The Effect of Swiss Chard Powder as a Curing Agent on Volatile Compound Profile and Other Qualitative Properties of Heat-Treated Sucuk
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. HTS Production
2.3. Microbiological Analyses
2.4. Physicochemical Analyses
2.4.1. pH and Water Activity (aw)
2.4.2. Thiobarbituric Acid Reactive Substances (TBARS)
2.4.3. Residual Nitrite
2.4.4. Instrumental Color
2.5. Sensory Analysis
2.6. Volatile Compounds
2.7. Statistical Analysis
3. Results and Discussion
3.1. The Effect of Using SCP on pH
3.2. The Effect of Using SCP on aw
3.3. The Effect of Using SCP on TBARS
3.4. The Effect of Using SCP on Residual Nitrite
3.5. The Effect of Using SCP on Instrumental Color
3.6. The Effect of Using SCP on Microbiological Properties
3.7. The Effect of Using SCP on Sensory Attributes
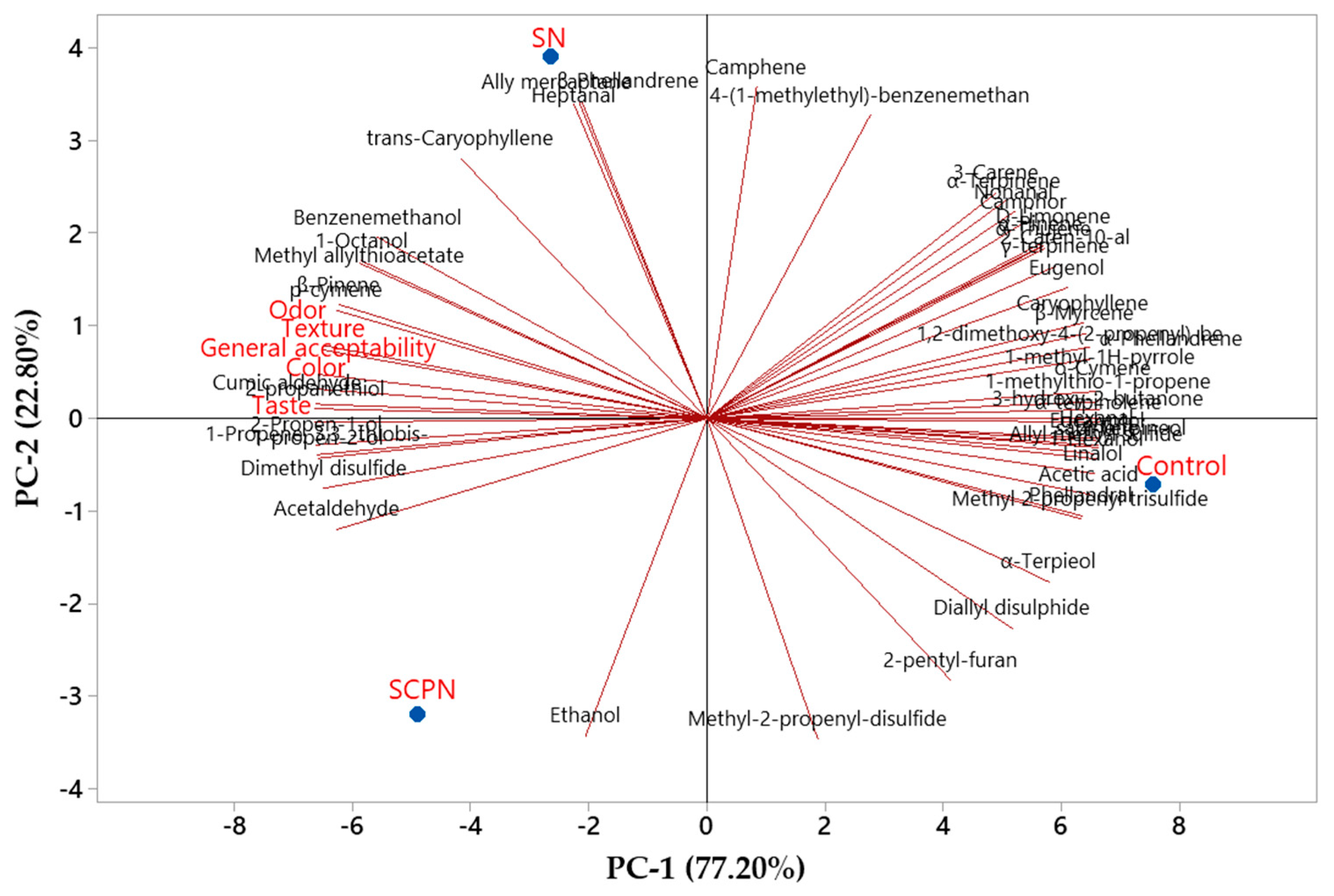
3.8. The Effect of Using SCP on Volatile Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armutçu, Ü.; Hazar, F.Y.; Yılmaz Oral, Z.F.; Kaban, G.; Kaya, M. Effects of different internal temperature applications on quality properties of heat-treated sucuk during production. J. Food Process. Preserv. 2020, 44, e14455. [Google Scholar] [CrossRef]
- Lücke, F.-K. Fermented sausages. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Springer Publishing: New York, NY, USA, 1998; pp. 441–483. ISBN 978-1-4613-0309-1. [Google Scholar]
- Jo, K.; Lee, S.; Yong, H.I.; Choi, Y.S.; Jung, S. Nitrite sources for cured meat products. LWT 2020, 129, 109583. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Bacus, J.N. Cured meat products without direct addition of nitrate or nitrite: What are the issues? Meat Sci. 2007, 77, 136–147. [Google Scholar] [CrossRef]
- Bertol, T.M.; Fiorentini, A.M.; Santos, M.J.H.D.; Sawitzki, M.C.; Kawski, V.L.; Agnes, I.B.L.; Costa, C.D.; Coldebella, A.; Lopes, L.D.S. Rosemary extract and celery-based products used as natural quality enhancers for colonial type salami with different ripening times. Food Sci. Technol. 2012, 32, 783–792. [Google Scholar] [CrossRef]
- Tsoukalas, D.S.; Katsanidis, E.; Marantidou, S.; Bloukas, J.G. Effect of freeze-dried leek powder (FDLP) and nitrite level on processing and quality characteristics of fermented sausages. Meat Sci. 2011, 87, 140–145. [Google Scholar] [CrossRef]
- Magrinya, N.; Bou, R.; Tres, A.; Rius, N.; Codony, R.; Guardiola, F. Effect of tocopherol extract, Staphylococcus carnosus culture, and celery concentrate addition on quality parameters of organic and conventional dry-cured sausages. J. Agricul. Food Chem. 2009, 57, 8963–8972. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green alternatives to synthetic antioxidants, antimicrobials, nitrates, and nitrites in clean label Spanish chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef]
- Eisinaite, V.; Vinauskiene, R.; Viskelis, P.; Leskauskaite, D. Effects of freeze-dried vegetable products on the technological process and the quality of dry fermented sausages. J. Food Sci. 2016, 81, C2175–C2182. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, M.M.; Munekata, P.E.; de Souza Lopes, A.; do Nascimento, M.D.S.; Pateiro, M.; Lorenzo, J.M.; Pollonio, M.A.R. Using chitosan and radish powder to improve stability of fermented cooked sausages. Meat Sci. 2020, 167, 108165. [Google Scholar] [CrossRef]
- Ozaki, M.M.; Dos Santos, M.; Ribeiro, W.O.; de Azambuja Ferreira, N.C.; Picone, C.S.F.; Domínguez, R.; Lorenzo, J.M.; Pollonio, M.A.R. Radish powder and oregano essential oil as nitrite substitutes in fermented cooked sausages. Food Res. Int. 2021, 140, 109855. [Google Scholar] [CrossRef]
