Sustainable Biopolymer Films from Amazonian Tambatinga Fish Waste: Gelatin Extraction and Performance for Food Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Separation and Cleaning of Tambatinga Skins
2.2.2. Tambatinga Skin Composition
2.2.3. Gelatin Production
2.2.4. Physicochemical Characterization of Tambatinga Skin Gelatin
Yield
pH
Amino Acid Content
Gel Strength (Bloom)
Gel Melting Temperature
2.2.5. Production of Tambatinga Gelatin Films
2.2.6. Characterization of Tambatinga Gelatin Films
Thickness and Moisture Content
Scanning Electron Microscopy (SEM)
Fourier-Transform Infrared Spectroscopy (FTIR)
Differential Scanning Calorimetry (DSC)
Mechanical Properties
Water Vapor Permeability (WVP)
Gloss
Color
UV/Visible-Light Barrier
2.3. Statistical Analysis
3. Results and Discussion
3.1. Tambatinga Skin and Gelatin Characterization
3.2. Tambatinga Gelatin Films Characterization
3.2.1. Visual Aspect and Scanning Electron Microscopy (SEM)
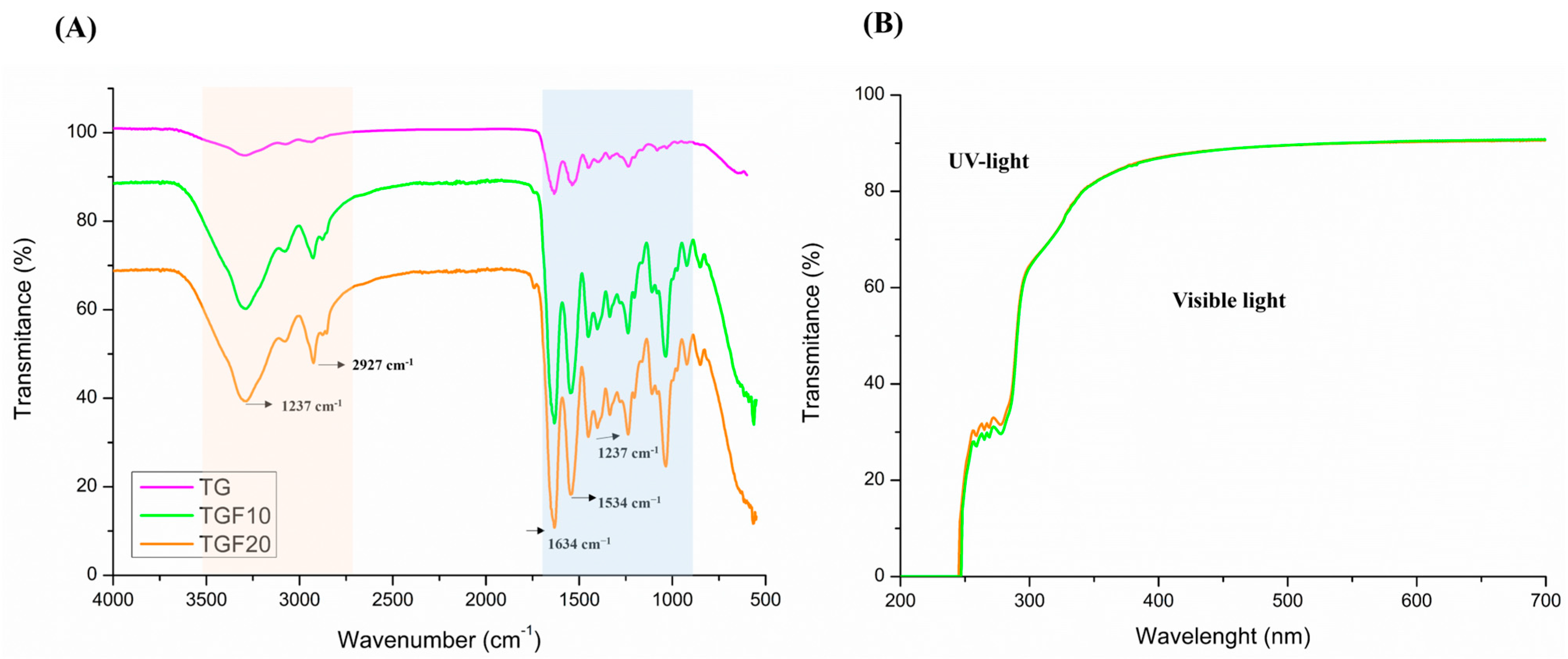
3.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2.3. Thickness and Moisture Content
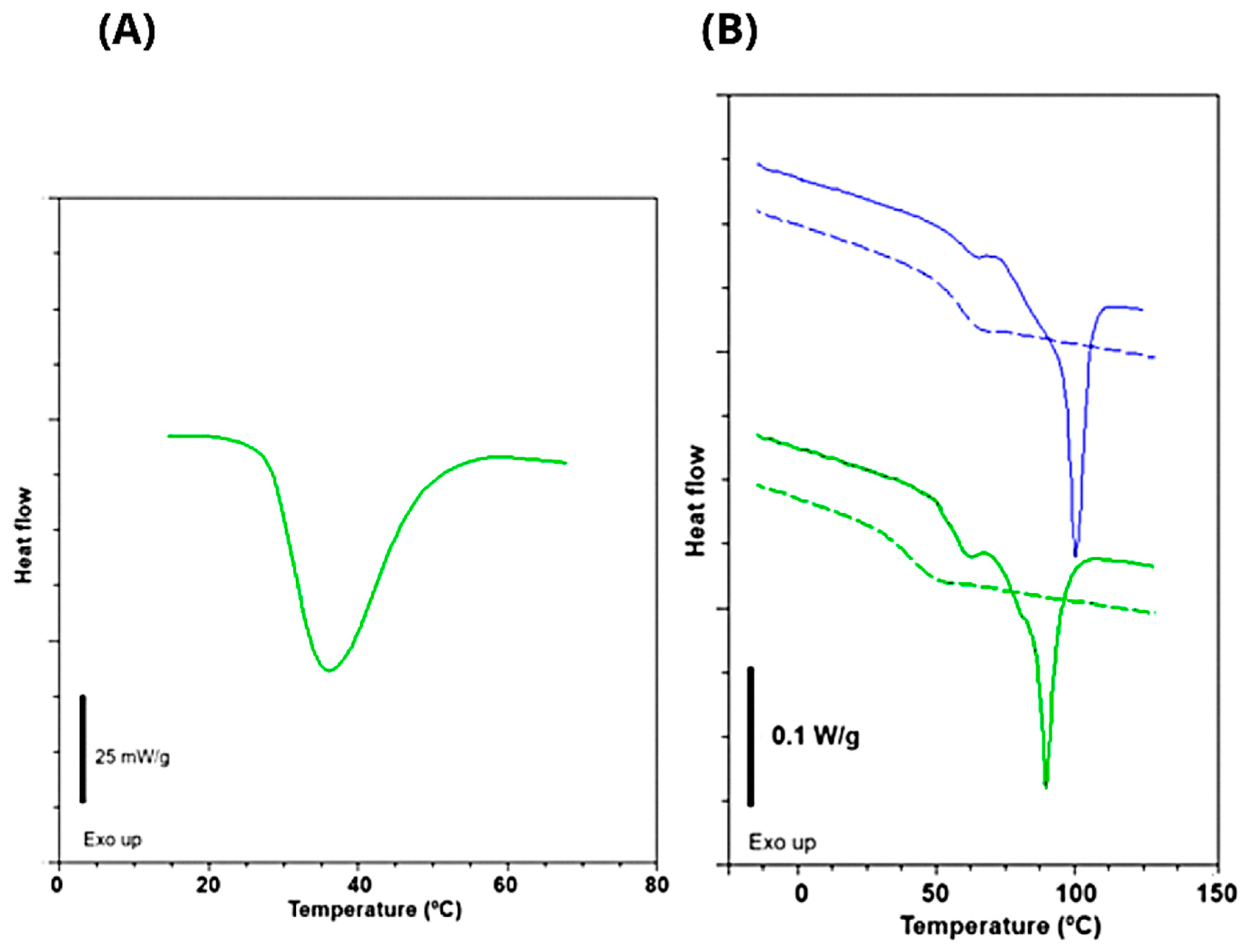
3.2.4. Differential Scanning Calorimetry
3.2.5. Mechanical Properties
3.2.6. Water Vapor Permeability (WVP)
3.2.7. Color and Gloss Properties
3.2.8. UV-Vis Light Barrier
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAO Yearbook: Fishery and Aquaculture Statistics 2019; FAO: Rome, Italy, 2021.
