Preparation, Characterization, and Mechanism of Hypoglycemic Action of a Goat Casein Peptide Delivery System Involving DPP-IV Inhibition and GLP-1 Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Preparation of Goat Casein Peptide (GCAPS)
2.3. Determination of Encapsulation EFFICIENCY (EE)
2.4. Characterization of GCAPS Encapsulation System
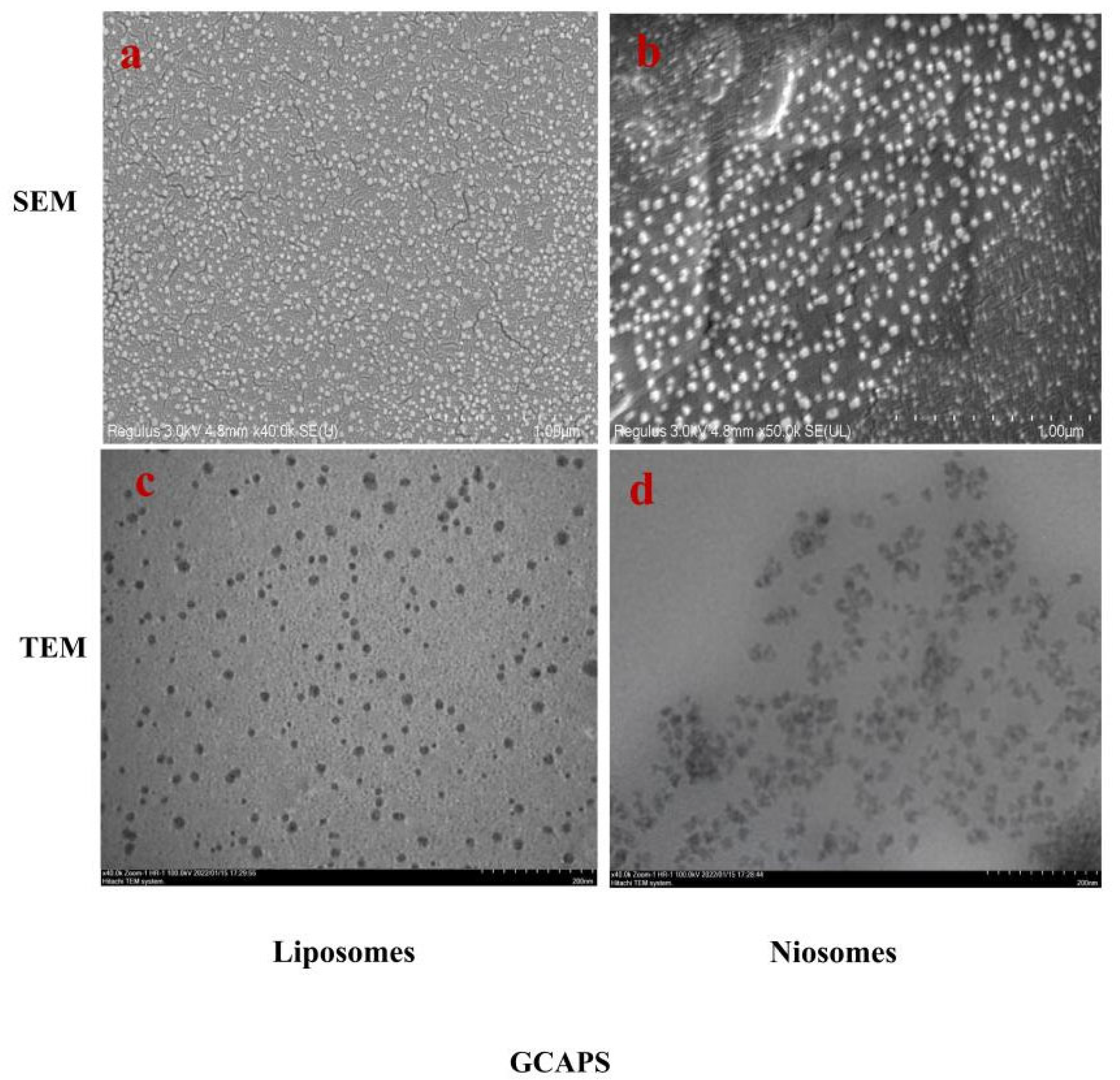
2.4.1. Characterization of Surface Morphology
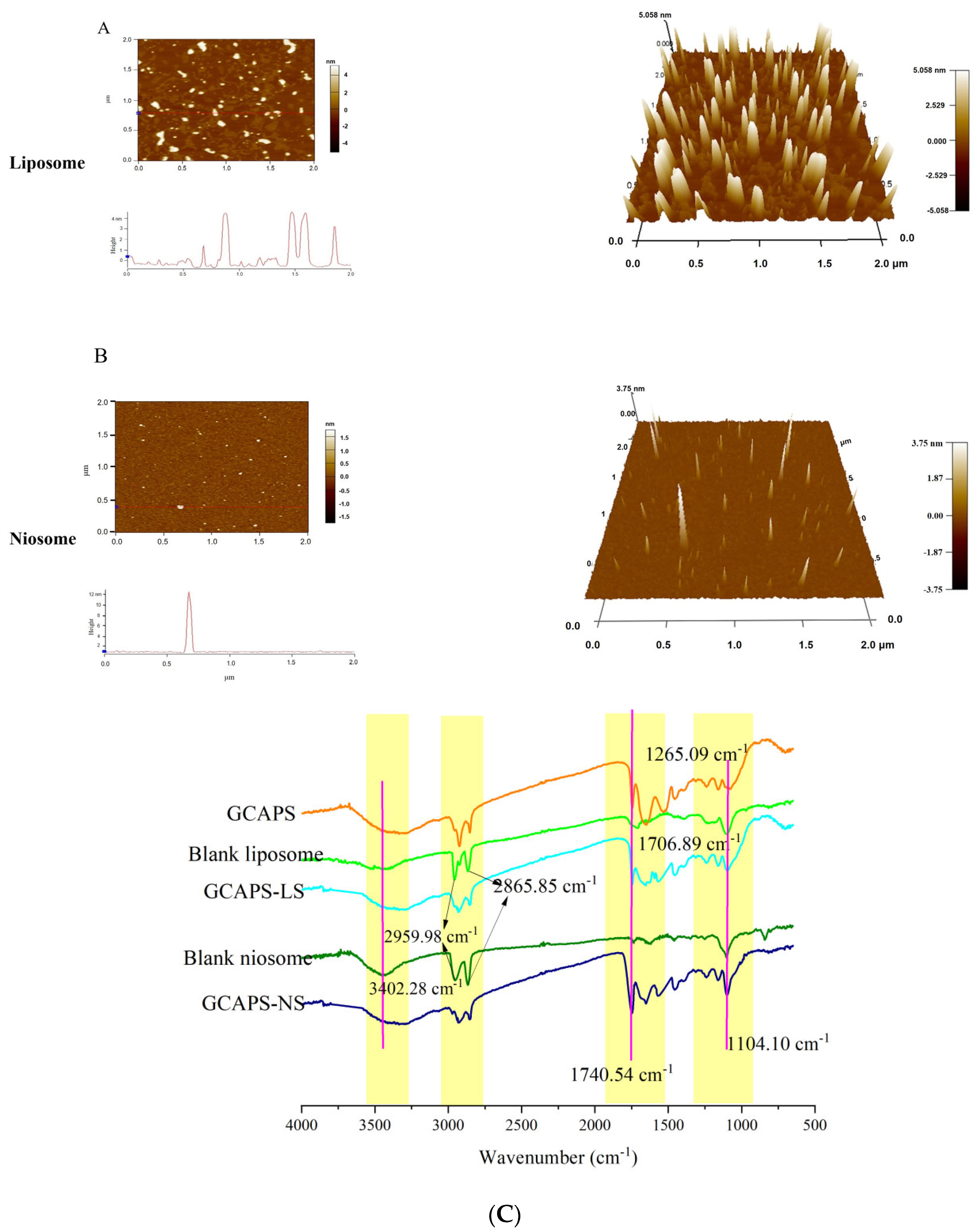
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
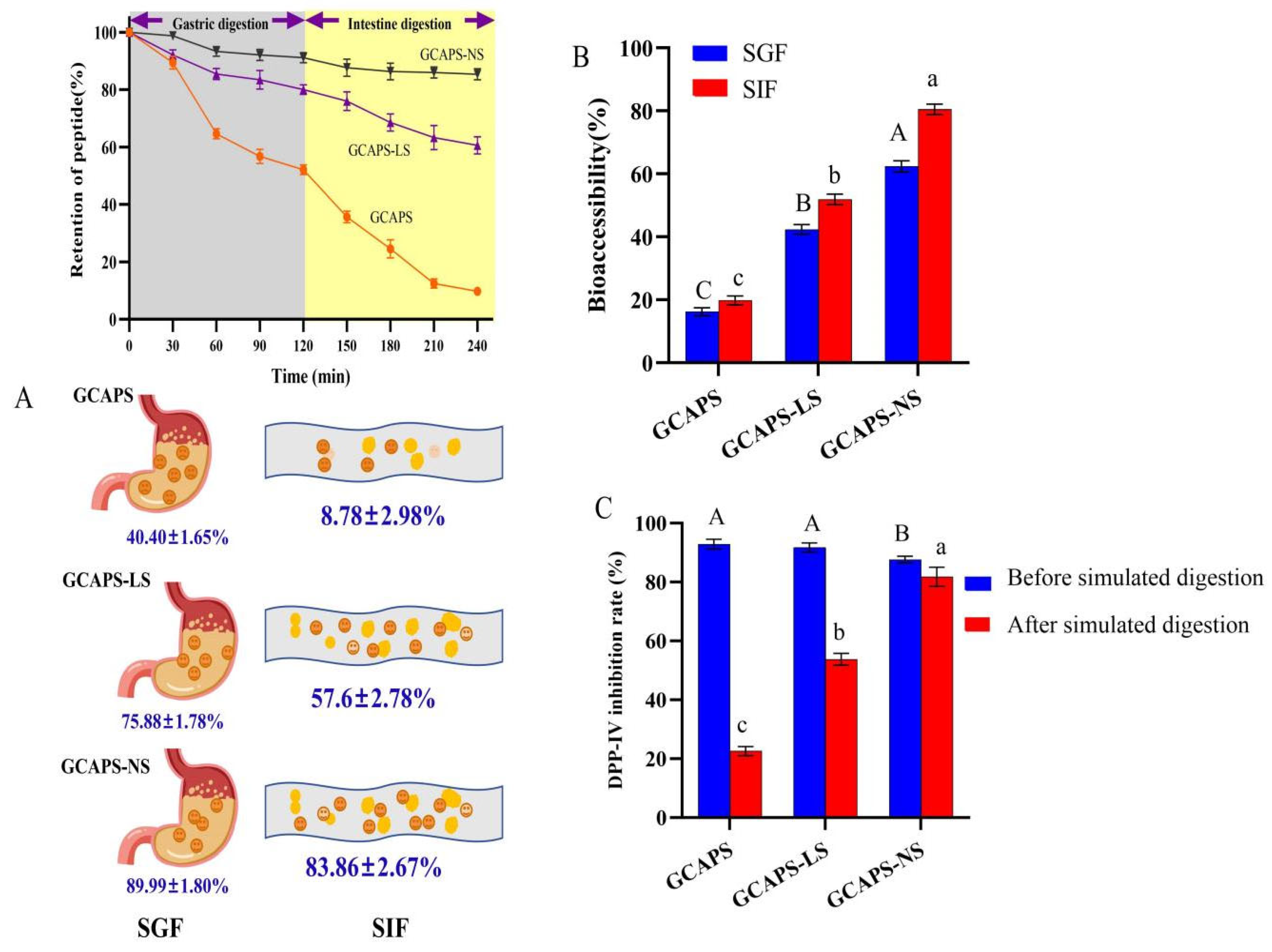
2.5. In Vitro Digestive Stability of the Nanoparticle System
2.6. Determination of DPP-IV Inhibition
2.7. In Vivo Bioavailability of GCAPS and GCAPS-NS
2.7.1. Animal Experiment
2.7.2. Establishment of Animal Model and Drug Intervention
2.7.3. Sample Collection from Experimental Animals
2.7.4. Determination of Serum and Hepatic Lipid Biochemical Parameters
2.7.5. Hematoxylin and Eosin Staining
2.7.6. Oral Glucose Tolerance Test (GTT)
2.7.7. Insulin Tolerance Test (ITT)
2.7.8. Determination of Fasting Blood Glucose Levels
2.7.9. Determination of Serum GLP-1 and hsCRP Levels
2.8. Analysis of Gut Microbiota in Mice
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation of GCAPS-Loaded Nanoparticle Systems (GCAPS-LS and GCAPS-NS)
3.2. Characterization of the GCAPS-Loaded Nanoparticle System
3.3. In Vitro Simulated Gastrointestinal Digestion Stability
3.4. Effects of GCAPS and GCAPS-NS Intervention on Fat Accumulation and Body Weight Gain in Mice
3.5. Effects of GCAPS and GCAPS-NS Intervention on White and Brown Adipose Tissue Indices in Mice
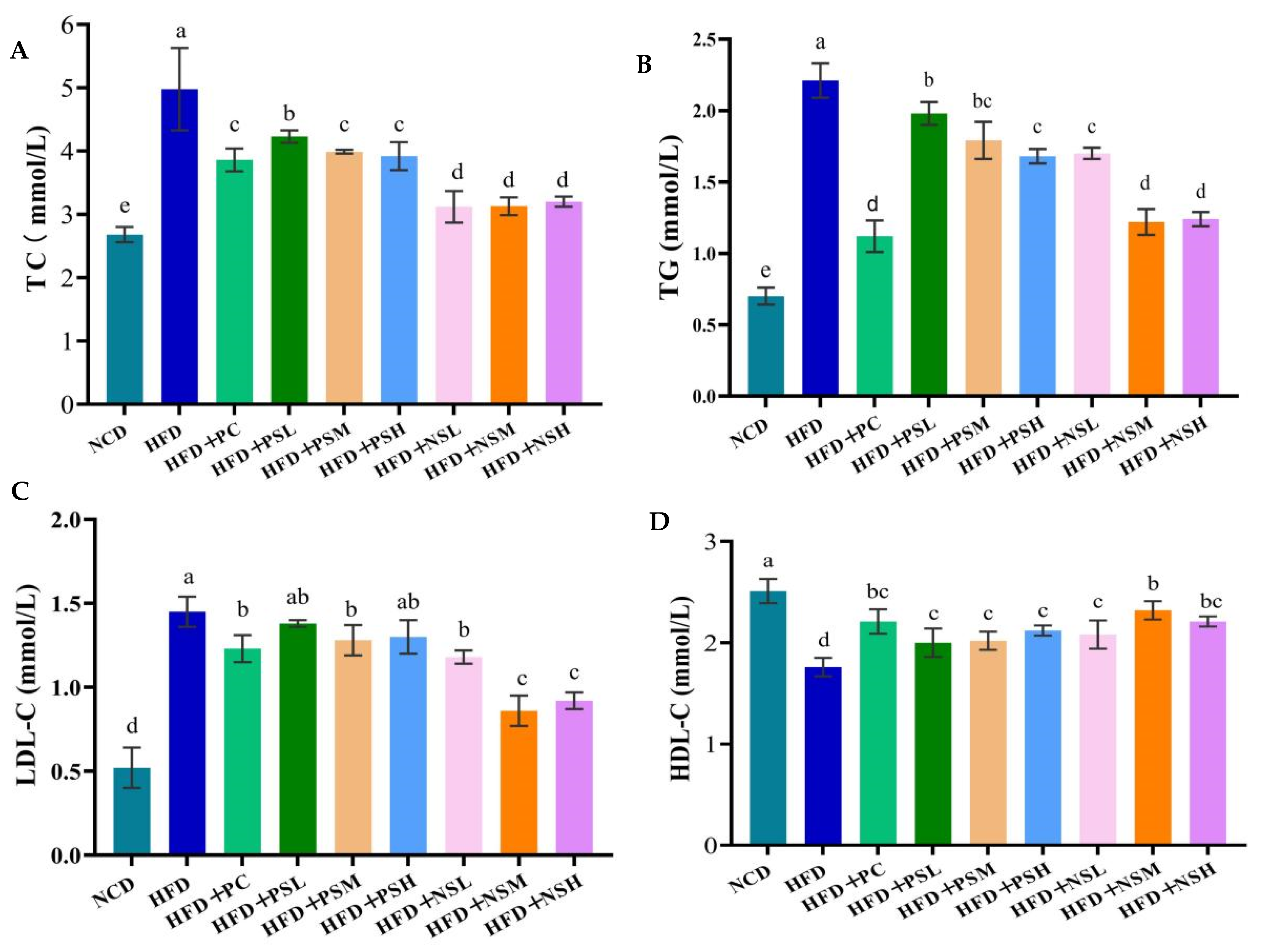
