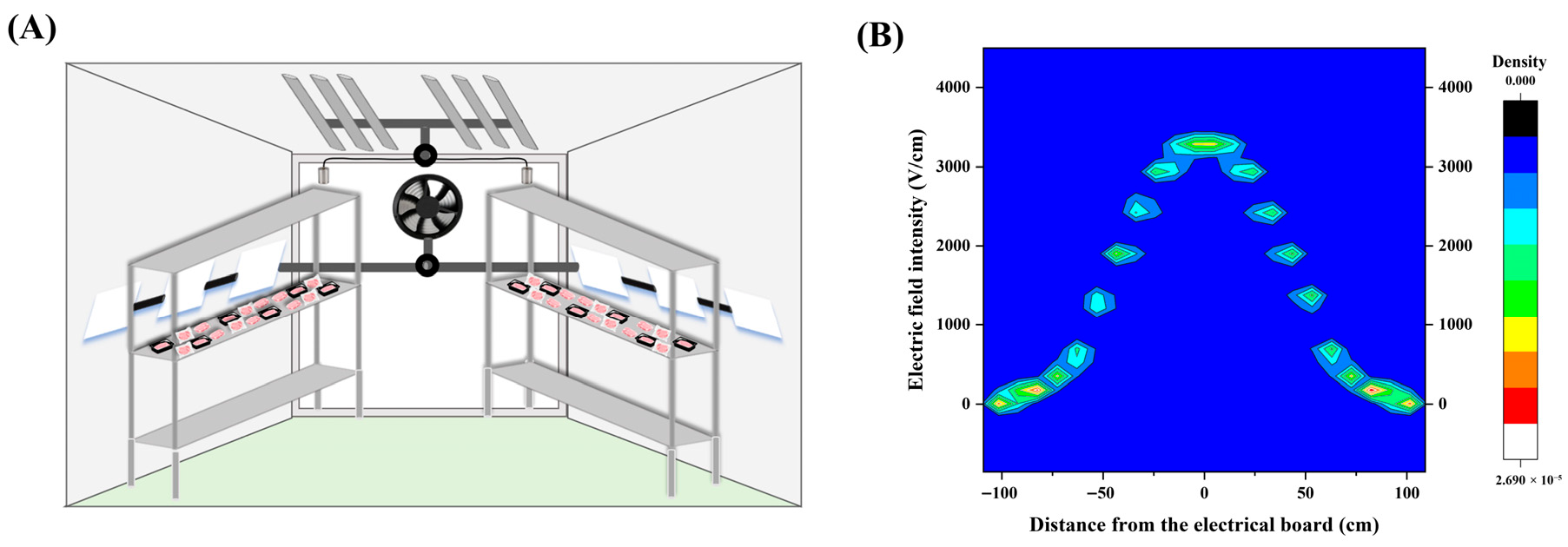
2.2. Cold Storage with the EF
2.3. WHC
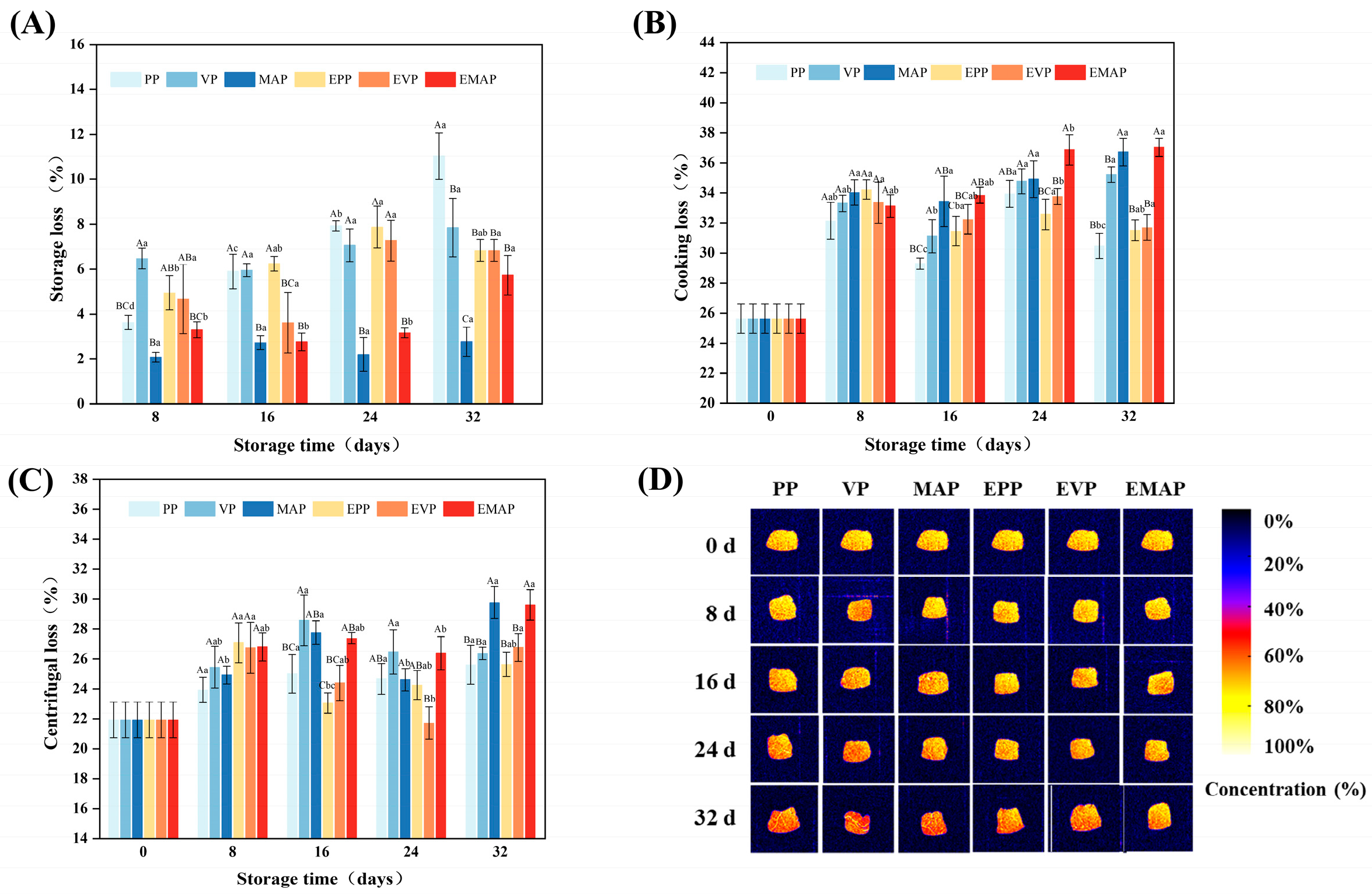
2.3.1. Storage Loss
2.3.2. Cooking Loss
2.3.3. Centrifuging Loss
2.3.4. Magnetic Resonance Imaging (MRI)
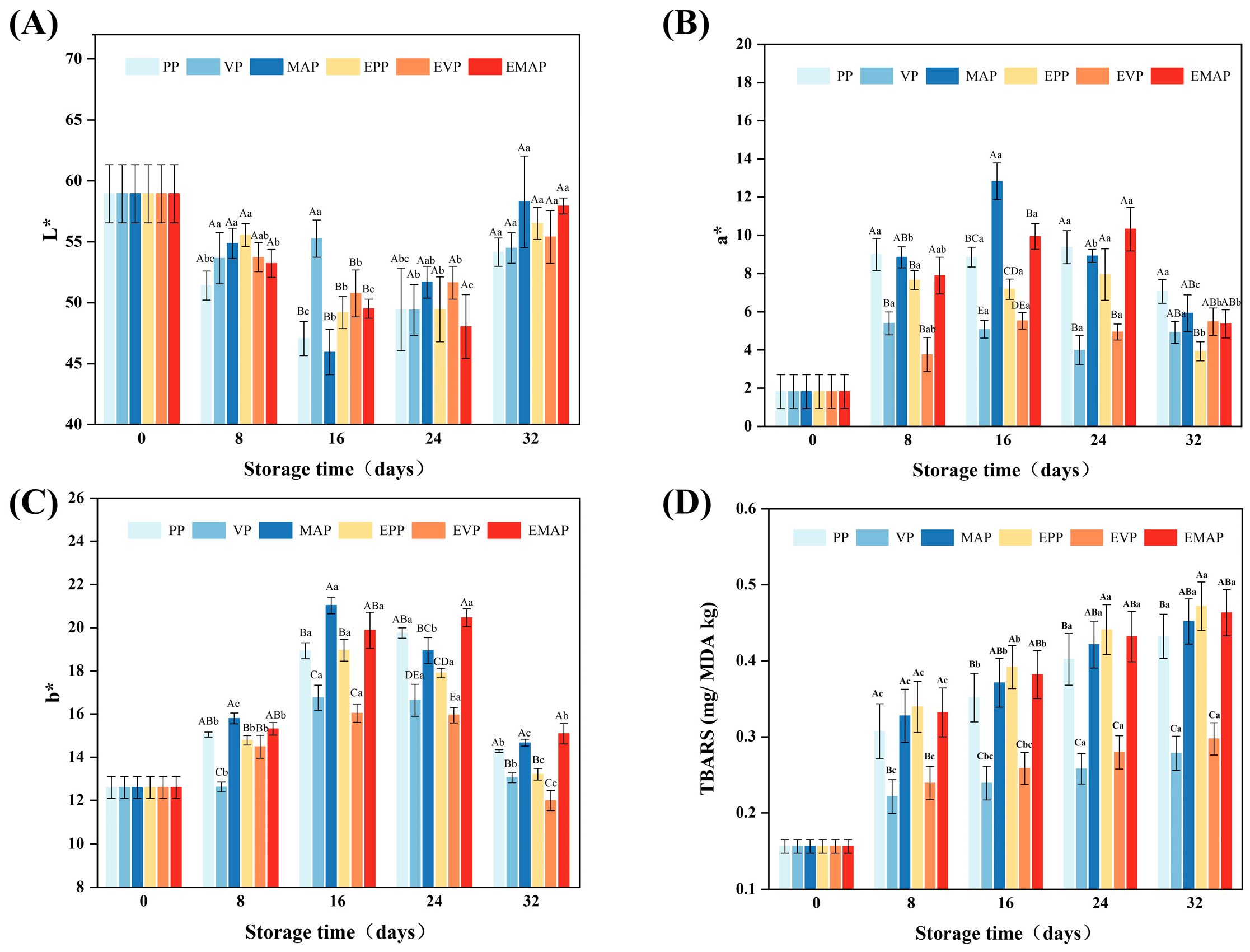
2.4. Color
2.5. TBARS
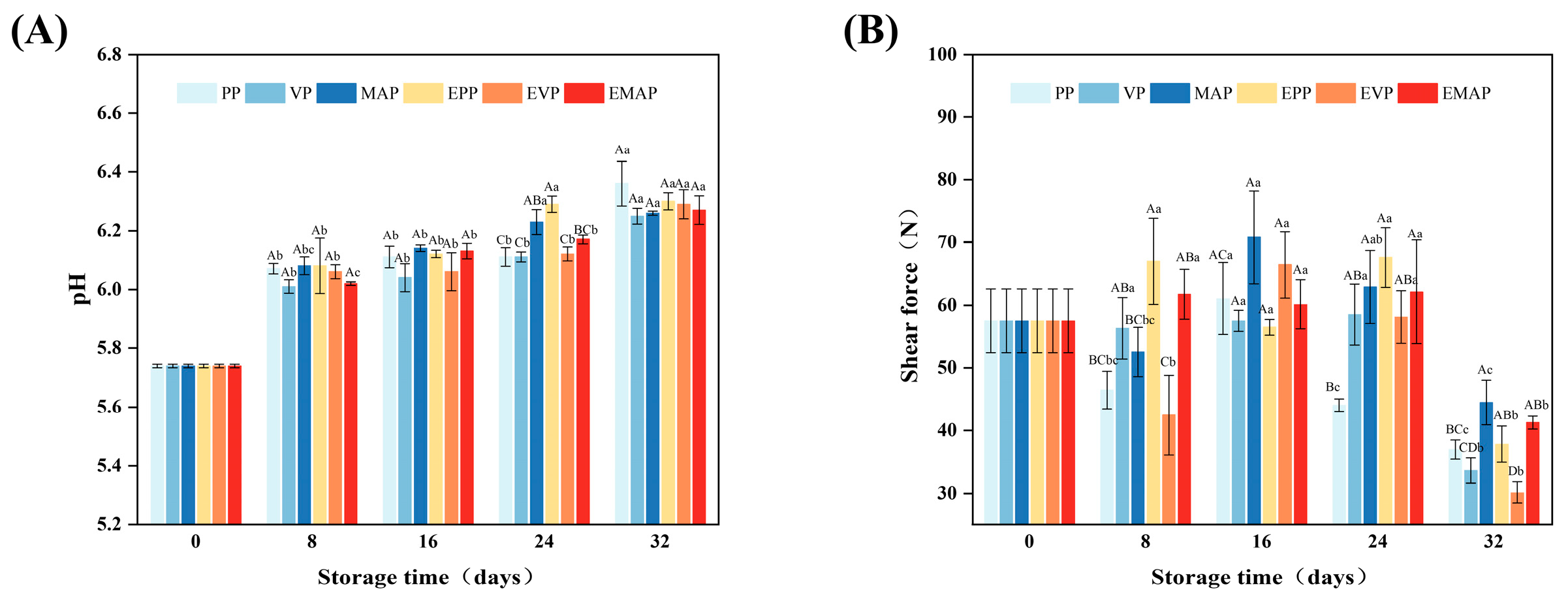
2.6. pH Value
2.7. Shear Force
2.8. Microbial Analysis
2.9. TVB-N
2.10. Microbial Community Characterization
2.11. Statistical Analysis
3. Results and Discussion
3.1. WHC
3.2. Changes in Color
3.3. Changes in TBARS
3.4. pH
3.5. Shear Force
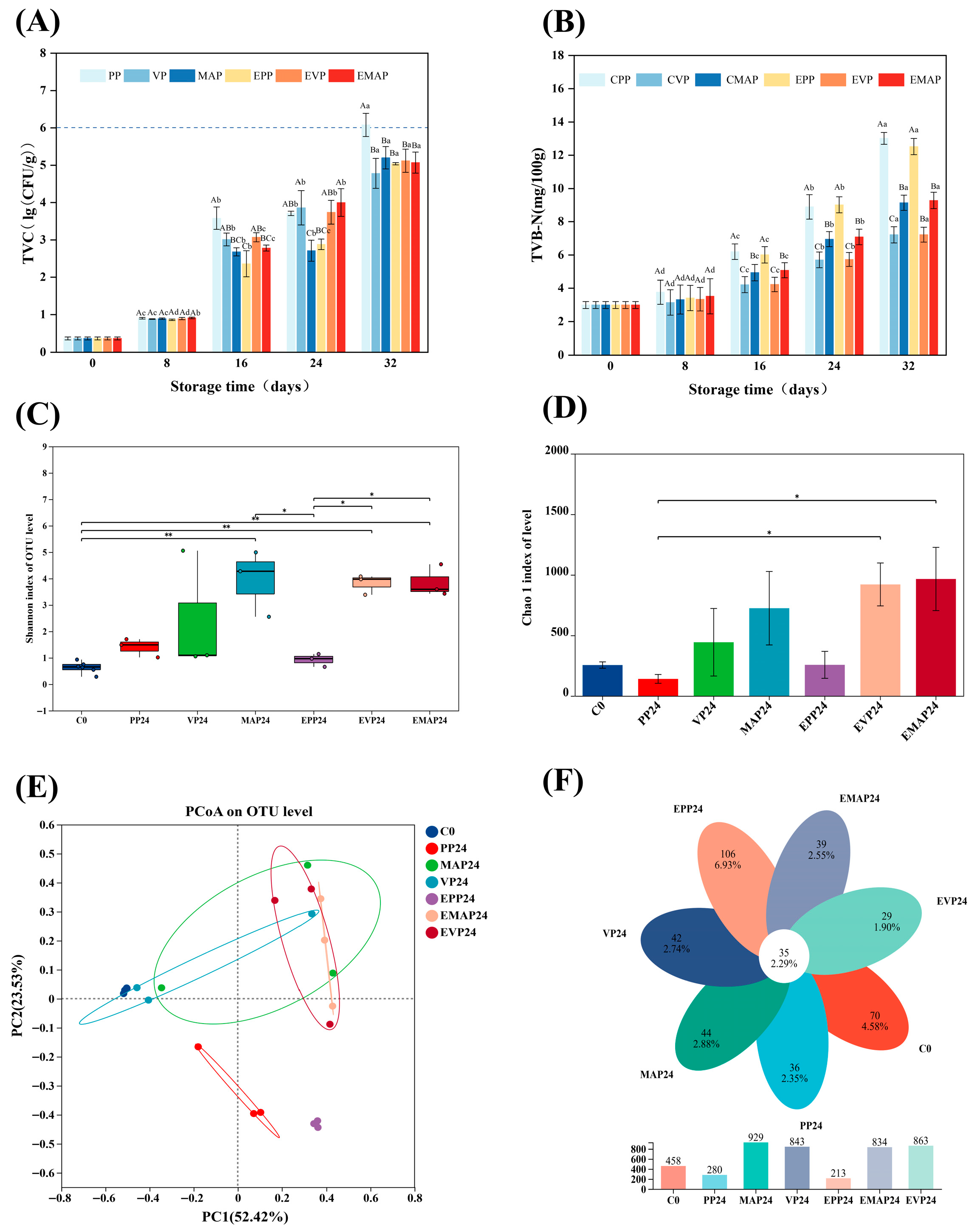
3.6. Microbiological Analyses
3.7. TVB-N
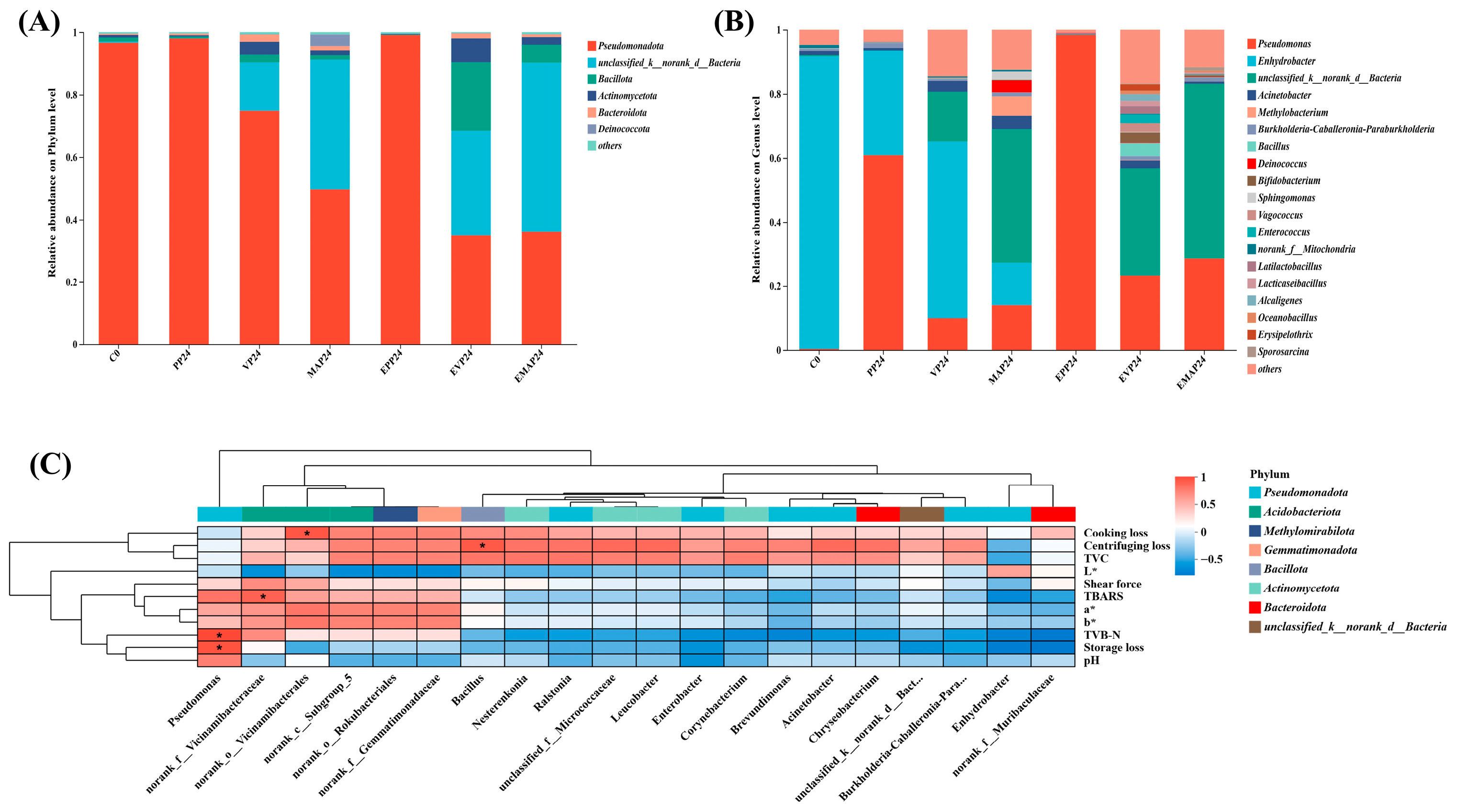
3.8. Microbial Abundance and Diversity
3.9. Correlation Between Microorganisms and Physiochemical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EF | Electrostatic Field |
| HVEF | High-Voltage Electrostatic Field |
| PP | Polyethylene Packaging |
| VP | Vacuum Packaging |
| MAP | Modified Atmosphere Packaging |
| EPP | Electrostatic Field Treatment for PP |
| EVP | Electrostatic Field Treatment for VP |
| EMAP | Electrostatic Field Treatment for MAP |
| TBARS | Thiobarbituric Acid Reactive Substances |
| WHC | Water-Holding Capacity |
| TVC | Total Viable Counts |
| TVB-N | Total Volatile Basic Nitrogen |
| MRI | Magnetic Resonance Imaging |
| PCoA | Principal Coordinate Analysis |
| ANOVAs | Analysis of Variances |
| OTUs | Operational Taxonomic Units |
References
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, J.; Li, R.; Ye, K. High-throughput sequencing analysis of the bacterial community for assessing the differences in extraction methods of bacteria separation from chilled pork. LWT 2020, 134, 110213. [Google Scholar] [CrossRef]
- Bellés, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. The combined effects of superchilling and packaging on the shelf life of lamb. Meat Sci. 2017, 133, 126–132. [Google Scholar] [CrossRef]
