Protective Effects of Quinic Acid Against Disuse-Induced Skeletal Muscle Atrophy via Regulation of Inflammation and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Micro-Computed Tomography (Micro-CT) Imaging
2.3. Histological Analysis
2.4. Grip Strength Test
2.5. Treadmill Test
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Cell Culture
2.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. QA Restored the DMA-Induced Reductions in Muscle Weights Along with Muscle Fiber CSA
3.2. QA Enhanced Grip Strength and Exercise Capacity in DMA-Induced Muscle Atrophy Mice
3.3. QA Inhibited Muscle-E3 Ubiquitin Ligases Along with the Modulation of Inflammatory Cytokines and Antioxidant Enzymes in the TA Muscle of DMA-Induced Mice
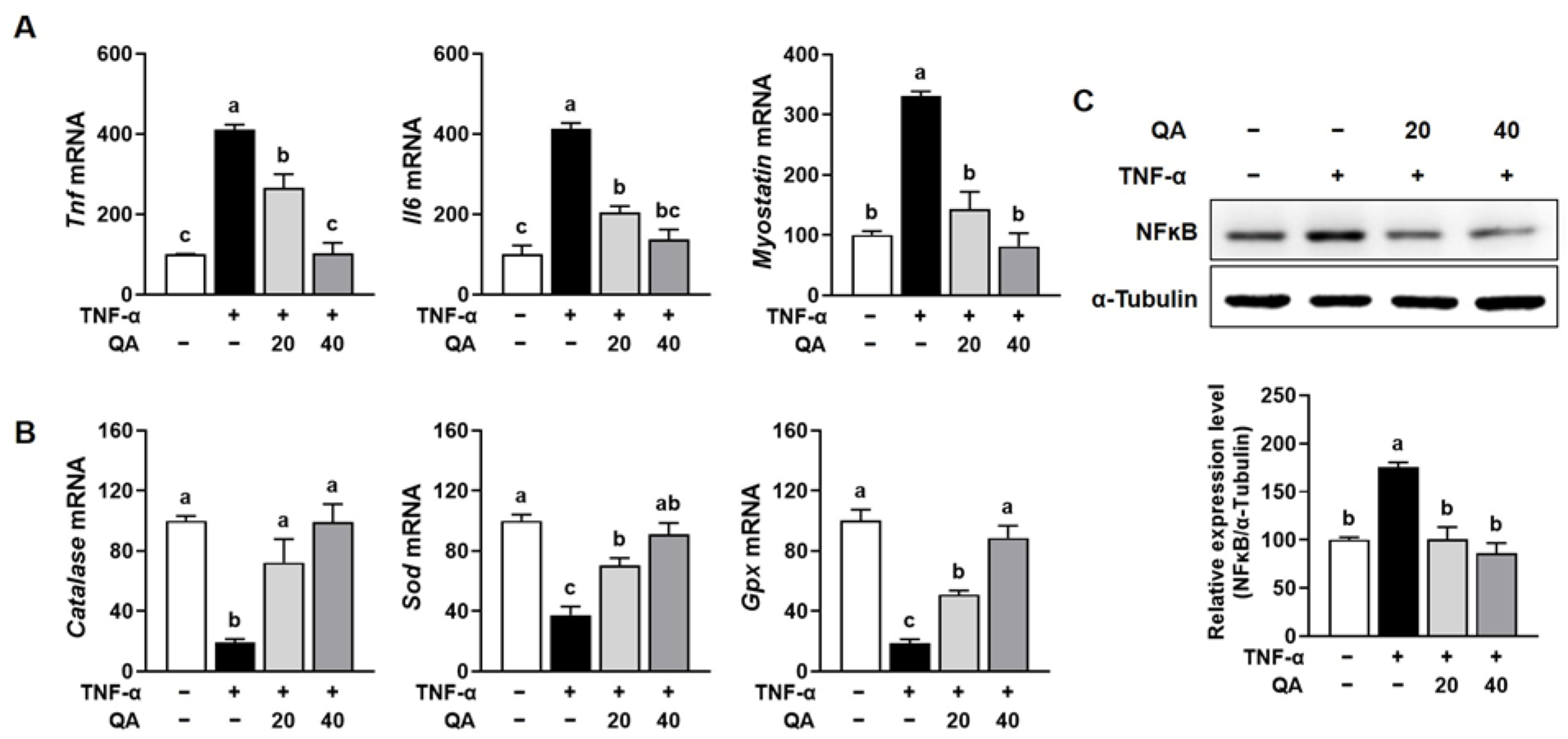
3.4. QA Suppressed NF-κB-Driven Pro-Inflammatory Cytokines and Increased Antioxidant Enzymes in TNF-α-Induced L6 Myotube Atrophy
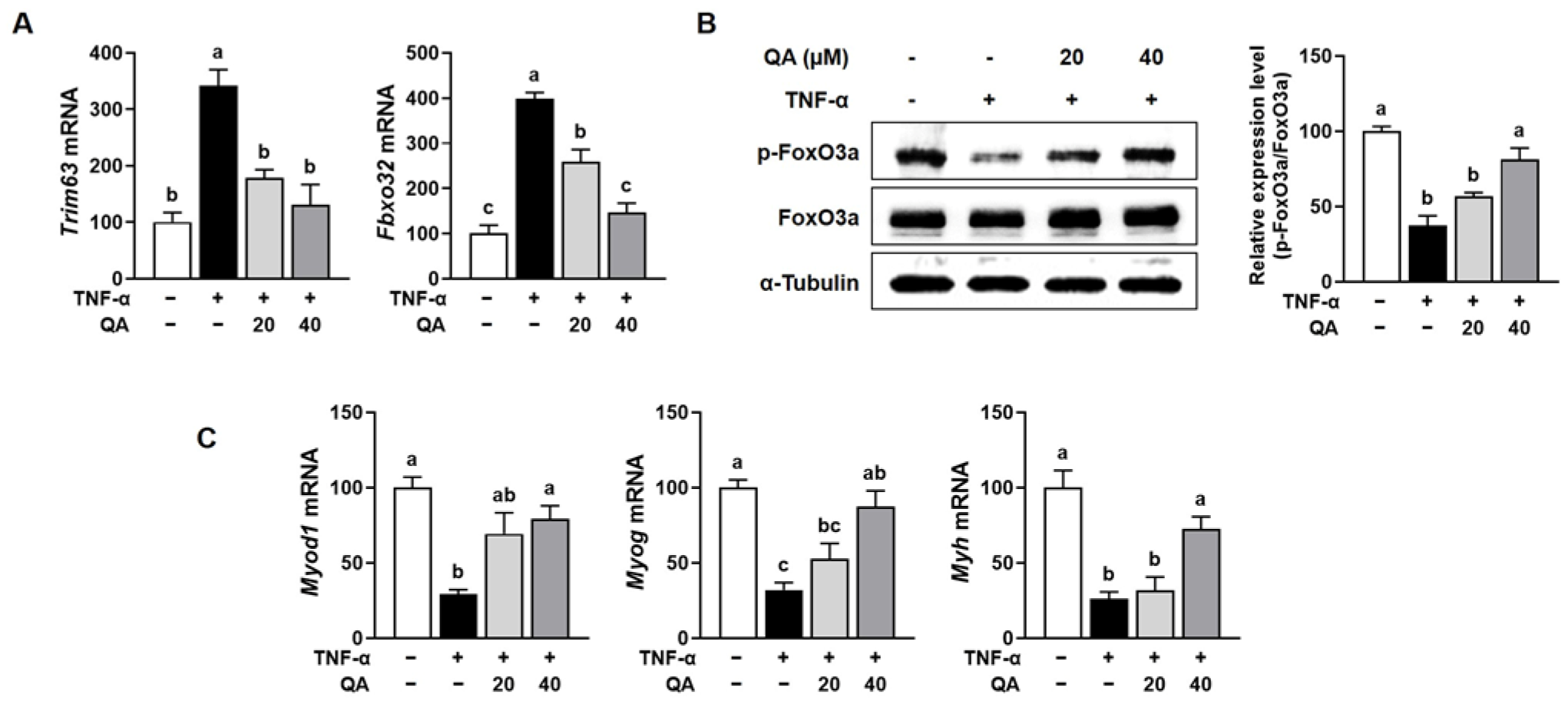
3.5. QA Attenuated Protein Degradation While Enhancing Protein Synthesis in TNF-α-Induced L6 Myotube Atrophy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DMA | disuse-induced muscle atrophy |
| EDL | extensor digitorum longus |
| FoXO3α | forkhead box protein O3α |
| GA | gastrocnemius |
| IL | interleukin |
| mTOR | mammalian target of rapamycin |
| MuRF1 | muscle ring finger 1 |
| NF-κB | nuclear factor kappa B |
| PI3K | phosphatidylinositol 3-kinase |
| p-4EBP1 | phosphorylated 4EBP1 |
| p-Akt | phosphorylated Akt |
| p-mTOR | phosphorylated mTOR |
| p-PI3K | phosphorylated PI3K |
| p-p70S6K | phosphorylated p70S6K |
| QA | quinic acid |
| ROS | reactive oxygen species |
| SOL | soleus |
| TA | tibialis anterior |
| TNF-α | tumor necrosis factor-α |
References
- Nunes, E.A.; Stokes, T.; McKendry, J.; Currier, B.S.; Phillips, S.M. Disuse-induced skeletal muscle atrophy in disease and nondisease states in humans: Mechanisms, prevention, and recovery strategies. Am. J. Physiol.-Cell Physiol. 2022, 322, C1068–C1084. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Cellular mechanism of immobilization-induced muscle atrophy: A mini review. Sports Med. Health Sci. 2019, 1, 19–23. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Bao, P.; Wang, Y.J.; Lu, J.; Liu, Y.J. H2S protects against immobilization-induced muscle atrophy via reducing oxidative stress and inflammation. Front. Physiol. 2022, 13, 844539. [Google Scholar] [CrossRef]
