Metabolomics Reveals the Regulatory Mechanism of Antibacterial Fiber Membrane Packaging on the Postharvest Quality of Wax Apple (Syzygium samarangense)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of a Polyethylene Oxide/Oxidized Tinctorial Gum (PEO/OSG) Composite Fiber Membrane Loaded with ε-PL
2.3. Determination of In Vitro Antibacterial Activity and Mechanism of Fiber Membranes
2.3.1. Cell Membrane Integrity
2.3.2. Cell Wall Integrity (AKP)
2.3.3. Cell Membrane Permeability
2.3.4. Observation of Bacterial Micromorphology
2.4. Application of a Composite Fiber Membrane in Wax Apple Preservation
2.4.1. Decay Index, Weight Loss
2.4.2. Firmness, Color
2.4.3. Determination of Respiratory Strength
2.4.4. Determination of Total Soluble Solids Content and Titratable Acid (TA) Content
2.4.5. Malondialdehyde (MDA) Content
2.4.6. Measurement of Polyphenol Oxidase (PPO) Activity
2.4.7. Measurement of Peroxidase (POD) Activity
2.4.8. Sensory Evaluation
2.5. Metabolomics Analysis
2.6. Statistical Analysis
3. Results and Discussion
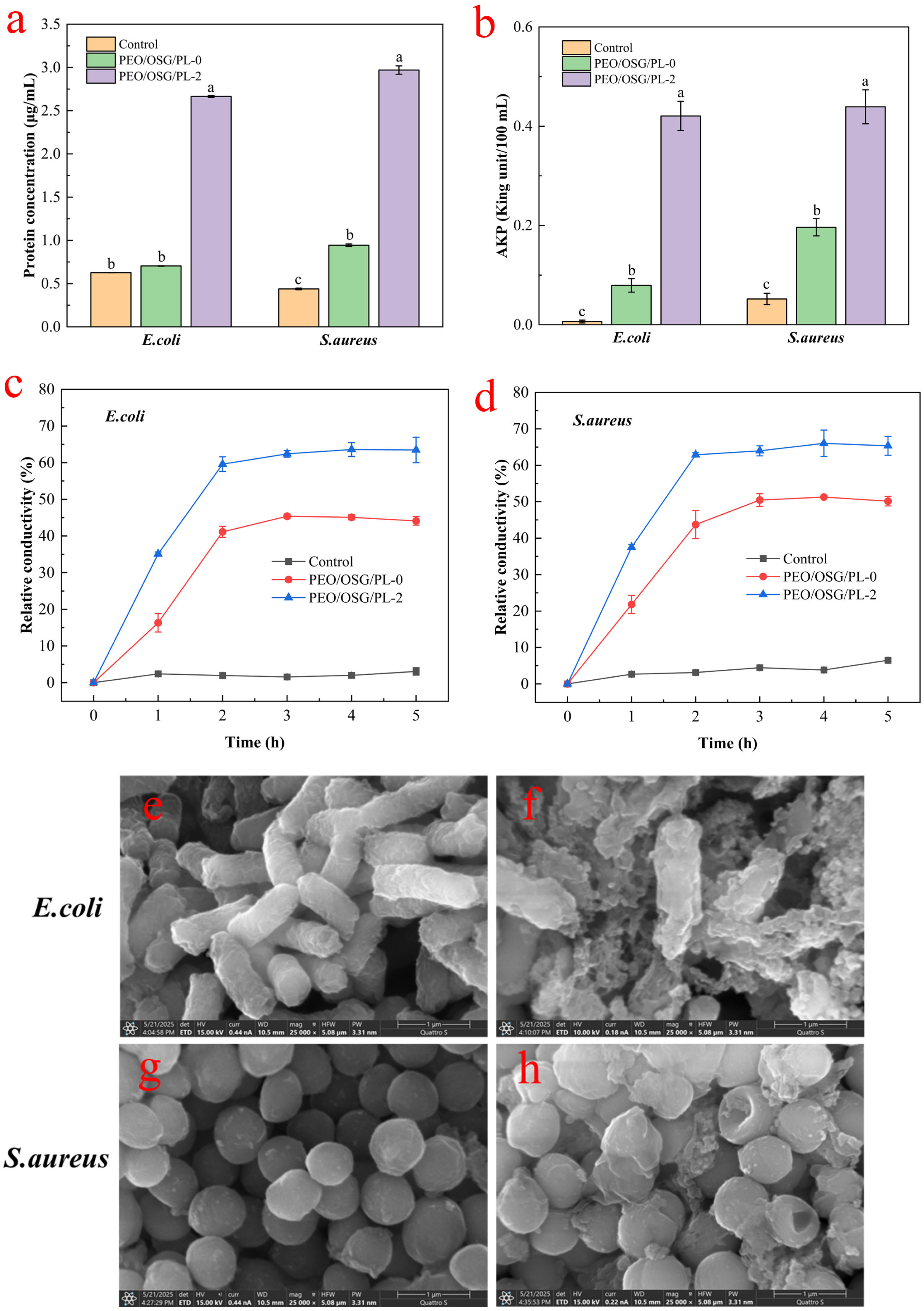
3.1. In Vitro Antibacterial Activity and Mechanism of Fiber Membranes
3.1.1. Analysis of Protein Leakage
3.1.2. Analysis of Intracellular AKP Levels
3.1.3. Permeability of the Cell Membrane
3.1.4. Microscopic Morphology of Bacteria
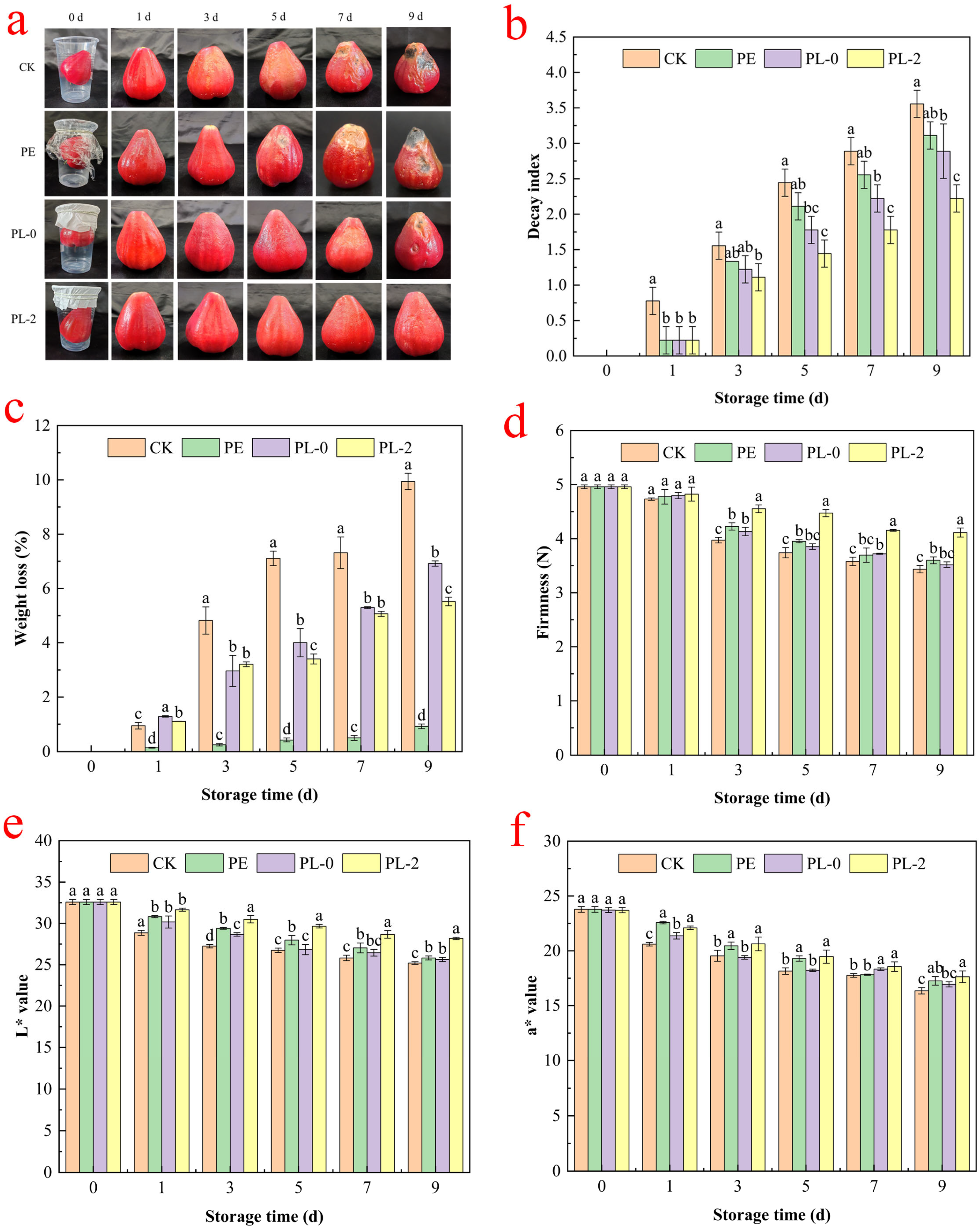
3.2. Experiments on Preserving the Freshness of Wax Apple Seed Pods Via Composite Fiber Membranes
