Listeria monocytogenes: A Continuous Global Threat in Ready-to-Eat (RTE) Foods
Abstract
1. Introduction
2. Listeria monocytogenes
2.1. Importance of L. monocytogenes Biofilms
2.2. Food Safety Risk of L. monocytogenes
2.3. Global Trends and Impact of Listeriosis
3. Ready-to-Eat (RTE) Foods
4. Significance of L. monocytogenes in RTE Supply Chain
4.1. National and International Food Recall Data
4.2. National Compliance and Prevalence of L. monocytogenes in RTE Foods
5. Current Intervention Strategies
5.1. Risk-Based Regulatory Framework
5.2. Antimicrobial Intervention Strategies to Meet Requirements
| Types of Intervention Strategies | Description | Product Type | Example of Efficacy | Reference | Limitation 1 |
|---|---|---|---|---|---|
| Physical intervention strategies: Thermal | |||||
| Pasteurization using conventional heating | Use of thermal heat above 72 °C to destroy L. monocytogenes. | RTE deli turkey | 1.95–3 log10 reduction at 93.3 °C for 2–5 min | [141] | High temperatures might affect the texture, flavor or nutritional quality. Possible issue with the packaging, as it will need to withstand high temperatures. |
| RTE deli meats | 3 log10 reduction within 266 min at 55 °C or 44 min at 60 °C or 23 min at 62.5 °C or 5 min at 65 °C | [142] | |||
| RTE chicken drumette | 7 log10 reduction at 54 min and 28 min at 60 °C and 70 °C, respectively, or at 18 min and 19 min at 80 °C and 90 °C, respectively | [143] | |||
| Lean sausage | 7 log10 reduction within 8.1 min at 68.9 °C | [130] | |||
| Fat sausage | 7 log10 reduction within 8.4 min at 68.9 °C | [130] | |||
| Lean ham | 7 log10 reduction within 6.3 min at 67.9 °C | [130] | |||
| Fat ham | 7 log10 reduction within 5.8 min at 68.9 °C | [130] | |||
| Pasteurization via infrared heating | Use of infrared radiation to kill L. monocytogenes. | Deli ham | 0.75–1.85 log10 reductions when processed for 45–75 s | [144] | Limited penetration capability for complete destruction of the bacteria [145]. |
| Roast beef | 3.8 log10 reductions when processed for 60–90 s | [144] | |||
| Turkey frankfurters | 3.5, 4.3, and 4.5 log10 reductions at temperatures of 70 °C, 75 °C, and 80 °C for 82 s, 92 s, and 103 s, respectively | [146] | |||
| Sliced ham | 4.16 log10 reduction after 50 s | [147] | |||
| Cooked chicken | 3.7 log10 reduction 62 °C within 9.3 min; 4.9 log10 reduction at 68 °C within 5.2 min; 6.0 log10 reduction at 75 °C within 3.6 min | [145] | |||
| Physical intervention strategies: Non-thermal | |||||
| High-pressure processing (HPP) | Use of high pressure (400–600 MPa) for microbial inactivation [148,149]. | Fresh Hispánico-type cheese | 3.8 log10 reduction at high pressure of 400 MPa for 3 min | [150] | High equipment cost and possible issue with the packaging, as it will need to withstand high pressure [151]. |
| Ripe Mahón cheese | 1 log10 reduction at 400 MPa for 18 min | [150] | |||
| RTE cooked chicken | 3.3 log10 reduction at pressure level of 600 MPa for 2 min | [152] | |||
| RTE sliced cooked ham | 4 log10 reduction at 504 MPa for 3 min | [153] | |||
| Sliced mortadella | 4 log10 reduction at 526 MPa for 3 min | [153] | |||
| Sliced dry cured ham (white pig) | 3.9 log10 reduction at 600 MPa for 5 min | [154] | |||
| Sliced dry cured ham (Iberian pig) | 1.9 log10 reduction at 600 MPa for 5 min | [154] | |||
| RTE cooked chicken | 1.4 log10 reduction at 600 MPa for 2 min | [155] | |||
| Dry cured ham | 0.92–3.92 log10 reduction at 450 MPa for 5 min | [156] | |||
| Dry cured ham | 6.28–6.82 log10 reduction at 600 MPa for 5 min | [156] | |||
| Dry cured ham | 5.26–7.96 log10 reduction at 750 MPa for 5 min | [156] | |||
| Mozzarella | 3–4 log10 reduction at 400 MPa for 10 min | [157] | |||
| Mozzarella | Not detected at 500 MPa for 10 min | [157] | |||
| Smoked salmon | 1.5 log10 reduction at 500 MPa for 10 min | [157] | |||
| Salchichon (dry cured sausage) | 3.42 log10 reduction at 600 MPa for 8 min | [158] | |||
| Dry cured loin | 3.08 log10 reduction at 600 MPa for 8 min | [158] | |||
| Bacon | 1.68 log10 reduction at 593 MPa for 5 min at 4.0 °C | [159] | |||
| Smoked rainbow trout | 1.0–1.82 log10 reduction at 500 MPa for 5 min at 4.4 °C | [160] | |||
| Smoked rainbow trout | 1.2–2.73 log10 reduction at 500 MPa for 5 min at 4.4 °C | [160] | |||
| Irradiation | Use of ionizing radiation to eliminate L. monocytogenes by destroying the DNA of L. monocytogenes, thereby leading to its inactivation. | Vacuum packed cooked ham | Achievement of FSC (102 CFU) and zero tolerance at doses of 1 kGy and 2.5 kGy of e-beam radiation | [161] | Possible issue with consumer acceptance, and expensive [162]. |
| Chicken breast Adobo | Complete elimination at a minimum dose of 25 kGy of gamma irradiation with total irradiation time of 4.6 h | [163] | |||
| Pulse light (PL) | Use of short flashes with wavelengths of 200–1100 nm, including UV light known as pulse light, which disrupts DNA transcription and replication in L. monocytogenes [164]. | Cooked ham | 2 log10 CFU/cm2 reduction at pulse light of 8.4 J/cm2 | [101] | Limited penetration capability [101]. Higher fluences may lead to change in sensory quality [101]. |
| Cooked bologna | 1 log10 CFU/cm2 reduction at pulse light of 8.4 J/cm2 | [101] | |||
| RTE cured meat products | 1.5–1.8 log10 CFU/cm2 reduction at pulse light of 11.9 J/cm2 | [165] | |||
| Ultrasound | Washing technique applied by using bath-type ultrasound between frequencies of 20–100 kHz to destroy and detach the microorganisms from the food surface [166,167,168,169]. | Lettuce | 0.42 log10 CFU/cm2 at 10 min treatment time | [170] | May increase lipid oxidation. Ultrasound parameters should be optimized for each product type [168]. |
| Lettuce | 0.39 log10 CFU/cm2 at 30 min treatment time | [170] | |||
| Lettuce | 0.40 log10 CFU/cm2 at 60 min treatment time | [170] | |||
| Chemical intervention strategies | |||||
| Chemical additives | The application of chemical substances, referred to as preservatives (e.g., sodium diacetate, sodium lactate) to reduce pH, therefore establishing an environment unfavorable to pathogens. | Vacuum-packaged frankfurters | Inhibited the growth and increased the storage time to 120 days when sodium lactate and sodium acetate used together (all at 0.25%) | [109] | Affects the sensory properties of the product [171,172]. May lead to heat resistance of L. monocytogenes when used in combination with salt [173]. |
| Weiners | Prevented the growth during storage at 4.5 °C for 60 days when ≥3% sodium acetate and combination of ≥1% lactate plus ≥ 0.1% diacetate was used | [137]. | |||
| Use of organic acids | Use of GRAS (generally recognized as safe) organic acid, such as such as acetic acid, lactic acid, citric acid, malic acid, and peracetic acid, to disrupt cell function. | Lettuce | 1.18 log10 CFU/cm2 when 2% acetic acid used for 10 min | [170] | May affect the organoleptic quality of the product due to residue organic acid left on the product and also due to organic acid-specific odor and taste |
| Lettuce | 1.87 log10 CFU/cm2 when 2% acetic acid used for 30 min | [170] | |||
| Lettuce | 2.68 log10 CFU/cm2 when 2% acetic acid used for 60 min | [170] | |||
| Lettuce | 1.21 log10 CFU/cm2 when 2% lactic acid used for 10 min | [170] | |||
| Lettuce | 2.12 log10 CFU/cm2 when 2% lactic acid used for 10 min | [170] | |||
| Lettuce | 2.99 log10 CFU/cm2 when 2% lactic acid used for 10 min | [170] | |||
| Lettuce | 6.01 log10 CFU/g when 1% acetic used for 0.5 min | [174] | |||
| Lettuce | 5.33 log10 CFU/g when 1% acetic used for 0.5 min | [174] | |||
| Lettuce | 5.04 log10 CFU/g when 1% acetic used for 1 min | [174] | |||
| Lettuce | 4.34 log10 CFU/g when 1% acetic used for 1 min | [174] | |||
| Biological intervention strategies | |||||
| Use of lactic acid bacteria (LAB) starter cultures | LAB inhibit L. monocytogenes by (i) acidification (pH reduction), (ii) competitive exclusion for nutrients and niches, and (iii) producing antimicrobials (organic acids, hydrogen peroxide, bacteriocins, such as nisin) [175,176]. | Cheese | 1.48–4.16 log10 for lactococci 1.96–4.21 for lactobacilli | [177] | Should be used as a part of hurdle technology, as this singly will not ensure complete safety [178]. Strains used should be GRAS-approved or assigned Qualified Presumption of Safety (QPS) [178,179]. Food matrix might influence the survival or replication of L. monocytogenes and LAB [179]. |
| Gorgonzola cheese | Not detected in 25 g after addition of two strains of lactic acid bacteria, Lactococcus lactis subsp. Cremoris FT27. | [180] | |||
| Soft cheese | 0.5–1 log10 CFU/g reduction when a mixture comprising three lactic acid bacteria was added | [181] | |||
| Dry-cured fermented sausage, “salchichon” | 1.6–2.2 log10 CFU/g reduction after addition of Lactobacillus sakei 205 | [182] | |||
| Bacteriophages | Use of bacteriophages, which are viruses that infect and replicate within bacteria, thus killing the host [183]. E.g., phages such as A511 and P100 were effective in controlling L. monocytogenes in RTE foods [184]. | Fresh-cut red delicious apples | 0.4 log10 reduction after application of nisin and phage mixture | [185] | Efficiency is dependent on intrinsic and extrinsic parameters, such as phage concentration, food matrix, and storage conditions. More efficient in liquid products. |
| Honeydew melons stored at 10 °C | 2.0–4.6 log10 reduction after application of nisin and phage mixture | [185] | |||
| Cooked turkey | 2.1 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 4 °C | [186] | |||
| Cooked turkey | 1.5 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 10 °C | [186] | |||
| Roast beef | 1.7 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 4 °C | [186] | |||
| Roast beef | 1.7 log10 CFU/cm2 reduction when PhageGuard ListexTM P100 was used at 10 °C | [186] | |||
| Cheese | 0.7 log10 reduction by using the commercially available phage, ListShield | [187] | |||
| Smoked salmon | 1 log10 reduction by using the commercially available phage, ListShield | [187] | |||
| Smoked salmon | 0.85, 2.4, 2.75, 2.34, and 1.58 log10 reduction at 1, 5, 10, 15, and 30 days after application of bacteriophage P100 | [188] | |||
| Hot dog | >2 log10 concentration reduction after addition of lactic acid bacteria | [189]. | |||
| Bacteriocins | Use of antimicrobial peptides (e.g., nisin) produced by certain bacteria, such as lactic acid bacteria, which attacks the cell membrane of the pathogen, resulting in damage to the membrane structure [190,191,192]. | RTE sliced pork ham | 2.83 log10 reduction by bacteriophage P100 (LISTEXTM P100) | [193] | Higher concentrations of bacteriocin are required to inhibit growth [139]. Intrinsic properties of food will affect the stability of bacteriocin, which is unstable to pH changes [139]. Country-specific restriction in the use of nisin in food [100,104,105,106,107]. Should be used as a part of hurdle technology. |
| Cooked ham | 1.85 log10 reduction by 200 AU/cm2 nisin A (N200) | [194] | |||
| Cooked ham | 1.80 log10 reduction by 200 AU/g nisin plus 1.8% potassium lactate | [194] | |||