- Phillips, S.M.; McGlory, C. CrossTalk proposal: The dominant mechanism causing disuse muscle atrophy is decreased protein synthesis. J. Physiol. 2014, 592, 5341–5343. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Criswell, D.S. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid. Redox Signal. 2011, 15, 2519–2528. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, X.; Zhang, J.; Dong, G.; Xiao, W.; Xu, X. Hydrogen sulfide alleviates skeletal muscle fibrosis via attenuating inflammation and oxidative stress. Front. Physiol. 2020, 11, 533690. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, F.; Yin, Q.; Wang, D.; Han, W.; Zhang, Y. Reactive oxygen species interact with NLRP3 inflammasomes and are involved in the inflammation of sepsis: From mechanism to treatment of progression. Front. Physiol. 2020, 11, 571810. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2024, 40, 3408–3437. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.; Dandstedt, G. Structure and configuration of quinic acid. Chem. Ber. 1932, 65, 1009. [Google Scholar]
- Lee, S.Y.; Moon, E.; Kim, S.Y.; Lee, K.R. Quinic acid derivatives from Pimpinella brachycarpa exert anti-neuroinflammatory activity in lipopolysaccharide-induced microglia. Bioorganic Med. Chem. Lett. 2013, 23, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Paing, Y.M.M.; Cho, N.; Kim, C.; Yoo, J.; Choi, J.W.; Lee, S.H. Quinic acid alleviates behavior impairment by reducing neuroinflammation and MAPK activation in LPS-treated mice. Biomol. Ther. 2024, 32, 309–318. [Google Scholar] [CrossRef]
- Jang, S.-A.; Park, D.W.; Kwon, J.E.; Song, H.S.; Park, B.; Jeon, H.; Sohn, E.-H.; Koo, H.J.; Kang, S.C. Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed. Pharmacother. 2017, 96, 563–571. [Google Scholar] [CrossRef]
- Soh, Y.; Kim, J.-A.; Sohn, N.W.; Lee, K.R.; Kim, S.Y. Protective effects of quinic acid derivatives on tetrahydropapaveroline-induced cell death in C6 glioma cells. Biol. Pharm. Bull. 2003, 26, 803–807. [Google Scholar] [CrossRef]
- Han, Z.; Wang, L.; Sun, P.; Huang, M.; Yu, F.; Liu, J.; Wu, Y.; He, P.; Tu, Y.; Li, B. Quinic acid as an inhibitor of α-glucosidase activity, nonenzymatic glycosylation, and glucose transport in Caco-2 cells. Food Front. 2024, 5, 2545–2555. [Google Scholar] [CrossRef]