- Ozaki, M.M.; Munekata, P.E.; Jacinto-Valderrama, R.A.; Efraim, P.; Pateiro, M.; Lorenzo, J.M.; Pollonio, M.A.R. Beetroot and radish powders as natural nitrite source for fermented dry sausages. Meat Sci. 2021, 171, 108275. [Google Scholar] [CrossRef]
- Pennisi, L.; Verrocchi, E.; Paludi, D.; Vergara, A. Effects of vegetable powders as nitrite alternative in Italian dry fermented sausage. Ital. J. Food Saf. 2020, 9, 8422. [Google Scholar] [CrossRef] [PubMed]
- Babaoğlu, A.S.; Karakaya, M. Investigation of the quality characteristics of naturally cured sucuks with dill, spinach and Swiss chard powders during refrigerated storage. Selçuk J. Agric. Food Sci. 2022, 36, 98–104. [Google Scholar] [CrossRef]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz Oral, Z.F. Effect of celery powder as an alternative nitrite source on some quality properties and nitrosamine formation in sucuk. Kafkas Univ. Vet. Fak. Derg. 2023, 29, 545–550. [Google Scholar] [CrossRef]
- Republic of Türkiye Ministry of Agriculture and Forestry. Turkish Food Codex Regulation on Food Additives (2013); Official Gazette; Republic of Türkiye Ministry of Agriculture and Forestry: Ankara, Turkey, 2013. [Google Scholar]
- Yılmaz Oral, Z.F.; Kaya, M.; Kaban, G. Using celery powder in a semi-dry fermented sausage ‘heat-treated sucuk’: Nitrosamine formation, lipid oxidation, and volatile compounds. Foods 2024, 13, 3306. [Google Scholar] [CrossRef]
- Öztürk Kerimoğlu, B.; Serdaroğlu, M. Residual nitrite content of heat-treated sucuk as affected by chard powder incorporation and processing. Gıda/J. Food 2020, 45, 825–835. [Google Scholar] [CrossRef]
- Yılmaz Oral, Z.F. Isıl işlem görmüş sucuk üretiminde kürleme ajanı olarak pazı tozu kullanımının nitrozamin oluşumuna ve kalite parametrelerine etkisi. Gıda/J. Food 2023, 48, 1036–1046. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Alizadeh Salmani, B.; Eskandari, S.; Arab, M. Effects of plant substitutes for nitrite on the technological characteristics of fermented sausages: A comprehensive review. Food Sci. Nutr. 2025, 13, e70186. [Google Scholar] [CrossRef]
- Kaban, G.; Kaya, M. Identification of lactic acid bacteria and Gram-positive catalase-positive cocci isolated from naturally fermented sausage (sucuk). J. Food Sci. 2008, 73, 385–388. [Google Scholar] [CrossRef]
- Kaya, M.; Sayın, B.; Çınar Topçu, K.; Karadayı, M.; Kamiloğlu, A.; Güllüce, M.; Kaban, G. Genotypic and technological characterization of lactic acid bacteria and coagulase-negative staphylococci isolated from Sucuk: A preliminary screening of potential starter cultures. Foods 2025, 14, 3495. [Google Scholar] [CrossRef]
- Baumgart, J.; Eigener, V.; Firnhaber, J.; Hildebrant, G.; Hoekstra, E.S.; Samson, R.A.; Spicher, G.; Timm, F.; Yarrow, D.; Zschaler, R. Mikrobiologische Untersuchung von Lebensmitteln, 3rd ed.; Behr’s Verlag: Hamburg, Germany, 1993. [Google Scholar]
- Kilic, B.; Richards, M.P. Lipid oxidation in poultry döner kebab: Pro-oxidative and anti-oxidative factors. J. Food Sci. 2003, 68, 686–689. [Google Scholar] [CrossRef]
- NMKL Standard 165; Nitrite and Nitrate in Foodstuffs by Ion Chromatography. Nordic-Baltic Committee on Food Analysis: Bergen, Norway, 2000.
- Kaban, G. Changes in the composition of volatile compounds and in microbiological and physicochemical parameters during pastırma processing. Meat Sci. 2009, 82, 17–23. [Google Scholar] [CrossRef]
- ChiPlot. Available online: https://www.chiplot.online/ (accessed on 16 September 2025).