| Cooked ham | 4 log10 reduction by interleavers (containing mixture of enterocins, sakacin, nisin, potassium lactate) | [194] | |||
| Cooked ham | 4 log10 reduction by interleavers (containing mixture of enterocins, sakacin, nisin, potassium lactate) | [194] | |||
| RTE Russian type salad | Not detected in 25 g until day 5 when 40 µg/g of enterocin AS-48 was used at 10 °C | [195] | |||
| RTE turkey ham | 4 log10 CFU/g reduction at 0.4% and 0.5% nisin treatment after 7 and 14 days of storage, respectively. | [196] | |||
| Sliced dry cured ham (white pig) | 0.80 log10 reduction when nisin was applied directly (200 AU/mL), and 3 log10 reduction after 60 days of storage | [154] | |||
| Sliced dry cured ham (Iberian pig) | 1.24 log10 reduction when nisin was applied directly (200 AU/mL), and 3 log10 reduction after 60 days of storage | [154] | |||
| Smoked salmon | 2, 3.4, 4.5, 4.25, and 4.25 log10 reductions at 1, 5, 10, 15, and 30 days after spraying with enterocin AS-48 | [188] | |||
| Gorgonzola cheese | Not detected after addition of two strains of lactic acid bacteria, Lactococcus lactis subsp. Cremoris FT27 | [180] | |||
| Soft cheese | 0.5–1 log10 CFU/g reduction when a mixture comprising three lactic acid bacteria was added. | [181] | |||
| Dry-cured fermented sausage, “salchichon” | 1.6–2.2 log10 CFU/g reductions after addition of Lactobacillus sakei 205 | [182] | |||
| Sardinian dairy products | 3–4 log10 CFU/g reductions when a mixture of lactic acid bacteria was added | [177] | |||
| Jben (a Moroccan fresh cheese) | 1.46 log10 CFU/g reduction) in comparison to control after addition of enterocin OS1 (200 AU/g) after 15 days of storage at 8 °C | [197] | |||
| Spices, herbs, and essential oils | Use of spices, herbs, and essential oil derived from plants (e.g., thyme, rosemary), to provide bioactive components, such as phenolic acids, terpenes, aldehydes, and flavonoids, which have antimicrobial properties that help damage the cellular structure of the pathogen [198]. | Ham slices | 2.5, 2.6, and 3.0 log10 reductions with addition of clove, rosemary, cassia bark, and licorice, and 2.9, 3.0, and 3.2 log10 reductions in MAP | [199] | May affect taste and aroma of products. |
| Cheese (curd) | No growth observed at concentration of 2000 ppm (day 1–5) | [200] | |||
| Smoked Salmon | No growth observed at concentration of 4000 ppm (day 1–3) | [200] | |||
| Italian mortadella | Growth was 2.29–2.79 log10 CFU/g less in samples treated with thyme and rosemary compared to the control samples after 30 days of storage | [201] | |||
5.2.1. Challenges and Considerations in Implementing Intervention Strategies
- (a)
- Influence of different types of RTE foods: Intervention strategies must be adapted depending on the characteristics of RTE foods, such as deli meats, cheeses, and salads. For example, thermal treatments above 72 °C are effective in destroying L. monocytogenes in deli meats without significantly affecting sensory quality [142]. However, for heat-sensitive products like RTE salads, such treatments can negatively impact texture and flavor. In these cases, non-thermal methods like high-pressure processing (HPP), which keeps the product temperatures below 45 °C, are more suitable [149,150]. Koutsoumanis et al. [127] reported that RTE salad can be processed at 400–600 MPa for 1.5–3.0 min at 4–8 °C to meet the microbiological criteria. Similarly, irradiation at 2.5 kGy may eliminate L. monocytogenes in RTE cooked meats but can cause undesirable odors, leading to consumer rejection [161]. This illustrates the importance of balancing microbial safety with sensory qualities, such as taste, texture, and appearance, which are critical for consumer acceptance [202].
- (b)
- Influence of food matrix properties: The intrinsic properties of RTE food, such as pH, aw, fat content, and nutrient composition play a crucial role in determining the effectiveness of intervention strategies. For example, Verheyen et al. [203] showed that fat enhanced L. monocytogenes inactivation in emulsions but offered protection in gelled emulsions (mimicking processed fish products), likely due to differences in heat transfer and shielding effects. Similarly, studies using various meat products show that fat content and aw can modulate microbial lethality, sometimes enhancing or limiting inactivation depending on the processing method [153,156,204]. In ground beef, fat reduced heat resistance at lower temperature (51.7 °C) but increased it when subjected at higher temperatures (57.2 °C and 62.8 °C) [204]. Furthermore, Bover-Cid et al. [156] compared two types of dry-cured ham during HPP treatment: one with a higher aw (0.92) and lower fat content (14.25%), and the other one with a lower aw (0.88) and higher fat content (33.26%). The former showed greater inactivation (5.26 log10 reduction at 750 MPa), while the latter was more resistant (0.92 log10 reduction at 450 MPa). Conversely, in cooked ham and mortadella, higher fat content was linked to lower HPP effectiveness [153]. Salt concentration, in combination with temperature, can also significantly influence L. monocytogenes survival and growth dynamics. In meat emulsions with 20% fat, different NaCl concentrations (2.5%, 5.0%, and 7.5%) produced markedly different growth outcomes depending on storage temperature (10, 20, and 30 °C) [205]. At higher temperatures (20–30 °C), lower salt levels (2.5% and 5.0%) allowed for faster growth, whereas 7.5% NaCl had an inhibitory effect [205]. However, at 10 °C, L. monocytogenes was able to grow even at 7.5% NaCl, reaching 6.8 log10 CFU/g after 12 days, indicating its ability to survive under cold, high-salt conditions [205]. Similarly, in a challenge test involving twelve RTE salad products, L. monocytogenes was able to grow in two types: tomato–cucumber without salt and lemon juice, and tahini salad at 4, 10, and 24 °C [206]. This was attributed to higher pH (>4.6) and release of nutrients from the tomato and cucumber [206]. In another study about Pinata (RTE Italian sausage), it has also been reported that the concentration of LAB and low aw (0.91) and pH (5.8–5.9) acted as the key hurdles to the growth of L. monocytogenes [207]. These examples illustrate how fat content interacts with other matrix factors, such as aw, pH, and nutrient composition, to influence microbial survival, emphasizing the need for tailored interventions that consider the specific properties of each RTE product.
- (c)
- Strain variability and stress resistance: Different L. monocytogenes strains exhibit varying degrees of tolerance to common stresses encountered in food processing and within the host, such as acid, osmotic, and thermal stress, as well as biofilm production [131,132,133,208,209]. The glutamate decarboxylase (GAD) system, a key acid-resistance mechanism, also varies between strains, with higher GAD activity correlating with improved survival in gastric juice [208,209]. Mechanistically, the L. monocytogenes GAD system comprises intracellular GAD (GADi) and an antiport arm (GADe), which are activated at different pH levels and differ by strain and medium, helping explain between-strain differences in acid tolerance. For example, when strains 10403S and LO28 were both exposed to pH 2.7, 10403S survived, whereas LO28 lost viability [210]. Notably, preliminary studies suggest that clinical L. monocytogenes isolates often possess significantly higher GAD activities compared to food isolates, indicating that strains more capable of causing human infection may be inherently more resistant to acidic conditions [208,211]. Additionally, clinical strains produced biofilm at higher incubation temperatures compared to strains isolated from food factories [209]. Consistent with this variability, Aryani et al. [212] reported D-values (time required at a specific temperature and condition to reduce the microbial population by one decimal) of twenty L. monocytogenes strains ranging from 9 to 30 min at 55 °C, 0.6 to 4.0 min at 60 °C, and 0.1 to 0.60 min at 65 °C, highlighting broad differences in thermal resistance. Similarly, Wang et al. [213] reported wide kinetic ranges across 33 L. monocytogenes strains, with maximum growth rates (µmax), lag times (λ), and (D60) time required for 1 log10 reduction at 60 °C ranging between 0.20 and 0.45 h−1, 0.24 and 3.36 h, and 0.52 and 3.93 min, respectively. They also showed that the mild acid adaptation (pH 5.5) increased heat resistance for most strains and produced survival curves with a shoulder during 60 °C inactivation, while leaving growth kinetics largely unchanged [213]. Heredia et al. [214] studied the inter-strain variability of twenty-six clinical and food L. monocytogenes isolates and concluded that strains from the meat category exhibited the lowest average pHmin (4.57), indicating potential acid adaptation. This inherent variability means that a universal approach to L. monocytogenes control may be inadequate, as highly resistant strains could persist despite interventions employed. Beyond strain-level variation, there are different serotypes of L. monocytogenes, out of which serotype 4b is associated with 50% of human outbreaks and serotypes 1/2a, 1/2b, and 1/2c are mostly isolated from food [68,71]. These differences in serotype distribution and virulence further underline that a universal approach to L. monocytogenes control may be inadequate, as certain serotypes and strains may persist or remain infectious despite interventions. A more nuanced understanding of both strain- and serotype-specific resistance is therefore critical to developing targeted and robust intervention strategies. A risk-based, virulence, and pathogenicity classification developed by the Joint FAO/WHO for L. monocytogenes should be considered similar to the approach used for Shiga toxin-producing E. coli (STEC) in food [215,216].
- (d)
- Other considerations: Beyond technical efficacy, factors such as consumer perception and cost can significantly influence the adoption of intervention strategies. For example, consumer concerns about irradiation and chemical preservatives have driven demand for clean-label, minimally processed products [217,218,219,220]. This shift has encouraged the food industry to explore novel biocontrol approaches for managing L. monocytogenes in RTE foods, such as bacteriophages, bacteriocins, spices, herbs, and essential oils [177,181,186,200,202]. Likewise, advanced technologies like HPP and pulsed electric fields (PEFs), while effective, may be prohibitively expensive for small and medium-sized producers [221]. Although these factors are outside the main scope of this section, they remain important in determining the real-world feasibility of intervention implementation.
5.2.2. Multi-Hurdle Approaches for Controlling L. monocytogenes
5.3. Future Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- New South Wales Government. Listeriosis. Available online: https://www.health.nsw.gov.au/Infectious/factsheets/Pages/listeriosis.aspx (accessed on 14 May 2025).
- World Health Organization. Listeriosis. Available online: http://who.int/news-room/fact-sheets/detail/listeriosis (accessed on 1 February 2025).
