Encapsulation of Allyl Isothiocyanate by Freeze- and Spray-Drying: Effects on Retention and Sensory Perception in Sodium-Reduced Soups
Abstract
1. Introduction
2. Materials and Methods
2.1. Encapsulation of the AITC
2.1.1. Materials
2.1.2. Allyl Isothiocyanate Emulsion and Microcapsules
2.1.3. Percent Surface Oil Content
2.1.4. AITC Quantification
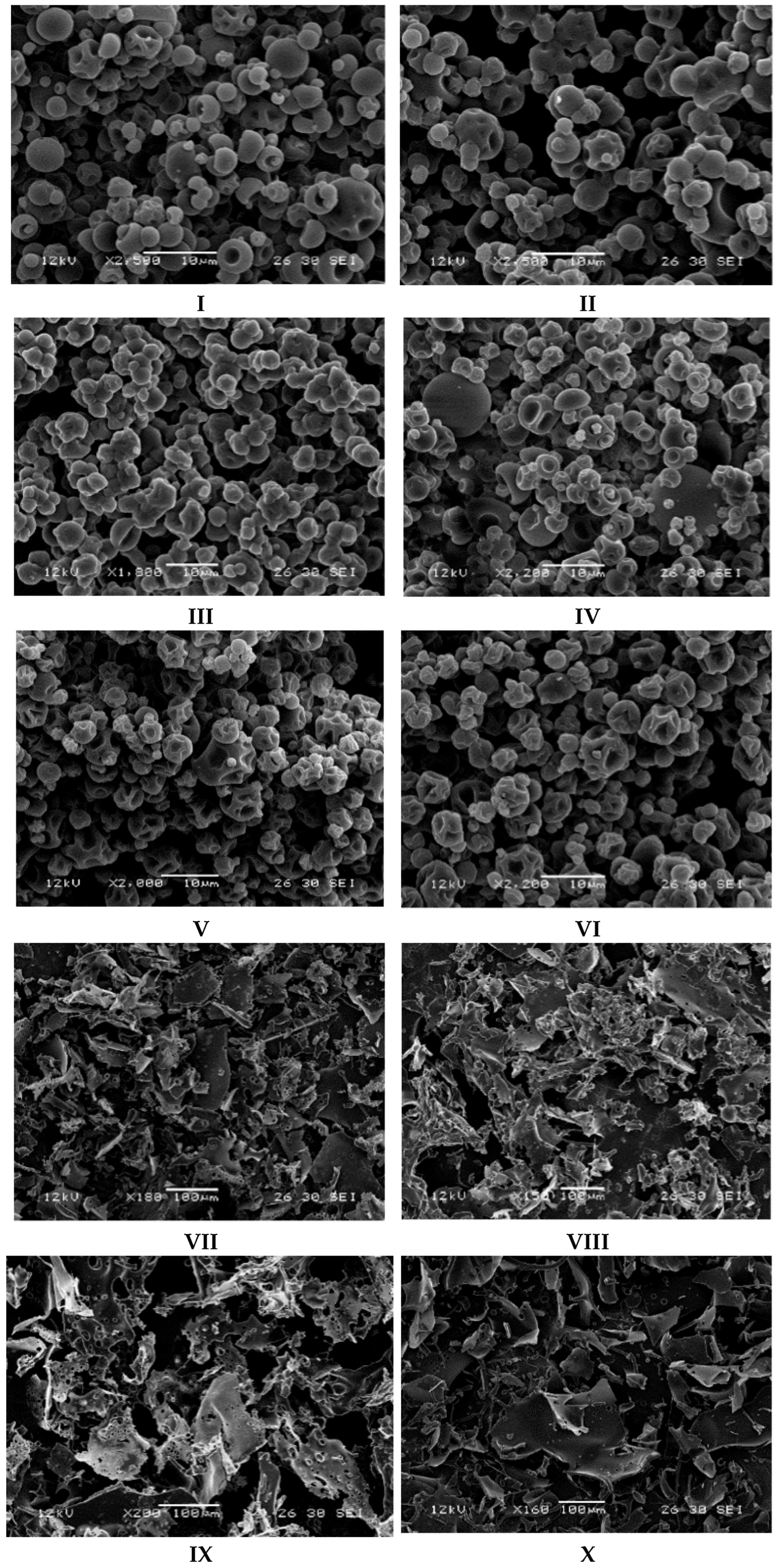
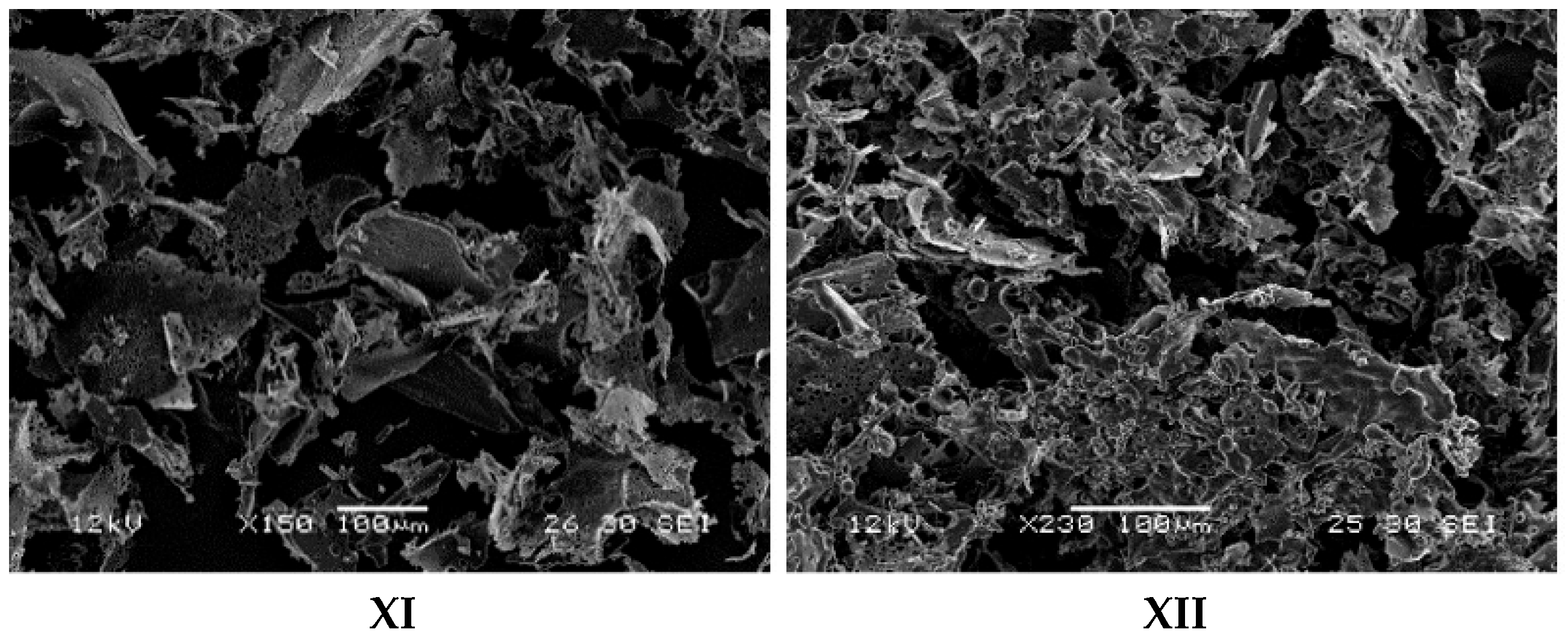
2.1.5. Scanning Electron Microscopy
2.1.6. Moisture Content of AITC Microcapsules
2.2. Sensory Evaluation
2.2.1. Participants
2.2.2. Samples
2.2.3. Sensory Evaluation
2.3. Statistical Analysis
2.3.1. Chemical Analysis
2.3.2. Sensory Evaluation
3. Results and Discussion
3.1. Physico-Chemical Characteristics
3.1.1. Encapsulation Efficiency
3.1.2. Surface Oil Percentage
3.1.3. Moisture Content
3.1.4. Scanning Electron Microscopy
3.2. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO, World Health Organization. Noncommunicable Diseases. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 2 April 2024).
- WHO, World Health Organisation. Reducing Salt Intake in Populations; Report of a WHO Forum and Technical Meeting; WHO Document Production Services: Geneva, Switzerland, 2007; Available online: https://iris.who.int/handle/10665/43653 (accessed on 2 April 2024).
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Vinceti, M. Sodium Intake and Risk of Hypertension: A Systematic Review and Dose–Response Meta-Analysis of Observational Cohort Studies. Curr. Hypertens. Rep. 2022, 24, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.-Y.; Jeong, Y.-J. Sodium Intake, Blood Pressure and Cardiovascular Disease. Korean Circ. J. 2020, 50, 555–571. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yeh, T.-L.; Shih, M.-C.; Tu, Y.-K.; Chien, K.-L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2020, 12, 2934. [Google Scholar] [CrossRef]
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diab. Rep. 2019, 19, 44. [Google Scholar] [CrossRef]
- Morrison, A.C.; Ness, R.B. Sodium Intake and Cardiovascular Disease. Annu. Rev. Public Health 2011, 32, 71–90. [Google Scholar] [CrossRef]
- Calliope, S.R.; Samman, N.C. Sodium Content in Commonly Consumed Foods and Its Contribution to the Daily Intake. Nutrients 2020, 12, 34. [Google Scholar] [CrossRef]
- Dötsch, M.; Busch, J.; Batenburg, M.; Liem, G.; Tareilus, E.; Mueller, R.; Meijer, G. Strategies to Reduce Sodium Consumption: A Food Industry Perspective. Crit. Rev. Food Sci. Nutr. 2009, 49, 841–851. [Google Scholar] [CrossRef]
- Lorén, N.; Niimi, J.; Höglund, E.; Albin, R.; Rytter, E.; Bjerre, K.; Nielsen, T. Sodium Reduction in Foods: Challenges and Strategies for Technical Solutions. J. Food Sci. 2023, 88, 885–900. [Google Scholar] [CrossRef]
- Gaudette, N.J.; Pietrasik, Z. The Sensory Impact of Salt Replacers and Flavor Enhancer in Reduced Sodium Processed Meats Is Matrix Dependent. J. Sens. Stud. 2017, 32, e12247. [Google Scholar] [CrossRef]
- Fellendorf, S.; Kerry, J.P.; O’Sullivan, M.G. Consumer Attitudes on Salt and Fat Reduced Foods in the Republic of Ireland. Food Nutr. Sci. 2018, 9, 880. [Google Scholar] [CrossRef]
