1. Introduction
Food safety has become a major concern worldwide. The consumption of food contaminated with potentially toxic elements (PTEs) is a worldwide concern due to their harmful impact on human health. Elements such as arsenic (As), aluminum (Al), barium (Ba), cadmium (Cd), chromium (Cr), lead (Pb), and titanium (Ti) are considered non-essential and potentially toxic. These elements can accumulate in rice grown in contaminated soil and water. As a result, rice consumption may substantially contribute to health risks related to exposure. In recent times, cumulative exposure to multiple contaminants has become a crucial concern in the field of food safety [
1,
2].
Food contamination by heavy metals has been regarded as a serious issue due to their stable, non-degradable, and persistent nature. Human exposure to heavy metals can occur through three main pathways: ingestion, inhalation, and skin contact; however, the most significant exposure to heavy metals in humans is food consumption. Heavy metal exposure can damage organs even at low levels. These metals (such as Pb, Cd, As, Hg) enter the environment from both natural sources and human activities, such as, e.g., industrial waste disposal, excessive and continuous use of pesticides and chemical fertilizers, mining/extraction activities and the dumping of household waste. All these activities cause accumulation in the soil, environment, and plants, entering the food chain and harming living organisms. Among various agricultural products, rice is particularly prone to accumulating heavy metals, which can cause serious health issues in humans [
3]. Prolonged exposure to potentially toxic elements (PTEs) such as lead (Pb), arsenic (As), nickel (Ni), and cadmium (Cd) has been linked to an increased risk of malignant brain tumors, cardiovascular and kidney system disorders, as well as cognitive impairments in children [
4]. Globalization has significantly contributed to soil and water pollution and contamination of harvested crops [
5,
6]. Since ubiquitous PTEs are chemically stable and non-biodegradable, humans are highly at risk of unavoidable exposure to these contaminants via food chains [
7,
8,
9,
10]. In addition to regional contamination, global climate change has been reported to influence the mobility and bioavailability of potentially toxic elements (PTEs) in agricultural soils, thereby increasing health risks associated with contaminated food crops in various regions worldwide [
11]. Their findings reinforce the need for region-specific monitoring and health risk assessment of heavy metals in staple crops such as rice.
The heavy metals are classified by the International Agency for Research on Cancer (IARC) as follows: cadmium is classified as a human carcinogen (Group 1), Pb is a possible human carcinogen (Group 2B), and its inorganic compounds are considered a probable human carcinogen (Group 2A), while iAs (inorganic arsenic) is classified as a Group 1 substance, meaning it is carcinogenic to humans [
12]. Arsenic (As) is a naturally occurring metalloid widely distributed in the environment and cannot be completely eliminated from ecosystems or the food chain [
13]. It occurs in three major forms: organic arsenic, inorganic arsenic (iAs), and arsine gas, each differing in toxicity. Among these, inorganic arsenic (iAs) is considered the most toxic species. The term total arsenic (tAs) refers to the sum of organic and inorganic arsenic concentrations. Globally, risk assessments of arsenic contamination in food and the environment primarily focus on iAs due to its higher toxicity and stronger association with adverse health effects. Acute exposure to iAs may result in symptoms such as nausea, vomiting, abdominal pain, diarrhea, bruising, numbness, muscle cramps, and, in extreme cases, death [
14]. Long-term exposure has been linked to chronic respiratory disorders, including laryngitis, bronchial infections, and an increased risk of lung cancer.