- US Centers for Disease Control and Prevention. People at Increased Risk for Listeria Infection. Available online: https://www.cdc.gov/listeria/risk-factors/index.html#cdc_risk_factors_who-pregnant-people-and-newborns (accessed on 8 June 2025).
- World Health Organization. Foodborne Illnesses Per 100,000 (Median, 95% Uncertainty Interval). Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/foodborne-illnesses-per-100-000--2010-(median--95--uncertainty-interval) (accessed on 12 May 2025).
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; De Silva, N.R.; Gargouri, N. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. About Listeria Infection. Available online: https://www.cdc.gov/listeria/about/ (accessed on 10 August 2025).
- US Centers for Disease Control and Prevention. Clinical Overview of Listeriosis. Available online: https://www.cdc.gov/listeria/hcp/clinical-overview/index.html (accessed on 12 August 2025).
- Department of Health Disability and Ageing. National Notifiable Diseases Surveillance System (NNDSS) Fortnightly Reports. Available online: https://www.health.gov.au/resources/collections/nndss-fortnightly-reports#2025 (accessed on 12 August 2025).
- Australian Bureau of Statistics. National, State and Territory Population. 2023. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/dec-2023#data-downloads (accessed on 31 August 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2023; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2025; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2023 (accessed on 15 September 2025).
- Possas, A.; Hernández, M.; Esteban-Carbonero, Ó.; Valero, A.; Rodríguez-Lázaro, D. Listeria monocytogenes survives better at lower storage temperatures in regular and low-salt soft and cured cheeses. Food Microbiol. 2022, 104, 103979. [Google Scholar] [CrossRef]
- US Department of Agriculture. Listeria monocytogenes, Listeriosis and You. Available online: https://www.usda.gov/about-usda/news/blog/listeria-monocytogenes-listeriosis-and-you (accessed on 14 April 2025).
- Ferreira, V.; Wiedmann, M.; Teixeira, P.; Stasiewicz, M.J. Listeria monocytogenes persistence in food-associated environments: Epidemiology, strain characteristics, and implications for public health. J. Food Prot. 2014, 77, 150–170. [Google Scholar]
- Di Ciccio, P.; Conter, M.; Zanardi, E.; Ghidini, S.; Vergara, A.; Paludi, D.; Festino, A.; Ianieri, A. Listeria monocytogenes: Biofilms in food processing. Ital. J. Food Sci. 2012, 24, 203–213. Available online: https://scholar.google.com/scholar_lookup?title=Listeria+monocytogenes:+Biofilms+in+food+processing&author=Di+Ciccio,+P.&author=Conter,+M.&author=Zanardi,+E.&author=Ghidini,+S.&author=Vergara,+A.&author=Paludi,+D.&author=Festino,+A.R.&author=Ianieri,+A.&publication_year=2012&journal=Ital.+J.+Food+Sci.&volume=24&pages=203%E2%80%93213 (accessed on 1 August 2025).
- Food Standards Australia New Zealand. Australia New Zealand Food Standards Code—Standard 3.2.2—Food Safety Practices and General Requirements (Australia Only); Food Standards Australia New Zealand: Canberra, Australia, 2000. [Google Scholar]
- Center for Food Safety and Applied Nutrition. Control of Listeria Monocytogenes in Ready-to-Eat Foods: Guidance for Industry (Draft Guidance); US Food and Drug Authority (FDA): Rockville, MD, USA, 2017. [Google Scholar]
- Buchanan, R.L.; Gorris, L.G.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- USDA Food Safety and Inspection Service. Annual Recall Summaries. Available online: https://www.fsis.usda.gov/food-safety/recalls-public-health-alerts/annual-recall-summaries (accessed on 8 March 2025).
- Food Standards Australia New Zealand. Australian Food Recall Statistics. Available online: https://www.foodstandards.gov.au/food-recalls/recallstats (accessed on 21 January 2025).
- European Commission. RASFF Window_ Notification 2024.1499 (Listeria monocytogenes in Cooked Ham). Available online: https://webgate.ec.europa.eu/rasff-window/screen/notification/667121 (accessed on 8 March 2025).
- Government of Canada. Statistics: Food Recall Incidents and Food Recalls. Available online: https://inspection.canada.ca/food-safety-for-consumers/canada-s-food-safety-system/food-recall-incidents-and-food-recalls/eng/1348756225655/1348756345745 (accessed on 8 March 2025).
- Department of Agriculture, Fisheries and Forestry. Imported Food Data Reports and Surveys from 2017–2023. Available online: https://www.agriculture.gov.au/biosecurity-trade/import/goods/food/inspection-testing/surveys-data (accessed on 1 June 2025).
- Food Standards Australia New Zealand. Listeria Monocytogenes in Cooked Prawns; Food Standards Australia New Zealand: Canberra, Australia, 2004. [Google Scholar]
- New South Wales Food Authority. Microbiological Quality of Packaged Sliced Ready-to-Eat Meat Products; New South Wales (NSW) Food Authority: Silverwater, Australia, 2009. [Google Scholar]
- Krsteski, R.; Rockliff, S. Microbiological Quality of Ready-to-Eat Foods: July 2014–June 2015; ACT Health Protection Service: Canberra, Australia, 2015. [Google Scholar]
- New South Wales Food Authority. Survey of Listeria monocytogenes in Sliced Pre-Packaged RTE Meats; NSW Food Authority: Silverwater, Australia, 2013. [Google Scholar]
- Codex Alimentarius Commission. Guidelines on the Application of General Principles of Food Hygiene to the Control of Listeria monocytogenes in Foods—CAC/GL 61—2007; Codex Alimentarius Commission: Rome, Italy, 2007. [Google Scholar]
- Aalto-Araneda, M.; Lunden, J.; Markkula, A.; Hakola, S.; Korkeala, H. Processing plant and machinery sanitation and hygiene practices associate with listeria monocytogenes occurrence in ready-to-eat fish products. Food Microbiol. 2019, 82, 455–464. [Google Scholar] [CrossRef]
- Gupta, P.; Adhikari, A. Novel Approaches to Environmental Monitoring and Control of Listeria monocytogenes in Food Production Facilities. Foods 2022, 11, 1760. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Tsaloumi, S.; Aspridou, Z.; Tassou, C.; Gougouli, M. Application of quantitative microbiological risk assessment (QMRA) to food spoilage: Principles and methodology. Trends Food Sci. Technol. 2021, 114, 189–197. [Google Scholar] [CrossRef]
- Victoria State Government. Listeriosis. Available online: https://www.health.vic.gov.au/infectious-diseases/listeriosis (accessed on 10 August 2025).
- FAO/WHO. Risk Assessment of Listeria monocytogenes in Ready-to-Eat Foods; Annali Della Facoltà di Medicina Veterinaria di: Pisa, Italy, 2004. [Google Scholar]
- Farber, J.M.; Peterkin, P. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991, 55, 476–511. [Google Scholar] [CrossRef] [PubMed]
- Petran, R.; Zottola, E. A study of factors affecting growth and recovery of Listeria monocytogenes Scott A. J. Food Sci. 1989, 54, 458–460. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Holch, A.; Webb, K.; Lukjancenko, O.; Ussery, D.; Rosenthal, B.M.; Gram, L. Genome sequencing identifies two nearly unchanged strains of persistent Listeria monocytogenes isolated at two different fish processing plants sampled 6 years apart. Appl. Environ. Microbiol. 2013, 79, 2944–2951. [Google Scholar] [CrossRef]
- Bonsaglia, E.; Silva, N.; Júnior, A.F.; Júnior, J.A.; Tsunemi, M.; Rall, V. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control 2014, 35, 386–391. [Google Scholar] [CrossRef]
- Blaschek, H.P.; Wang, H.H.; Agle, M.E. Biofilms in the Food Environment; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes–how this pathogen survives in food-production environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, X.; Wang, Q.; Yang, J.; Zhong, Q. The mixed biofilm formed by Listeria monocytogenes and other bacteria: Formation, interaction and control strategies. Crit. Rev. Food Sci. Nutr. 2024, 64, 8570–8586. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Breidt, F., Jr.; Kathariou, S. Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Appl. Environ. Microbiol. 2006, 72, 7711–7717. [Google Scholar] [CrossRef]
- Zhang, H.; Que, F.; Xu, B.; Sun, L.; Zhu, Y.; Chen, W.; Ye, Y.; Dong, Q.; Liu, H.; Zhang, X. Identification of Listeria monocytogenes contamination in a ready-to-eat meat processing plant in China. Front. Microbiol. 2021, 12, 628204. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, B.; Cerf, O. Persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 2011, 145, 1–8. [Google Scholar] [CrossRef]
- Leong, D.; Alvarez-Ordóñez, A.; Jordan, K. Monitoring occurrence and persistence of Listeria monocytogenes in foods and food processing environments in the Republic of Ireland. Front. Microbiol. 2014, 5, 436. [Google Scholar] [CrossRef]
- Tompkin, R.B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef]
- da Silva, D.A.L.; de Melo Tavares, R.; Camargo, A.C.; Yamatogi, R.S.; De Martinis, E.C.P.; Nero, L.A. Biofilm growth by Listeria monocytogenes on stainless steel and expression of biofilm-related genes under stressing conditions. World J. Microbiol. Biotechnol. 2021, 37, 119. [Google Scholar] [CrossRef]
- Samelis, J.; Metaxopoulos, J. Incidence and principal sources of Listeria Spp. And Listeria monocytogenes contamination in processed meats and a meat processing plant. Food Microbiol. 1999, 16, 465–477. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Chang, Z.; Liu, X.; Chen, W.; Yu, Y.; Wang, X.; Dong, Q.; Ye, Y.; Zhang, X. Listeria monocytogenes contamination characteristics in two ready-to-eat meat plants from 2019 to 2020 in Shanghai. Front. Microbiol. 2021, 12, 729114. [Google Scholar] [CrossRef]
- Muhterem-Uyar, M.; Dalmasso, M.; Bolocan, A.S.; Hernandez, M.; Kapetanakou, A.E.; Kuchta, T.; Manios, S.G.; Melero, B.; Minarovičová, J.; Nicolau, A.I. Environmental sampling for Listeria monocytogenes control in food processing facilities reveals three contamination scenarios. Food Control 2015, 51, 94–107. [Google Scholar] [CrossRef]
- Ho, A.; Lappi, V.; Wiedmann, M. Longitudinal monitoring of Listeria monocytogenes contamination patterns in a farmstead dairy processing facility. J. Dairy Sci. 2007, 90, 2517–2524. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.P.; De Martinis, E.C.P. Current knowledge and perspectives on biofilm formation: The case of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2013, 97, 957–968. [Google Scholar] [CrossRef]
- Lasa, I. Towards the identification of the common features of bacterial biofilm development. Int. Microbiol. 2006, 9, 21–28. [Google Scholar] [PubMed]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. The One Health approach in food safety: Challenges and opportunities. Food Front. 2024, 5, 1837–1865. [Google Scholar] [CrossRef]
- Lotoux, A.; Milohanic, E.; Bierne, H. The viable but non-culturable state of Listeria monocytogenes in the one-health continuum. Front. Cell. Infect. Microbiol. 2022, 12, 849915. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, A.R. Assessment of bacterial viability: A comprehensive review on recent advances and challenges. Microbiology 2019, 165, 593–610. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.-W.; Ding, T. Current perspectives on viable but non-culturable state in foodborne pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Panera-Martínez, S.; Capita, R.; García-Fernández, C.; Alonso-Calleja, C. Viability and Virulence of Listeria monocytogenes in Poultry. Microorganisms 2023, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Panera-Martinez, S.; Rodriguez-Melcon, C.; Del Campo, C.; Alonso-Calleja, C.; Capita, R. Prevalence and levels of cells of Salmonella spp. and Listeria monocytogenes in various physiological states naturally present in chicken meat. Food Control 2025, 167, 110770. [Google Scholar] [CrossRef]