3.6. Effects of GCAPS and GCAPS-NS Intervention on Serum Lipid and Hepatic Lipid Levels in Mice
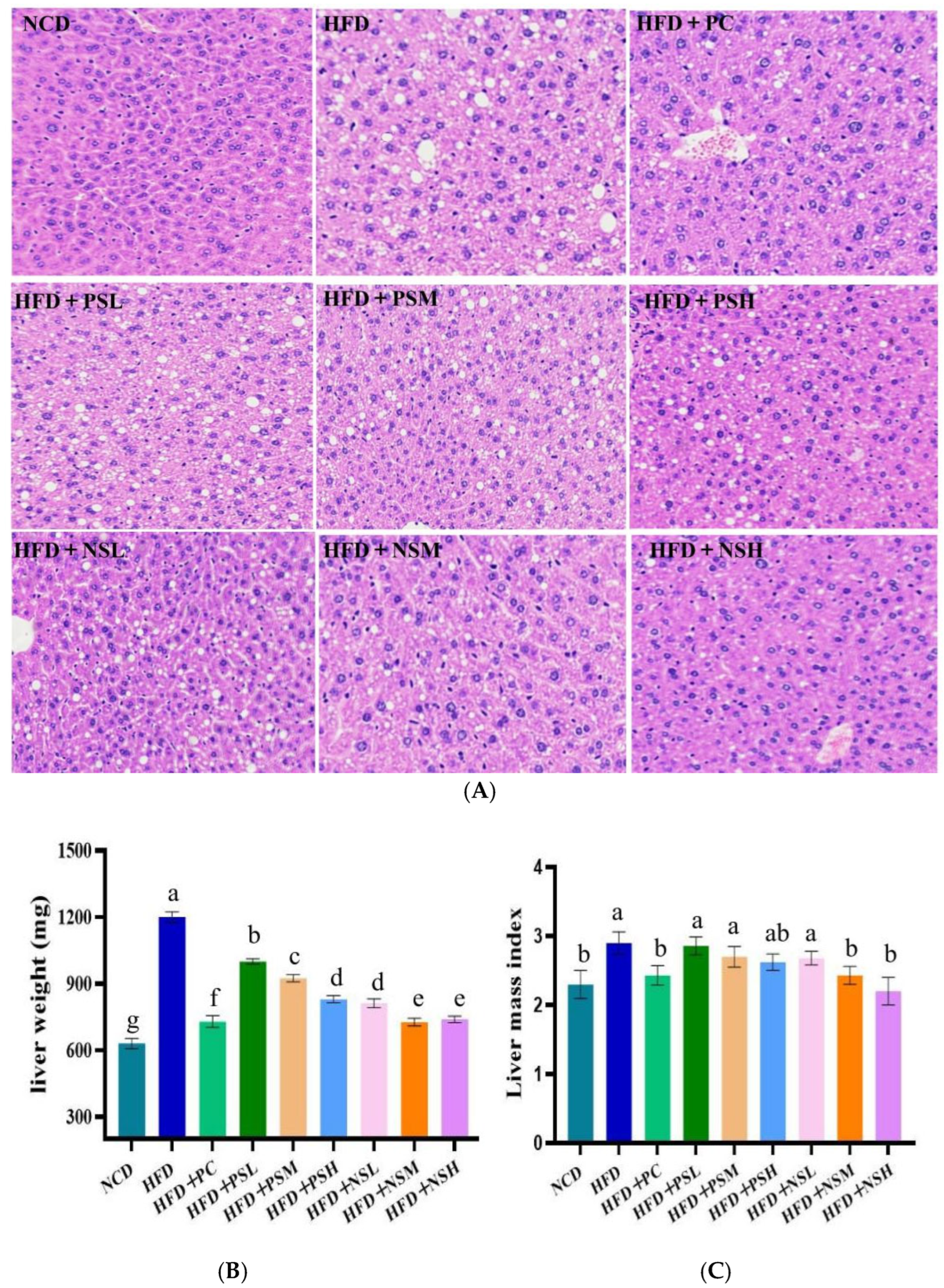
3.7. Effects of GCAPS and GCAPS-NS Intervention on Liver Tissue in Mice
3.8. Effects of GCAPS and GCAPS-NS Intervention on GTT and ITT
3.9. Effects of GCAPS and GCAPS-NS Intervention on Serum Glucose, hsCRP, and GLP-1 Levels in Mice
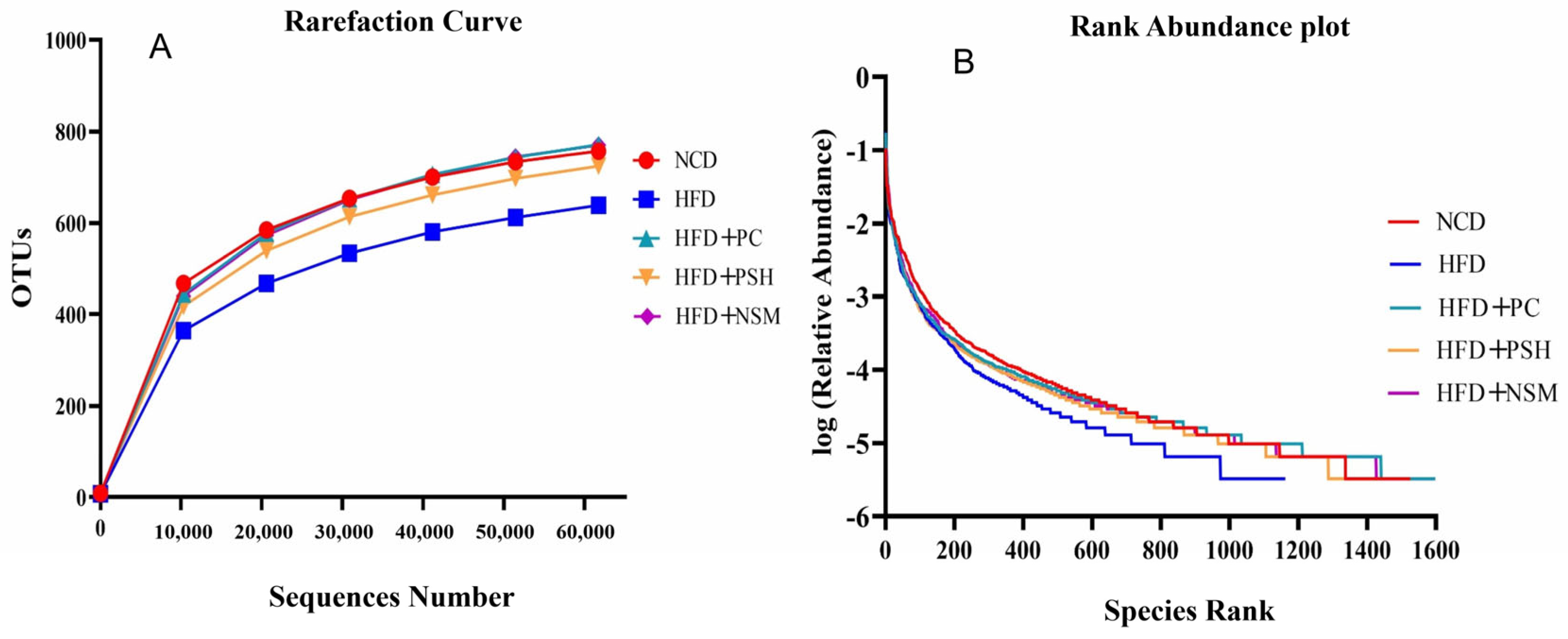
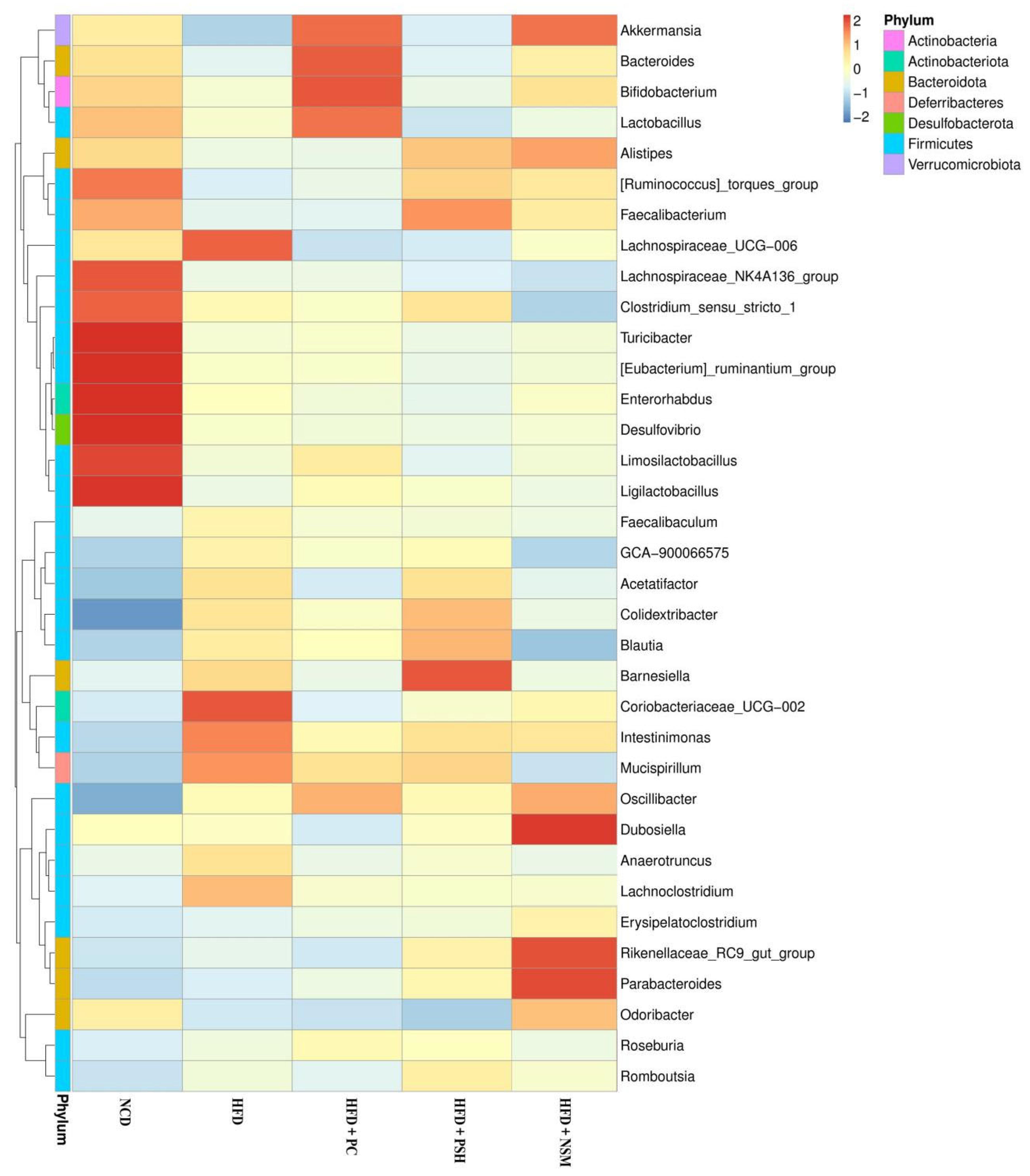
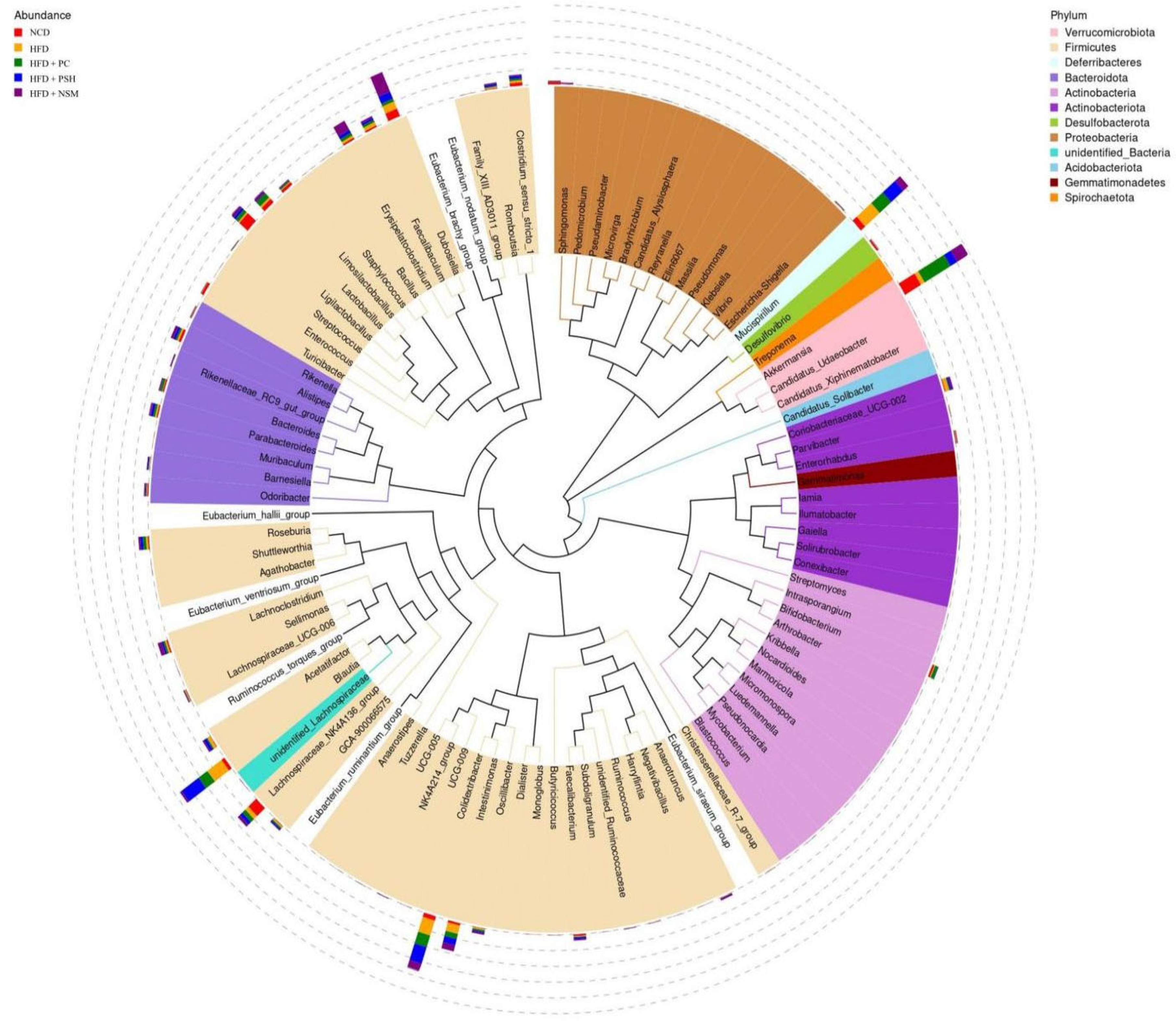
3.10. Effects of GCAPS and GCAPS-NS Intervention on Gut Microbiota Composition in Mice
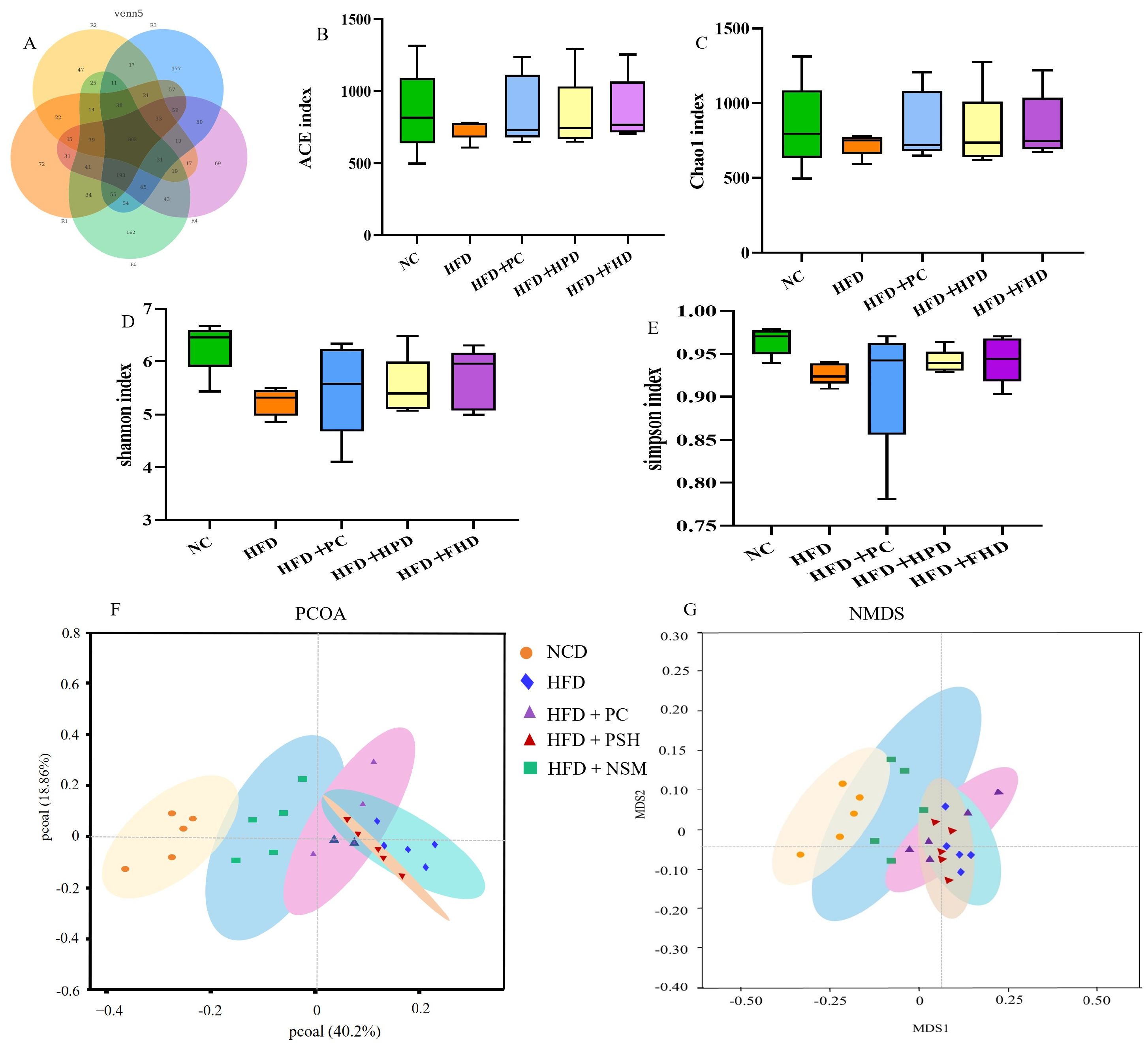
3.11. Effects of Different Intervention Groups on Gut Microbial Communities in Mice
3.12. Effects of Different Intervention Groups on the Gut Microbiota Structure in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Mil, S.; Biter, L.; Van de Geijn, G.; Birnie, E.; Dunkelgrun, M.; IJzermans, J.; Meulen, N.; Mannaerts, G.; Cabezas, M. Contribution of Type 2 Diabetes Mellitus to Subclinical Atherosclerosis in Subjects with Morbid Obesity. Obes. Surg. 2018, 28, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Nayak, A.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.; Battula, S.-N.; Swarnalath, G.; Pushpadass, H.; Naik, L.N.; Franklin, M. Preparation of casein biopeptide-loaded niosomes by high shear homogenization and their characterization. J. Agric. Food Chem. 2021, 69, 4371–4380. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; McClements, D.; Cheng, C.; Xie, Y.; Liang, R.; Liu, J.; Zou, L. Encapsulation of bitter peptides in water-in-oil high internal phase emulsions reduces their bitterness and improves gastrointestinal stability. Food Chem. 2022, 386, 132787. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Karaca, A.C.; Rezaei, A.; Qamar, M.; Assadpour, E.; Esatbeyoglu, T.; Jafari, S. Lipid-based nanodelivery systems of curcumin: Recent advances, approaches, and applications. Food Chem. 2025, 463, 141193. [Google Scholar] [CrossRef]
- Moutinho, C.; Matos, C.; Teixeira, J. Nanocarrier possibilities for functional targeting of bioactive peptides and proteins: State-of-the-art. J. Drug Target. 2012, 20, 114–141. [Google Scholar] [CrossRef]
- Liu, M.; Wang, F.; Pu, C.; Tang, W.; Sun, Q. Nanoencapsulation of lutein within lipid-based delivery systems:Characterization and comparison of zein peptide stabilized nanoemulsion, solid lipid nanoparticle, and nano-structured lipid carrier. Food Chem. 2021, 358, 129840. [Google Scholar] [CrossRef]
- Hsiao, L.; Lai, Y.; Lai, J.; Hsu, C.; Wang, N.; Wang, S.; Jan, J.S. Cross-linked polypeptide-based gel particles by emulsion for efficient protein encapsulation. Polymer 2017, 115, 261–272. [Google Scholar] [CrossRef]
- Ramezanzad, L.; Hosseini, S.; Akbari-Adergani, B.; Yaghmur, A. Cross-linked chitosancoate liposomes for encapsulation of fish-derived peptide. LWT 2021, 150, 112057. [Google Scholar] [CrossRef]
- Dumont, C.; Jannin, V.; Miolane, C.; Lelong, Q.; Valour, J.-P.; Urbaniak, S.; Fessi, H.; Bourgeois, S. A proof-of-concept for developing oral lipidized peptide nanostructured lipid carrier formulations. J. Drug Deliv. Sci. Technol. 2019, 54, 1012394. [Google Scholar] [CrossRef]