3.2.1. Decay Index and Weight Loss Rate
3.2.2. Firmness
3.2.3. Color
3.2.4. Respiratory Strength
3.2.5. Total Soluble Solids Content
3.2.6. Titratable Acid Content
3.2.7. MDA Content
3.2.8. PPO Activity
3.2.9. POD Activity
3.2.10. Sensory Analysis
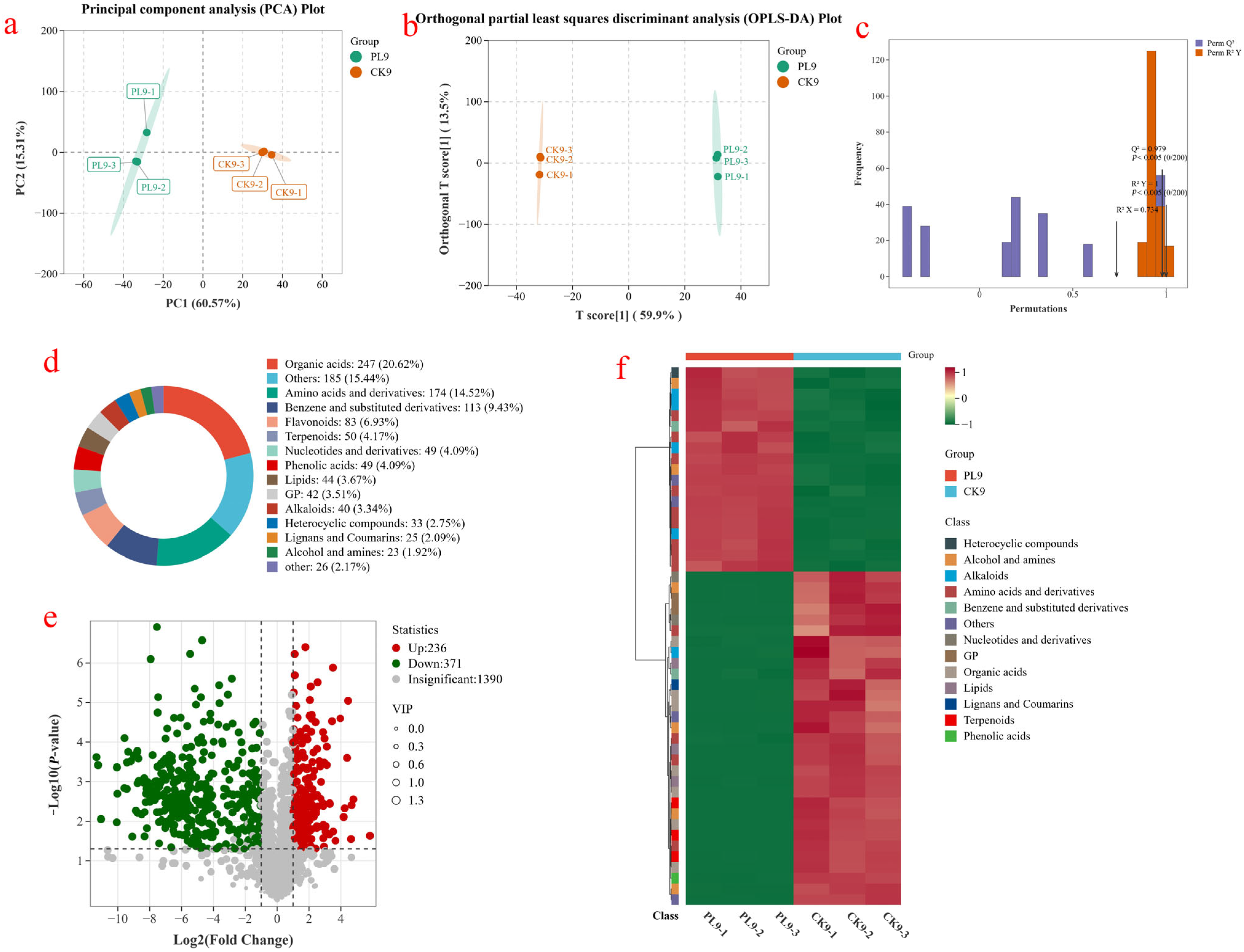
3.3. Metabolomics Analysis
3.3.1. Multivariate Statistical Analysis
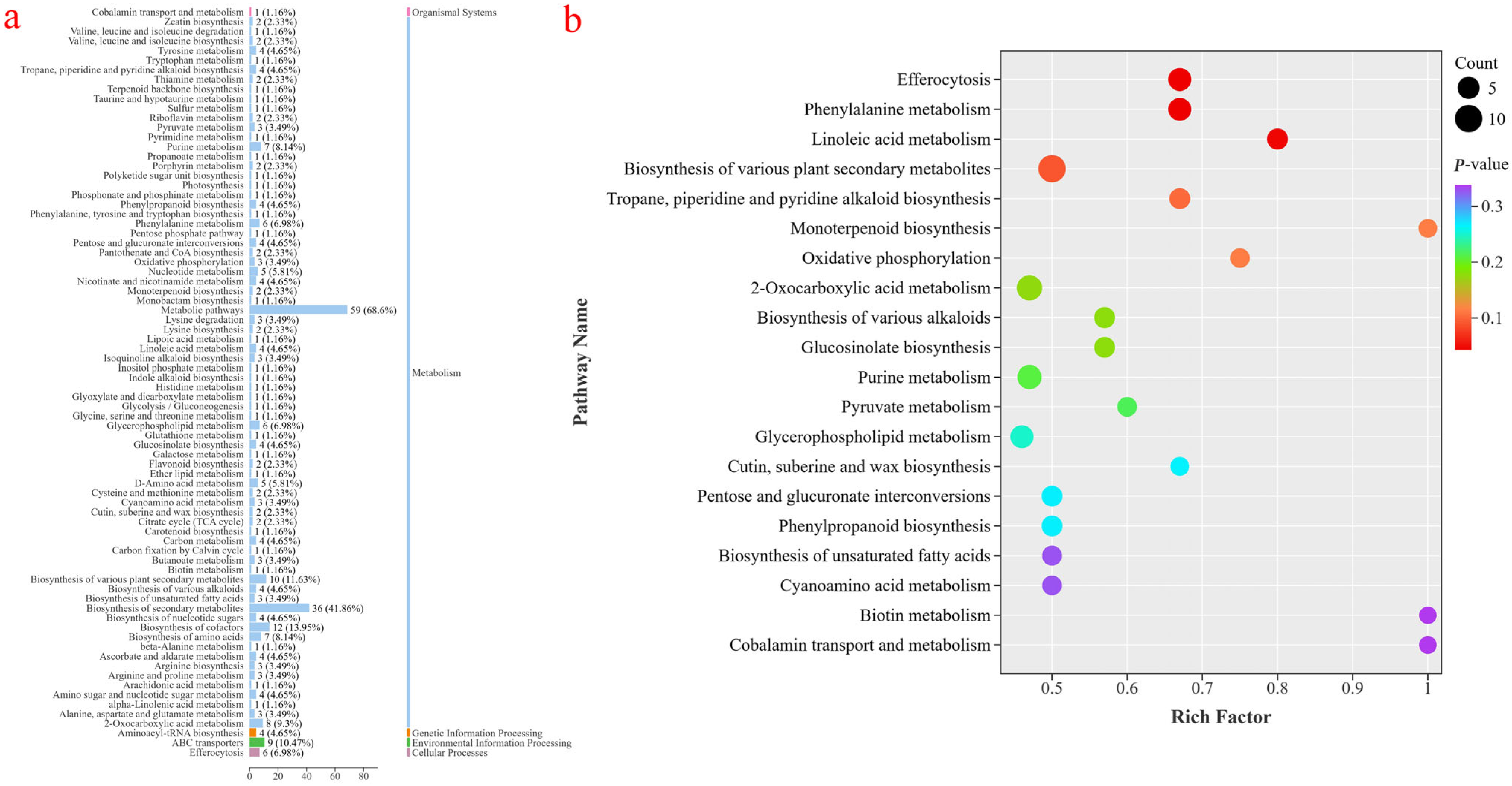
3.3.2. KEGG Enrichment Analysis of Differential Metabolites
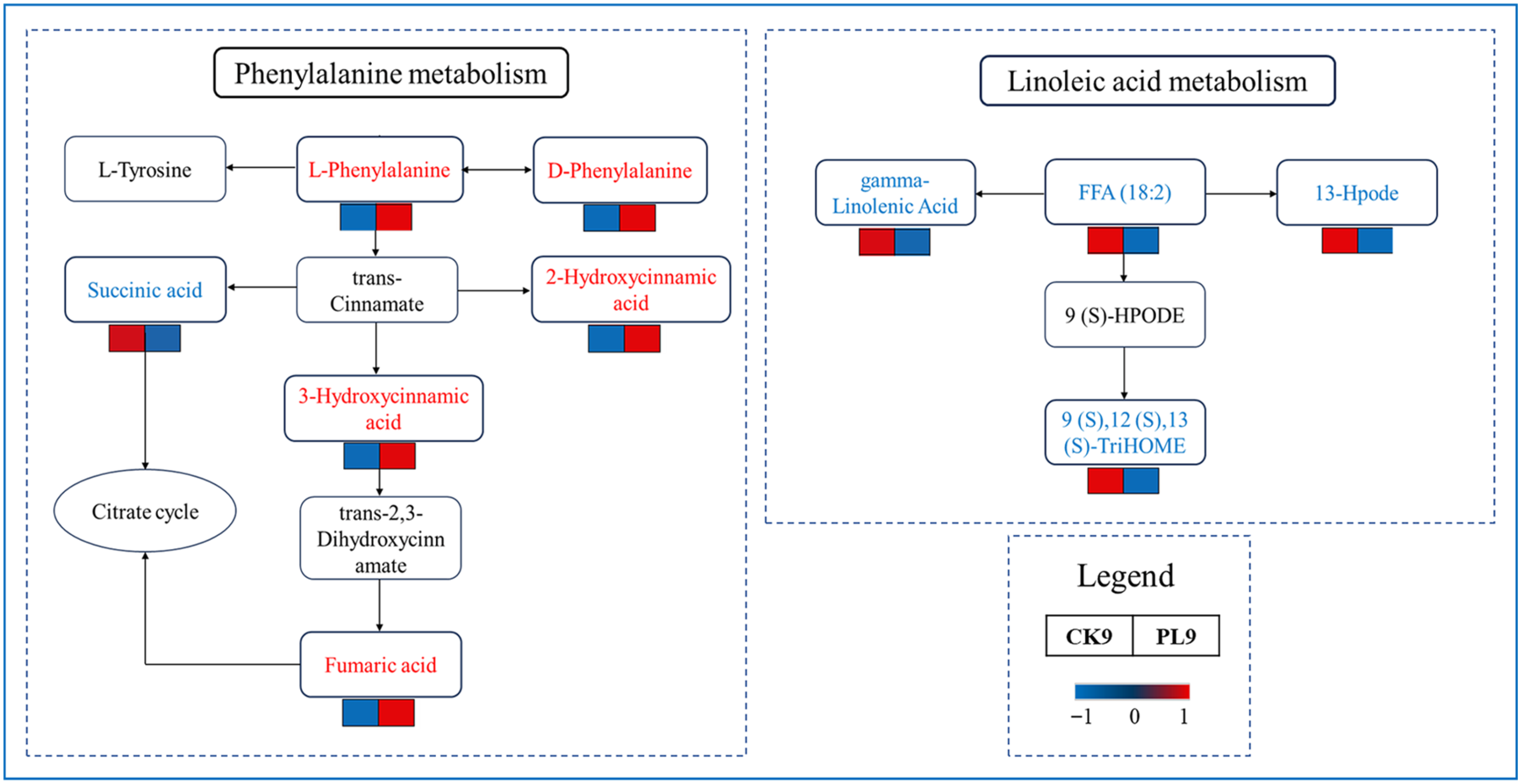
3.3.3. Analysis of Main Metabolic Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.-x.; Wang, Y.; Chen, F.-h.; He, F.; Wu, G.-b.; Zhang, S.; Lin, H.-t. Exogenous nitric oxide inhibits the respiratory metabolism of postharvest wax apple fruit and its role in the delayed cottony softening. Sci. Hortic. 2023, 317, 112043. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, N.; Li, B.; Zhao, T.; Cheng, C.; Chen, C.; Deng, H.; Yan, R. Simulated logistical transport effects on textural change of wax apple using different packaging types during storage time. Food Packag. Shelf Life 2024, 43, 101285. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Z.; Goksen, G.; Khan, M.R.; Ahmad, N.; Zhang, W.; Deng, H. Application of tannic acid and Fe3+ crosslinking-enhanced chitosan films for wax apple (Syzygium samarangense) preservation. Int. J. Biol. Macromol. 2025, 321, 146318. [Google Scholar] [CrossRef]