- Nychas, G.J.E.; Skandamis, P.N. 22—Fresh meat spoilage and modified atmosphere packaging (MAP). In Improving the Safety of Fresh Meat; Sofos, J.N., Ed.; Woodhead Publishing: Cambridge, UK, 2005; pp. 461–502. [Google Scholar]
- Borch, E.; Kant-Muermans, M.-L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, G.; Ye, K.; Wang, S.; Xu, X.; Li, C. Microbial changes in vacuum-packed chilled pork during storage. Meat Sci. 2015, 100, 145–149. [Google Scholar] [CrossRef]
- Gan, J.L.; Mukaddas, M.; Tao, Y.; Liu, H.Q.; Ye, K.P.; Zhou, G.H. High-voltage electrostatic field with 35 kV-15 min could reduce Pseudomonas spp. to maintain the quality of pork during −1 °C storage. Innov. Food Sci. Emerg. Technol. 2024, 94, 103700. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Pu, A.; Tian, J.; Yang, Z.; Feng, Y.; Zhang, Y.; Liu, G. A preliminary exploration of the synergistic preservation effect of electrostatic field and superchilling on muscle foods: Mechanisms, influencing factors, applications, and challenges. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70066. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, D.; Xie, F.; Li, X.; Schroyen, M.; Chen, L.; Hou, C. Changes in water holding capacity of chilled fresh pork in controlled freezing-point storage assisted by different modes of electrostatic field action. Meat Sci. 2023, 204, 109269. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, D.; Wen, X.; Xu, Y.; Leng, D.; Yang, Q.; Hou, C.; Li, X.; Zhang, D. Variable-intensity electrostatic fields inhibit specific spoilage organisms in fresh meat: Mechanisms and antimicrobial efficacy. Food Chem. 2025, 495, 146593. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, X.; Zhang, D.; Schroyen, M.; Wang, D.; Li, X.; Hou, C. Changes in the Freshness and Bacterial Community of Fresh Pork in Controlled Freezing Point Storage Assisted by Different Electrostatic Field Usage Frequencies. Food Bioprocess Technol. 2024, 17, 939–954. [Google Scholar] [CrossRef]