- Danesi, F.; Gómez-Caravaca, A.; Di Biase, D.; Verardo, V.; Bordoni, A. New insight into the cholesterol-lowering effect of phytosterols in rat cardiomyocytes. Food Res. Int. 2016, 89, 1056–1063. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal Stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631. [Google Scholar] [CrossRef]
- Mahale, N.; Thakkar, P.; Mali, R.; Walunj, D.; Chaudhari, S.-R. Niosomes:Novel sustained release nonionic stable vesicular systems-an overview. Adv. Colloid Interface Sci. 2012, 15, 181–184. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A.; Omidfar, K. Formulation and characterization of bovine serum albumin loaded noisome. AAPS Pharm. Sci. Technol. 2017, 18, 27–33. [Google Scholar] [CrossRef]
- Du, X.; Huang, X.; Wang, L.; Mo, L.; Jing, H.; Bai, X.; Wang, H.-X. Nanosized niosomes as effective delivery device to improve the stability and bioaccessibility of goat milk whey protein peptide. Food Res. Int. 2022, 161, 111729. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Huang, X.; Xing, S.; Zhong, X.; Wang, H.; Su, W.; Song, Y.; Tan, M. Dual-targeted astaxanthin nanoparticles cloaked in bacterial ghosts for alleviation of alcoholic fatty liver disease. Food Biosci. 2025, 68, 106465. [Google Scholar] [CrossRef]
- Han, C.; Kong, X.; Xia, X.; Huang, X.; Mao, Z.; Han, J.; Shi, F.; Liang, Y.; Wang, A.; Zhang, F. Effects of ginseng peptides on the hypoglycemic activity and gut microbiota of a type 2 diabetes mellitus mice model. J. Funct. Foods 2023, 111, 105897. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.; Su, J.; Zhu, B.; Wang, S.; Liu, K.; Wang, H.; Shi, S.; Zhang, Q.; Qin, L.; et al. Roles of gut microbiota and metabolites in a homogalacturonan type pectic polysaccharide from Ficus pumila Linn. Fruits mediated amelioration of obesity. Carbohydr. Polym. 