- Sinopoli, D.A.; Lawless, H.T. Taste Properties of Potassium Chloride Alone and in Mixtures with Sodium Chloride Using a Check-All-That-Apply Method. J. Food Sci. 2012, 77, S319–S322. [Google Scholar] [CrossRef]
- Lawless, H.T.; Rapacki, F.; Horne, J.; Hayes, A. The Taste of Calcium and Magnesium Salts and Anionic Modifications. Food Qual. Prefer. 2003, 14, 319–325. [Google Scholar] [CrossRef]
- Kuo, W.-Y.; Lee, Y. Effect of Food Matrix on Saltiness Perception—Implications for Sodium Reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 906–923. [Google Scholar] [CrossRef]
- Lawless, H.; Gillette, M. Sensory Responses to Oral Chemical Heat. In Characterization and Measurement of Flavor Compounds; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; Volume 289, pp. 26–42. ISBN 978-0-8412-0944-2. [Google Scholar]
- Viana, F. Chemosensory Properties of the Trigeminal System. ACS Chem. Neurosci. 2011, 2, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food; Food Science Text Series; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-6487-8. [Google Scholar]
- Han, P.; Müller, L.; Hummel, T. Peri-Threshold Trigeminal Stimulation with Capsaicin Increases Taste Sensitivity in Humans. Chemosens. Percept. 2022, 15, 1–7. [Google Scholar] [CrossRef]
- Utama-ang, N.; Cheewinworasak, T.; Simawonthamgul, N.; Samakradhamrongthai, R.S. Influence of Garlic and Pepper Powder on Physicochemical and Sensory Qualities of Flavoured Rice Noodle. Sci. Rep. 2020, 10, 8538. [Google Scholar] [CrossRef]
- Rhyu, M.-R.; Kim, Y.; Lyall, V. Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1. Int. J. Mol. Sci. 2021, 22, 3360. [Google Scholar] [CrossRef]
- He, W.; Liang, L.; Zhang, Y. Pungency Perception and the Interaction with Basic Taste Sensations: An Overview. Foods 2023, 12, 2317. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zhao, L.; Zhang, Q.-B.; Shi, B.-L.; Zhong, K.; Wang, H.-Y.; Xie, R.; Liu, L.-Y. The Effect of the Pungent Sensation Elicited by Sichuan Pepper Oleoresin on the Sensory Perception of Saltiness throughout Younger and Older Age Groups. Food Qual. Prefer. 2020, 86, 103987. [Google Scholar] [CrossRef]
- Narukawa, M.; Sasaki, S.; Watanabe, T. Effect of Capsaicin on Salt Taste Sensitivity in Humans. Food Sci. Technol. Res. 2011, 17, 167–170. [Google Scholar] [CrossRef]
- Amyoony, J.; Gorman, M.; Dabas, T.; Moss, R.; McSweeney, M.B. The Effect of Allyl Isothiocyanate Addition on Consumers’ Saltiness Perception. Int. J. Food Sci. Technol. 2024, 59, 950–958. [Google Scholar] [CrossRef]
- Le, B.; Yu, B.; Amin, M.S.; Liu, R.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Salt Taste Receptors and Associated Salty/Salt Taste-Enhancing Peptides: A Comprehensive Review of Structure and Function. Trends Food Sci. Technol. 2022, 129, 657–666. [Google Scholar] [CrossRef]
- Sindhu, S.; Maya, P.; Indira, T.N. A Method for Preparation of Mustard (Brassica Juncea) Powder with Retained Pungency and Reduced Bitterness. LWT 2012, 49, 42–47. [Google Scholar] [CrossRef]