- Ercoşkun, H.; Tağı, Ş.; Ertaş, A.H. The effect of different fermentation intervals on the quality characteristics of heat-treated and traditional sucuks. Meat Sci. 2010, 85, 174–181. [Google Scholar] [CrossRef]
- Medyński, A.; Pospiech, E.; Kniat, R. Effect of various concentrations of lactic acid and sodium chloride on selected physico-chemical meat traits. Meat Sci. 2000, 55, 285–290. [Google Scholar] [CrossRef]
- Wang, D.; Hu, G.; Wang, H.; Wang, L.; Zhang, Y.; Zou, Y.; Zhao, L.; Liu, F.; Jin, Y. Effect of mixed starters on proteolysis and formation of biogenic amines in dry fermented mutton sausages. Foods 2021, 10, 2939. [Google Scholar] [CrossRef] [PubMed]
- Kurćubić, V.S.; Mašković, P.Z.; Vujić, J.M.; Vranić, D.V.; Vesković-Moračanin, S.M.; Okanović, Đ.G.; Lilić, S.V. Antioxidant and antimicrobial activity of Kitaibelia vitifolia extract as alternative to the added nitrite in fermented dry sausage. Meat Sci. 2014, 97, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Canonico, I.; Allen, P. Effects of organic tomato pulp powder and nitrite level on the physicochemical, textural and sensory properties of pork luncheon roll. Meat Sci. 2013, 95, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef]
- Sallan, S.; Kaban, G.; Oğraş, Ş.Ş.; Çelik, M.; Kaya, M. Nitrosamine formation in a semi-dry fermented sausage: Effects of nitrite, ascorbate and starter culture and role of cooking. Meat Sci. 2020, 159, 107917. [Google Scholar] [CrossRef]
- Horsch, A.M.; Sebranek, J.G.; Dickson, J.S.; Niebuhr, S.E.; Larson, E.M.; Lavieri, N.A.; Wilson, L.A. The effect of pH and nitrite concentration on the antimicrobial impact of celery juice concentrate compared with conventional sodium nitrite on Listeria monocytogenes. Meat Sci. 2014, 96, 400–407. [Google Scholar] [CrossRef]
- Xi, Y.; Sullivan, G.A.; Jackson, A.L.; Zhou, G.H.; Sebranek, J.G. Use of natural antimicrobials to improve the control of Listeria monocytogenes in a cured cooked meat model system. Meat Sci. 2011, 88, 503–511. [Google Scholar] [CrossRef]
- Djeri, N.; Williams, S.K. Celery juice powder used as nitrite substitute in sliced vacuum-packaged turkey bologna stored at 4 °C for 10 weeks under retail display light. J. Food Qual. 2014, 37, 361–370. [Google Scholar] [CrossRef]
- Kayaardı, S.; Gök, V. Effect of replacing beef fat with olive oil on quality characteristics of Turkish soudjouk (sucuk). Meat Sci. 2004, 66, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, H.; Bayram, M. Colour and textural attributes of sucuk during ripening. Meat Sci. 2006, 73, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Ercoşkun, H.; Özkal, S.G. Kinetics of traditional Turkish sausage quality aspects during fermentation. Food Cont. 2011, 22, 165–172. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Pérez-Alvarez, J.A.; Sayas-Barberá, M.E.; Fernández-López, J.; Aranda-Catalá, V. Physicochemical characteristics of Spanish-type dry-cured sausage. Food Res. Int. 1999, 32, 599–607. [Google Scholar] [CrossRef]