2020, 248, 116780. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Nagata, T.; Furuya, F.; Nishizawa, M.; Mukai, E. White-skinned sweet potato (Ipomoea batatas L.) acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in normal rats. Heliyon 2023, 9, e14719. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Ye, H.; Gao, X.; Wang, H.; Li, Q.; Hu, J.; Zhang, F.; Luo, J. An acidic polysaccharide promoting GLP-1 secretion from Dendrobium huoshanense protocorm-like bodies: Structure validation and activity exploration. Int. J. Biol. Macromol. 2024, 278, 134783. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Peng, S.; McClements, D.; Chen, L.; Lin, S.; Wang, W. Enhancing stability of curcumin-loaded casein nanoparticles by adding liposomal nanoparticles. LWT 2023, 189, 115405. [Google Scholar] [CrossRef]
- Yang, Y.; Zhan, J.; Cheng, J.; Cao, Y.; Wu, C. Coptis chinensis shows distinct effects on hyperlipidemia and gut microbiota in high-fat diet induced mice with cold or hot syndrome. Chin. Herb. Med. 2025, 17, 529–538. [Google Scholar] [CrossRef]
- Shang, X.; Hu, G.; Zhang, M.; Zuo, J.; Wang, M.; Wu, J.; Lv, Q.; Zhou, Y.; Shao, T.; Wang, G. Therapeutic potential of polysaccharides in type 2 diabetes mellitus via multi-target modulation of gut microbiota. Chin. Herb. Med. 2025. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Grecco, G.; Gao, Y.; Gao, H.; Liu, Y.; Atwood, B. Prenatal opioid administration induces shared alterations to the maternal and offspring gut microbiome: A preliminary analysis. Drug Alcohol Depend. 2021, 227, 108914. [Google Scholar] [CrossRef]