- Qian, C.; Pan, H.; Shao, H.; Yu, Q.; Lou, Y.; Li, Y. Effects of HVEF treatment on the physicochemical properties and bacterial communities of Larimichthys crocea fillets during refrigerated storage. Int. J. Food Sci. Technol. 2022, 57, 7691–7700. [Google Scholar] [CrossRef]
- Valdez-Miranda, J.I.; Robles-López, M.R.; Robles-de-la-Torre, R.R.; Alamilla-Beltrán, L.; Hernández-Sánchez, H.; Gutiérrez-López, G.F. Effect of high-voltage electrostatic field treatments on bananas (Musa paradisiaca var. sapientum) on their postharvest quality, enzymatic activity and morphological changes. Food Bioprod. Process. 2024, 146, 135–146. [Google Scholar] [CrossRef]
- Kao, N.-Y.; Tu, Y.-F.; Sridhar, K.; Tsai, P.-J. Effect of a high voltage electrostatic field (HVEF) on the shelf-life of fresh-cut broccoli (Brassica oleracea var. italica). LWT 2019, 116, 108532. [Google Scholar] [CrossRef]
- Ko, W.-C.; Yang, S.-Y.; Chang, C.-K.; Hsieh, C.-W. Effects of adjustable parallel high voltage electrostatic field on the freshness of tilapia (Orechromis niloticus) during refrigeration. LWT—Food Sci. Technol. 2016, 66, 151–157. [Google Scholar] [CrossRef]
- Bellés, M.; Alonso, V.; Roncalés, P.; Beltrán, J.A. A review of fresh lamb chilling and preservation. Small Rumin. Res. 2017, 146, 41–47. [Google Scholar] [CrossRef]
- Chen, S.; Liu, L.; Ma, Y.; He, Y.; Chen, Y.; Yang, X.; Chen, J.; Chen, W. Preservation of Chilled Pork Quality by Electromagnetic Field–Assisted Modified Atmosphere Packaging During Cold Storage. Food Bioprocess Technol. 2025, 1–15. [Google Scholar] [CrossRef]