- European Food Safety Authority. Request for updating the former SCVPH opinion on Listeria monocytogenes risk related to ready-to-eat foods and scientific advice on different levels of Listeria monocytogenes in ready-to-eat foods and the related risk for human illness-scientific opinion of the panel on biological hazards. EFSA J. 2008, 6, 599. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Expert Consultation on Risk Assessment of Microbiological Hazards in Foods; World Health Organization and Food and Agriculture Organization of the United Nations: Rome, Italy, 2000. [Google Scholar]
- Swaminathan, B.; Gerner-Smidt, P. The Epidemiology of human listeriosis. Microbes Infect. 2007, 9, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.L.; Killinger, A. Listeria monocytogenes and listeric infections. Bacteriol. Rev. 1966, 30, 309–382. [Google Scholar] [CrossRef] [PubMed]
- Lyytikäinen, O.; Autio, T.; Maijala, R.; Ruutu, P.; Honkanen-Buzalski, T.; Miettinen, M.; Hatakka, M.; Mikkola, J.; Anttila, V.-J.; Johansson, T. An outbreak of Listeria monocytogenes serotype 3a infections from butter in Finland. J. Infect. Dis. 2000, 181, 1838–1841. [Google Scholar] [CrossRef]
- Yezli, S.; Otter, J.A. Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food Environ. Virol. 2011, 3, 1–30. [Google Scholar] [CrossRef]
- Notermans, S.; Dufrenne, J.; Teunis, P.; Chackraborty, T. Studies on the risk assessment of Listeria monocytogenes. J. Food Prot. 1998, 61, 244–248. [Google Scholar] [CrossRef]
- Sprong, R.C.; Hulstein, M.F.; Van der Meer, R. High intake of milk fat inhibits intestinal colonization of Listeria but not of Salmonella in rats. J. Nutr. 1999, 129, 1382–1389. [Google Scholar] [CrossRef]
- Heras, V.; Clooney, A.G.; Ryan, F.J.; Cabrera-Rubio, R.; Casey, P.G.; Hueston, C.M.; Pinheiro, J.; Rudkin, J.K.; Melgar, S.; Cotter, P. Short-term consumption of a high-fat diet increases host susceptibility to Listeria monocytogenes infection. Microbiol. Soc. 2020, 7, 7. [Google Scholar] [CrossRef]
- Burall, L.S.; Grim, C.J.; Datta, A.R. A clade of Listeria monocytogenes serotype 4b variant strains linked to recent listeriosis outbreaks associated with produce from a defined geographic region in the US. PLoS ONE 2017, 12, e0176912. [Google Scholar] [CrossRef] [PubMed]
- Kramarenko, T.; Roasto, M.; Meremäe, K.; Kuningas, M.; Põltsama, P.; Elias, T. Listeria monocytogenes prevalence and serotype diversity in various foods. Food Control 2013, 30, 24–29. [Google Scholar] [CrossRef]
- Pontello, M.; Guaita, A.; Sala, G.; Cipolla, M.; Gattuso, A.; Sonnessa, M.; Gianfranceschi, M.V. Listeria monocytogenes serotypes in human infections (Italy, 2000–2010). Ann. Dell’ist. Super. Sanità 2012, 48, 146–150. [Google Scholar] [CrossRef]
- Maury, M.M.; Tsai, Y.-H.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A. uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef]
- Orsi, R.H.; den Bakker, H.C.; Wiedmann, M. Listeria monocytogenes lineages: Genomics, evolution, ecology, and phenotypic characteristics. Int. J. Med. Microbiol. 2011, 301, 79–96. [Google Scholar] [CrossRef]
- Roberts, A.; Nightingale, K.; Jeffers, G.; Fortes, E.; Kongo, J.M.; Wiedmann, M. Genetic and phenotypic characterization of listeria monocytogenes lineage III. Microbiology 2006, 152, 685–693. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Listeria Monocytogenes in Ready-to-Eat (RTE) Food: Attribution, Characterization and Monitoring: Meeting Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- European Food Safety Authority. The European Union One Health 2021 zoonoses report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- Nepomuceno, F.V.; Akutsu, R.d.C.C.d.A.; Draeger, C.L.; da Silva, I.C.R. Foodborne diseases: A study before and during the covid-19 pandemic in Brazil. Nutrients 2023, 16, 60. [Google Scholar] [CrossRef]
- Department of Health Disability and Ageing. Australia’s Notifiable Disease Status, 2015—Annual Report of the National Notifiable Diseases Surveillance System; Department of Health Disability and Ageing: Canberra, ACT, Australia, 2019. [Google Scholar]
- Amy, B.; NNDSS Annual Report Working Group; O’Dwyer, M.R.; Trungove, M. Australia’s Notifiable Disease Status, 2016: Annual Report of the National Notifiable Diseases Surveillance System. Commun. Dis. Intell. 2021, 45. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. National, State and Territory Population. Available online: https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population (accessed on 8 October 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2009 [2007 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2009; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2009-2007-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2010 [2008 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2010; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2010-2008-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2011 [2009 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2011; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2011-2009-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2012 [2010 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2013; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2012-2010-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Annual Epidemiological Report 2013 [2011 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2013; Available online: https://www.ecdc.europa.eu/en/publications-data/annual-epidemiological-report-2013-2011-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report 2016 [2014 Data]; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2017; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2016-2014-data (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2015; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2015 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2016; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2016 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2017; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2017 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2018; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2018 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2019; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2019 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2020; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2023; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2020 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2021; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2021 (accessed on 15 September 2025).
- European Centre for Disease Prevention and Control. Listeriosis. In Annual Epidemiological Report for 2022; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/listeriosis-annual-epidemiological-report-2022 (accessed on 15 September 2025).
- Australian National University; Food Standards Australia New Zealand. The Annual Cost of Foodborne Illness in Australia; Food Standards Australia New Zealand: Canberra, Australia, 2022. [Google Scholar]
- Hoffman, S.; Maculloch, B.; Batz, M. Economic Burden of Major Foodborne Illnesses Acquired in the United States; U.S. Department of Agriculture, Economic Research Service: Washingtn, DC, USA, 2015. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- Health Canada. Policy on Listeria Monocytogenes in Ready-to-Eat Foods; Health Canada: Ottawa, ON, Canada, 2023. [Google Scholar]
- Piet, J.; Kieran, J.; Dara, L. Listeria monocytogenes in food: Control by monitoring the food processing environment. Afr. J. Microbiol. Res. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Australia New Zealand Food Standards Code—Standard 1.6.1—Microbiological Limits in Food; Food Standards Australia New Zealand: Canberra, ACT, Australia, 2015. [Google Scholar]
- Health Canada. Health Canada’s Proposal to Enable the Use of a New Food Additive, Nisin, as an Antimicrobial Preservative in or on Various Foods; Health Canada: Ottawa, ON, Canada, 2017. [Google Scholar]
- Hierro, E.; Barroso, E.; De la Hoz, L.; Ordóñez, J.A.; Manzano, S.; Fernández, M. Efficacy of pulsed light for shelf-life extension and inactivation of Listeria monocytogenes on ready-to-eat cooked meat products. Innov. Food Sci. Emerg. Technol. 2011, 12, 275–281. [Google Scholar] [CrossRef]
- Watson. E325 Sodium Lactate. Available online: https://food-detektiv.de/en/additives/?enummer=Sodium%20lactate (accessed on 12 August 2025).
- Food-Info. E325: Sodium Lactate. Available online: https://www.food-info.net/uk/e/e325.htm (accessed on 12 August 2015).
- Hudson, J.A.; McIntyre, L.; Billington, C. Application of bacteriophages to control pathogenic and spoilage bacteria in food processing and distribution. In Bacteriophages in the Control of Food and Waterborne Pathogens; Wiley: Hoboken, NJ, USA, 2010; pp. 119–135. [Google Scholar]
- Food Standards Australia New Zealand. Final Assessment Report- Use of Nisin in Processed Meat Products; Food Standards Australia New Zealand: Canberra, Australia, 2007. [Google Scholar]
- Rhodia Inc. Gras Notice No. Grn 000065: Nisin—Use in Frankfurter Casings and Cooked RTE Meat and Poultry Products; Rhodia Inc.: La Défense, France, 2001. [Google Scholar]
- European Food Safety Authority. Safety of Nisin (E 234) as a Food Additive in the Light of New Toxicological Data and the Proposed Extension of Use; European Food Safety Authority: Parma, Italy, 2017. [Google Scholar]
- Samelis, J.; Bedie, G.K.; Sofos, J.N.; Belk, K.E.; Scanga, J.A.; Smith, G.C. Control of Listeria monocytogenes with combined antimicrobials after postprocess contamination and extended storage of frankfurters at 4 ℃ in vacuum packages. J. Food Prot. 2002, 65, 299–307. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- South Australia Health. Guideline for the Control of Listeria in Food Service to Vulnerable Persons; South Australia Health: Adelaide, Australia, 2019. [Google Scholar]
- USDA Food Safety and Inspection Service. FSIS Compliance Guideline: Controlling Listeria monocytogenes in Post-Lethality Exposed Ready-to-Eat Meat and Poultry Products; USDA Food Safety and Inspection Service: Washington, DC, USA, 2014. [Google Scholar]
- Uyttendaele, M.; Busschaert, P.; Valero, A.; Geeraerd, A.; Vermeulen, A.; Jacxsens, L.; Goh, K.; De Loy, A.; Van Impe, J.; Devlieghere, F. Prevalence and challenge tests of Listeria monocytogenes in Belgian Produced and retailed mayonnaise-based deli-salads, cooked meat products and smoked fish between 2005 and 2007. Int. J. Food Microbiol. 2009, 133, 94–104. [Google Scholar] [CrossRef]
- Stessl, B.; Ruppitsch, W.; Wagner, M. Listeria monocytogenes post-outbreak management-when could a food production be considered under control again? Int. J. Food Microbiol. 2022, 379, 109844. [Google Scholar] [CrossRef]
- Chilled Food Association Ltd. Shelf Life of Ready to Eat Food in Relation to L. monocytogenes—Guidance for Food Business Operators; Chilled Food Association Ltd.: Kettering, UK, 2010. [Google Scholar]
- Victoria State Government. Notification Procedures for Infectious Diseases. Available online: https://www.health.vic.gov.au/infectious-diseases/notification-procedures-for-infectious-diseases (accessed on 14 May 2025).
- Food Standards Australia New Zealand. Food Recalls- What Is Food Recalls? Available online: https://www.foodstandards.gov.au/industry/foodrecalls/recalls/pages/whatisafoodrecall.aspx (accessed on 21 August 2025).
- Government of Canada. Toolkit for Food Businesses New to the Safe Food for Canadians Regulations. Available online: https://inspection.canada.ca/en/food-safety-industry/toolkit-food-businesses (accessed on 7 July 2025).
- Food Standards Australia New Zealand. How to Recall Food. Available online: https://www.foodstandards.gov.au/food-recalls/how-to-recall-food (accessed on 7 July 2025).
- US Food & Drug Administration. Post-Outbreak Response and Prevention Strategies to Enhance Food Safety (Updated 17 January 2025). Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/post-outbreak-response-and-prevention-strategies-enhance-food-safety-updated-january-17-2025 (accessed on 7 July 2025).