- Eib, S.; Gajek, S.R.; Schneider, D.J.; Hensel, O.; Seuss-Baum, I. Determination of Detection Thresholds of Sinigrin in Water-Based Matrix and Allyl Isothiocyanate in Water- and Oil-Based Matrices. J. Sens. Stud. 2020, 35, e12571. [Google Scholar] [CrossRef]
- Ko, J.A.; Jeon, J.Y.; Park, H.J. Preparation and Characterization of Allyl Isothiocyanate Microcapsules by Spray Drying. J. Food Biochem. 2012, 36, 255–261. [Google Scholar] [CrossRef]
- Liu, T.-T.; Yang, T.-S. Stability and Antimicrobial Activity of Allyl Isothiocyanate during Long-Term Storage in an Oil-in-Water Emulsion. J. Food Sci. 2010, 75, C445–C451. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A Review on the Encapsulation of Bioactive Components Using Spray-Drying and Freeze-Drying Techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An Overview of Encapsulation of Active Compounds Used in Food Products by Drying Technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Chacon, P.A.; Buffo, R.A.; Holley, R.A. Inhibitory Effects of Microencapsulated Allyl Isothiocyanate (AIT) against Escherichia Coli O157:H7 in Refrigerated, Nitrogen Packed, Finely Chopped Beef. Int. J. Food Microbiol. 2006, 107, 231–237. [Google Scholar] [CrossRef]
- Li, Y.; Teng, Z.; Chen, P.; Song, Y.; Luo, Y.; Wang, Q. Enhancement of Aqueous Stability of Allyl Isothiocyanate Using Nanoemulsions Prepared by an Emulsion Inversion Point Method. J. Colloid Interface Sci. 2015, 438, 130–137. [Google Scholar] [CrossRef]
- Grimes, C.A.; Riddell, L.J.; Campbell, K.J.; Beckford, K.; Baxter, J.R.; He, F.J.; Nowson, C.A. Dietary Intake and Sources of Sodium and Potassium among Australian Schoolchildren: Results from the Cross-Sectional Salt and Other Nutrients in Children (SONIC) Study. BMJ Open 2017, 7, e016639. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of Avocado Oil by Spray Drying Using Whey Protein and Maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Lyu, C.; Hendriks, A.; Geary, L.N.; Forde, C.G.; Stieger, M. Getting Hot: Effect of Chili Pepper Addition on Sensory Perception of Liquid and Solid Foods. J. Food Sci. 2023, 88, A158–A171. [Google Scholar] [CrossRef]
- Amyoony, J.; Gorman, M.; Moss, R.; McSweeney, M.B. A Consumer Evaluation of Salt-Reduced Tomato Soup and Vegetable Juice Made with Grape Pomace. J. Food Sci. 2024, 89, 2438–2449. [Google Scholar] [CrossRef]
- Leygeber, S.; Grossmann, J.L.; Diez-Simon, C.; Karu, N.; Dubbelman, A.-C.; Harms, A.C.; Westerhuis, J.A.; Jacobs, D.M.; Lindenburg, P.W.; Hendriks, M.M.W.B.; et al. Flavor Profiling Using Comprehensive Mass Spectrometry Analysis of Metabolites in Tomato Soups. Metabolites 2022, 12, 1194. [Google Scholar] [CrossRef]
- Ozturk, G.; Dogan, M.; Said Toker, O. Physicochemical, Functional and Sensory Properties of Mellorine Enriched with Different Vegetable Juices and TOPSIS Approach to Determine Optimum Juice Concentration. Food Biosci. 2014, 7, 45–55. [Google Scholar] [CrossRef]