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent Developments in Valorisation of Bioactive Ingredients in Discard/Seafood Processing by-Products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Peixe BR. Anuário Brasileiro da Piscicultura; Brazilian Fish Farming Association: Brasília, Brazil, 2022; pp. 1–79. [Google Scholar]
- Silva-Acuña, A.; Guevara, M. Evaluación de dos dietas comerciales sobre el crecimiento del hÃbrido de Colossoma macropomum x Piaractus brachypomus. Zootec. Trop. 2002, 20, 449–459. [Google Scholar]
- Feltes, M.M.C.; Correia, J.F.G.; Beirão, L.H.; Block, J.M.; Ninow, J.L.; Spiller, V.R. Alternativas Para a Agregação de Valor Aos Resíduos da Industrialização de Peixe. Rev. Bras. Eng. Agrícola Ambient. 2010, 14, 669–677. [Google Scholar] [CrossRef]
- Sousa, S.; Vázquez, J.; Pérez-Martín, R.; Carvalho, A.; Gomes, A. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules 2017, 22, 1545. [Google Scholar] [CrossRef]
- Lv, L.C.; Huang, Q.Y.; Ding, W.; Xiao, X.H.; Zhang, H.Y.; Xiong, L.X. Fish Gelatin: The Novel Potential Applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2009; pp. 142–163. [Google Scholar]
- Sionkowska, A. Collagen Blended with Natural Polymers: Recent Advances and Trends. Prog. Polym. Sci. 2021, 122, 101452. [Google Scholar] [CrossRef]
- Hennet, T. Collagen Glycosylation. Curr. Opin. Struct. Biol. 2019, 56, 131–138. [Google Scholar] [CrossRef]
- Grand View Research. Gelatin Market Size, Share & Trends Analysis Report by Source (Bovine, Porcine), by Function (Stabilizer, Thickener), by Application (Food & Beverages), by Region, and Segment Forecasts, 2022–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/gelatin-market-analysis (accessed on 4 February 2025).
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the Circular Economy: An Analysis of 114 Definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Toniciolli Rigueto, C.V.; Rosseto, M.; Alessandretti, I.; de Oliveira, R.; Wohlmuth, D.A.R.; Ferreira Menezes, J.; Loss, R.A.; Dettmer, A.; Pizzutti, I.R. Gelatin Films from Wastes: A Review of Production, Characterization, and Application Trends in Food Preservation and Agriculture. Food Res. Int. 2022, 162, 112114. [Google Scholar] [CrossRef]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and Characterization of Acid Soluble Collagen from Fish Waste: Development of Collagen-Chitosan Blend as Food Packaging Film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Getachew, A.T.; Ahmad, R.; Park, J.-S.; Chun, B.-S. Fish Skin Gelatin Based Packaging Films Functionalized by Subcritical Water Extract from Spent Coffee Ground. Food Packag. Shelf Life 2021, 29, 100735. [Google Scholar] [CrossRef]
- Li, Y.; Shan, P.; Yu, F.; Li, H.; Peng, L. Fabrication and Characterization of Waste Fish Scale-Derived Gelatin/Sodium Alginate/Carvacrol Loaded ZIF-8 Nanoparticles Composite Films with Sustained Antibacterial Activity for Active Food Packaging. Int. J. Biol. Macromol. 2023, 230, 123192. [Google Scholar] [CrossRef] [PubMed]
- Thirukumaran, R.; Anu Priya, V.K.; Krishnamoorthy, S.; Ramakrishnan, P.; Moses, J.A.; Anandharamakrishnan, C. Resource Recovery from Fish Waste: Prospects and the Usage of Intensified Extraction Technologies. Chemosphere 2022, 299, 134361. [Google Scholar] [CrossRef] [PubMed]
- Patwary, M.d.A.; Yasin, M.d.; Azam, M.d.S.; Hossain, M.A. Extraction and Industrial Valorization of Collagen, Gelatin, and Hydroxyapatite from Freshwater Fish Scales: A Review. Food Bioprod. Process. 2025, 154, 319–333. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and Physico-Chemical Characterisation of Nile Perch (Lates niloticus) Skin and Bone Gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, K.; Zhu, Y.; Zhang, W.; Xie, Y.; Wang, Z.; Zhou, H.; Yang, T.; Zhang, Q.; Xu, B. Collagen and Its Derivatives: From Structure and Properties to Their Applications in Food Industry. Food Hydrocoll. 2022, 131, 107748. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- BCAF. Physical-Chemical Methods n. 5, 12, 45, 53. In Physical Methods in Chemical Analysis; Academic Press: Cambridge, MA, USA, 2017; pp. 247–248. [Google Scholar]
- ASTM D2868-17; Standard Test Method for Nitrogen Content (Kjeldahl) and Hide Substance Content of Leather, Wet Blue and Wet White. ASTM International: West Conshohocken, PA, USA, 2017.