- European Commission. Regulation (EC) No 178/2002 General food law regulation. In Article 50 (Rapid Alert System); European Commission: Brussels, Belgium, 2002. [Google Scholar]
- European Commission, and Directorate-General SANTE. Alert and Cooperation Network (ACN) Annual Reports and Publications. Available online: https://food.ec.europa.eu/safety/acn/reports-and-publications_en (accessed on 20 September 2025).
- USDA Food Safety and Inspection Service. Frequently Requested Records; US Department of Agriculture (USDA): Annapolis, MD, USA, 2025. Available online: https://www.fsis.usda.gov/about-fsis/freedom-information-act-foia/frequently-requested-records (accessed on 15 June 2025).
- USDA Food Safety and Inspection Service. Understanding FSIS Food Recalls. Available online: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/food-safety-basics/understanding-fsis-food-recalls (accessed on 8 March 2025).
- Koutsoumanis, K.P.; Aspridou, Z. Moving towards a risk-based food safety management. Curr. Opin. Food Sci. 2016, 12, 36–41. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Principles and Guidelines for the Conduct of Microbiological Risk Assessment CAC/GL 30-1999; Codex Alimentarius Commission: Rome, Italy, 1999. [Google Scholar]
- Teunis, P.; Schijven, J.F. Generic Guidance to Quantitative Microbial Risk Assessment for Food and Water; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2019. [Google Scholar]
- Koutsoumanis, K.; Pavlis, A.; Nychas, G.-J.E.; Xanthiakos, K. Probabilistic model for Listeria monocytogenes growth during distribution, retail storage, and domestic storage of pasteurized milk. Appl. Environ. Microbiol. 2010, 76, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Risk assessment of Listeria monocytogenes in Ready-to-Eat Foods: Technical Report: Food & Agriculture Org; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- US Food & Drug Administration. CPG Sec 555.320 Listeria monocytogenes; US Food & Drug Administration: Silver Spring, MD, USA, 2008. [Google Scholar]
- Ahn, J.; Lee, H.-Y.; Knipe, L.; Balasubramaniam, V. Effect of a post-packaging pasteurization process on inactivation of a Listeria innocua surrogate in meat products. Food Sci. Biotechnol. 2014, 23, 1477–1481. [Google Scholar] [CrossRef]
- Sibanda, T.; Buys, E.M. Listeria monocytogenes pathogenesis: The role of stress adaptation. Microorganisms 2022, 10, 1522. [Google Scholar] [CrossRef]
- Lakicevic, B.Z.; Den Besten, H.M.; De Biase, D. Landscape of stress response and virulence genes among Listeria monocytogenes strains. Front. Microbiol. 2022, 12, 738470. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Pan, Y.; Zhao, Y.; Liu, H. Phenotypic heterogeneity and pathogenicity of Listeria monocytogenes under complex salinities of bile salts and sodium salts stress. Arch. Microbiol. 2025, 207, 101. [Google Scholar] [CrossRef]
- Aalto-Araneda, M.; Pöntinen, A.; Pesonen, M.; Corander, J.; Markkula, A.; Tasara, T.; Stephan, R.; Korkeala, H. Strain variability of Listeria monocytogenes under NaCl stress elucidated by a high-throughput microbial growth data assembly and analysis protocol. Appl. Environ. Microbiol. 2020, 86, e02378-19. [Google Scholar] [CrossRef]
- Myintzaw, P.; Pennone, V.; McAuliffe, O.; Begley, M.; Callanan, M. Variability in cold tolerance of food and clinical Listeria monocytogenes isolates. Microorganisms 2022, 11, 65. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to stress conditions encountered in food and food processing environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef]
- Glass, K.A.; Granberg, D.A.; Smith, A.L.; Mcnamara, A.M.; Hardin, M.; Mattias, J.; Ladwig, K.; Johnson, E.A. Inhibition of Listeria monocytogenes by sodium diacetate and sodium lactate on wieners and cooked bratwurst. J. Food Prot. 2002, 65, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.C.; Silva, S.P.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Muriana, P.; Gande, N.; Robertson, W.; Jordan, B.; Mitra, S. Effect of prepackage and postpackage pasteurization on postprocess elimination of Listeria monocytogenes on deli turkey products. J. Food Prot. 2004, 67, 2472–2479. [Google Scholar] [CrossRef]
- Selby, T.; Berzins, A.; Gerrard, D.; Corvalan, C.; Grant, A.; Linton, R. Microbial heat resistance of Listeria monocytogenes and the impact on ready-to-eat meat quality after post-package pasteurization. Meat Sci. 2006, 74, 425–434. [Google Scholar] [CrossRef]
- Li, M.; Pradhan, A.; Cooney, L.; Mauromoustakos, A.; Crandall, P.; Slavik, M.; Li, Y. A predictive model for the inactivation of Listeria innocua in cooked poultry products during postpackage pasteurization. J. Food Prot. 2011, 74, 1261. [Google Scholar] [CrossRef]
- Gande, N.; Muriana, P. prepackage surface pasteurization of ready-to-eat meats with a radiant heat oven for reduction of Listeria monocytogenes. J. Food Prot. 2003, 66, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sites, J. Elimination of Listeria monocytogenes on cooked chicken breast meat surfaces by near-infrared surface pasteurization prior to final packaging. J. Food Process Eng. 2012, 35, 1–15. [Google Scholar] [CrossRef]
- Huang, L. Infrared surface pasteurization of turkey frankfurters. Innov. Food Sci. Emerg. Technol. 2004, 5, 345–351. [Google Scholar] [CrossRef]
- Ha, J.-W.; Ryu, S.-R.; Kang, D.-H. Evaluation of near-infrared pasteurization in controlling Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes in ready-to-eat sliced ham. Appl. Environ. Microbiol. 2012, 78, 6458–6465. [Google Scholar] [CrossRef]
- European Food Safety Authority. High-Pressure Processing: Food Safety Without Compromising Quality. Available online: https://www.efsa.europa.eu/en/news/high-pressure-processing-food-safety-without-compromising-quality (accessed on 15 June 2025).
- Koutsoumanis, K.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; Lindqvist, R.; et al. The efficacy and safety of high-pressure processing of food. EFSA J. 2022, 20, e07128. [Google Scholar] [CrossRef]
- Morales, P.; Calzada, J.; Rodríguez, B.; De Paz, M.; Gaya, P.; Nuñez, M. Effect of cheese water activity and carbohydrate content on the barotolerance of Listeria monocytogenes Scott A. J. Food Prot. 2006, 69, 1328–1333. [Google Scholar] [CrossRef]
- Rastogi, N.; Raghavarao, K.; Balasubramaniam, V.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Patterson, M.; Mackle, A.; Linton, M. Effect of high pressure, in combination with antilisterial agents, on the growth of Listeria monocytogenes during extended storage of cooked chicken. Food Microbiol. 2011, 28, 1505–1508. [Google Scholar] [CrossRef]
- Hereu, A.; Dalgaard, P.; Garriga, M.; Aymerich, T.; Bover-Cid, S. Modeling the high pressure inactivation kinetics of Listeria monocytogenes on RTE cooked meat products. Innov. Food Sci. Emerg. Technol. 2012, 16, 305–315. [Google Scholar] [CrossRef]
- Hereu, A.; Bover-Cid, S.; Garriga, M.; Aymerich, T. High hydrostatic pressure and biopreservation of dry-cured ham to meet the Food Safety Objectives for Listeria monocytogenes. Int. J. Food Microbiol. 2011, 154, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Stratakos, A.C.; Delgado-Pando, G.; Linton, M.; Patterson, M.F.; Koidis, A. Synergism between high-pressure processing and active packaging against Listeria monocytogenes in ready-to-eat chicken breast. Innov. Food Sci. Emerg. Technol. 2015, 27, 41–47. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Belletti, N.; Aymerich, T.; Garriga, M. Modeling the Protective effect of aw and fat content on the high pressure resistance of Listeria monocytogenes in dry-cured ham. Food Res. Int. 2015, 75, 194–199. [Google Scholar] [CrossRef]