The Ministry of Agriculture of the Republic of Indonesia and the National Standardization Agency have regulated the maximum limits of heavy metal contamination in rice through the Minister of Agriculture Regulation No. 53 of 2018 concerning the Safety and Quality of Fresh Plant-Based Food [
15] and SNI 6128:2020 [
16] on rice, with the maximum limits set at 100 µg/kg for Cd, 200 µg/kg for Pb, and 200 µg/kg for inorganic arsenic as regulated in CXS_193e [
17]. The global food body, Codex Alimentarius, regulates heavy metal contamination in Standard 193-1995 [
17], which outlines the General Standard for Contaminants and Toxins in Food and Feed. Recommendations from the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in Codex Standard 193-1995 also set toxicity values for each type of heavy metal, with the Provisional Tolerable Monthly Intake (PTMI) for cadmium set at 25 µg/kg bw/day. For inorganic arsenic, the lower reference dose for increased lung cancer incidence (BMDL
0.5) was determined from epidemiological studies with a value of 3.0 µg/kg bw/day, using various assumptions to estimate total exposure to inorganic arsenic from drinking water and food. The European Food Safety Authority (EFSA) identified three health effects of lead exposure that provide sufficient consistent evidence for risk analysis: (1) neurodevelopmental disruption in toddlers, measured by a decrease in IQ; (2) cardiovascular effects in adults, measured by an increase in systolic blood pressure; and (3) an increased incidence of chronic kidney disease in adults, measured by a decrease in Glomerular Filtration Rate (GFR) [
18]. Exposure to lead is associated with a wide range of effects, including various neurodevelopmental effects, mortality (mainly due to cardiovascular diseases), impaired renal function, hypertension, impaired fertility and adverse pregnancy outcomes. For adults, the adverse effects associated with the blood lead concentrations, for which the weight of evidence is greatest and most consistent, is a lead-associated increase in systolic blood pressure [
19].
One of the main food crop commodities in the world is rice (
Oryza sativa L.). Rice is an important source of energy, vitamins, and essential nutrients and is a staple food for more than half of the world’s population, spread across Europe, America, and Asia [
3]. Indonesia ranks as the fourth-largest rice consumer globally, with a total consumption reaching 35.3 million metric tons in 2023, which equals 125 kg per capita [
20]. Rice is a crop with a strong capacity to absorb cadmium, making it crucial to understand the pattern of cadmium contamination in the rice supply chain through predictive methods and to prevent and control it promptly. Heavy metals, particularly cadmium (Cd), arsenic (As), and lead (Pb), are highly bioavailable and tend to accumulate in rice grains due to the plant’s efficient uptake mechanisms. These toxic elements can be readily absorbed from contaminated soil and water, where they bind to soil particles or dissolve in water and then enter the rice plant through the roots [
21].
Indonesia is an agrarian country that economically depends on agriculture. As mentioned, rice is the staple food in Indonesia, with an annual consumption of approximately 30.90 million tons [
20]. With the increasing anthropogenic activities, such as irrigation with wastewater, as well as the use of agricultural fertilizers, pesticides, and organic manure in farming, rice may become a primary source of PTEs in the diet, particularly Cd, Pb, and As. Research focusing on heavy metal contamination in rice and its associated risk assessment in Indonesia is still limited, with existing studies mostly focused on specific regions that do not represent the entire country. Given the high consumption rate and food safety concerns, PTEs content in rice is an important issue to study both in Indonesia and globally. The potential health risks from long-term rice consumption containing PTEs need to be addressed [
22].
This study aims to assess the exposure to PTEs (Cd, Pb and As) from rice in Indonesia by examining (i) the levels of Cd, Pb, and As in rice in Indonesia; (ii) the effect of rice processing into cooked rice on the levels of Cd, Pb, and As; (iii) the levels of exposure to PTEs Cd, Pb, and As from rice by age groups 0–59 months, 5–12 years, 13–18 years, 19–55 years, >55 years; (iv) to assess the health risk due to exposure to Cd, Pb, and As through the consumption of rice and cooked rice in Indonesia by age groups 0–59 months, 5–12 years, 13–18 years, 19–55 years, >55 years.
2. Materials and Methods
2.1. Chemicals and Materials
Cadmium, Lead, and Arsenic are standard solutions from Merck (Darmstadt, Germany). Ultrapure water was generated from a water purification system (Direct-Q® 5UV, Merck, Darmstadt, Germany). Suprapur 60% nitric acid (HNO3) and suprapur 30% hydrogen peroxide (H2O2) were purchased from Merck (Darmstadt, Germany). A Working standard solution was prepared by serial dilution of the multi-element calibration standard-2A solution. Tuning solution for ICP-MS containing 1 μg/mL of Ba, Bi, Ce, Co, In, Li, and U in 2.5% HNO3 0.5% HCl (Inorganic Ventures, Christianburg, VA, USA). All glassware and TFM vessels were cleaned by soaking in a 20% (v/v) HNO3 reagent grade for at least 24 h and rinsed with deionized water.