- Diniz, G.S.; Barbarino, E.; Oiano-Neto, J.; Pacheco, S.; Lourenço, S.O. Gross Chemical Profile and Calculation of Nitrogen-to-Protein Conversion Factors for Nine Species of Fishes from Coastal Waters of Brazil. Lat. Am. J. Aquat. Res. 2017, 41, 254–264. [Google Scholar] [CrossRef]
- AOCS. Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction. Oficial Procedure. Am. Oil Chem. Soc. 2017, Am 5-04, 1–4. [Google Scholar]
- Ramalho Procopio, F.; Valéria de Campos, J.; Mattos, A.L.A.; de Sá Moreira de Souza Filho, M.; Furtado, A.A.L.; de Araujo Nogueira, A.R.; Antonio Chagas Jacintho, M. An Enhancement to Development, Characterization and Potential Application of Gelatin Extracted from Native Brazilian Fish Skin (Colossoma macropomum). J. Aquat. Food Product. Technol. 2024, 33, 205–220. [Google Scholar] [CrossRef]
- White, J.A.; Hart, R.J.; Fry, J.C. An Evaluation of the Waters Pico-Tag System for the Amino-Acid Analysis of Food Materials. J. Autom. Chem. 1986, 8, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.R.; Frost, B.; Augustin, J. Precolumn Phenylisothiocyanate Derivatization and Liquid Chromatography of Amino Acids in Food. J. Assoc. Off. Anal. Chem. 1989, 72, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Spies, J.R. Determination of Tryptophan in Proteins. Anal. Chem. 1967, 39, 1412–1416. [Google Scholar] [CrossRef]
- Gelatin Manufacturers Institute of America. Standard Methods for the Testing of Edible Gelatin; GMIA: Chandler, AZ, USA, 2019; pp. 1–32. [Google Scholar]
- Sobral, P.J.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, Water Vapor Barrier and Thermal Properties of Gelatin Based Edible Films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Bergo, P.; Moraes, I.C.F.; Sobral, P.J.d.A. Effects of Moisture Content on Structural and Dielectric Properties of Cassava Starch Films. Starch-Stärke 2012, 64, 835–839. [Google Scholar] [CrossRef]
- ASTM D882/12; Standard Test Methods for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials; ASTM International: West Conshohocken, PA, USA, 2001.
- ASTM E96/E96M; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 1989.
- Bonilla, J.; Sobral, P.J.A. Investigation of the Physicochemical, Antimicrobial and Antioxidant Properties of Gelatin-Chitosan Edible Film Mixed with Plant Ethanolic Extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Jamilah, B.; Tan, K.W.; Umi Hartina, M.R.; Azizah, A. Gelatins from Three Cultured Freshwater Fish Skins Obtained by Liming Process. Food Hydrocoll. 2011, 25, 1256–1260. [Google Scholar] [CrossRef]
- Giacomelli da Silva, C.; Souza Rodrigues, A.; Carolina Lima, A.; de Oliveira Mello, R.; Dal Pont Morisso, F.; Cristina Prestes Dornelles, R.; Hashime Kubota, E. Gelatin Extracted from Jundiá Skin (Rhamdia quelen): An Alternative to the Discarded by-Product. Food Res. Int. 2022, 161, 111829. [Google Scholar] [CrossRef]
- Binsi, P.K.; Shamasundar, B.A.; Dileep, A.O.; Badii, F.; Howell, N.K. Rheological and Functional Properties of Gelatin from the Skin of Bigeye Snapper (Priacanthus hamrur) Fish: Influence of Gelatin on the Gel-Forming Ability of Fish Mince. Food Hydrocoll. 2009, 23, 132–145. [Google Scholar] [CrossRef]
- Arnesen, J.; Gildberg, A. Extraction and Characterisation of Gelatine from Atlantic Salmon (Salmo salar) Skin. Bioresour. Technol. 2007, 98, 53–57. [Google Scholar] [CrossRef]