- Silva, E.K.; Meireles, M.A.A.; Saldaña, M.D.A. Supercritical Carbon Dioxide Technology: A Promising Technique for the Non-Thermal Processing of Freshly Fruit and Vegetable Juices. Trends Food Sci. Technol. 2020, 97, 381–390. [Google Scholar] [CrossRef]
- Singh, A.; Seo, H.-S. Sample Temperatures Can Modulate Both Emotional Responses to and Sensory Attributes of Tomato Soup Samples. Food Qual. Prefer. 2020, 86, 104005. [Google Scholar] [CrossRef]
- Ströhla, L.C.; Hidangmayum, K.S.; Waehrens, S.S.; Orlien, V.; Petersen, M.A. Effect of Processing and Accelerated Storage on the Volatile Composition and Sensory Profile of a Tomato Soup. Food Qual. Saf. 2022, 6, fyac024. [Google Scholar] [CrossRef]
- Gonzalez-Estanol, K.; Orr, R.E.; Hort, J.; Stieger, M. Can Flavour and Texture Defects of Plant-Based Burger Patties Be Mitigated by Combining Them with a Bun and Tomato Sauce? Food Qual. Prefer. 2023, 109, 104920. [Google Scholar] [CrossRef]
- Nguyen, H.; Wismer, W.V. The Influence of Companion Foods on Sensory Attribute Perception and Liking of Regular and Sodium-Reduced Foods. J. Food Sci. 2020, 85, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Uekane, T.M.; Costa, A.C.P.; Pierucci, A.P.T.R.; da Rocha-Leão, M.H.M.; Rezende, C.M. Sulfur Aroma Compounds in Gum Arabic/Maltodextrin Microparticles. LWT 2016, 70, 342–348. [Google Scholar] [CrossRef]
- Ratanasiriwat, P.; Worawattanamateekul, W.; Klaypradit, W. Properties of Encapsulated Wasabi Flavour and Its Application in Canned Food. Int. J. Food Sci. Technol. 2013, 48, 749–757. [Google Scholar] [CrossRef]
- Jin, F.; Ding, R.; Ding, K.; Han, T.; Chen, X. Preparation of Allyl Isothiocyanate Microencapsulation and Its Application in Pork Preservation. J. Food Process. Preserv. 2020, 44, e14709. [Google Scholar] [CrossRef]
- Zhong, L.; Fu, Z.; Bai, X.; Zhu, H.; Zhu, P.; Huang, H.; Liu, Y. Effect of Spray-Drying or Freeze-Drying on the Physicochemical Parameters, Antioxidant Activity, and Volatile Components of Craft Beer. Int. J. Food Sci. Technol. 2023, 58, 6222–6233. [Google Scholar] [CrossRef]
- Chindapan, N.; Puangngoen, C. Comparative Evaluation of Volatile Aroma Compounds Content of Freeze-Dried and Spray-Dried Instant Coffees. Burapha Sci. J. 2024, 29, 647–671. [Google Scholar]
- Xin, X.; Essien, S.; Dell, K.; Woo, M.W.; Baroutian, S. Effects of Spray-Drying and Freeze-Drying on Bioactive and Volatile Compounds of Smoke Powder Food Flavouring. Food Bioprocess Technol. 2022, 15, 785–794. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and Stability of Spray-Dried and Freeze-Dried Microcapsules Co-Encapsulated with Fish Oil, Phytosterol Esters, and Limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Yaman, D.M.; Koçak Yanık, D.; Elik Demir, A.; Uzun Karka, H.; Güçlü, G.; Selli, S.; Kelebek, H.; Göğüş, F. Effect of Encapsulation Techniques on Aroma Retention of Pistacia Terebinthus L. Fruit Oil: Spray Drying, Spray Freeze Drying, and Freeze Drying. Foods 2023, 12, 3244. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Hernández, X.; Wandersleben, T.; Barahona, T.; Medina, C.; Quiroz, A.; Rubilar, M. Influence of Multilayer O/W Emulsions Stabilized by Proteins from a Novel Lupin Variety AluProt-CGNA and Ionic Polysaccharides on d-Limonene Retention during Spray-Drying. Colloids Surf. Physicochem. Eng. Asp. 2018, 536, 234–241. [Google Scholar] [CrossRef]