- Meng, C.-R.; Zhang, Q.; Yang, Z.-F.; Geng, K.; Zeng, X.-Y.; Thilini Chethana, K.; Wang, Y. Lasiodiplodia syzygii sp. nov. (Botryosphaeriaceae) causing post-harvest water-soaked brown lesions on Syzygium samarangense in Chiang Rai, Thailand. Biodivers. Data J. 2021, 9, e60604. [Google Scholar] [CrossRef]
- Health, E.P.o.P.; Bragard, C.; Baptista, P.; Chatzivassiliou, E.; Di Serio, F.; Gonthier, P.; Jaques Miret, J.A.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Pest categorisation of Pestalotiopsis microspora. EFSA J. 2023, 21, e8493. [Google Scholar] [CrossRef]
- Esua, J.O.; Chin, N.L.; Yusof, Y.A.; Sukor, R. Antioxidant Bioactive Compounds and Spoilage Microorganisms of Wax Apple (Syzygium samarangense) during Room Temperature Storage. Int. J. Fruit Sci. 2017, 17, 188–201. [Google Scholar] [CrossRef]
- Khandaker, M.M.; Boyce, A.N. Growth, distribution and physiochemical properties of wax apple (Syzygium samarangense): A Review. Aust. J. Crop Sci. 2016, 10, 1640–1648. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Chang, C.-W.; Hsu, M.-C.; Chung, H.-Y.; Liang, Y.S. Effects of different concentrations of oxygen used for storage on the postharvest physiology and quality of wax apple (Syzygium samarangense Blume Merr. & L. M. perry cv. pink). Sci. Hortic. 2023, 313, 111906. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Nawaz, G.; Zhao, C.; Li, Y.; Dong, T.; Zhu, M.; Du, X.; Zhang, L.; Li, Z.; et al. Exogenous Melatonin Attenuates Post-Harvest Decay by Increasing Antioxidant Activity in Wax Apple (Syzygium samarangense). Front. Plant Sci. 2020, 11, 569779. [Google Scholar] [CrossRef]