- Indriani, S.; Srisakultiew, N.; Boonchuen, P.; Kingwascharapong, P.; Sai-Ut, S.; Benjakul, S.; Pongsetkul, J. Investigating the relationship between microbial community dynamics and flavor profiles in Korat chicken breast fillets under varied packaging conditions. Int. J. Food Microbiol. 2025, 435, 111157. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.D.; Smith, J.P.; Dodds, K.L. Shelf life extension and microbiological safety of fresh meat—A review. Food Microbiol. 1991, 8, 267–297. [Google Scholar] [CrossRef]
- Beterams, A.; Tolksdorf, T.; Martin, A.; Stingl, K.; Bandick, N.; Reich, F. Change of Campylobacter, Escherichia coli and Salmonella counts in packaged broiler breast meat stored under modified atmosphere and vacuum conditions at 4 and 10 °C based on cultural and molecular biological quantification. Food Control 2023, 145, 109337. [Google Scholar] [CrossRef]
- Xu, Y.; Leng, D.; Li, X.; Wang, D.; Chai, X.; Schroyen, M.; Zhang, D.; Hou, C. Effects of different electrostatic field intensities assisted controlled freezing point storage on water holding capacity of fresh meat during the early postmortem period. Food Chem. 2024, 439, 138096. [Google Scholar] [CrossRef]
- Mousakhani-Ganjeh, A.; Hamdami, N.; Soltanizadeh, N. Impact of high voltage electric field thawing on the quality of frozen tuna fish (Thunnus albacares). J. Food Eng. 2015, 156, 39–44. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, M.; Shen, D.; Liu, Y. A new strategy to improve the quality of frozen chicken wings: High voltage electrostatic field combined with phosphorus-free water retaining agent. Food Res. Int. 2024, 188, 114479. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guan, W.; Zhu, M.; Cui, L.; He, X.; Song, Y.; Zhang, H. Effects of Continuous High-Voltage Alternating Electric Field Treatment on the Color, Lipid Oxidation, and Myofibrillar Protein of Pork During Cold Storage. J. Food Sci. 2025, 90, e70452. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Lin, H.X.; Guan, W.Q.; Song, Y.; He, X.X.; Zhang, D.Q. Effect of static magnetic field-assisted thawing on the quality, water status, and myofibrillar protein characteristics of frozen beef steaks. Food Chem. 2024, 436, 137709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, Q.; Tian, M.; Yang, W.; Min, C.; Fan, S.; Wang, D.; Li, X.; Zhang, D.; Hou, C. Sustainable nanofiber films based on polylactic acid/modified cellulose nanocrystals containing various types of polyphenols, exhibiting antioxidant activity and high stability. Food Chem. 2025, 477, 143514. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, W.; Yang, Q.; Zhu, C.; Tian, M.; Richel, A.; Fauconnier, M.-L.; Hou, C.; Zhang, D. Reinforcement of chitosan/polyvinyl alcohol film by quercetin self-assembled nanocrystals for fresh meat preservation. Food Chem. 2025, 493, 145826. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Li, X.; Zhang, C. Effects of oxygen concentration in modified atmosphere packaging on water holding capacity of pork steaks. Meat Sci. 2019, 148, 189–197. [Google Scholar] [CrossRef]
- He, Y.; Sun, G.; Koga, K.; Xu, L. Electrostatic field-exposed water in nanotube at constant axial pressure. Sci. Rep. 2014, 4, 6596. [Google Scholar] [CrossRef]
- Li, W.L.; Yang, X.Q.; Wang, J.W.; Dong, Y.; Xu, X.L.; Wang, H.H. Water holding-capacity and flavor improvement of prepared meat patties induced by magnetic field-assisted marinating and preheating. J. Food Eng. 2024, 381, 112194. [Google Scholar] [CrossRef]
- Laurent, W.; Bonny, J.M.; Renou, J.P. Muscle characterisation by NMR imaging and spectroscopic techniques. Food Chem. 2000, 69, 419–426. [Google Scholar] [CrossRef]
- Fallah-Joshaqani, S.; Hamdami, N.; Keramat, J. Qualitative attributes of button mushroom (Agaricus bisporus) frozen under high voltage electrostatic field. J. Food Eng. 2021, 293, 110384. [Google Scholar] [CrossRef]