- Misiou, O.; van Nassau, T.J.; Lenz, C.A.; Vogel, R.F. The preservation of Listeria-critical foods by a combination of endolysin and high hydrostatic pressure. Int. J. Food Microbiol. 2018, 266, 355–362. [Google Scholar] [CrossRef]
- Cava, R.; Higuero, N.; Ladero, L. High-pressure processing and storage temperature on Listeria monocytogenes, microbial counts and oxidative changes of two traditional dry-cured meat products. Meat Sci. 2021, 171, 108273. [Google Scholar] [CrossRef] [PubMed]
- Cetin-Karaca, H.; Cruzen, S.M.; Ebbing, D. Survival of Listeria monocytogenes on cooked and high pressure treated bacon. Appl. Food Res. 2023, 3, 100259. [Google Scholar] [CrossRef]
- Kafle, R.; Fouladkhah, A.C. Effects of thermally-assisted and high-pressure processing on background microbiota and the Listeria monocytogenes load of a minimally processed commodity. Microorganisms 2024, 12, 1858. [Google Scholar] [CrossRef]
- Cabeza, M.C.; Cambero, I.; de la Hoz, L.; Ordóñez, J.A. Optimization of e-beam irradiation treatment to eliminate Listeria monocytogenes from ready-to-eat (RTE) cooked ham. Innov. Food Sci. Emerg. Technol. 2007, 8, 299–305. [Google Scholar] [CrossRef]
- Tauxe, R.V. Food safety and irradiation: Protecting the public from foodborne infections. Emerg. Infect. Dis. 2001, 7, 516. [Google Scholar] [CrossRef]
- Feliciano, C.P.; De Guzman, Z.M.; Tolentino, L.M.M.; Cobar, M.L.C.; Abrera, G.B. Radiation-treated ready-to-eat (RTE) chicken breast adobo for immuno-compromised patients. Food Chem. 2014, 163, 142–146. [Google Scholar] [CrossRef]
- Wang, T.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Pulsed ultra-violet inactivation spectrum of Escherichia coli. Water Res. 2005, 39, 2921–2925. [Google Scholar] [CrossRef]
- Ganan, M.; Hierro, E.; Hospital, X.F.; Barroso, E.; Fernández, M. Use of pulsed light to increase the safety of ready-to-eat cured meat products. Food Control 2013, 32, 512–517. [Google Scholar] [CrossRef]
- Sagong, H.-G.; Lee, S.-Y.; Chang, P.-S.; Heu, S.; Ryu, S.; Choi, Y.-J.; Kang, D.-H. Combined effect of ultrasound and organic acids to reduce Escherichia Coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh lettuce. Int. J. Food Microbiol. 2011, 145, 287–292. [Google Scholar] [CrossRef]
- Scouten, A.; Beuchat, L. Combined effects of chemical, heat and ultrasound treatments to kill Salmonella and Escherichia Coli O157: H7 on alfalfa seeds. J. Appl. Microbiol. 2002, 92, 668–674. [Google Scholar] [PubMed]
- Gómez-Salazar, J.A.; Galván-Navarro, A.; Lorenzo, J.M.; Sosa-Morales, M.E. Ultrasound effect on salt reduction in meat products: A review. Curr. Opin. Food Sci. 2021, 38, 71–78. [Google Scholar] [CrossRef]
- Paul, I.D.; Jayachandran, L.E.; Kumar, P. Ultrasound and high-pressure processing of RTE: Recent trends. In Recent Advances in Ready-to-Eat Food Technology; CRC Press: Boca Raton, FL, USA, 2024; pp. 52–64. [Google Scholar]
- Turhan, E.U.; Polat, S.; Erginkaya, Z.; Konuray, G. Investigation of synergistic antibacterial effect of organic acids and ultrasound against pathogen biofilms on lettuce. Food Biosci. 2022, 47, 101643. [Google Scholar] [CrossRef]
- Ruusunen, M.; Vainionpää, J.; Puolanne, E.; Lyly, M.; Lähteenmäki, L.; Niemistö, M.; Ahvenainen, R. Effect of sodium citrate, carboxymethyl cellulose and carrageenan levels on quality characteristics of low-salt and low-fat bologna type sausages. Meat Sci. 2003, 64, 371–381. [Google Scholar] [CrossRef]
- Bloukas, J.; Paneras, E.; Fournitzis, G. Sodium lactate and protective culture effects on quality characteristics and shelf-life of low-fat frankfurters produced with olive oil. Meat Sci. 1997, 45, 223–238. [Google Scholar] [CrossRef]
- Juneja, V.; Mukhopadhyay, S.; Marks, H.; Mohr, T.B.; Warning, A.; Datta, A. Predictive thermal inactivation model for effects and interactions of temperature, NaCl, sodium pyrophosphate, and sodium lactate on Listeria monocytogenes in ground beef. Food Bioprocess Technol. 2014, 7, 437–446. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, M.R.; Park, J.W.; Park, K.H.; Chung, M.S.; Ryu, S.; Kang, D.H. Use of organic acids to inactivate Escherichia Coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes on organic fresh apples and lettuce. J. Food Sci. 2011, 76, M293–M298. [Google Scholar] [CrossRef] [PubMed]
- Kasra-Kermanshahi, R.; Mobarak-Qamsari, E. Inhibition effect of lactic acid bacteria against foodborne pathogen, Listeria monocytogenes. Appl. Food Biotechnol. 2015, 2, 11–19. [Google Scholar] [CrossRef]
- Webb, L.; Ma, L.; Lu, X. Impact of lactic acid bacteria on the control of Listeria monocytogenes in ready-to-eat foods. Food Qual. Saf. 2022, 6, fyac045. [Google Scholar] [CrossRef]
- Pisano, M.B.; Fadda, M.E.; Viale, S.; Deplano, M.; Mereu, F.; Blažić, M.; Cosentino, S. Inhibitory effect of Lactiplantibacillus Plantarum and Lactococcus Lactis autochtonous strains against Listeria monocytogenes in a laboratory cheese model. Foods 2022, 11, 715. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for biocontrol of Listeria monocytogenes using lactic acid bacteria and their metabolites in ready-to-eat meat-and dairy-ripened products. Foods 2022, 11, 542. [Google Scholar] [CrossRef]
- Yap, P.-C.; MatRahim, N.-A.; AbuBakar, S.; Lee, H.Y. Antilisterial potential of lactic acid bacteria in eliminating Listeria monocytogenes in host and ready-to-eat food application. Microbiol. Res. 2021, 12, 234–257. [Google Scholar] [CrossRef]
- Morandi, S.; Silvetti, T.; Vezzini, V.; Morozzo, E.; Brasca, M. How we can improve the antimicrobial performances of lactic acid bacteria? a new strategy to control Listeria monocytogenes in gorgonzola cheese. Food Microbiol. 2020, 90, 103488. [Google Scholar] [CrossRef]
- Panebianco, F.; Giarratana, F.; Caridi, A.; Sidari, R.; De Bruno, A.; Giuffrida, A. Lactic acid bacteria isolated from traditional italian dairy products: Activity against Listeria monocytogenes and modelling of microbial competition in soft cheese. LWT-Food Sci. Technol. 2021, 137, 110446. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Sánchez-Montero, L.; Padilla, P.; Córdoba, J.J. Effect of the dry-cured fermented sausage “salchichón” processing with a selected lactobacillus sakei in Listeria monocytogenes and microbial population. Foods 2021, 10, 856. [Google Scholar] [CrossRef] [PubMed]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of phage-based inhibition of Listeria monocytogenes in food products and food processing environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent bacteriophage for efficient biocontrol of Listeria monocytogenes in ready-to-eat foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef]
- Leverentz, B.; Conway, W.S.; Camp, M.J.; Janisiewicz, W.J.; Abuladze, T.; Yang, M.; Saftner, R.; Sulakvelidze, A. Biocontrol of Listeria monocytogenes on fresh-cut produce by treatment with lytic bacteriophages and a bacteriocin. Appl. Environ. Microbiol. 2003, 69, 4519–4526. [Google Scholar] [CrossRef] [PubMed]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage Listex™ P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Perera, M.N.; Abuladze, T.; Li, M.; Woolston, J.; Sulakvelidze, A. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiol. 2015, 52, 42–48. [Google Scholar] [CrossRef]
- Baños, A.; García-López, J.D.; Núñez, C.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Biocontrol of Listeria monocytogenes in fish by enterocin as-48 and listeria lytic bacteriophage p100. LWT-Food Sci. Technol. 2016, 66, 672–677. [Google Scholar] [CrossRef]