2.2. Sample Collection and Sample Preparation
The samples for this study were taken from the 30 largest rice-producing provinces in Indonesia based on rice production data from 2022 provided by Statistics Indonesia (BPS) [
20], namely East Java, West Java, Central Java, South Sulawesi, South Sumatra, Lampung, North Sumatra, Banten, Aceh, West Nusa Tenggara, West Sumatra, South Kalimantan, East Nusa Tenggara, Central Sulawesi, West Kalimantan, Special Region of Yogyakarta, Southeast Sulawesi, West Sulawesi, Central Kalimantan, Bengkulu, Jambi, North Sulawesi, Gorontalo, Kalimantan, Riau, Papua, Maluku, Bangka Belitung, and North Kalimantan. Rice samples were taken from two cities or districts, each province’s largest rice production centers. Rice samples were collected by the Agricultural Quality Control Officers and Plant Pest Control Officers in each province, with 1 kg of rice from each location. Large laboratory samples were subsampled using the quartering or Koning technique to reduce the sample size; then, the test samples were homogenized by grinding/blending.
The rice samples to be tested were processed from 10 rice samples from 10 randomly selected provinces using two different cooking methods: with a rice cooker and by steaming using a pot and an aluminum steamer. The Indonesian population commonly uses these rice cooking methods. The cooking process used bottled drinking water to avoid heavy metal contamination from the water used to cook the rice. The Cd, Pb, and As levels in the water were below the LoD, which were 0.001 mg/L. The cooked rice from the 10 rice samples was then tested for heavy metals using a microwave digestion system and ICP-MS. The changes in the Cd, Pb, and As levels in rice after cooking were used as the processing factors for exposure calculations.
2.3. Microwave Digestion System and ICP-MS Analysis
The testing method used in this study refers to AOAC 2015.01 Heavy Metals in Food Inductively Coupled Plasma–Mass Spectrometry [
23]. A 0.25 g sample was weighed into a vessel of the microwave digestion system, and 4 mL of Suprapur HNO
3 65% and 1 mL of H
2O
2 30% were added. The vessel was placed into a Milestone Ethos Up microwave digestion system, and the digestion conditions were set at ramp temperature of 180 °C for 20 min, with a hold time of 15 min. The contents of the vessel were poured into a 20 mL volumetric flask, the vessel was rinsed, and the volumetric flask was brought to volume with ultrapure water. The solution was then diluted four times.
The prepared sample solution was analyzed quantitatively by ICP-MS. The Cd, Pb, and As levels in rice were analyzed using a Thermo ICAP RQ ICP-MS with the following conditions and ICP-MS settings: ion identifiers for 111Cd, 208Pb, and 75As. RF power was set at 27 MHz, with a nebulizer gas flow rate of 0.9 L/min, auxiliary gas flow rate of 0.8 L/min, and coolant gas flow rate of 14.0 L/min, using the KED operation mode, with a QCell flow rate of 4.5 mL/min and a spray chamber temperature of 3 °C. The Pb test was performed using ICP-MS with the following Limits of Detection (LoD): Cd 0.024 mg/kg, Pb 0.021 mg/kg, and As 0.03 mg/kg, and the Limits of Quantification (LoQ): Cd 0.08 mg/kg, Pb 0.07 mg/kg, and As 0.1 mg/kg.