- Lin, H.; Zhao, S.; Han, X.; Guan, W.; Liu, B.; Chen, A.; Sun, Y.; Wang, J. Effect of static magnetic field extended supercooling preservation on beef quality. Food Chem. 2022, 370, 131264. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y.; Wang, Y.; Hao, S.; Zhang, K.; Tian, J.; Jin, Y. Effect of changes in the structure of myoglobin on the color of meat products. Food Mater. Res. 2024, 4, e011. [Google Scholar] [CrossRef]
- Krell, J.; Poveda-Arteaga, A.; Weiss, J.; Witte, F.; Terjung, N.; Gibis, M. Influence of different storage atmospheres in packaging on color stability of beef. J. Food Sci. 2024, 89, 5774–5787. [Google Scholar] [CrossRef]
- Li, S.; Guo, X.; Shen, Y.; Pan, J.; Dong, X. Effects of oxygen concentrations in modified atmosphere packaging on pork quality and protein oxidation. Meat Sci. 2022, 189, 108826. [Google Scholar] [CrossRef]
- Cayuela, J.M.; Gil, M.D.; Bañón, S.; Garrido, M.D. Effect of vacuum and modified atmosphere packaging on the quality of pork loin. Eur. Food Res. Technol. 2004, 219, 316–320. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.J.; Lee, H.C.; Yoo, S.S.; Shim, J.H.; Chin, K.B. Effects of pork meat cut and packaging type on lipid oxidation and oxidative products during refrigerated storage (8 °C). J. Food Sci. 2008, 73, C127–C134. [Google Scholar] [CrossRef]
- Arroyo, C.; Eslami, S.; Brunton, N.P.; Arimi, J.M.; Noci, F.; Lyng, J.G. An assessment of the impact of pulsed electric fields processing factors on oxidation, color, texture, and sensory attributes of turkey breast meat. Poult. Sci. 2015, 94, 1088–1095. [Google Scholar] [CrossRef]