- Shi, C.; Fang, D.; Huang, C.; Zhou, A.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Li, W. Active electrospun nanofiber packaging maintains the preservation quality and antioxidant activity of blackberry. Postharvest Biol. Technol. 2023, 199, 112300. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, S.; Liu, Q.; Kong, B.; Wang, H. Fabrication and characterization of cinnamaldehyde loaded polysaccharide composite nanofiber film as potential antimicrobial packaging material. Food Packag. Shelf Life 2020, 26, 100600. [Google Scholar] [CrossRef]
- Devkar, P.; Nangare, S.; Zawar, L.; Shirsath, N.; Bafna, P.; Jain, P. Design of polyacrylamide grafted sesbania gum-mediated pH-responsive IPN-based microbeads for delivery of diclofenac sodium: In-vitro-in-vivo characterizations. Int. J. Biol. Macromol. 2023, 230, 123360. [Google Scholar] [CrossRef]
- Zhu, Y.; Cui, H.; Li, C.; Lin, L. A novel polyethylene oxide/Dendrobium officinale nanofiber: Preparation, characterization and application in pork packaging. Food Packag. Shelf Life 2019, 21, 100329. [Google Scholar] [CrossRef]
- Yong, Y.; Gu, Y.; Ahmad, H.N.; Wang, L.; Wang, R.; Zhu, J. Design and characterization of tannic acid/E-polylysine biocomposite packaging films with excellent antibacterial and antioxidant properties for beef preservation. Food Chem. 2024, 439, 138155. [Google Scholar] [CrossRef]