- Dong, J.; Zheng, H.; Zeng, Q.; Zhang, X.; Du, L.; Bais, S. Protective effect of D-(−)-quinic acid as food supplement in modulating AMP-activated protein kinase signalling pathway activation in HFD induced obesity. Hum. Exp. Toxicol. 2022, 41, 09603271221119804. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef] [PubMed]
- Mortelé, O.; Jörissen, J.; Spacova, I.; Lebeer, S.; van Nuijs, A.L.; Hermans, N. Demonstrating the involvement of an active efflux mechanism in the intestinal absorption of chlorogenic acid and quinic acid using a Caco-2 bidirectional permeability assay. Food Funct. 2021, 12, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, M.-B.; Hwang, J.-K. Red bean extract inhibits immobilization-induced muscle atrophy in C57BL/6N mice. J. Med. Food 2020, 23, 29–36. [Google Scholar] [CrossRef]
- Sa, B.-K.; Kim, C.; Kim, M.-B.; Hwang, J.-K. Panduratin A prevents tumor necrosis factor-alpha-induced muscle atrophy in L6 rat skeletal muscle cells. J. Med. Food 2017, 20, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Deng, C.; Qiu, J.; Wang, K.; Chang, M.; Zhou, S.; Gu, Y.; Shen, Y.; Wang, W.; et al. Potential Therapeutic Strategies for Skeletal Muscle Atrophy. Antioxidants 2022, 12, 44. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Wang, Y. Factors, mechanisms and improvement methods of muscle strength loss. Front. Cell Dev. Biol. 2024, 12, 1509519. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Esser, K.A. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 230–235. [Google Scholar] [CrossRef]
- Charge, S.B.; Rudnicki, M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004, 84, 209–238. [Google Scholar] [CrossRef]
- Zumbaugh, M.D.; Johnson, S.E.; Shi, T.H.; Gerrard, D.E. Molecular and biochemical regulation of skeletal muscle metabolism. J. Anim. Sci. 2022, 100, skac035. [Google Scholar] [CrossRef] [PubMed]
- Ommati, M.M.; Farshad, O.; Mousavi, K.; Khalili, M.; Jamshidzadeh, A.; Heidari, R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia 2020, 75, 1221–1230. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Xue, L.; Sun, Y.; Zhang, K.; Fan, M.; Qian, H.; Li, Y.; Wang, L. Chlorogenic Acid Improves High-Fat Diet-Induced Skeletal Muscle Metabolic Disorders by Regulating Mitochondrial Function and Lactate Metabolism. J. Agric. Food Chem. 2025, 73, 10347–10357. [Google Scholar] [CrossRef] [PubMed]