- Tasker, A.L.; Hitchcock, J.P.; He, L.; Baxter, E.A.; Biggs, S.; Cayre, O.J. The Effect of Surfactant Chain Length on the Morphology of Poly(Methyl Methacrylate) Microcapsules for Fragrance Oil Encapsulation. J. Colloid Interface Sci. 2016, 484, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, H.; Ghorbani, M.; Jafari, S.M.; Sadeghi Mahoonak, A.; Rajabzadeh, G. Retention of Saffron Bioactive Components by Spray Drying Encapsulation Using Maltodextrin, Gum Arabic and Gelatin as Wall Materials. Food Hydrocoll. 2015, 51, 327–337. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, F.; Ma, R.; Tian, Y. Advances in Gum Arabic Utilization for Sustainable Applications as Food Packaging: Reinforcement Strategies and Applications in Food Preservation. Trends Food Sci. Technol. 2023, 142, 104215. [Google Scholar] [CrossRef]
- Do, H.T.T.; Nguyen, H.V.H. Effects of Spray-Drying Temperatures and Ratios of Gum Arabic to Microcrystalline Cellulose on Antioxidant and Physical Properties of Mulberry Juice Powder. Beverages 2018, 4, 101. [Google Scholar] [CrossRef]
- Igual, M.; Cebadera, L.; Cámara, R.M.; Agudelo, C.; Martínez-Navarrete, N.; Cámara, M. Novel Ingredients Based on Grapefruit Freeze-Dried Formulations: Nutritional and Bioactive Value. Foods 2019, 8, 506. [Google Scholar] [CrossRef]
- Di Battista, C.A.; Constenla, D.; Ramírez-Rigo, M.V.; Piña, J. The Use of Arabic Gum, Maltodextrin and Surfactants in the Microencapsulation of Phytosterols by Spray Drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis labrusca Var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-Drying Technique for Microencapsulation of Elsholtzia Ciliata Ethanolic Extract Using Different Coating Materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Gadhave, A. Determination of Hydrophilic-Lipophilic Balance Value. Int. J. Sci. Res. IJSR 2014, 3, 573–575. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, Q. Food-Derived Biopolymers for Nutrient Delivery. In Nutrient Delivery; Grumezescu, A.M., Ed.; Nanotechnology in the Agri-Food Industry; Academic Press: Salt Lake City, UT, USA, 2017; pp. 251–291. ISBN 978-0-12-804304-2. [Google Scholar]
- Nurhad, B.; Andoyo, R.; Mahani; Indiarto, R. Study the Properties of Honey Powder Produced from Spray Drying and Vacuum Drying Method. Int. Food Res. J. 2012, 19, 907. [Google Scholar]
- Boel, E.; Koekoekx, R.; Dedroog, S.; Babkin, I.; Vetrano, M.R.; Clasen, C.; Van den Mooter, G. Unraveling Particle Formation: From Single Droplet Drying to Spray Drying and Electrospraying. Pharmaceutics 2020, 12, 625. [Google Scholar] [CrossRef]
- Osman, A.; Shahidzadeh, N.; Stitt, H.; Shokri, N. Morphological Transformations during Drying of Surfactant-Nanofluid Droplets. J. Ind. Eng. Chem. 2018, 67, 92–98. [Google Scholar] [CrossRef]