- Abuqwider, J.; Altamimi, M.; Mauriello, G. Limosilactobacillus reuteri in Health and Disease. Microorganisms 2022, 10, 522. [Google Scholar] [CrossRef]
- Lynch, J.; Gonzalez, E.; Choy, K.; Faull, K.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.; Paramo, J.; Hsiao, E. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef]
- Goodrich, J.; Waters, J.; Poole, A.; Sutter, J.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.-Y.; Liu, B.; Zhou, Y.; Lv, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Liao, Z.; Zhou, J.; Lin, J.; Zhu, Y.; Feng, J.; Cheng, Z.; Li, X.; Li, Z.; Nie, S.-P. Supplementation of Akkermansia muciniphila improves diabetic kidney disease by regulating gut microbiome in db/db mice. Food Biosci. 2025, 69, 107042. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, X.; Chen, X.; Liu, S.; Cheng, L.; Kang, Y.; Lin, F. Lactobacillus casein prevents hippocampal atrophy and cognitive impairment in rats with type 2 diabetes by regulating blood glucose levels. Brain Res. 2025, 1850, 149407. [Google Scholar] [CrossRef]
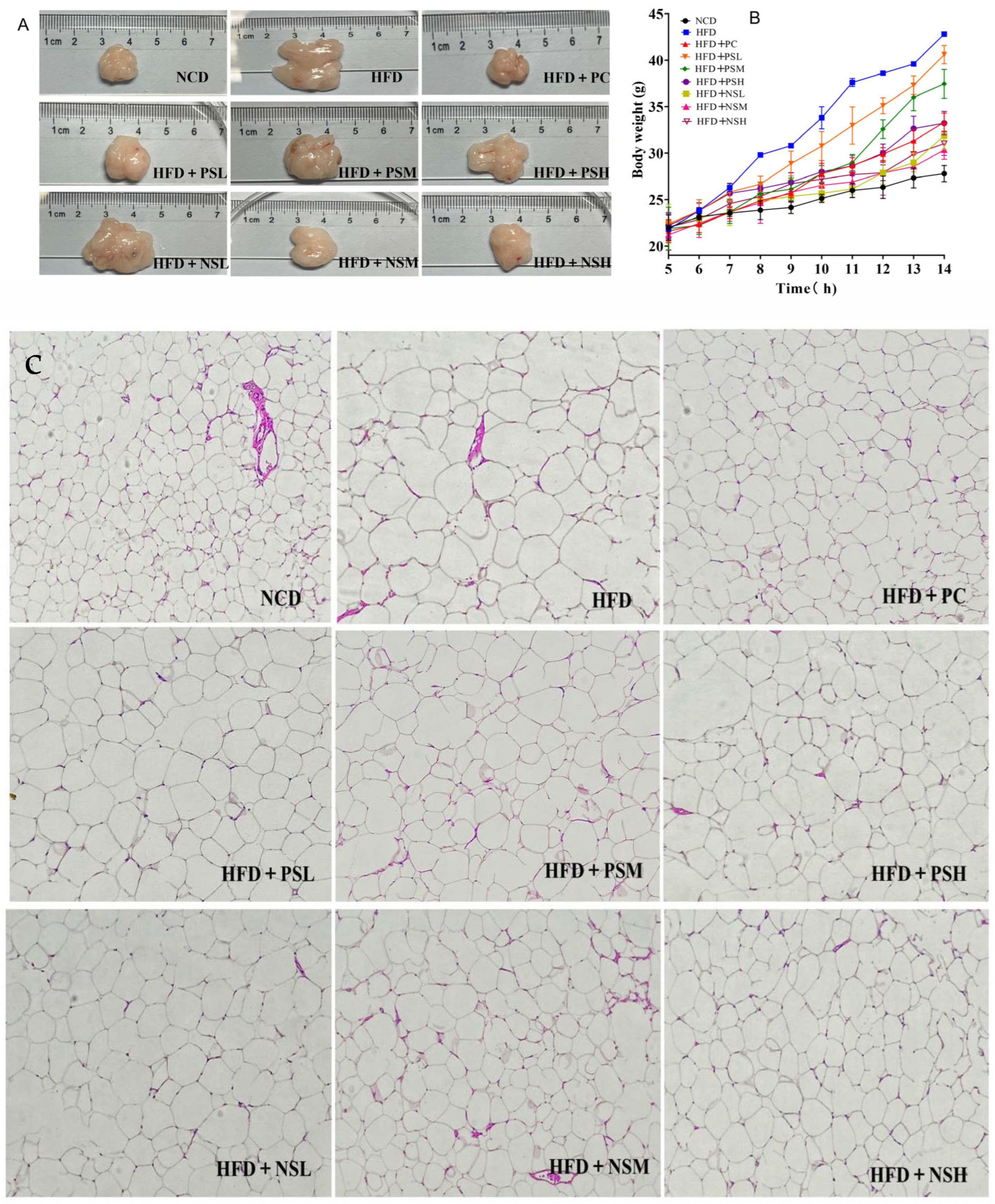



| Samples | Epididymal Fat Index | Perirenal Fat Index | Brown Adipose Tissue Index | Inguinal Fat Index |
|---|---|---|---|---|
| NCD | 14.65 ± 1.65 d | 4.69 ± 0.03 e | 5.55 ± 0.33 a | 6.07 ± 0.02 f |
| HFD | 53.88 ± 1.89 a | 18.69 ± 0.98 a | 2.63 ± 0.58 h | 28.31 ± 0.03 a |
| HFD+PC | 36.48 ± 2.02 bc | 13.96 ± 0.76 c | 3.94 ± 0.05 d | 13.77 ± 0.05 d |
| HFD+PSL | 40.97 ± 1.33 b | 15.78 ± 1.01 b | 3.78 ± 0.01 e | 19.59 ± 0.04 b |