- Meador, B.M.; Mirza, K.A.; Tian, M.; Skelding, M.B.; Reaves, L.A.; Edens, N.K.; Tisdale, M.J.; Pereira, S.L. The Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCg) Attenuates Skeletal Muscle Atrophy in a Rat Model of Sarcopenia. J. Frailty Aging 2015, 4, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; Bennett, B.T.; Wilson, J.C.; Edens, N.K.; Pereira, S.L. Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats. Exp. Gerontol. 2014, 50, 82–94. [Google Scholar] [CrossRef]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W.; et al. Inflammation: Roles in Skeletal Muscle Atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Zhang, H.; Qi, G.; Wang, K.; Yang, J.; Shen, Y.; Yang, X.; Chen, X.; Yao, X.; Gu, X.; Qi, L.; et al. Oxidative stress: Roles in skeletal muscle atrophy. Biochem. Pharmacol. 2023, 214, 115664. [Google Scholar] [CrossRef]
- Shen, Y.C.; Yen, J.C.; Liou, K.T. Ameliorative Effects of Caffeic Acid Phenethyl Ester on an Eccentric Exercise-Induced Skeletal Muscle Injury by Down-Regulating NF-κB Mediated Inflammation. Pharmacology 2013, 91, 219–228. [Google Scholar] [CrossRef]
- Le, N.H.; Kim, C.S.; Park, T.; Park, J.H.; Sung, M.K.; Lee, D.G.; Hong, S.M.; Choe, S.Y.; Goto, T.; Kawada, T.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef]
- Sun, L.J.; Sun, Y.N.; Chen, S.J.; Liu, S.; Jiang, G.R. Resveratrol attenuates skeletal muscle atrophy induced by chronic kidney disease via MuRF1 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 487, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Rzhepakovsky, I.; Piskov, S.; Avanesyan, S.; Dudchik, N.; Sizonenko, M.; Timchenko, L.; Kurchenko, V.; Osadchy, A.; Nagdalian, A.; Jafari, S.M. Functional and health-promoting bioactivities of fractions derived from chicken embryo hydrolysates. Food Res. Int. 2025, 219, 117058. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, Y.; Jia, G.; Zhao, H.; Liu, G.; Huang, Z. Ferulic acid regulates muscle fiber type formation through the Sirt1/AMPK signaling pathway. Food Funct. 2019, 10, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ushio, H. Ferulic Acid Promotes Hypertrophic Growth of Fast Skeletal Muscle in Zebrafish Model. Nutrients 2017, 9, 1066. [Google Scholar] [CrossRef]
- Jang, Y.J.; Son, H.J.; Kim, J.S.; Jung, C.H.; Ahn, J.; Hur, J.; Ha, T.Y. Coffee consumption promotes skeletal muscle hypertrophy and myoblast differentiation. Food Funct. 2018, 9, 1102–1111. [Google Scholar] [CrossRef]
- Wang, D.T.; Yin, Y.; Yang, Y.J.; Lv, P.J.; Shi, Y.; Lu, L.; Wei, L.B. Resveratrol prevents TNF-alpha-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014, 19, 206–213. [Google Scholar] [CrossRef]