- Lallbeeharry, P.; Tian, Y.; Fu, N.; Wu, W.D.; Woo, M.W.; Selomulya, C.; Chen, X.D. Effects of Ionic and Nonionic Surfactants on Milk Shell Wettability during Co-Spray-Drying of Whole Milk Particles. J. Dairy Sci. 2014, 97, 5303–5314. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, M.; Verdurmen, R.E.M.; Straatsma, M.; Gunsing, M.; Blei, S.; Sommerfeld, M. Modelling Agglomeration in Spray Drying Installations. Acta Hortic. 2005, 674, 131–138. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, L.; Ma, X.; Xu, Q.; Tian, W.; Li, Z. Microstructure of Spray Freezing Dried Powders Affected by the Presence of Inert Particles. Int. J. Food Eng. 2020, 16, 20190334. [Google Scholar] [CrossRef]
- Lawless, H.; Rozin, P.; Shenker, J. Effects of Oral Capsaicin on Gustatory, Olfactory and Irritant Sensations and Flavor Identification in Humans Who Regularly or Rarely Consume Chili Pepper. Chem. Senses 1985, 10, 579–589. [Google Scholar] [CrossRef]
- Lawless, H.; Stevens, D.A. Effects of Oral Chemical Irritation on Taste. Physiol. Behav. 1984, 32, 995–998. [Google Scholar] [CrossRef]
- Tran, H.T.T.; Stetter, R.; Herz, C.; Spöttel, J.; Krell, M.; Hanschen, F.S.; Schreiner, M.; Rohn, S.; Behrens, M.; Lamy, E. Allyl Isothiocyanate: A TAS2R38 Receptor-Dependent Immune Modulator at the Interface Between Personalized Medicine and Nutrition. Front. Immunol. 2021, 12, 669005. [Google Scholar] [CrossRef]
- Gibbs, B.F.; Kermasha, S.; Alli, I.; Mulligan, C.N. Encapsulation in the Food Industry: A Review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [CrossRef]
- Dang, Y.T.; Tran, H.; Kha, T.C. Encapsulation of W/O/W Acerola Emulsion by Spray Drying: Optimization, Release Kinetics, and Storage Stability. Foods 2024, 13, 1463. [Google Scholar] [CrossRef]
- Spence, C. What Is the Relationship between the Presence of Volatile Organic Compounds in Food and Drink Products and Multisensory Flavour Perception? Foods 2021, 10, 1570. [Google Scholar] [CrossRef]
- Lyu, C.; Schijvens, D.; Hayes, J.E.; Stieger, M. Capsaicin Burn Increases Thickness Discrimination Thresholds Independently of Chronic Chili Intake. Food Res. Int. 2021, 149, 110702. [Google Scholar] [CrossRef]
- Green, B.G.; Hayes, J.E. Individual Differences in Perception of Bitterness from Capsaicin, Piperine and Zingerone. Chem. Senses 2004, 29, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; Delahunty, C.M. Examination of Chemical Irritation and Textural Influence on Food Preferences in Two Age Cohorts Using Complex Food Systems. Food Qual. Prefer. 2002, 13, 571–581. [Google Scholar] [CrossRef]
- Simons, C.T.; Klein, A.H.; Carstens, E. Chemogenic Subqualities of Mouthfeel. Chem. Senses 2019, 44, 281–288. [Google Scholar] [CrossRef]
- Lv, L.; Zhuang, Y.; Zhang, H.; Tian, N.; Dang, W.; Wu, S. Capsaicin-Loaded Folic Acid-Conjugated Lipid Nanoparticles for Enhanced Therapeutic Efficacy in Ovarian Cancers. Biomed. Pharmacother. 2017, 91, 999–1005. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of Polyphenols—A Review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Popescu, G.S.; Radu, F.; Velciov, A.B.; Pîrvulescu, L.; Cozma, A.; Stănciugelu, M.M.; Marcu, D.-F.; Hădărugă, N.G. A Review: Water and Methods Employed for Moisture Determination in Food. J. Agroaliment. Process. Technol. 2022, 28, 310. [Google Scholar]