- Nychas, G.-J.E.; Skandamis, P.N.; Tassou, C.C.; Koutsoumanis, K.P. Meat spoilage during distribution. Meat Sci. 2008, 78, 77–89. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Zhang, C.; Chen, Y.; Zhou, W.; Zhang, C. Amine-Responsive Bilayer Indicator Films Based on Black Wolfberry Anthocyanin and Hydrophobically Modified TiO2 for Real-Time Meat Freshness Monitoring. Food Bioprocess Technol. 2025, 18, 9351–9368. [Google Scholar] [CrossRef]
- Shackelford, S.D.; Wheeler, T.L.; Koohmaraie, M. Technical Note: Use of belt grill cookery and slice shear force for assessment of pork longissimus tenderness. J. Anim. Sci. 2004, 82, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; Allen, P.; O’Neill, E.E.; Kerry, J.P. Investigation of the effects of commercial carcass suspension (24 and 48 h) on meat quality in high oxygen modified atmosphere packed beef steaks during chill storage. Food Res. Int. 2010, 43, 277–284. [Google Scholar] [CrossRef]
- Zakrys-Waliwander, P.I.; O’Sullivan, M.G.; O’Neill, E.E.; Kerry, J.P. The effects of high oxygen modified atmosphere packaging on protein oxidation of bovine M. longissimus dorsi muscle during chilled storage. Food Chem. 2012, 131, 527–532. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Zhang, D.; Zhu, L.; Jiang, Q.; Ge, X.; Fang, Y.; Peng, J.; Liu, Y. Real-time and rapid prediction of TVB-N of livestock and poultry meat at three depths for freshness evaluation using a portable fluorescent film sensor. Food Chem. 2023, 400, 134041. [Google Scholar] [CrossRef]
- Lei, Y.; Huang, J.; Cheng, Y.; Zhang, Y.; Huang, T.; Huang, M. Changes in bacterial communities and the volatilome of braised chicken with different packaging stored at 4 °C. Food Res. Int. 2022, 155, 111056. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, X.; Hadiatullah, H.; Zhang, J.; Zhao, G. Determination of microbial diversities and aroma characteristics of Beitang shrimp paste. Food Chem. 2021, 344, 128695. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, H.; Yang, Y.; Cui, L.; Chisoro, P.; Yang, C.; Wu, G.; Li, Q.; Li, J.; Zhang, C.; et al. Exploring the role of static magnetic field in supercooling storage from the viewpoint of meat quality and microbial community. Food Res. Int. 2024, 195, 114884. [Google Scholar] [CrossRef] [PubMed]