- Zhao, J.; Huan, H.; Yang, T.; Chen, J.; Yao, G. Fibrous membranes of poly(ethylene oxide)/Sesbania gum oxide/ε-poly(lysine): An influence on its structure. Food Chem. 2025, 470, 142753. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Ling, S.; Yuan, M.; Sun, Q.; Dong, X. Antibacterial mechanism of chitooligosaccharides against specific spoilage organisms in chilled processed fish paste products. Food Control 2025, 172, 111148. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Guo, L.; Wang, L.; Liu, Y. Antibacterial mechanism of rose essential oil against Pseudomonas putida isolated from white Hypsizygus marmoreus at cellular and metabolic levels. Ind. Crops Prod. 2023, 196, 116523. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, M.; Wang, L.; Tao, N.; Li, L.; Zhu, W.; Xu, C.; Deng, S.; Wang, Y. Mechanism of antimicrobials immobilized on packaging film inhabiting foodborne pathogens. LWT 2022, 169, 114037. [Google Scholar] [CrossRef]
- Ding, J.; Dwibedi, V.; Huang, H.; Ge, Y.; Li, Y.; Li, Q.; Sun, T. Preparation and antibacterial mechanism of cinnamaldehyde/tea polyphenol/polylactic acid coaxial nanofiber films with zinc oxide sol to Shewanella putrefaciens. Int. J. Biol. Macromol. 2023, 237, 123932. [Google Scholar] [CrossRef]
- SB/T 10886-2012; Specification for the Circulation of Wax Apples. China Standards Press: Beijing, China, 2012.