| HFD+PSM | 39.87 ± 1.95 bc | 15.33 ± 1.34 bc | 3.32 ± 0.03 g | 18.77 ± 1.01 b |
| HFD+PSH | 38.66 ± 2.01 bc | 14.63 ± 0.54 bc | 3.42 ± 0.02 f | 17.45 ± 1.21 bc |
| HFD+NSL | 36.54 ± 1.62 bc | 12.22 ± 1.38 cd | 4.02 ± 0.05 d | 15.43 ± 1.34 c |
| HFD+NSM | 35.58 ± 1.15 c | 9.67 ± 2.45 d | 4.54 ± 0.01 b | 13.23 ± 0.21 e |
| HFD+NSH | 36.36 ± 1.01 c | 8.98 ± 1.05 d | 4.31 ± 0.05 c | 13.54 ± 0.51 d |
| Samples | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|
| NCD | 5.8 ± 0.02 eF | 10.8 ± 0.07 aH | 10.0 ± 0.13 bH | 8.2 ± 0.21 cI | 6.4 ± 0.04 dH |
| HFD | 8.6 ± 0.23 eA | 17.8 ± 0.12 aA | 16.7 ± 0.08 bA | 15.8 ± 0.01 cA | 13.3 ± 0.23 dA |
| HFD+PC | 7.9 ± 0.02 eC | 16.4 ± 0.14 aB | 15.2 ± 0.08 bB | 14.0 ± 0.12 cB | 10.8 ± 0.20 dC |
| HFD+PSL | 8.2 ± 0.23 eAB | 15.6 ± 0.14 aC | 13.6 ± 0.08 bC | 11.8 ± 0.04 cE | 10.9 ± 0.12 dC |
| HFD+PSM | 8.3 ± 0.16 eAB | 14.8 ± 0.10 aD | 13.4 ± 0.07 bD | 12.2 ± 0.06 cD | 10.2 ± 0.02 dD |
| HFD+PSH | 7.9 ± 0.12 eB | 13.7 ± 0.05 aE | 13.0 ± 0.03 bF | 12.6 ± 0.12 cC | 12.0 ± 0.16 dB |
| HFD+NSL | 7.4 ± 0.13 eE | 12.2 ± 0.08 bF | 13.3 ± 0.17 aD | 10.6 ± 0.11 cF | 9.8 ± 0.08 dE |
| HFD+NSM | 7.8 ± 0.03 dD | 10.6 ± 0.02 bI | 12.4 ± 0.14 aG | 9.8 ± 0.06 cG | 7.8 ± 0.14 dG |
| HFD+NSH | 8.1 ± 0.08 eBC | 11.6 ± 0.12 bG | 13.2 ± 0.10 aE | 9.4 ± 0.05 cH | 8.3 ± 0.02 dF |
| Samples | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|
| NCD | 8.00 ± 0.01 aC | 5.80 ± 0.02 bG | 4.30 ± 0.03 eH | 4.86 ± 0.02 dI | 5.00 ± 0.01 cG |
| HFD | 9.90 ± 0.02 aA | 8.80 ± 0.12 dA | 8.50 ± 0.13 eA | 9.37 ± 0.07 bA | 8.96 ± 0.02 cA |
| HFD+PC | 8.23 ± 0.04 aB | 6.89 ± 0.04 bF | 6.22 ± 0.12 dF | 5.83 ± 0.04 eB | 6.65 ± 0.14 cB |