| Wall Material | Encapsulation Method | Emulsifier | EE mg AITC/g Powder | ±SE | z | p |
|---|---|---|---|---|---|---|
| GA | FD | None | 13.97 | 0.27 | - | - |
| GA | FD | T20 | 7.12 | 2.16 | −0.932 | 0.999 |
| GA | FD | T80 | 5.58 | 0.71 | −1.141 | 0.993 |
| GA | SD | None | 0.90 | 0.01 | - | - |
| GA | SD | T20 | 136.71 | 4.32 | 18.483 | <0.001 * |
| GA | SD | T80 | 76.71 | 2.83 | 10.317 | <0.001 * |
| MD | FD | None | 3.40 | 0.13 | - | - |
| MD | FD | T20 | 13.36 | 1.33 | 1.354 | 0.972 |
| MD | FD | T80 | 8.44 | 0.47 | 0.685 | 1.000 |
| MD | SD | None | 0.60 | 0.01 | - | - |
| MD | SD | T20 | 12.31 | 0.76 | 1.493 | 0.912 |
| MD | SD | T80 | 85.32 | 17.01 | 11.529 | <0.001 * |
| Wall Material | Encapsulation Method | Emulsifier | % Surface Oil | ±SE | z | p |
|---|---|---|---|---|---|---|
| GA | FD | None | 83.5 | 2.43 | - | - |
| GA | FD | T20 | 89.3 | 1.77 | 2.887 | 0.1451 |
| GA | FD | T80 | 94.0 | 2.02 | 5.258 | <0.01 * |
| GA | SD | None | 84.9 | 0.838 | - | - |
| GA | SD | T20 | 87.8 | 0.794 | 1.468 | 0.9493 |
| GA | SD | T80 | 90.3 | 1.11 | 2.695 | 0.2286 |
| MD | FD | None | 89.5 | 1.30 | - | - |
| MD | FD | T20 | 94.6 | 0.680 | 2.573 | 0.2951 |
| MD | FD | T80 | 90.2 | 1.57 | 0.338 | 1 |
| MD | SD | None | 92.8 | 1.32 | - | - |
| MD | SD | T20 | 89.3 | 1.14 | −1.771 | 0.8341 |
| MD | SD | T80 | 89.3 | 0.602 | −1.767 | 0.836 |
| Wall Material | Encapsulation Method | Surfactant | % Change in Moisture Content | ±SE | z | p |
|---|---|---|---|---|---|---|
| GA | None | None | 8.27 | 0.177 | - | - |
| MD | None | None | 4.71 | 0.874 | −1.45 | 0.977 |
| GA | FD | None | 5.35 | 1.34 | - | - |
| GA | FD | T20 | 24.4 | 3.80 | 7.74 | <0.01 * |
| GA | FD | T80 | 11.5 | 3.23 | 2.50 | 0.409 |
| GA | SD | None | 6.96 | 0.600 | - | - |
| GA | SD | T20 | 4.15 | 1.793 | −1.14 | 0.997 |
| GA | SD | T80 | 0.696 | 0.0569 | −2.50 | 0.379 |
| MD | FD | None | 5.05 | 0.581 | - | - |
| MD | FD | T20 | 2.56 | 1.74 | −1.01 | 0.999 |
| MD | FD | T80 | 6.78 | 2.72 | 0.700 | 1 |
| MD | SD | None | 1.07 | 0.251 | - | - |
| MD | SD | T20 | 6.37 | 0.567 | 2.15 | 0.666 |
| MD | SD | T80 | 4.45 | 0.487 | 1.37 | 0.985 |
| Sample | Salty | Sweet | Bitter | Sour | Savoury |
|---|---|---|---|---|---|
| Spray Dried Sensory Trial (n = 79) | |||||
| Control | 2.7 a1,2 ± 1.1 | 3.6 a ± 1.1 | 0.9 a ± 0.6 | 1.5 a ± 0.7 | 3.1 a ± 1.2 |
| GA-SD | 3.1 a ± 1.2 | 3.5 a ± 1.0 | 1.2 a ± 1.0 | 1.3 a ± 0.7 | 3.0 a ± 1.0 |
| GA-SD-T20 | 2.9 a ± 1.0 | 3.5 a ± 1.2 | 1.1 a ± 0.6 | 1.5 a ± 0.8 | 2.9 a ± 1.1 |
| MD-SD-T20 | 2.8 a ± 1.3 | 3.8 a ± 1.0 | 1.1 a ± 0.7 | 1.4 a ± 1.0 | 3.2 a ± 1.2 |
| MD-SD-T80 | 2.9 a ± 1.4 | 3.7 a ± 1.4 | 1.2 a ± 0.6 | 1.4 a ± 0.4 | 3.0 a ± 1.0 |
| Freeze-Dried Sensory Trial (n = 93) | |||||
| Control | 2.8 a ± 1.3 | 3.5 a ± 1.7 | 1.1 a ± 1.4 | 1.4 a ± 0.8 | 2.6 a ± 1.1 |
| GA-FD | 3.3 a ± 1.5 | 3.5 a ± 1.6 | 1.2 a ± 1.6 | 1.6 a ± 0.6 | 2.9 a ± 1.2 |