- Wang, T.; Zhai, X.; Huang, X.; Li, Z.; Zhang, X.; Zou, X.; Shi, J. Effect of different coating methods on coating quality and mango preservation. Food Packag. Shelf Life 2023, 39, 101133. [Google Scholar] [CrossRef]
- Li, X.; Peng, S.; Yu, R.; Li, P.; Zhou, C.; Qu, Y.; Li, H.; Luo, H.; Yu, L. Co-Application of 1-MCP and Laser Microporous Plastic Bag Packaging Maintains Postharvest Quality and Extends the Shelf-Life of Honey Peach Fruit. Foods 2022, 11, 1733. [Google Scholar] [CrossRef]
- Wang, M.; Huang, D.; Sun, Y.; Yao, G.; Huan, H.; Chen, J. Antibacterial Activity of Modified Sesbania Gum Composite Film and Its Preservation Effect on Wampee Fruit (Clausena lansium (Lour.) Skeels). Foods 2024, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, X.; Liang, J.; Fu, Y.; Wang, J.; Jiang, M.; Pan, L. Cell wall and reactive oxygen metabolism responses of strawberry fruit during storage to low voltage electrostatic field treatment. Postharvest Biol. Technol. 2022, 192, 110208. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, S.; Xi, F.; Yang, W.; Zhou, L.; Zhang, G.; Zhu, H.; Zhang, Q. Preservation effect of plasma-activated water (PAW) treatment on fresh walnut kernels. Innov. Food Sci. Emerg. Technol. 2023, 85, 103304. [Google Scholar] [CrossRef]
- Gautam, A.; Gill, P.P.S.; Singh, N.; Jawandha, S.K.; Arora, R.; Singh, A.; Ajay. Composite coating of xanthan gum with sodium nitroprusside alleviates the quality deterioration in strawberry fruit. Food Hydrocoll. 2024, 155, 110208. [Google Scholar] [CrossRef]
- Chang, H.; Li, K.; Ye, J.; Chen, J.; Zhang, J. Effect of Dual-Modified Tapioca Starch/Chitosan/SiO2 Coating Loaded with Clove Essential Oil Nanoemulsion on Postharvest Quality of Green Grapes. Foods 2024, 13, 3735. [Google Scholar] [CrossRef]
- Cui, R.; Liu, F.-Y.; Wu, H. Enhancement of antibacterial activity of cinnamaldehyde against Bacillus cereus by nanoemulsion and its application in milk. Food Biosci. 2025, 64, 105931. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Wang, J.; Jiang, J.; Zhai, X.; Wang, W.; Hou, H. Influence of starch content on the physicochemical and antimicrobial properties of starch/PBAT/ε-polylysine hydrochloride blown films. Food Packag. Shelf Life 2022, 34, 101005. [Google Scholar] [CrossRef]
- Wang, S.; Liu, S.; Hao, G.; Zhao, L.; Lü, X.; Wang, H.; Wang, L.; Zhang, J.; Ge, W. Antimicrobial activity and mechanism of isothiocyanate from Moringa oleifera seeds against Bacillus cereus and Cronobacter sakazakii and its application in goat milk. Food Control 2022, 139, 109067. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, L.; Zhu, J.; Wu, H.; Li, W.; Sun, Q. Antibacterial properties and mechanism of biopolymer-based films functionalized by CuO/ZnO nanoparticles against Escherichia coli and Staphylococcus aureus. J. Hazard. Mater. 2021, 402, 123542. [Google Scholar] [CrossRef]
- Qi, J.; Xu, H.; Wu, X.; Lv, Z.; Yue, T.; Yang, H.; Yuan, Y. Antimicrobial activity and mechanism of protocatechualdehyde against Alicyclobacillus spp. And its effect on apple juice physicochemical properties. Food Control 2025, 175, 111289. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Wang, Q.; Guo, T.; Yuan, Y.; Yue, T.; Jia, H.; Ge, Q.; Zhao, Z.; Cai, R. Antimicrobial activity and mechanism of preservatives against Alicyclobacillus acidoterrestris and its application in apple juice. Int. J. Food Microbiol. 2023, 386, 110039. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Wang, J.; Li, Y.; Zhang, J.; Wu, Z.; Jin, P.; Cao, S.; Zheng, Y. Recent advances in high relative humidity strategy for preservation of postharvest fruits and vegetables: A comprehensive review. Food Chem. 2025, 481, 144130. [Google Scholar] [CrossRef]