- Sishi, B.J.; Engelbrecht, A.M. Tumor necrosis factor alpha (TNF-alpha) inactivates the PI3-kinase/PKB pathway and induces atrophy and apoptosis in L6 myotubes. Cytokine 2011, 54, 173–184. [Google Scholar] [CrossRef]
- Farah, A.; de Paula Lima, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee chlorogenic acids incorporation for bioactivity enhancement of foods: A review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Heikkilä, E.; Hermant, A.; Thevenet, J.; Bermont, F.; Kulkarni, S.S.; Ratajczak, J.; Santo-Domingo, J.; Dioum, E.H.; Canto, C.; Barron, D. The plant product quinic acid activates Ca2+-dependent mitochondrial function and promotes insulin secretion from pancreatic beta cells. Br. J. Pharmacol. 2019, 176, 3250–3263. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Al-Obaidi, M.M.J.; Shahid, N.; Noordin, M.I.B.; Looi, C.Y.; Wong, W.F.; Khaing, S.L.; Mustafa, M.R. Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney and pancreas tissues of STZ-induced diabetic rats: A mechanistic study. Food Chem. Toxicol. 2014, 71, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Inbathamizh, L.; Padmini, E. Quinic acid as a potent drug candidate for prostate cancer—A comparative pharmacokinetic approach. Asian J. Pharm. Clin. Res. 2013, 6, 106–112. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-B.; Lee, H.; Kang, J.; Kim, B.; Hwang, J.-K. Protective Effects of Quinic Acid Against Disuse-Induced Skeletal Muscle Atrophy via Regulation of Inflammation and Oxidative Stress. Foods 2025, 14, 3833. https://doi.org/10.3390/foods14223833
Kim M-B, Lee H, Kang J, Kim B, Hwang J-K. Protective Effects of Quinic Acid Against Disuse-Induced Skeletal Muscle Atrophy via Regulation of Inflammation and Oxidative Stress. Foods. 2025; 14(22):3833. https://doi.org/10.3390/foods14223833
Chicago/Turabian StyleKim, Mi-Bo, Hyerin Lee, Junhui Kang, Bohkyung Kim, and Jae-Kwan Hwang. 2025. "Protective Effects of Quinic Acid Against Disuse-Induced Skeletal Muscle Atrophy via Regulation of Inflammation and Oxidative Stress" Foods 14, no. 22: 3833. https://doi.org/10.3390/foods14223833
APA StyleKim, M.-B., Lee, H., Kang, J., Kim, B., & Hwang, J.-K. (2025). Protective Effects of Quinic Acid Against Disuse-Induced Skeletal Muscle Atrophy via Regulation of Inflammation and Oxidative Stress. Foods, 14(22), 3833. https://doi.org/10.3390/foods14223833