- Alves, A.L.; Fraguas, F.J.; Carvalho, A.C.; Valcárcel, J.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A.; Silva, T.H. Characterization of Codfish Gelatin: A Comparative Study of Fresh and Salted Skins and Different Extraction Methods. Food Hydrocoll. 2022, 124, 107238. [Google Scholar] [CrossRef]
- Yang, L.; Yang, M.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Structural and Emulsion Stabilization Comparison of Four Gelatins from Two Freshwater and Two Marine Fish Skins. Food Chem. 2022, 371, 131129. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Pérez-Mateos, M.; Gómez-Estaca, J.; López-Caballero, E.; Giménez, B.; Montero, P. Fish Gelatin: A Renewable Material for Developing Active Biodegradable Films. Trends Food Sci. Technol. 2009, 20, 3–16. [Google Scholar] [CrossRef]
- Fan, H.Y.; Dumont, M.-J.; Simpson, B.K. Preparation and Physicochemical Characterization of Films Prepared with Salmon Skin Gelatin Extracted by a Trypsin-Aided Process. Curr. Res. Food Sci. 2020, 3, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Montero, P.; Fernández-Martín, F.; Gómez-Guillén, M.C. Physico-Chemical and Film-Forming Properties of Bovine-Hide and Tuna-Skin Gelatin: A Comparative Study. J. Food Eng. 2009, 90, 480–486. [Google Scholar] [CrossRef]
- Bonilla, J.; Paiano, R.B.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.A. Biodegradation of Films Based on Natural and Synthetic Biopolymers Using an Aquatic System from Active Sludge. J. Polym. Environ. 2021, 29, 1380–1395. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Liu, Y.; Qin, Y.; Kan, J.; Liu, J. Preparation and Characterization of Active and Intelligent Films Based on Fish Gelatin and Haskap Berries (Lonicera caerulea L.) Extract. Food Packag. Shelf Life 2019, 22, 100417. [Google Scholar] [CrossRef]
- Tessaro, L.; Luciano, C.G.; Quinta Barbosa Bittante, A.M.; Lourenço, R.V.; Martelli-Tosi, M.; José do Amaral Sobral, P. Gelatin and/or Chitosan-Based Films Activated with “Pitanga” (Eugenia uniflora L.) Leaf Hydroethanolic Extract Encapsulated in Double Emulsion. Food Hydrocoll. 2021, 113, 106523. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, J.; Kang, X.; Wang, B.; Liu, P.; Cui, B.; Abd El-Aty, A.M. Development and Characterization of Starch Films Prepared by Extrusion Blowing: The Synergistic Plasticizing Effect of Water and Glycerol. LWT 2021, 148, 111820. [Google Scholar] [CrossRef]
- Tabatabaei, S.D.; Ghiasi, F.; Hashemi Gahruie, H.; Hosseini, S.M.H. Effect of Emulsified Oil Droplets and Glycerol Content on the Physicochemical Properties of Persian Gum-Based Edible Films. Polym. Test. 2022, 106, 107427. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Plasticizers; ChemTec Publishing: Toronto, ON, Canada, 2004. [Google Scholar]
- Mirzapour-Kouhdasht, A.; Moosavi-Nasab, M.; Yousefi, R.; Eun, J.-B. Bio/Multi-Functional Peptides Derived from Fish Gelatin Hydrolysates: Technological and Functional Properties. Biocatal. Agric. Biotechnol. 2021, 36, 102152. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The Effect of Plasticizers on the Functional Properties of Biodegradable Gelatin-Based Film: A Review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Emulsion Film Based on Fish Skin Gelatin and Palm Oil: Physical, Structural and Thermal Properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.; Psomiadou, E.; Nakayama, A.; Aiba, S.; Yamamoto, N. Edible Films Made from Gelatin, Soluble Starch and Polyols, Part 3. Food Chem. 1997, 60, 593–604. [Google Scholar] [CrossRef]