- Yang, S.; Miao, Q.; Huang, Y.; Jian, P.; Wang, X.; Tu, M. Preparation of cinnamaldehyde-loaded polyhydroxyalkanoate/chitosan porous microspheres with adjustable controlled-release property and its application in fruit preservation. Food Packag. Shelf Life 2020, 26, 100596. [Google Scholar] [CrossRef]
- Shen, C.; Yang, X.; Wang, D.; Li, J.; Zhu, C.; Wu, D.; Chen, K. Carboxymethyl chitosan and polycaprolactone-based rapid in-situ packaging for fruit preservation by solution blow spinning. Carbohydr. Polym. 2024, 326, 121636. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, H.; Niu, B.; Jiang, L. Antibacterial activities of R-(+)-Limonene emulsion stabilized by Ulva fasciata polysaccharide for fruit preservation. Int. J. Biol. Macromol. 2018, 111, 1273–1280. [Google Scholar] [CrossRef]
- Xiao, M.; Leung, A.Y.K.; Zhu, Z.; Zhu, L.; Xiao, K. Optimization of the extraction of duckweed protein and its application as an antioxidant composite membrane in blueberry preservation. Int. J. Biol. Macromol. 2025, 311, 143847. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Stability, solubility, mechanical and barrier properties of cassava starch—Carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocoll. 2012, 28, 59–67. [Google Scholar] [CrossRef]
- Liang, F.; Liu, C.; Geng, J.; Chen, N.; Lai, W.; Mo, H.; Liu, K. Chitosan–fucoidan encapsulating cinnamaldehyde composite coating films: Preparation, pH-responsive release, antibacterial activity and preservation for litchi. Carbohydr. Polym. 2024, 333, 121968. [Google Scholar] [CrossRef]
- Geng, C.; Liu, X.; Ma, J.; Ban, H.; Bian, H.; Huang, G. High strength, controlled release of curcumin-loaded ZIF-8/chitosan/zein film with excellence gas barrier and antibacterial activity for litchi preservation. Carbohydr. Polym. 2023, 306, 120612. [Google Scholar] [CrossRef]
- Yang, H.; Li, L.; Li, C.; Xu, Z.; Tao, Y.; Lu, J.; Xia, X.; Tan, M.; Du, J.; Wang, H. Multifunctional and antimicrobial carboxymethyl cellulose-based active hydrogel film for fruits packaging and preservation. Food Biosci. 2024, 59, 104005. [Google Scholar] [CrossRef]
- Yang, Z.; Guan, C.; Zhou, C.; Pan, Q.; He, Z.; Wang, C.; Liu, Y.; Song, S.; Yu, L.; Qu, Y.; et al. Amphiphilic chitosan/carboxymethyl gellan gum composite films enriched with mustard essential oil for mango preservation. Carbohydr. Polym. 2023, 300, 120290. [Google Scholar] [CrossRef]
- Huang, J.; Wu, W.; Niu, B.; Fang, X.; Chen, H.; Wang, Y.; Gao, H. Characterization of Zizania latifolia polysaccharide-corn starch composite films and their application in the postharvest preservation of strawberries. Lwt-Food Sci. Technol. 2023, 173, 114332. [Google Scholar] [CrossRef]
- Xin, Y.; Yang, C.; Zhang, J.; Xiong, L. Application of Whey Protein-Based Emulsion Coating Treatment in Fresh-Cut Apple Preservation. Foods 2023, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Hao, Y.; Liu, B.; Chen, Y.; Li, L. Development and Application of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Film Containing Salicylic Acid for Banana Preservation. Foods 2023, 12, 3397. [Google Scholar] [CrossRef]
- Liu, G.; Chen, B.; Liu, H.; Wang, X.; Zhang, Y.; Wang, C.; Liu, C.; Zhong, Y.; Qiao, Y. Effects of Hydroxyethyl Cellulose and Sulfated Rice Bran Polysaccharide Coating on Quality Maintenance of Cherry Tomatoes during Cold Storage. Foods 2023, 12, 3156. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Li, J.; Lei, H.; Zhen, X.; Gou, D.; Liu, T. Preparation of chitosan/Enoki mushroom foot polysaccharide composite cling film and its application in blueberry preservation. Int. J. Biol. Macromol. 2023, 246, 125567. [Google Scholar] [CrossRef]
- Tan, Y.; Gao, M.; Li, L.; Jiang, H.; Liu, Y.; Gu, T.; Zhang, J. Functional components and antioxidant activity were improved in ginger fermented by Bifidobacterium adolescentis and Monascus purpureus. LWT 2024, 197, 115931. [Google Scholar] [CrossRef]