- Ndoye, B.; Dicko, M.H.; Gill, C. Exploring the spoilage microbiota of raw and processed meats under different storage environments and its related profiling methods: A review. Cogent Food Agric. 2025, 11, 2562177. [Google Scholar] [CrossRef]
- Walayat, N.; Tang, W.; Wang, X.; Yi, M.; Guo, L.; Ding, Y.; Liu, J.; Ahmad, I.; Ranjha, M. Quality evaluation of frozen and chilled fish: A review. eFood 2023, 4, e67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, L.; Tian, M.; Sun, X.; Shi, R.; Li, J.; Wang, D.; Yang, Q.; Zhang, D.; Hou, C. Synergistic Preservation of Fresh Pork: Coupling Electrostatic Field and Packaging During Controlled Freezing-Point Storage. Foods 2025, 14, 3890. https://doi.org/10.3390/foods14223890
Wang W, Liu L, Tian M, Sun X, Shi R, Li J, Wang D, Yang Q, Zhang D, Hou C. Synergistic Preservation of Fresh Pork: Coupling Electrostatic Field and Packaging During Controlled Freezing-Point Storage. Foods. 2025; 14(22):3890. https://doi.org/10.3390/foods14223890
Chicago/Turabian StyleWang, Wenxin, Le Liu, Ming Tian, Xiaotong Sun, Ruixin Shi, Jiarui Li, Debao Wang, Qingfeng Yang, Dequan Zhang, and Chengli Hou. 2025. "Synergistic Preservation of Fresh Pork: Coupling Electrostatic Field and Packaging During Controlled Freezing-Point Storage" Foods 14, no. 22: 3890. https://doi.org/10.3390/foods14223890
APA StyleWang, W., Liu, L., Tian, M., Sun, X., Shi, R., Li, J., Wang, D., Yang, Q., Zhang, D., & Hou, C. (2025). Synergistic Preservation of Fresh Pork: Coupling Electrostatic Field and Packaging During Controlled Freezing-Point Storage. Foods, 14(22), 3890. https://doi.org/10.3390/foods14223890