- Vijayakumar, P.P.; Muriana, P.M. Inhibition of Listeria monocytogenes on ready-to-eat meats using bacteriocin mixtures based on mode-of-action. Foods 2017, 6, 22. [Google Scholar] [CrossRef]
- Muriana, P.M. Bacteriocins for control of Listeria spp. In Food. J. Food Prot. 1996, 59, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Abee, T.; Krockel, L.; Hill, C. Bacteriocins: Modes of action and potentials in food preservation and control of food poisoning. Int. J. Food Microbiol. 1995, 28, 169–185. [Google Scholar] [CrossRef]
- Furlaneto-Maia, L.; Ramalho, R.; Rocha, K.R.; Furlaneto, M.C. Antimicrobial activity of enterocins against Listeria sp. and other food spoilage bacteria. Biotechnol. Lett. 2020, 42, 797–806. [Google Scholar] [CrossRef]
- Figueiredo, A.C.L.; Almeida, R.C. Antibacterial efficacy of nisin, bacteriophage p100 and sodium lactate against Listeria monocytogenes in ready-to-eat sliced pork ham. Braz. J. Microbiol. 2017, 48, 724–729. [Google Scholar] [CrossRef]
- Jofre, A.; Garriga, M.; Aymerich, T. Inhibition of Listeria monocytogenes in cooked ham through active packaging with natural antimicrobials and high-pressure processing. J. Food Prot. 2007, 70, 2498–2502. [Google Scholar] [CrossRef]
- Molinos, A.C.; Abriouel, H.; López, R.L.; Omar, N.B.; Valdivia, E.; Gálvez, A. Enhanced bactericidal activity of enterocin as-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad. Food Chem. Toxicol. 2009, 47, 2216–2223. [Google Scholar] [CrossRef]
- Ruiz, A.; Williams, S.; Djeri, N.; Hinton, A., Jr.; Rodrick, G. Nisin affects the growth of Listeria monocytogenes on ready-to-eat turkey ham stored at four degrees celsius for sixty-three days. Poult. Sci. 2010, 89, 353–358. [Google Scholar] [CrossRef]
- Ananou, S.; Bouraqqadi, M.; Zouhri, N.; El Kinany, S.; Manni, L. Control of Listeria monocytogenes in a fresh cheese using aromatic and medicinal plants and enterocin: A comparative study. Lett. Appl. Microbiol. 2023, 76, ovad076. [Google Scholar] [CrossRef]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. Potential application of essential oils for mitigation of Listeria monocytogenes in meat and poultry products. Front. Nutr. 2020, 7, 577287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, B.; Xiong, Y.L.; Sun, X. Antimicrobial activities of spice extracts against pathogenic and spoilage bacteria in modified atmosphere packaged fresh pork and vacuum packaged ham slices stored at 4 °C. Meat Sci. 2009, 81, 686–692. [Google Scholar] [CrossRef]
- Takahashi, H.; Kashimura, M.; Koiso, H.; Kuda, T.; Kimura, B. Use of ferulic acid as a novel candidate of growth inhibiting agent against Listeria monocytogenes in ready-to-eat food. Food Control 2013, 33, 244–248. [Google Scholar] [CrossRef]
- Giarratana, F.; Muscolino, D.; Ragonese, C.; Beninati, C.; Sciarrone, D.; Ziino, G.; Mondello, L.; Giuffrida, A.; Panebianco, A. Antimicrobial activity of combined thyme and rosemary essential oils against Listeria monocytogenes in Italian mortadella packaged in modified atmosphere: Thyme & rosemary EOs vs L monocytogenes. J. Essent. Oil Res. 2016, 28, 467–474. [Google Scholar] [CrossRef]
- Emma, M.-L.; Palou, E.; López-Malo, A. Biopreservatives as agents to prevent food spoilage. In Microbial Contamination and Food Degradation; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Verheyen, D.; Govaert, M.; Seow, T.K.; Ruvina, J.; Mukherjee, V.; Baka, M.; Skåra, T.; Van Impe, J.F. The complex effect of food matrix fat content on thermal inactivation of Listeria monocytogenes: Case study in emulsion and gelled emulsion model systems. Front. Microbiol. 2020, 10, 3149. [Google Scholar] [CrossRef]
- Fain, A.R., Jr.; Line, J.E.; Moran, A.B.; Martin, L.M.; Lechowich, R.V.; Carosella, J.M.; Brown, W.L. Lethality of heat to Listeria monocytogenes Scott A: D-value and Z-value determinations in ground beef and turkey. J. Food Prot. 1991, 54, 756–761. [Google Scholar] [CrossRef]
- Karina, P.; Julio, C.; Leda, G.; Noemi, Z. Behavior of Listeria monocytogenes type1 355/98 (85) in meat emulsions as affected by temperature, pH, water activity, fat and microbial preservatives. Food Control 2011, 22, 1573–1581. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Ghoush, M.A.; Al-Holy, M.; Hilal, H.A.; Al-Nabulsi, A.A.; Osaili, T.M.; Ayyash, M.; Holley, R.A. Survival and growth of Listeria monocytogenes and Staphylococcus aureus in ready-to-eat mediterranean vegetable salads: Impact of storage temperature and food matrix. Int. J. Food Microbiol. 2021, 346, 109149. [Google Scholar] [CrossRef] [PubMed]
- Polese, P.; Del Torre, M.; Venir, E.; Stecchini, M.L. A simplified modelling approach established to determine the Listeria monocytogenes behavior during processing and storage of a traditional (Italian) ready-to-eat food in accordance with the European commission regulation n 2073/2005. Food Control 2014, 36, 166–173. [Google Scholar] [CrossRef]
- Hill, C.; Cotter, P.D.; Sleator, R.D.; Gahan, C.G. Bacterial stress response in Listeria monocytogenes: Jumping the hurdles imposed by minimal processing. Int. Dairy J. 2002, 12, 273–283. [Google Scholar] [CrossRef]
- Nilsson, R.E.; Ross, T.; Bowman, J.P. Variability in biofilm production by Listeria monocytogenes correlated to strain origin and growth conditions. Int. J. Food Microbiol. 2011, 150, 14–24. [Google Scholar] [CrossRef]
- Karatzas, K.-A.G.; Suur, L.; O’Byrne, C.P. Characterization of the intracellular glutamate decarboxylase system: Analysis of its function, transcription, and role in the acid resistance of various strains of Listeria monocytogenes. Appl. Environ. Microbiol. 2012, 78, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Olier, M.; Rousseaux, S.; Piveteau, P.; Lemaıtre, J.-P.; Rousset, A.; Guzzo, J. Screening of glutamate decarboxylase activity and bile salt resistance of human asymptomatic carriage, clinical, food, and environmental isolates of Listeria monocytogenes. Int. J. Food Microbiol. 2004, 93, 87–99. [Google Scholar] [CrossRef]
- Aryani, D.; Den Besten, H.; Hazeleger, W.; Zwietering, M. Quantifying strain variability in modeling growth of Listeria monocytogenes. Int. J. Food Microbiol. 2015, 208, 19–29. [Google Scholar] [CrossRef]
- Wang, X.; Tian, S.; Wu, Y.; Li, H.; Bai, L.; Liu, H.; Zhang, X.; Dong, Q. Strain variability in growth and thermal inactivation characteristics of Listeria monocytogenes strains after acid adaptation. J. Food Prot. 2021, 84, 2229–2236. [Google Scholar] [CrossRef]
- Heredia, S.M.S.; Le Marc, Y.; Martín, J.S.; Possas, A.; Jiménez, E.C.; Díaz, A.V. Inter-strain variability on the cardinal parameters (pH and aw) of clinical and food isolates of Listeria monocytogenes using turbidimetric measurements. Int. J. Food Microbiol. 2024, 411, 110521. [Google Scholar] [CrossRef]
- FAO/WHO. Hazard identification and characterization: Criteria for categorizing shiga toxin–producing Escherichia coli on a risk basis. J. Food Prot. 2019, 82, 7–21. [Google Scholar] [CrossRef]
- FAO/WHO. Shiga Toxin-Producing Escherichia coli (STEC) and food: Attribution, Characterization, and Monitoring; WHO: Rome, Italy, 2018. [Google Scholar]
- Eustice, R.F.; Bruhn, C.M. Consumer acceptance and marketing of irradiated foods. In Food Irradiation Research and Technology; Wiley: Hoboken, NJ, USA, 2012; pp. 173–195. [Google Scholar]