- Liu, W.; Tian, Z.; Li, C.; Li, G. Thermal Denaturation of Fish Collagen in Solution: A Calorimetric and Kinetic Analysis. Thermochim. Acta 2014, 581, 32–40. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, Morphological and Thermal Behaviour Characterisations of Fish Gelatin Film Incorporated with Basil and Citronella Essential Oils as Affected by Surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Avena-Bustillos, R.J.; Bechtel, P.J.; Imam, S.H.; Glenn, G.M.; Orts, W.J. Effects of Drying Temperature on Barrier and Mechanical Properties of Cold-Water Fish Gelatin Films. J. Food Eng. 2009, 95, 327–331. [Google Scholar] [CrossRef]
- Wasswa, J.; Tang, J.; Gu, X. Utilization of Fish Processing By-Products in the Gelatin Industry. Food Rev. Int. 2007, 23, 159–174. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of Structural, Functional and Antioxidant Properties of Fish Gelatin Films Using Maillard Reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Kusznierewicz, B.; Malinowska-Pańczyk, E.; Sinkiewicz, I.; Gottfried, K.; Kołodziejska, I. Fish Gelatin Films Containing Aqueous Extracts from Phenolic-Rich Fruit Pomace. LWT 2020, 117, 108613. [Google Scholar] [CrossRef]
- Morais, S.K.Q.; Felipe, K.H.; de Souza Silva, J.D.; Santos, N.C.; de Farias Araújo, M.S.; Vieira, P.P.F.; Gusmão, T.A.S.; Gouveia, D.S. Development of Nile Tilapia Gelatin Films with Grape Pomace Extract for Fish Fillet Storage: Hydrophobic, Functional Properties, and Biodegradability. Food Biosci. 2025, 68, 106675. [Google Scholar] [CrossRef]
- Wu, J.; Song, G.; Huang, R.; Yan, Y.; Li, Q.; Guo, X.; Shi, X.; Tian, Y.; Wang, J.; Wang, S. Fish Gelatin Films Incorporated with Cinnamaldehyde and Its Sulfobutyl Ether-β-Cyclodextrin Inclusion Complex and Their Application in Fish Preservation. Food Chem. 2023, 418, 135871. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Zhang, A.; Xu, L.; Li, X.; Hu, T.; Shi, X. Characterization of Fish Gelatin-Artemisia Sphaerocephala Krasch Gum Films Prepared by Adding Bamboo Leaf Flavonoids as an Active Substrate: Application in Chilled Pork Preservation. LWT 2025, 231, 118367. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Salem, A.; Kechaou, H.; Nasri, M.; Menchari, Y. Physicochemical, Antioxidant and Antibacterial Properties of Fish Gelatin-Based Edible Films Enriched with Orange Peel Pectin: Wrapping Application. Food Hydrocoll. 2020, 103, 105688. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Iriany; Yustira, A.; Julianti, E.; Jaafar, M. Innovative Edible Food Wraps from Tilapia Fish Bone Gelatin and Passion Fruit Peel Extract. Case Stud. Chem. Environ. Eng. 2024, 10, 100990. [Google Scholar] [CrossRef]
- Nurilmala, M.; Suryamarevita, H.; Husein Hizbullah, H.; Jacoeb, A.M.; Ochiai, Y. Fish Skin as a Biomaterial for Halal Collagen and Gelatin. Saudi J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-69933-0. [Google Scholar]

| Fish Species (Skin) | Moisture (%) | Protein (%) * | Lipids (%) * | Ash (%) * | Reference |
|---|---|---|---|---|---|
| Tambatinga | 69.85 | 84.2 ± 1.4 | 13.1 ± 0.7 | 0.7 ± 0.1 | |