- Riel, G.; Boulaaba, A.; Popp, J.; Klein, G. Effects of parsley extract powder as an alternative for the direct addition of sodium nitrite in the production of mortadella-type sausages—Impact on microbiological, physicochemical and sensory aspects. Meat Sci. 2017, 131, 166–175. [Google Scholar] [CrossRef]
- Shin, D.M.; Hwang, K.E.; Lee, C.W.; Kim, T.K.; Park, Y.S.; Han, S.G. Effect of Swiss chard (Beta vulgaris var. cicla) as nitrite replacement on color stability and shelf-life of cooked pork patties during refrigerated storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Gómez, M.; Purriños, L.; Fonseca, S. Effect of commercial starter cultures on volatile compound profile and sensory characteristics of dry-cured foal sausage. J. Sci. Food Agric. 2016, 96, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Eisinaitė, V.; Tamkutė, L.; Vinauskienė, R.; Leskauskaitė, D. Freeze-dried celery as an indirect source of nitrate in cold-smoked sausages: Effect on safety and color formation. LWT 2020, 129, 109586. [Google Scholar] [CrossRef]
- Yılmaz Oral, Z.F.; Kaban, G. Effects of autochthonous strains on volatile compounds and technological properties of heat-treated sucuk. Food Biosci. 2021, 43, 101140. [Google Scholar] [CrossRef]
- Öz, E.; Kabil, E.; Kaban, G.; Kaya, M. Effect of autochthonous Pediococcus acidilactici on volatile profile and some properties of heat-treated sucuk. J. Food Process. Preserv. 2018, 42, e13752. [Google Scholar] [CrossRef]
- Ekici, L.; Ozturk, I.; Karaman, S.; Caliskan, O.; Tornuk, F.; Sagdic, O.; Yetim, H. Effects of black carrot concentrate on some physicochemical, textural, bioactive, aroma and sensory properties of sucuk, a traditional Turkish dry-fermented sausage. LWT—Food Sci. Technol. 2015, 62, 718–726. [Google Scholar] [CrossRef]
- Kaban, G.; Bayrak, D. The effects of using turkey meat on qualitative properties of heat-treated sucuk. Czech J. Food Sci. 2015, 33, 367–372. [Google Scholar] [CrossRef]
- Jin, S.K.; Choi, J.S.; Yang, H.S.; Park, T.S.; Yim, D.G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Guo, X.; Lu, S.; Wang, Y.; Dong, J.; Ji, H.; Wang, Q. Correlations among flavor compounds, lipid oxidation indices, and endogenous enzyme activity during the processing of Xinjiang dry-cured mutton ham. J. Food Process. Preserv. 2019, 43, e14199. [Google Scholar] [CrossRef]