- Bu, H.; Huang, X.; Huang, Q.; Cheng, P.; Dong, T.; Yun, X. Integrated proteomics and metabolomics analysis reveals the molecular mechanism of PP/PBAT-modified atmosphere packaging for the preservation of Allium mongolicum Regel. Postharvest Biol. Technol. 2024, 213, 112915. [Google Scholar] [CrossRef]
- Monaci, L.; De Angelis, E.; Montemurro, N.; Pilolli, R. Comprehensive overview and recent advances in proteomics MS based methods for food allergens analysis. TrAC Trends Anal. Chem. 2018, 106, 21–36. [Google Scholar] [CrossRef]

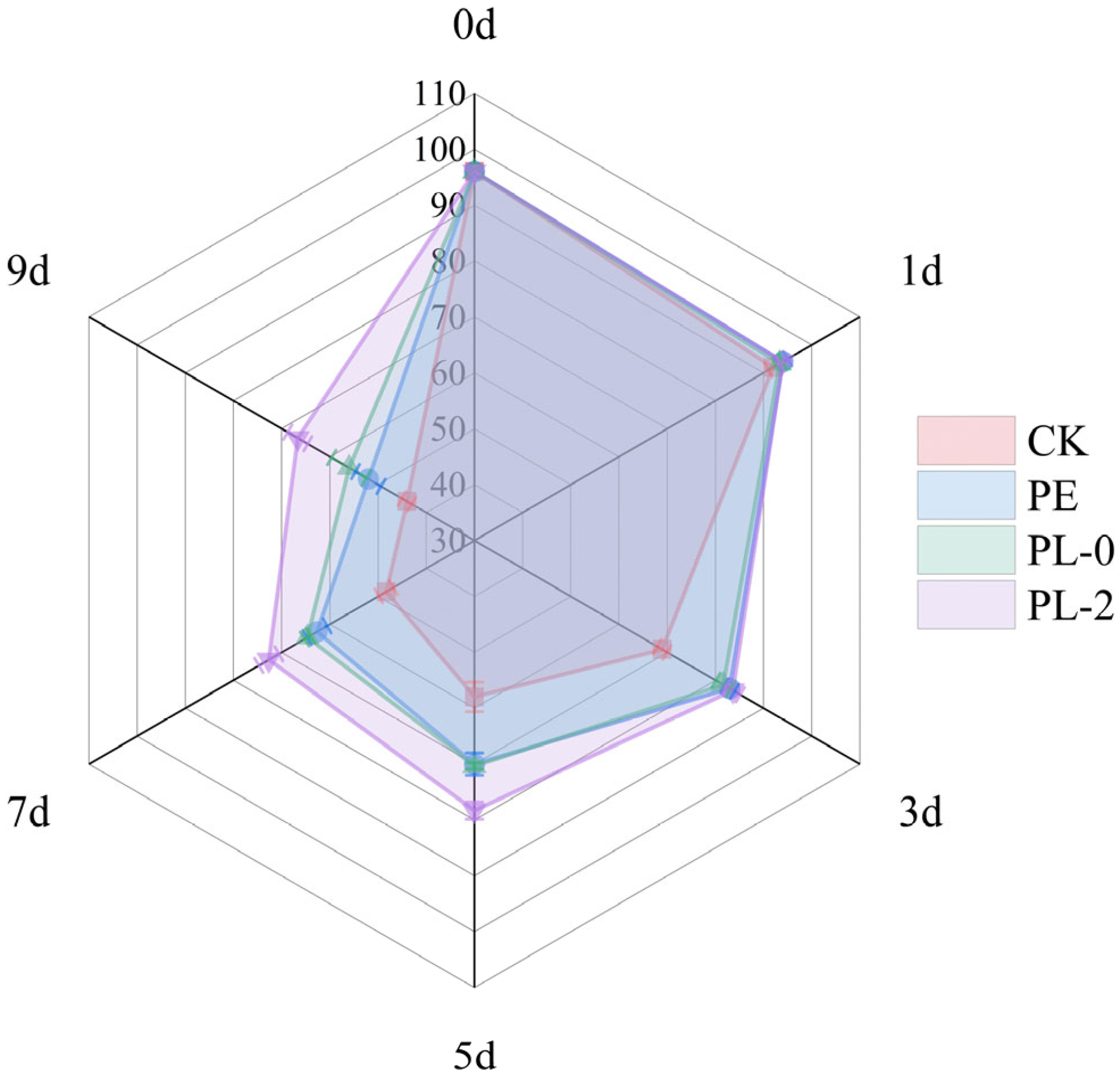
| Parameter | Score Range | Description |
|---|---|---|
| Appearance | 21–25 | Fruit is plump, well-formed, with a smooth surface, showing no wrinkling, depressions, or disease spots. |
| 16–20 | Fruit is generally plump, with slight irregularity in shape. The surface may exhibit very slight wrinkling or a few minor spots. | |
| 11–15 | Slightly dehydrated fruit with noticeable wrinkling or deformation, and a few minor blemishes. | |
| 0–10 | Severely wrinkled, shriveled, or collapsed fruit with extensive blemishes that significantly impair appearance. | |
| Color | 21–25 | Bright red skin with vivid, uniform coloration and glossy sheen, free of browning. |
| 16–20 | Relatively bright color, but slightly uneven or slightly dulled, with reduced gloss and very slight browning. | |
| 11–15 | Noticeably dulled or lost luster, with distinct brown spots or uneven coloring. | |
| 0–10 | Dull color, extensive browning or abnormal yellowish-brown discoloration, completely lacking luster. | |
| Aroma | 21–25 | Exhibits a rich, fresh aroma characteristic of wax apples, with no off-flavors. |
| 16–20 | Features a distinct wax apple aroma, though relatively faint, or may carry a very slight grassy note. | |
| 11–15 | Aroma is weak, or may exhibit noticeable grassy or alcoholic notes. | |
| 0–10 | Absence of fruit aroma, accompanied by pronounced rancid, alcoholic, musty, or other unpleasant off-flavors. | |
| Taste | 21–25 | Crisp texture, abundant juice, balanced sweetness and acidity, pure and rich flavor with no off-flavors. |
| 16–20 | Fairly crisp texture, adequate juice, slightly sour or bland, good flavor. | |
| 11–15 | Soft and mushy texture, insufficient juiciness, uneven sweetness and acidity, weak flavor, or slight off-flavor. | |
| 0–10 | Soft and mushy or dry and astringent texture, very little juice, excessively sour, bitter, or other strong off-flavors, unfit for normal consumption. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Yao, G.; Huang, D.; Sun, Y.; Chen, J.; Huan, H. Metabolomics Reveals the Regulatory Mechanism of Antibacterial Fiber Membrane Packaging on the Postharvest Quality of Wax Apple (Syzygium samarangense). Foods 2025, 14, 3794. https://doi.org/10.3390/foods14213794
Zhao J, Yao G, Huang D, Sun Y, Chen J, Huan H. Metabolomics Reveals the Regulatory Mechanism of Antibacterial Fiber Membrane Packaging on the Postharvest Quality of Wax Apple (Syzygium samarangense). Foods. 2025; 14(21):3794. https://doi.org/10.3390/foods14213794
Chicago/Turabian StyleZhao, Jiale, Guanglong Yao, Dongfen Huang, Yue Sun, Jian Chen, and Hengfu Huan. 2025. "Metabolomics Reveals the Regulatory Mechanism of Antibacterial Fiber Membrane Packaging on the Postharvest Quality of Wax Apple (Syzygium samarangense)" Foods 14, no. 21: 3794. https://doi.org/10.3390/foods14213794
APA StyleZhao, J., Yao, G., Huang, D., Sun, Y., Chen, J., & Huan, H. (2025). Metabolomics Reveals the Regulatory Mechanism of Antibacterial Fiber Membrane Packaging on the Postharvest Quality of Wax Apple (Syzygium samarangense). Foods, 14(21), 3794. https://doi.org/10.3390/foods14213794