| GA-FD-T20 | 3.2 a ± 1.4 | 3.4 a ± 1.0 | 1.1 a ± 1.5 | 1.5 a ± 0.7 | 2.8 a ± 1.0 |
| MD-FD-T20 | 3.0 a ± 1.4 | 3.2 a ± 1.4 | 0.9 a ± 1.0 | 1.5 a ± 0.7 | 2.7 a ± 1.6 |
| MD-FD-T80 | 3.2 a ± 1.5 | 3.6 a ± 1.4 | 1.2 a ± 1.0 | 1.6 a ± 0.7 | 3.2 a ± 1.4 |
| Sample | Spicy | Tomato Flavour | Thick | Creamy | Metallic |
|---|---|---|---|---|---|
| Spray Dried Sensory Trial (n = 79) | |||||
| Control | 0.5 a1,2 ± 0.4 | 4.8 a ± 1.5 | 3.9 a ± 1.2 | 3.1 a ± 1.0 | 1.1 a ± 1.0 |
| GA-SD | 0.5 a ± 0.5 | 4.7 a ± 1.3 | 3.0 a ± 1.2 | 2.7 a ± 1.6 | 1.2 a ± 1.2 |
| GA-SD-T20 | 0.5 a ± 0.2 | 4.4 a ± 1.4 | 3.4 a ± 1.4 | 2.9 a ± 1.7 | 1.2 a ± 1.2 |
| MD-SD-T20 | 0.4 a ± 0.6 | 4.8 a ± 1.6 | 3.7 a ± 1.5 | 3.3 a ± 1.2 | 1.0 a ± 1.1 |
| MD-SD-T80 | 0.5 a ± 0.4 | 4.5 a ± 1.0 | 3.0 a ± 1.0 | 2.7 a ± 1.0 | 1.1 a ± 1.3 |
| Freeze-Dried Sensory Trial (n = 93) | |||||
| Control | 0.7 a ± 0.2 | 3.7 a ± 1.1 | 2.6 c ± 1.1 | 2.4 b ±1.0 | 1.2 a ± 1.2 |
| GA-FD | 0.9 a ± 0.3 | 4.5 ab ± 1.6 | 4.1 ab ± 1.7 | 3.1 a ± 1.4 | 1.1 a ± 0.8 |
| GA-FD-T20 | 0.8 a ± 0.5 | 4.3 ab ± 1.5 | 3.2 c ± 1.3 | 2.7 ab ± 1.5 | 1.0 a ± 0.7 |
| MD-FD-T20 | 0.7 a ± 0.5 | 4.1 ab ± 1.4 | 2.6 c ± 1.3 | 2.2 b ± 1.6 | 1.0 a ± 0.5 |
| MD-FD-T80 | 0.9 a ± 0.2 | 4.7 b ± 1.3 | 3.6 bc ± 1.3 | 3.0 ab ± 1.2 | 0.9 a ± 0.7 |
| Sample | Overall Liking | Flavour | Texture |
|---|---|---|---|
| Spray Dried Sensory Trial (n = 79) | |||
| Control | 6.4 a1,2 ± 1.4 | 6.4 a ± 1.0 | 6.3 a ± 1.9 |
| GA-SD | 6.2 a ± 1.3 | 6.1 a ± 1.2 | 6.4 a ± 2.0 |
| GA-SD-T20 | 5.8 a ± 1.4 | 5.8 a ± 1.6 | 6.2 a ± 2.1 |
| MD-SD-T20 | 6.2 a ± 1.6 | 6.3 a ± 1.9 | 6.6 a ± 1.7 |
| MD-SD-T80 | 6.2 a ± 1.1 | 6.3 a ± 2.0 | 6.7 a ± 1.8 |
| Freeze-Dried Sensory Trial (n = 93) | |||
| Control | 5.5 b ± 1.4 | 5.5 b ± 1.4 | 5.6 b ± 1.9 |
| GA-FD | 5.9 ab ± 1.4 | 6.0 ab ± 1.4 | 5.9 ab ± 1.9 |
| GA-FD-T20 | 5.7 b ± 1.9 | 5.8 b ± 1.3 | 5.9 ab ± 1.4 |
| MD-FD-T20 | 5.8 b ± 2.1 | 5.7 b ± 1.5 | 6.0 ab ± 1.3 |
| MD-FD-T80 | 6.6 a ± 1.9 | 6.6 a ± 1.6 | 6.6 a ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolan, E.; Faraone, N.; McSweeney, M.B. Encapsulation of Allyl Isothiocyanate by Freeze- and Spray-Drying: Effects on Retention and Sensory Perception in Sodium-Reduced Soups. Foods 2025, 14, 3810. https://doi.org/10.3390/foods14223810
Dolan E, Faraone N, McSweeney MB. Encapsulation of Allyl Isothiocyanate by Freeze- and Spray-Drying: Effects on Retention and Sensory Perception in Sodium-Reduced Soups. Foods. 2025; 14(22):3810. https://doi.org/10.3390/foods14223810
Chicago/Turabian StyleDolan, Emily, Nicoletta Faraone, and Matthew B. McSweeney. 2025. "Encapsulation of Allyl Isothiocyanate by Freeze- and Spray-Drying: Effects on Retention and Sensory Perception in Sodium-Reduced Soups" Foods 14, no. 22: 3810. https://doi.org/10.3390/foods14223810
APA StyleDolan, E., Faraone, N., & McSweeney, M. B. (2025). Encapsulation of Allyl Isothiocyanate by Freeze- and Spray-Drying: Effects on Retention and Sensory Perception in Sodium-Reduced Soups. Foods, 14(22), 3810. https://doi.org/10.3390/foods14223810