- Ivanović, L.; Milašević, I.; Topalović, A.; Ðurović, D.; Mugoša, B.; Knežević, M.; Vrvić, M. Nutritional and phytochemical content of Swiss chard from Montenegro, under different fertilization and irrigation treatments. Br. Food J. 2019, 121, 411–425. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Liu, Y.; Li, Y.; Gao, L.; Zhao, J.J. Alpha-lipoic acid attenuates insulin resistance and improves glucose metabolism in high fat diet-fed mice. Acta Pharmacol. Sin. 2014, 35, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Kołota, E.; Adamczewska-Sowińska, K.; Czerniak, K. Yield and nutritional value of Swiss chard grown for summer and autumn harvest. J. Agric. Sci. 2010, 2, 120–124. [Google Scholar] [CrossRef][Green Version]
- Sidira, M.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Effect of immobilized Lactobacillus casei on volatile compounds of heat treated probiotic dry-fermented sausages. Food Chem. 2015, 178, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, K.A.; Tran, T.B.T.; Vogel, R.F. Production of volatile compounds by Lactobacillus sakei from branched chain α-keto acids. Food Microbiol. 2012, 29, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Misharina, T.A.; Andreenkov, V.A.; Vashchuk, E.A. Changes in the composition of volatile compounds during aging of dry-cured sausages. Appl. Biochem. Microbiol. 2001, 37, 413–418. [Google Scholar] [CrossRef]
- Sha, K.; Lang, Y.M.; Sun, B.Z.; Su, H.W.; Li, H.P.; Zhang, L.; Zhang, Y. Changes in lipid oxidation, fatty acid profile and volatile compounds of traditional Kazakh dry-cured beef during processing and storage. J. Food Process. Preserv. 2017, 41, e13059. [Google Scholar] [CrossRef]
- Alarcón, M.; Pérez-Coello, M.S.; Díaz-Maroto, M.C.; Alañón, M.E.; García-Ruiz, A.; Soriano, A. Inactive dry yeast to improve the oxidative stability of Spanish dry-fermented sausage “salchichón”. LWT 2021, 146, 111385. [Google Scholar] [CrossRef]
- Yin, X.; Lv, Y.; Wen, R.; Wang, Y.; Chen, Q.; Kong, B. Characterization of selected Harbin red sausages on the basis of their flavour profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108345. [Google Scholar] [CrossRef]
- Yılmaz Oral, Z.; Kaban, G. The effect of the combination of rosemary extract and green tea extract on nitrosamine content, microbiological, physicochemical and sensorial properties of heat-treated sucuk. Kafkas Univ. Vet. Fak. Derg. 2023, 29, 705–715. [Google Scholar] [CrossRef]
- Sallan, S.; Kaban, G.; Kaya, M. The effects of nitrite, sodium ascorbate and starter culture on volatile compounds of a semi-dry fermented sausage. LWT 2022, 153, 112540. [Google Scholar] [CrossRef]
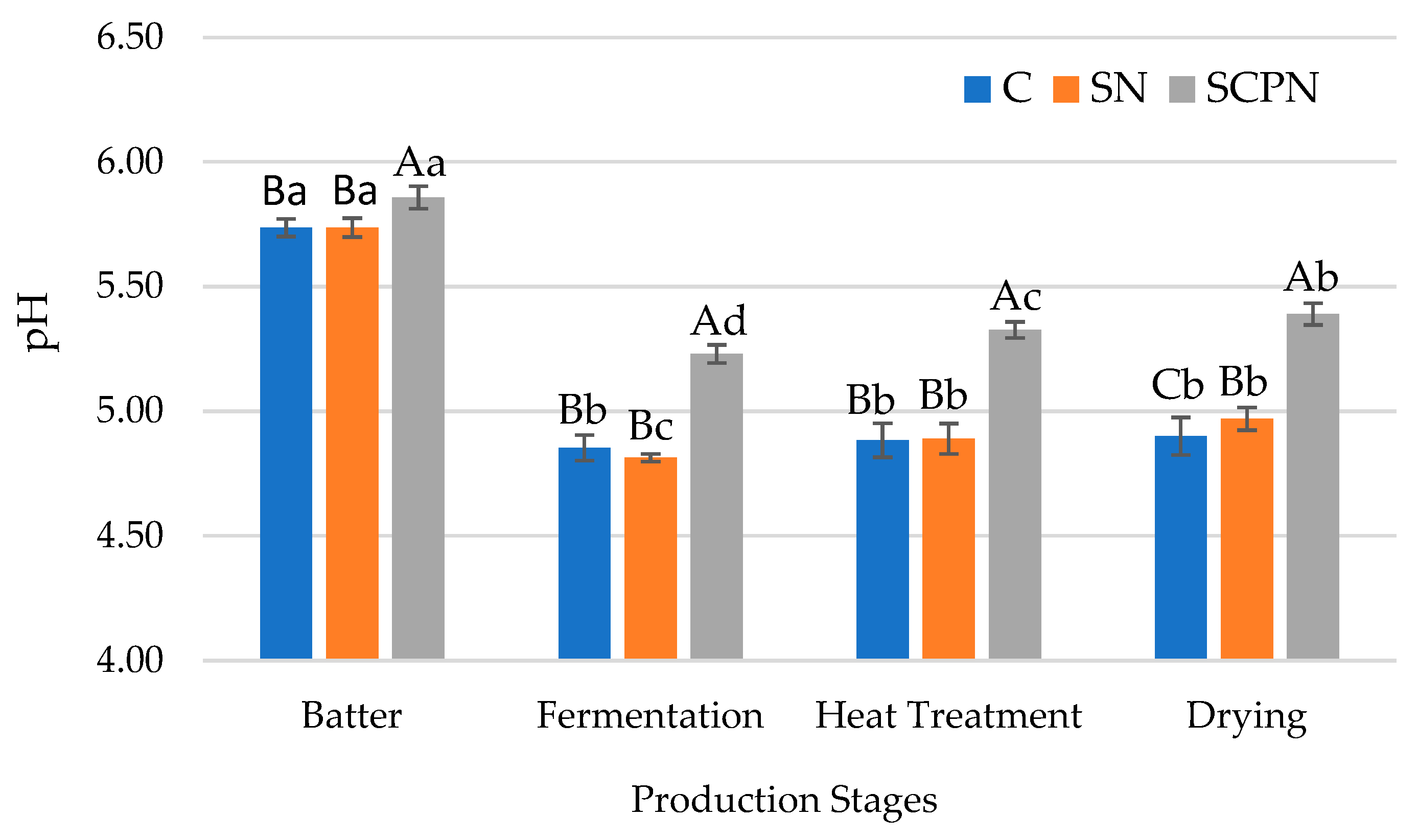

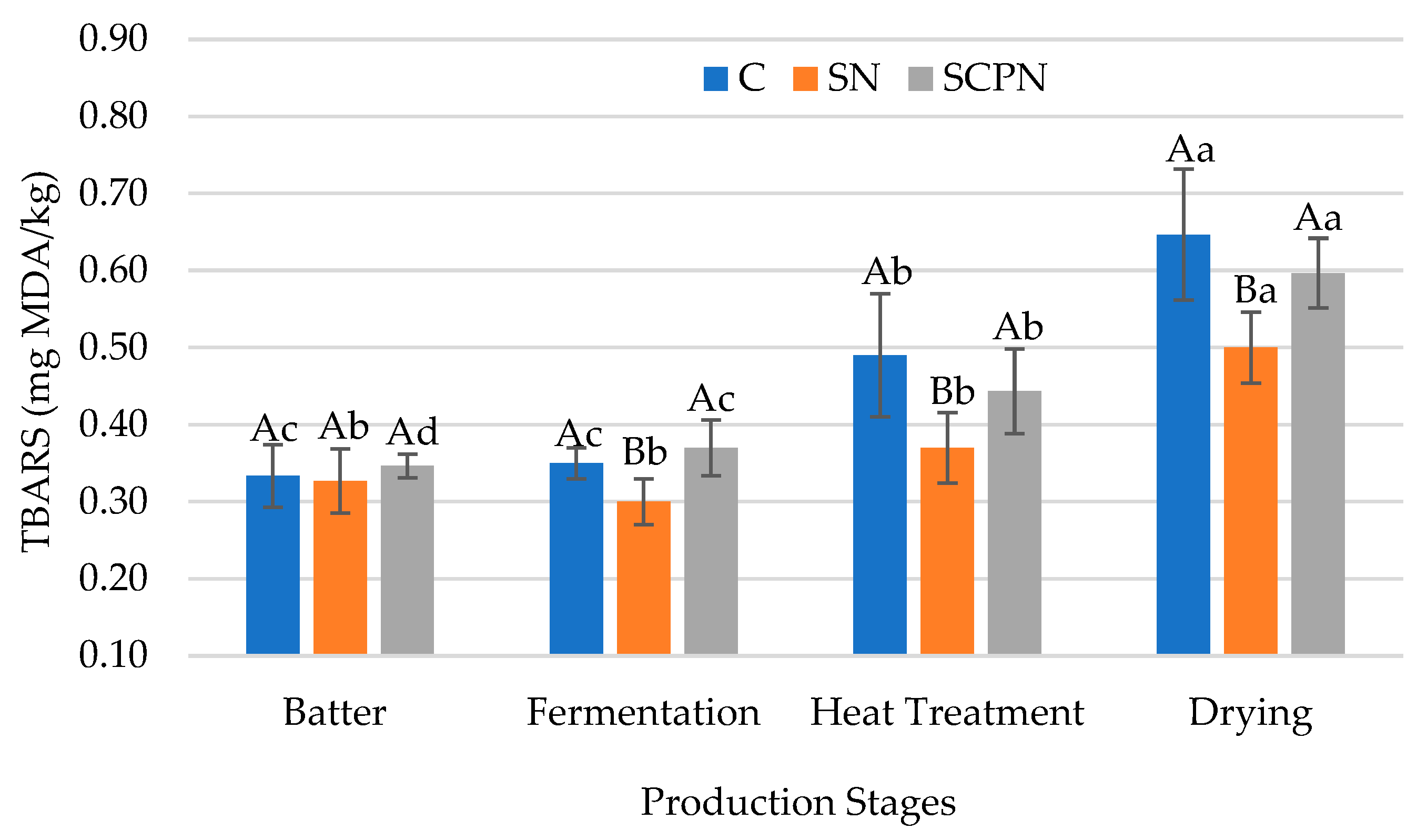
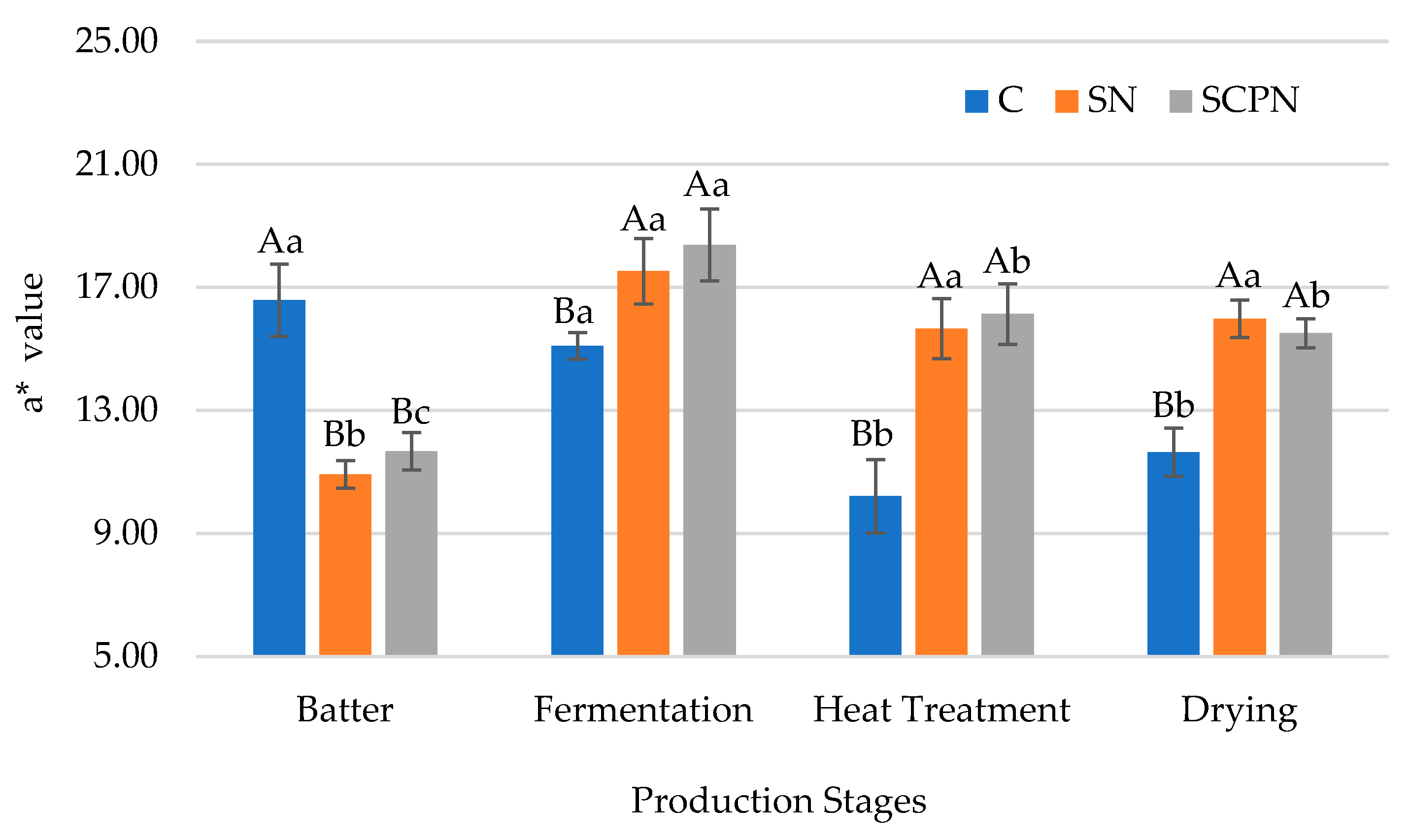


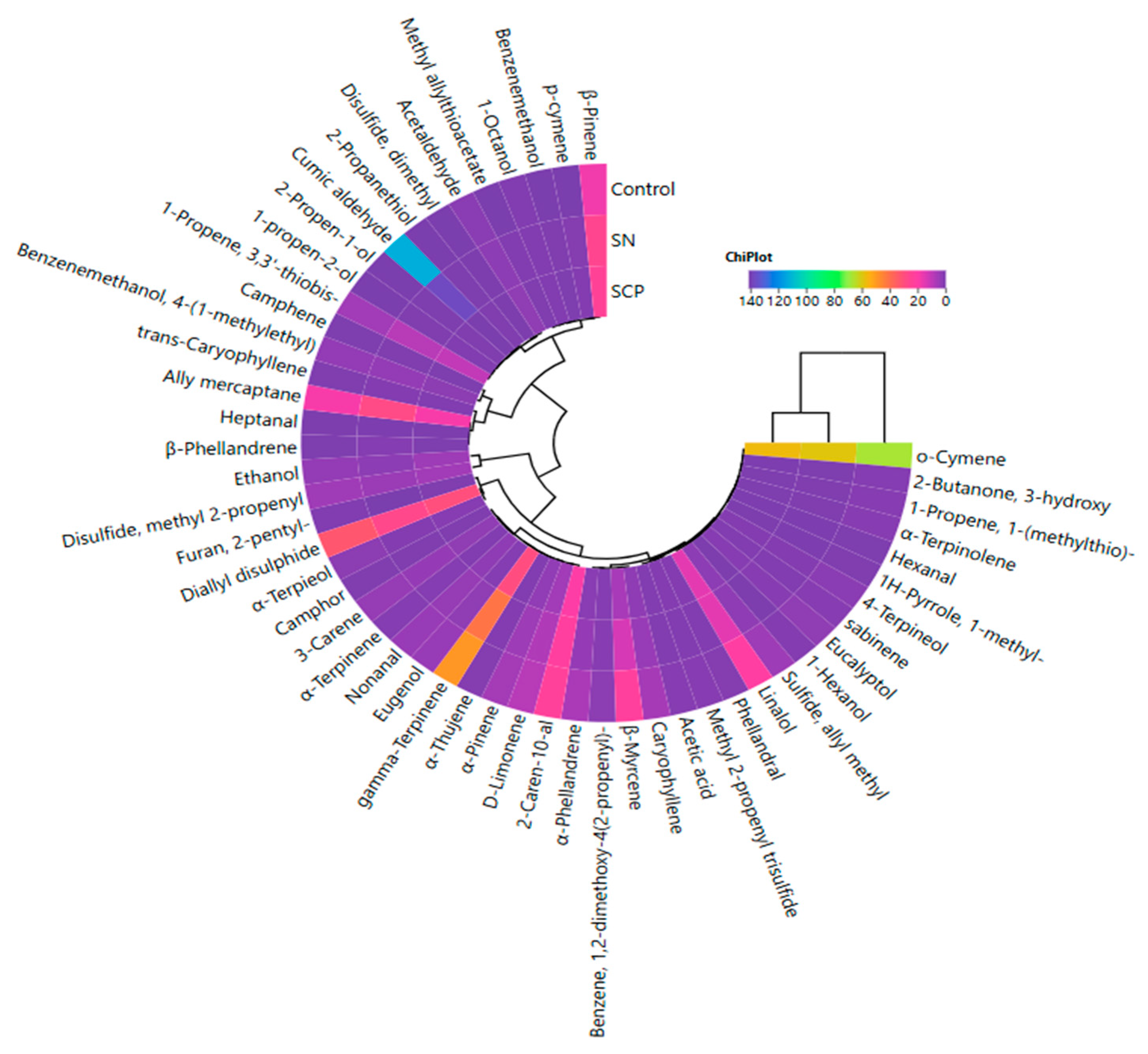
| Factors | pH | aw | TBARS (mg MDA/kg) | L* | a* | b* | Residual Nitrite (mg/kg) x |