| Jundiá | 70.6 ± 0.8 | 89.3 ± 2.9 | 5.9 ± 0.4 | 3.4 ± 0.3 | [37] |
| Red Tilapia | 70.43 ± 0.2 | 29.07 ± 0.31 | - | 0.51 ± 0.09 | [36] |
| Walking catfish | 62.47 ± 0.34 | 31.01 ± 0.48 | - | 0.52 ± 0.23 | [6] |
| Bigeye snapper | 52.79 ± 0.53 | 25.19 ± 0.85 | 1.2 ± 0.06 | 20.2 ± 0.64 | [38] |
| Properties | Tambatinga Skin Gelatin |
|---|---|
| Yield (%) | 35.5 ± 3.5 |
| Turbidity (NFU) | 13.8 ± 0.3 |
| pH | 5.18 ± 0.16 |
| Gel strength (Bloom) | 263.9 ± 5.3 |
| Melting temperature (°C) | 36.6 ± 0.6 |
| Melting enthalpy (J/g) | 7.0 ± 1.3 |
| Amino Acid | g/100 g of Total Amino Acids |
|---|---|
| Aspartic acid | 5.39 ± 0.04 |
| Glutamic acid | 9.74 ± 0.03 |
| Serine | 3.23 ± 0.01 |
| Glycine | 23.44 ± 0.09 |
| Histidine * | 0.67 ± 0.03 |
| Arginine | 8.70 ± 0.13 |
| Threonine * | 2.34 ± 0.07 |
| Alanine | 9.76 ± 0.03 |
| Proline | 12.47 ± 0.05 |
| Tyrosine | 0.41 ± 0.02 |
| Valine * | 2.12 ± 0.04 |
| Methionine * | 1.10 ± 0.08 |
| Cysteine | 0.47 ± 0.02 |
| Isoleucine * | 1.25 ± 0.02 |
| Leucine * | 2.62 ± 0.01 |
| Phenylalanine * | 1.80 ± 0.03 |
| Lysine * | 3.57 ± 0.02 |
| Hydroxyproline | 9.84 ± 0.01 |
| Tryptophan * | ND < 0.05 |
| Properties | TGF20 | TGF10 |
|---|---|---|
| Thickness (µm) | 72.2 ± 8.0 a | 70.4 ± 7.1 a |
| Moisture (%) | 11.6 ± 0.0 a | 11.1 ± 0.7 a |
| Tg1 (°C) 1st scan | 58.3 | 60.1 |
| Tg2 (°C) 1st scan | 77.9 | 80.7 |
| Tm (°C) 1st scan | 89.7 | 100.3 |
| ΔHm (J/g) 1st scan | 19.8 | 23.8 |
| Tg (°C) 2nd scan | 41.5 | 59.7 |
| Tensile strength (MPa) | 37.9 ± 3.3 b | 59.4 ± 5.8 a |
| Elongation at break (%) | 159.1 ± 3.2 a | 116.0 ± 3.6 b |
| Elastic modulus (MPa) | 4.9 ± 0.7 b | 13.8 ± 1.9 a |
| Water vapor permeability (g·mm/cm2·h·kPa) | 0.028 ± 0.00 a | 0.025 ± 0.00 b |
| Gloss units (G.U.) at 20° | 77.1 ± 14.6 a | 81.4 ± 5.7 a |
| Gloss units (G.U.) at 60° | 145.3 ± 3.3 B | 152.5 ± 2.2 A |
| L* | 89.9 ± 0.2 a | 90.2 ± 0.1 a |
| a* | −1.1 ± 0.0 a | −1.1 ± 0.0 a |
| b* | 2.7 ± 0.2 a | 2.8 ± 0.3 a |
| ΔE* | 3.8 ± 0.2 a | 3.6 ± 0.2 a |
| Opacity (%) | 0.4 ± 0.1 a | 0.6 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procopio, F.R.; Lourenço, R.V.; Bitante, A.M.Q.B.; Sobral, P.J.d.A.; Jacintho, M.A.C. Sustainable Biopolymer Films from Amazonian Tambatinga Fish Waste: Gelatin Extraction and Performance for Food Packaging Applications. Foods 2025, 14, 3866. https://doi.org/10.3390/foods14223866
Procopio FR, Lourenço RV, Bitante AMQB, Sobral PJdA, Jacintho MAC. Sustainable Biopolymer Films from Amazonian Tambatinga Fish Waste: Gelatin Extraction and Performance for Food Packaging Applications. Foods. 2025; 14(22):3866. https://doi.org/10.3390/foods14223866
Chicago/Turabian StyleProcopio, Fernanda Ramalho, Rodrigo Vinícius Lourenço, Ana Mônica Q. B. Bitante, Paulo José do Amaral Sobral, and Manuel Antônio Chagas Jacintho. 2025. "Sustainable Biopolymer Films from Amazonian Tambatinga Fish Waste: Gelatin Extraction and Performance for Food Packaging Applications" Foods 14, no. 22: 3866. https://doi.org/10.3390/foods14223866
APA StyleProcopio, F. R., Lourenço, R. V., Bitante, A. M. Q. B., Sobral, P. J. d. A., & Jacintho, M. A. C. (2025). Sustainable Biopolymer Films from Amazonian Tambatinga Fish Waste: Gelatin Extraction and Performance for Food Packaging Applications. Foods, 14(22), 3866. https://doi.org/10.3390/foods14223866