- Maherani, B.; Hossain, F.; Criado, P.; Ben-Fadhel, Y.; Salmieri, S.; Lacroix, M. World market development and consumer acceptance of irradiation technology. Foods 2016, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Bodie, A.R.; Wythe, L.A.; Dittoe, D.K.; Rothrock, M.J., Jr.; O’Bryan, C.A.; Ricke, S.C. Alternative additives for organic and natural ready-to-eat meats to control spoilage and maintain shelf life: Current perspectives in the United States. Foods 2024, 13, 464. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D. Cost analysis and environmental impact of pulsed electric fields and high pressure processing in comparison with thermal pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model-high pressure processing assisted by bacteriophage P100 and bacteriocinogenic pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef] [PubMed]
- Bleoancă, I.; Saje, K.; Mihalcea, L.; Oniciuc, E.-A.; Smole-Mozina, S.; Nicolau, A.I.; Borda, D. Contribution of high pressure and thyme extract to control Listeria monocytogenes in fresh cheese-a hurdle approach. Innov. Food Sci. Emerg. Technol. 2016, 38, 7–14. [Google Scholar] [CrossRef]
- Australian Meat Industry Council. Guidelines for the Safe Manufacture of Smallgoods, 3rd ed.; Meat & Livestock Australia: North Sydney, Australia, 2025. [Google Scholar]
- Bolumar, T.; Orlien, V.; Sikes, A.; Aganovic, K.; Bak, K.H.; Guyon, C.; Stübler, A.S.; de Lamballerie, M.; Hertel, C.; Brüggemann, D.A. High-pressure processing of meat: Molecular impacts and industrial applications. Compr. Rev. Food Sci. Food Saf. 2021, 20, 332–368. [Google Scholar] [CrossRef]
- Chhabra, A.; Carter, W.; Linton, R.; Cousin, M. A Predictive model to determine the effects of ph, milkfat, and temperature on thermal inactivation of Listeria monocytogenes. J. Food Prot. 1999, 62, 1143–1149. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Carrasco, E.; Bover-Cid, S.; Jofré, A.; Valero, A. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready-to-eat (RTE) foods: Activity 2, a quantitative risk characterization on L. monocytogenes in RTE foods; starting from the retail stage. EFSA Support. Publ. 2017, 14, 1252E. [Google Scholar] [CrossRef]
- Foerster, C.; Figueroa, G.; Evers, E. Risk assessment of Listeria monocytogenes in poultry and beef. Br. Food J. 2015, 117, 779–792. [Google Scholar] [CrossRef]
- Possas, A.; Valdramidis, V.; García-Gimeno, R.M.; Pérez-Rodríguez, F. High hydrostatic pressure processing of sliced fermented sausages: A quantitative exposure assessment for Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2019, 52, 406–419. [Google Scholar] [CrossRef]
- Mejlholm, O.; Dalgaard, P. Development and validation of an extensive growth and growth boundary model for Listeria monocytogenes in lightly preserved and ready-to-eat shrimp. J. Food Prot. 2009, 72, 2132–2143. [Google Scholar] [CrossRef]
- Mejlholm, O.; Bøknæs, N.; Dalgaard, P. Development and validation of a stochastic model for potential growth of Listeria monocytogenes in naturally contaminated lightly preserved seafood. Food Microbiol. 2015, 45, 276–289. [Google Scholar] [CrossRef]
- Mejlholm, O.; Gunvig, A.; Borggaard, C.; Blom-Hanssen, J.; Mellefont, L.; Ross, T.; Leroi, F.; Else, T.; Visser, D.; Dalgaard, P. predicting growth rates and growth boundary of Listeria monocytogenes—an international validation study with focus on processed and ready-to-eat meat and seafood. Int. J. Food Microbiol. 2010, 141, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Mejlholm, O.; Dalgaard, P. Modelling and predicting the simultaneous growth of Listeria monocytogenes and psychrotolerant lactic acid bacteria in processed seafood and mayonnaise-based seafood salads. Food Microbiol. 2015, 46, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Orsi, R.H.; Guariglia-Oropeza, V.; Wiedmann, M. Development of a modeling tool to assess and reduce regulatory and recall risks for cold-smoked salmon due to Listeria monocytogenes contamination. J. Food Prot. 2022, 85, 1335–1354. [Google Scholar] [CrossRef] [PubMed]
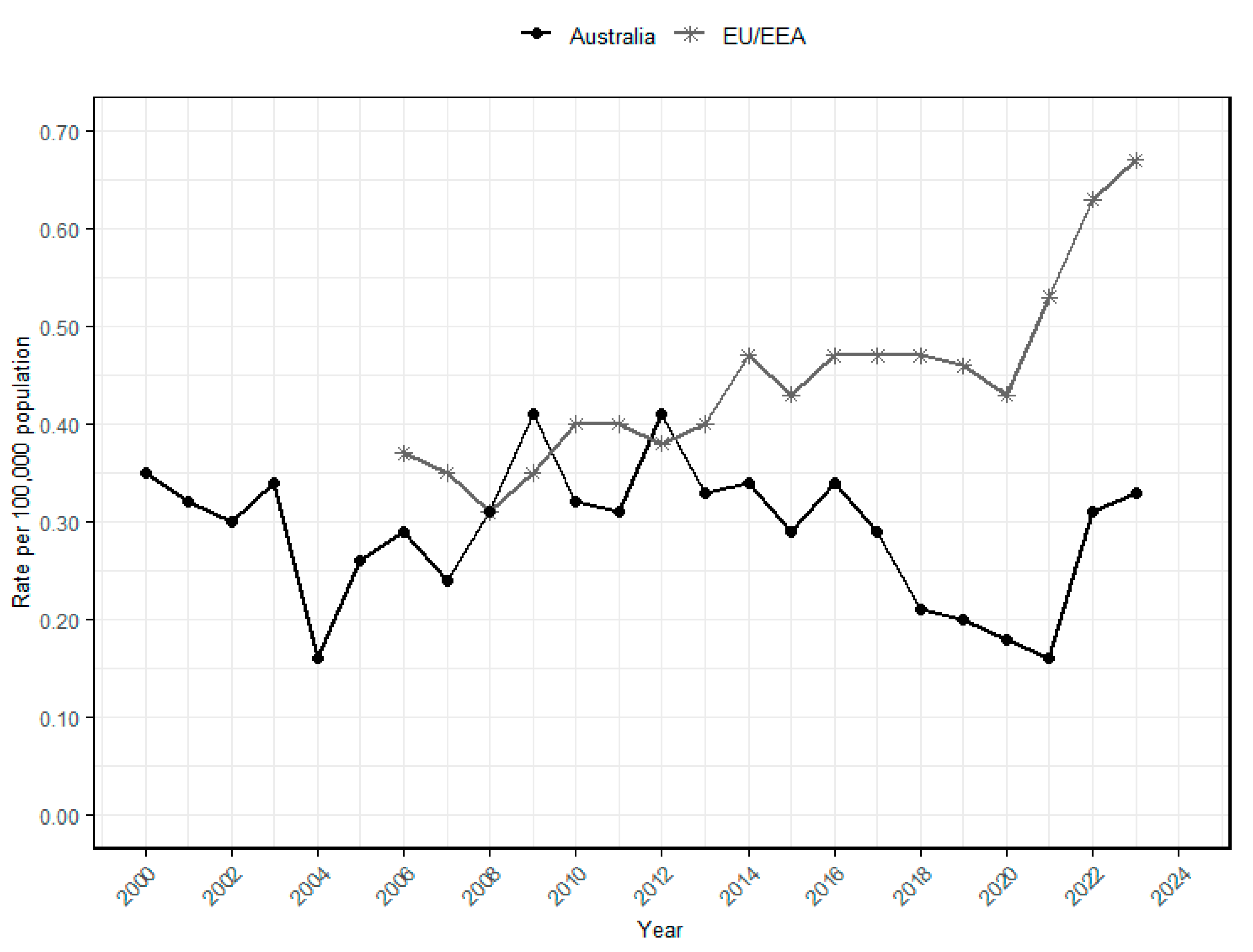
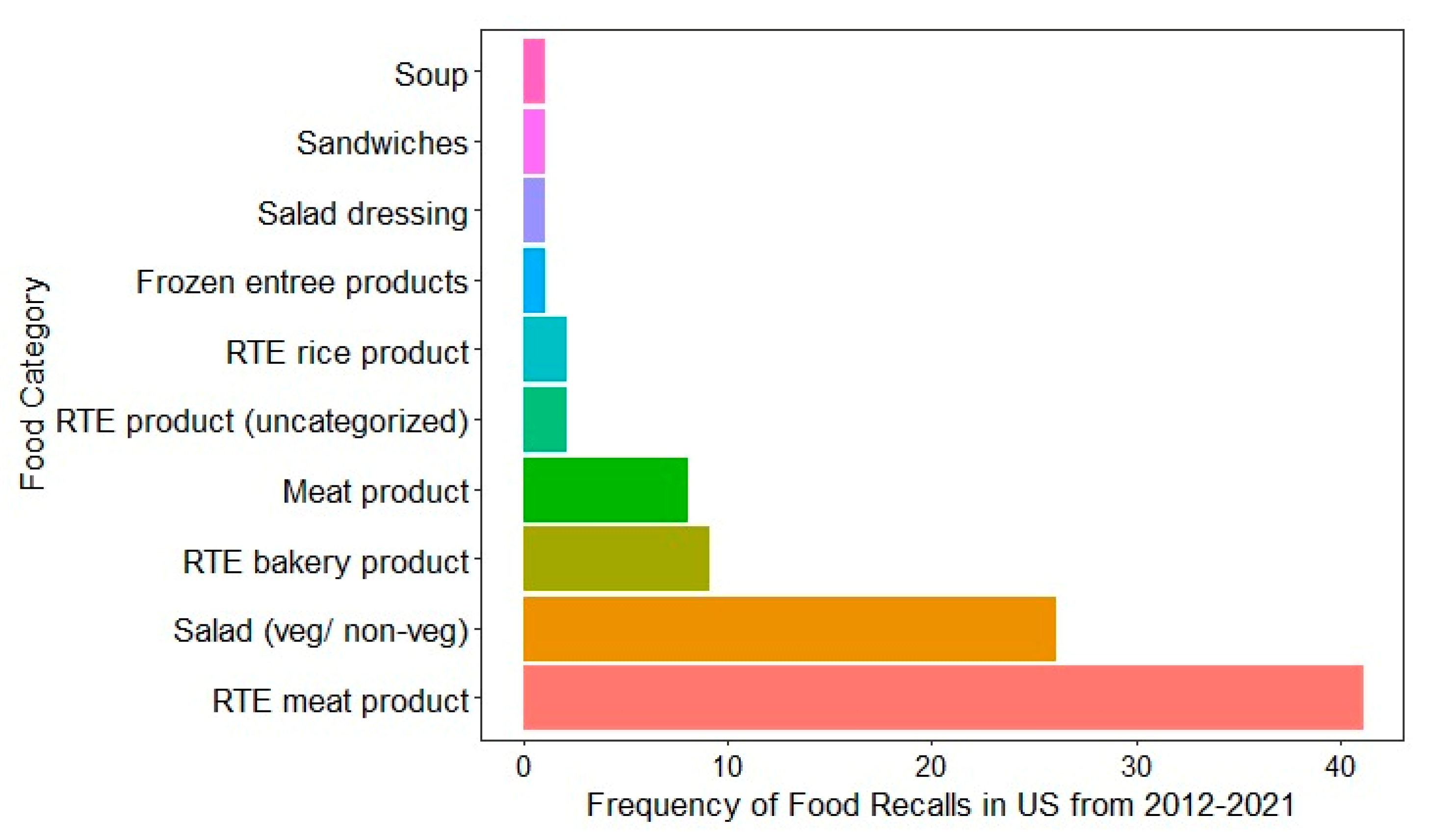
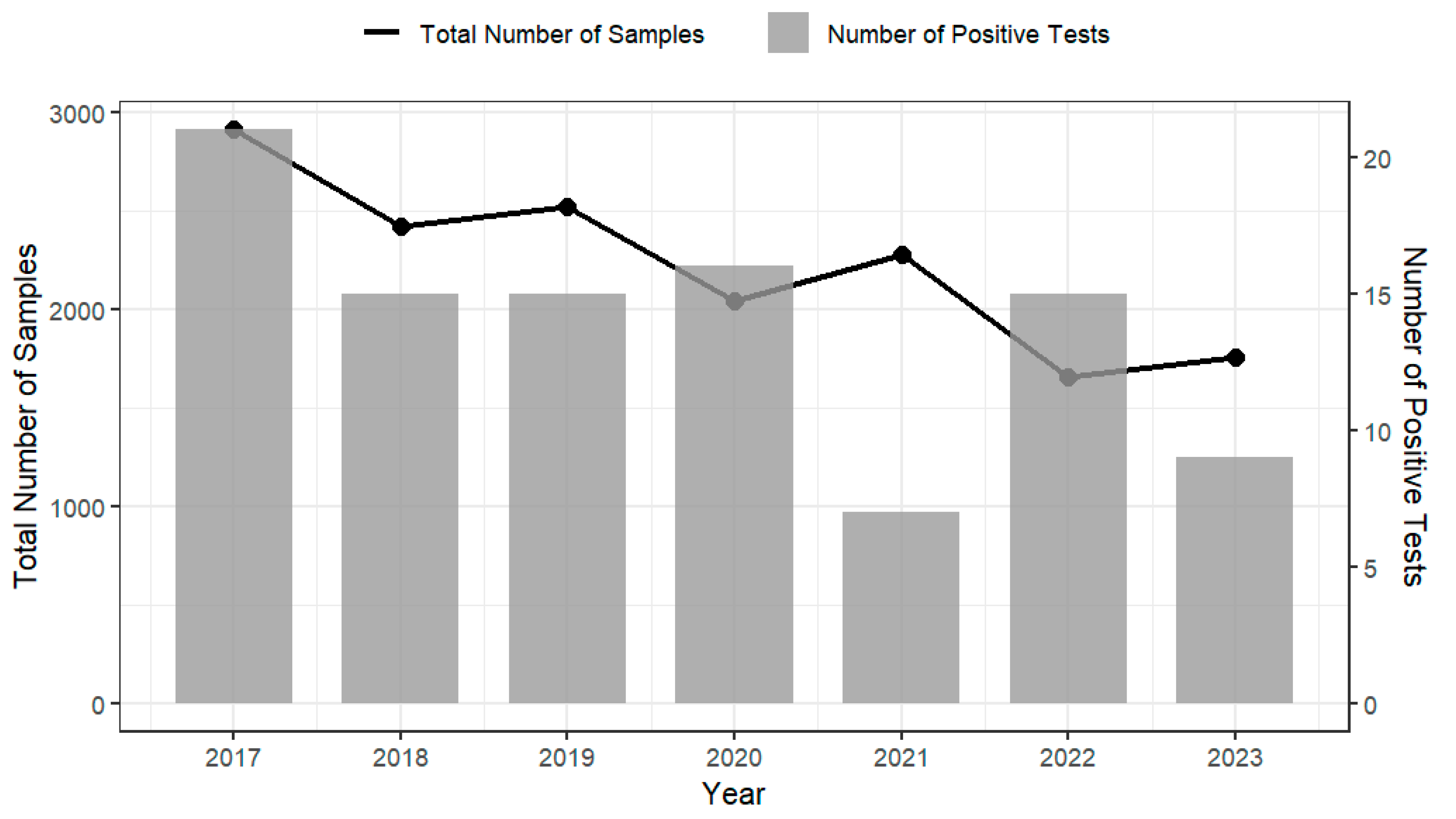
| Category of RTE Foods | Regulatory Thresholds (1) | Examples | Rationale | |||
|---|---|---|---|---|---|---|
| Region | pH | aw | Reference | |||
| High risk/Category 1 | Australia | ≥4.4 | NA (2) | [99] | Soft cheeses (Brie, Feta, Ricotta), deli meats, smoked seafood, cooked prawns, pre-packaged salads, fresh-cut fruit | High moisture and near-neutral pH allow L. monocytogenes to grow during storage |
| - | ≥0.92 | |||||
| ≥5.0 | ≥0.94 | |||||
| Codex | ≥4.4 | - | [27] | |||
| - | ≥0.92 | |||||
| ≥5.0 | ≥0.94 | |||||
| Canada | ≥4.4 | - | [97] | |||
| - | ≥0.92 | |||||
| ≥5.0 | ≥0.94 | |||||
| Europe | >4.4 | - | [96] | |||
| - | >0.92 | |||||
| >5.0 | >0.94 | |||||
| US | >4.4 | - | [16] | |||
| - | >0.92 | |||||
| Low risk/Category 2 | Australia | <4.4 | - | [99] | Pickled vegetables, dry crackers, hard cheeses, jam, canned soups, fermented meats (salami, pepperoni), chocolate, vinegar foods | Low pH and/or water activity inhibit L. monocytogenes growth |
| - | <0.92 | |||||
| <5.0 | <0.94 | |||||
| Codex | <4.4 | <0.92 | [27] | |||
| <5.0 | <0.94 | |||||
| Canada | <4.4 | - | [97] | |||
| - | <0.92 | |||||
| <5.0 | <0.94 | |||||
| Europe | ≤4.4 | - | [96] | |||
| - | ≤0.92 | |||||
| ≤5.0 | <0.94 | |||||
| US | ≤4.4 | - | [16] | |||
| - | ≤0.92 | |||||
| Country | Year of Recall | Total Number of Recall Events | Recalls Incidents Due to Microbiological Contamination | Recalls Due to L. monocytogenes | Reference |
|---|---|---|---|---|---|
| Australia | 2020–2024 | 446 | 90 (20.2%) | 28 (31.1%) | [19] |
| Canada | 2018–2023 | 821 | 316 (38.5%) | NA * | [21] |
| EU + | 2013–2022 | 11,684 | 6510 (55.7%) | 654 (10.0%) | [20,121] |
| US | 2012–2021 | 1093 | 213 (19.4%) | 92 (43.2%) | [18] |
| Agency | Products | Year | Total no. of Samples | No. of Positive for L. monocytogenes | Positive Samples (%) | Reference |
|---|---|---|---|---|---|---|
| FSANZ | Cooked prawns | 2003 | 230 | 4 | 2.0 | [23] |
| NSW Food Authority | Packaged sliced RTE meats | 2008 | 154 | 6 | 3.9 | [24] |
| NSW Food Authority | RTE meat products (including poultry) | 2011–2012 | NA * | 2 | 2.0 | [26] |
| ACT Health Service | RTE sandwiches, rolls, and baked goods | 2014–2016 | NA | 1 | 0.4 | [25] |
| Region | Regulation/Document Name | Microbiological Criteria * | Reference | |
|---|---|---|---|---|
| High Risk/Category 1 | Low Risk/Category 2 | |||
| Australia | Schedule 27 of the Australia New Zealand Food Standards Code | Absence in 25 g | ≤100 CFU/g | [99] |
| Canada | Policy on L. monocytogenes in RTE foods, 2023 | Absence in 25 g | ≤100 CFU/g | [97] |
| Codex | CXC/GL 61-2207 | Absence in 25 g | ≤100 CFU/g | [27] |
| European Union | Regulation (EC) No. 2073/2005 | Absence in 25 g | ≤100 CFU/g ** | [96] |
| US | US FDA Compliance policy guide (CPG), Article 1, Sec 555.320 | Absence in 25 g | Absence in 25 g | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yangchen, J.; Sarkar, D.; Rood, L.; Vaskoska, R.; Kocharunchitt, C. Listeria monocytogenes: A Continuous Global Threat in Ready-to-Eat (RTE) Foods. Foods 2025, 14, 3664. https://doi.org/10.3390/foods14213664
Yangchen J, Sarkar D, Rood L, Vaskoska R, Kocharunchitt C. Listeria monocytogenes: A Continuous Global Threat in Ready-to-Eat (RTE) Foods. Foods. 2025; 14(21):3664. https://doi.org/10.3390/foods14213664
Chicago/Turabian StyleYangchen, Jamyang, Dipon Sarkar, Laura Rood, Rozita Vaskoska, and Chawalit Kocharunchitt. 2025. "Listeria monocytogenes: A Continuous Global Threat in Ready-to-Eat (RTE) Foods" Foods 14, no. 21: 3664. https://doi.org/10.3390/foods14213664
APA StyleYangchen, J., Sarkar, D., Rood, L., Vaskoska, R., & Kocharunchitt, C. (2025). Listeria monocytogenes: A Continuous Global Threat in Ready-to-Eat (RTE) Foods. Foods, 14(21), 3664. https://doi.org/10.3390/foods14213664