|---|---|---|---|---|---|---|---|
| Treatment (T) | |||||||
| C | 5.09 ± 0.39 b | 0.956 ± 0.015 a | 0.46 ± 0.14 a | 44.05 ± 4.65 b | 13.38 ± 2.79 b | 18.10 ± 1.81 a | 4.58 ± 1.01 b |
| SN | 5.10 ± 0.45 b | 0.957 ± 0.016 a | 0.38 ± 0.09 b | 45.43 ± 4.29 a | 15.02 ± 2.67 a | 17.47 ± 3.13 a | 12.78 ± 1.82 a |
| SCPN | 5.45 ± 0.25 a | 0.948 ± 0.020 b | 0.43 ± 0.12 a | 43.48 ± 3.05 b | 15.42 ± 2.62 a | 18.66 ± 3.33 a | 13.14 ± 2.36 a |
| Significance | ** | ** | ** | * | ** | NS | ** |
| Production Stages (PS) | |||||||
| Batter | 5.78 ± 0.07 a | 0.967 ± 0.004 a | 0.33 ± 0.03 c | 41.93 ± 2.15 c | 13.06 ± 2.75 c | 21.71 ± 1.88 a | - |
| Fermentation | 4.96 ± 0.20 d | 0.964 ± 0.002 b | 0.34 ± 0.04 c | 40.06 ± 2.21 d | 17.00 ± 1.68 a | 18.56 ± 1.92 b | - |
| Heat Treatment | 5.03 ± 0.22 c | 0.957 ± 0.003 c | 0.43 ± 0.07 b | 48.61 ± 2.05 a | 14.00 ± 2.99 b | 16.72 ± 0.85 c | - |
| Drying | 5.09 ± 0.23 b | 0.927 ± 0.008 d | 0.58 ± 0.08 a | 46.67 ± 1.84 b | 14.37 ± 2.13 b | 15.33 ± 0.80 d | - |
| Significance | ** | ** | ** | ** | ** | ** | - |
| T × PS | ** | ** | * | NS | ** | * | - |
| Factors | Lactic Acid Bacteria | Micrococcus/ Staphylococcus | Enterobacteriaceae | Yeast-Mold |
|---|---|---|---|---|
| Treatment (T) | ||||
| C | 6.26 ± 2.01 a | 5.62 ± 1.47 a | <2 | <2 |
| SN | 6.12 ± 2.11 a | 5.78 ± 1.04 a | <2 | <2 |
| SCPN | 6.04 ± 2.11 a | 5.27 ± 1.51 b | <2 | <2 |
| Significance | NS | * | NS | NS |
| Production Stages (PS) | ||||
| Batter | 7.28 ± 0.15 b | 6.40 ± 0.14 b | <2 | <2 |
| Fermentation | 8.77 ± 0.07 a | 6.93 ± 0.12 a | <2 | <2 |
| Heat Treatment | 4.60 ± 0.34 c | 5.18 ± 0.27 c | <2 | <2 |
| Drying | 3.91 ± 0.46 d | 3.71 ± 0.88 d | <2 | <2 |
| Significance | ** | ** | NS | NS |
| T × PS | * | NS | NS | NS |
| Factors | Odor | Color | Taste | Texture | General Acceptability |
|---|---|---|---|---|---|
| Treatment (T) | |||||
| C | 5.61 ± 0.23 b | 4.59 ± 0.02 b | 5.44 ± 0.17 b | 5.59 ± 0.14 b | 5.48 ± 0.29 b |
| SN | 7.05 ± 0.17 a | 7.69 ± 0.24 a | 7.12 ± 0.12 a | 7.31 ± 0.36 a | 7.25 ± 0.30 a |
| SCPN | 6.97 ± 0.59 a | 8.00 ± 0.38 a | 7.52 ± 0.34 a | 7.24 ± 0.62 a | 7.33 ± 0.62 a |
| Significance | * | ** | ** | ** | * |
| Volatile Compounds | RT | KI | RI | Treatment | |||
|---|---|---|---|---|---|---|---|
| C | SN | SCPN | Sign. | ||||
| Alcohols | |||||||
| Ethanol | 8.680 | 539 | A | 3.44 ± 0.82 a | 2.59 ± 0.60 b | 4.86 ± 1.65 a | ** |
| 1-propen-2-ol | 9.927 | 543 | B | 0.00 ± 0.00 c | 0.59 ± 0.19 b | 0.83 ± 0.14 a | ** |
| 2-propen-1-ol | 10.401 | 581 | B | 0.00 ± 0.00 c | 0.46 ± 0.11 b | 0.62 ± 0.75 a | ** |
| 1-hexanol | 33.789 | 930 | B | 1.07 ± 0.21 a | 0.22 ± 0.05 b | 0.17 ± 0.03 b | ** |
| 1-octanol | 42.767 | 1127 | B | 0.80 ± 0.12 b | 1.04 ± 0.14 a | 0.96 ± 0.13 a | * |