| HFD+PSL | 8.34 ± 0.23 aB | 7.88 ± 0.02 bB | 6.90 ± 0.08 cB | 6.65 ± 0.04 dE | 6.58 ± 0.12 dB |
| HFD+PSM | 8.00 ± 0.03 aC | 7.67 ± 0.12 bCD | 6.75 ± 0.04 cC | 6.28 ± 0.10 dD | 6.10 ± 0.06 eC |
| HFD+PSH | 7.89 ± 0.02 aD | 7.53 ± 0.11 bCD | 6.56 ± 0.03 cD | 5.84 ± 0.12 dC | 5.43 ± 0.06 eD |
| HFD+NSL | 7.80 ± 0.04 aC | 7.65 ± 0.02 bC | 6.44 ± 0.05 cE | 5.43 ± 0.10 dF | 5.38 ± 0.14 dD |
| HFD+NSM | 8.01 ± 0.13 aC | 7.12 ± 0.07 bE | 5.68 ± 0.12 cG | 5.11 ± 0.03 eG | 5.21 ± 0.02 dF |
| HFD+NSH | 8.10 ± 0.09 aBC | 7.44 ± 0.12 bD | 6.23 ± 0.11 cF | 5.34 ± 0.06 cH | 5.28 ± 0.02 dE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Niu, W.; Wang, H. Preparation, Characterization, and Mechanism of Hypoglycemic Action of a Goat Casein Peptide Delivery System Involving DPP-IV Inhibition and GLP-1 Release. Foods 2025, 14, 3795. https://doi.org/10.3390/foods14213795
Du X, Niu W, Wang H. Preparation, Characterization, and Mechanism of Hypoglycemic Action of a Goat Casein Peptide Delivery System Involving DPP-IV Inhibition and GLP-1 Release. Foods. 2025; 14(21):3795. https://doi.org/10.3390/foods14213795
Chicago/Turabian StyleDu, Xiaojing, Wenlin Niu, and Hongxin Wang. 2025. "Preparation, Characterization, and Mechanism of Hypoglycemic Action of a Goat Casein Peptide Delivery System Involving DPP-IV Inhibition and GLP-1 Release" Foods 14, no. 21: 3795. https://doi.org/10.3390/foods14213795
APA StyleDu, X., Niu, W., & Wang, H. (2025). Preparation, Characterization, and Mechanism of Hypoglycemic Action of a Goat Casein Peptide Delivery System Involving DPP-IV Inhibition and GLP-1 Release. Foods, 14(21), 3795. https://doi.org/10.3390/foods14213795