| Aldehydes | |||||||
| Acetaldehyde | 5.212 | 523 | B | 2.13 ± 1.08 a | 2.57 ± 0.87 a | 2.99 ± 0.91 a | |
| Hexanal | 28.762 | 850 | A | 0.44 ± 0.10 a | 0.32 ± 0.09 b | 0.30 ± 0.06 b | * |
| Heptanal | 34.982 | 955 | A | 0.18 ± 0.05 a | 0.31 ± 0.15 a | 0.17 ± 0.04 a | NS |
| Nonanal | 43.714 | 1143 | A | 4.11 ± 0.68 a | 3.82 ± 0.88 a | 2.76 ± 0.68 b | ** |
| Ketones | |||||||
| 3-hydroxy-2-butanone | 24.728 | 779 | A | 1.31 ± 0.35 a | 0.48 ± 0.09 b | 0.30 ± 0.12 b | ** |
| Acids | |||||||
| Acetic acid | 19.032 | 710 | A | 1.51 ± 1.13 a | 1.00 ± 0.11 a | 1.03 ± 0.38 a | NS |
| Sulfur Compounds | |||||||
| 2-propanethiol | 10.401 | 570 | B | 0.00 ± 0.00 b | 0.47 ± 0.16 a | 0.56 ± 0.10 a | ** |
| Allyl mercaptan | 13.970 | 574 | B | 18.70 ± 6.79 b | 28.05 ± 7.22 a | 18.39 ± 2.98 b | * |
| Allyl methyl sulfide | 20.655 | 730 | B | 4.79 ± 1.49 a | 2.79 ± 0.63 b | 2.64 ± 0.81 b | ** |
| 1-methylthio-1-propene | 23.315 | 732 | C | 1.52 ± 0.97 a | 0.68 ± 0.16 b | 0.43 ± 0.12 b | ** |
| Dimethyl disulfide | 24.592 | 764 | C | 0.00 ± 0.00 b | 0.16 ± 0.06 a | 0.26 ± 0.12 a | ** |
| 3,3′-thiobis-1-propene | 31.789 | 888 | B | 5.35 ± 1.50 b | 8.66 ± 1.29 a | 9.98 ± 0.83 a | ** |
| Methyl-2-propenyl-disulfide | 35.790 | 958 | B | 5.28 ± 1.02 a | 4.30 ± 0.94 a | 5.36 ± 1.09 a | NS |
| Diallyl disulfide | 43.169 | 1038 | A | 33.20 ± 7.01 a | 27.02 ± 4.14 a | 30.03 ± 5.35 a | NS |
| Methyl allyl thioacetate | 43.477 | 1045 | C | 0.00 ± 0.00 c | 0.96 ± 0.17 a | 0.66 ± 0.18 b | ** |
| Methyl-2-propenyl-trisulfide | 45.164 | 1214 | C | 1.65 ± 0.83 a | 1.05 ± 0.17 a | 1.13 ± 0.23 a | NS |
| Terpenes | |||||||
| α-thujene | 34.800 | 944 | B | 1.29 ± 0.38 a | 1.17 ± 0.16 a | 0.95 ± 0.26 a | NS |
| α-pinene | 35.384 | 950 | B | 5.63 ± 1.79 a | 4.83 ± 1.27 a | 3.18 ± 0.73 b | * |
| Camphene | 36.521 | 970 | B | 0.43 ± 0.22 a | 0.54 ± 0.17 a | 0.34 ± 0.11 a | NS |
| Sabinene | 37.807 | 981 | B | 1.74 ± 0.74 a | 0.87 ± 0.08 b | 0.78 ± 0.18 b | ** |
| β-pinene | 38.094 | 996 | B | 17.70 ± 6.45 a | 25.80 ± 5.48 a | 24.28 ± 3.54 a | NS |
| β-myrcene | 38.170 | 998 | B | 22.65 ± 5.38 a | 13.14 ± 4.35 b | 6.33 ± 0.69 c | ** |
| 3-carene | 39.270 | 1026 | B | 3.85 ± 0.75 a | 3.71 ± 0.43 ab | 2.88 ± 0.59 b | * |
| α-phellandrene | 39.456 | 1035 | B | 6.80 ± 1.96 a | 3.67 ± 1.66 b | 2.00 ± 1.03 b | ** |
| α-terpinene | 39.845 | 1042 | B | 1.40 ± 0.43 a | 1.31 ± 0.49 ab | 0.91 ± 0.30 b | * |
| D-limonene | 40.310 | 1054 | B | 8.67 ± 2.83 a | 7.55 ± 1.86 ab | 5.31 ± 1.21 b | * |
| o-cymene | 40.492 | 1059 | B | 67.36 ± 9.92 a | 59.15 ± 20.82 a | 57.01 ± 10.05 a | NS |
| β-phellandrene | 40.614 | 1065 | B | 0.37 ± 0.19 b | 1.42 ± 1.42 a | 0.36 ± 0.08 b | ** |
| Eucalyptol | 40.872 | 1075 | B | 2.92 ± 0.33 a | 1.69 ± 0.46 b | 1.54 ± 0.28 b | ** |
| γ-terpinene | 41.405 | 1103 | B | 49.20 ± 11.27 a | 41.38 ± 4.00 a | 29.43 ± 6.26 b | ** |
| α-terpinolene | 42.492 | 1105 | B | 1.64 ± 0.67 a | 0.60 ± 0.12 b | 0.39 ± 0.08 b | ** |
| p-cymene | 43.038 | 1147 | B | 0.00 ± 0.00 c | 1.29 ± 0.19 a | 1.07 ± 0.23 b | ** |
| Linalool | 43.587 | 1161 | B | 21.01 ± 4.44 a | 15.24 ± 2.67 b | 15.21 ± 2.82 b | ** |
| Camphor | 45.985 | 1230 | B | 0.69 ± 0.17 a | 0.66 ± 0.17 a | 0.55 ± 0.09 a | NS |
| 4-terpineol | 46.171 | 1233 | B | 2.23 ± 0.67 a | 1.19 ± 0.28 b | 1.07 ± 0.26 b | ** |
| α-terpineol | 46.712 | 1256 | B | 2.36 ± 0.43 a | 1.14 ± 0.15 b | 1.56 ± 0.42 b | ** |
| Cumin aldehyde | 48.805 | 1334 | B | 112.98 ± 7.29 b | 137.72 ± 14.31 a | 141.67 ± 18.56 a | ** |
| Phellandral | 50.023 | 1354 | C | 1.27 ± 0.29 a | 1.04 ± 0.62 a | 1.07 ± 0.34 a | ** |
| 2-carene-10-al | 50.450 | 1390 | C | 23.14 ± 1.70 a | 21.99 ± 2.29 ab | 19.69 ± 2.59 b | NS |
| Eugenol | 52.590 | 1456 | B | 4.18 ± 0.69 a | 2.88 ± 0.46 b | 1.33 ± 0.25 c | ** |
| Trans-caryophyllene | 53.389 | 1473 | B | 0.68 ± 0.26 b | 1.14 ± 0.14 a | 0.83 ± 0.18 b | ** |
| Caryophyllene | 54.244 | 1490 | B | 6.03 ± 0.54 a | 4.19 ± 0.43 b | 2.72 ± 0.29 c | ** |
| Furans | |||||||
| 2-pentylfuran | 38.483 | 1021 | B | 0.84 ± 0.23 a | 0.00 ± 0.00 b | 0.57 ± 0.28 a | ** |
| Aromatic Hydrocarbons | |||||||
| 1-methyl-1H-pyrrole | 25.023 | 786 | B | 0.66 ± 0.41 a | 0.29 ± 0.08 b | 0.16 ± 0.03 b | ** |
| Benzenemethanol | 42.915 | 1132 | B | 0.00 ± 0.00 c | 0.64 ± 0.18 a | 0.39 ± 0.10 b | ** |
| 4-(1-methylethyl)-benzenemethanol | 50.230 | 1380 | B | 3.21 ± 1.32 a | 3.49 ± 0.76 a | 2.45 ± 0.26 a | NS |
| 1,2-dimethoxy-4-(2-propenyl)-benzene | 53.182 | 1482 | B | 2.34 ± 0.72 a | 1.77 ± 0.59 ab | 1.42 ± 0.50 b | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katmer, B.; Kaya, M. The Effect of Swiss Chard Powder as a Curing Agent on Volatile Compound Profile and Other Qualitative Properties of Heat-Treated Sucuk. Foods 2025, 14, 3785. https://doi.org/10.3390/foods14213785
Katmer B, Kaya M. The Effect of Swiss Chard Powder as a Curing Agent on Volatile Compound Profile and Other Qualitative Properties of Heat-Treated Sucuk. Foods. 2025; 14(21):3785. https://doi.org/10.3390/foods14213785
Chicago/Turabian StyleKatmer, Betül, and Mükerrem Kaya. 2025. "The Effect of Swiss Chard Powder as a Curing Agent on Volatile Compound Profile and Other Qualitative Properties of Heat-Treated Sucuk" Foods 14, no. 21: 3785. https://doi.org/10.3390/foods14213785
APA StyleKatmer, B., & Kaya, M. (2025). The Effect of Swiss Chard Powder as a Curing Agent on Volatile Compound Profile and Other Qualitative Properties of Heat-Treated Sucuk. Foods, 14(21), 3785. https://doi.org/10.3390/foods14213785