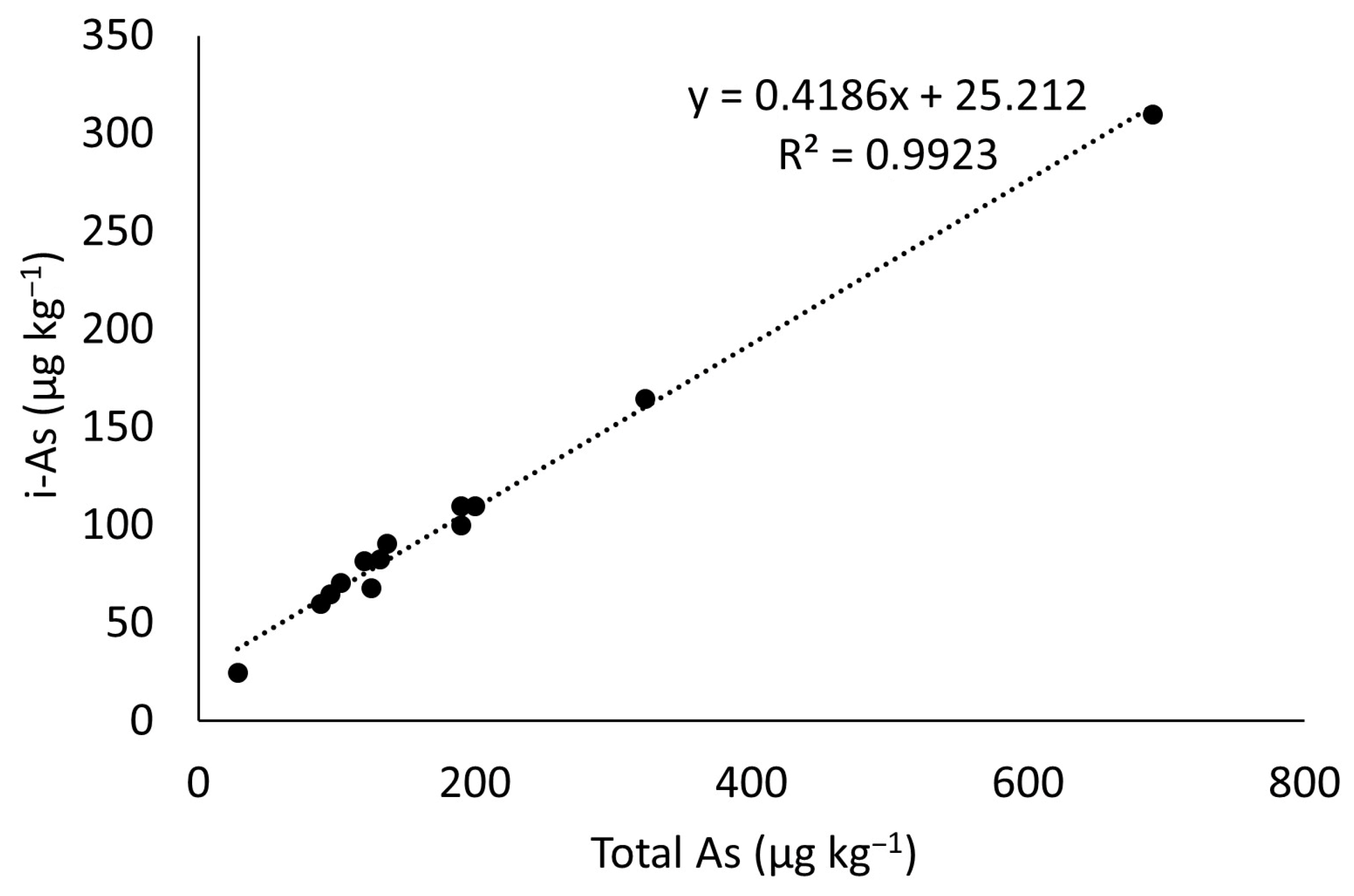
The concentration of inorganic arsenic (iAs) in rice samples was estimated using a linear regression model. The regression equation was developed based on data extracted from 13 paired observations of total arsenic (tAs) and inorganic arsenic (iAs) concentrations in rice reported in published studies from various Asian countries (
Table 1).
2.4. Human Risk Assessment and Exposure to Toxic Metals
2.4.1. Estimated Daily Intake (EDI)
The daily exposure values for Cd, Pb, and As were calculated using a deterministic approach. This deterministic approach was chosen for this study based on the FAO/WHO exposure assessment framework (2009) [
31], due to the availability of average food consumption data and average body weight data by age group obtained from the Individual Food Consumption Survey, Ministry of Health of the Republic of Indonesia [
22]. The exposure level calculation was conducted for the average Cd, Pb, and As concentrations; the maximum value and exposure levels were also calculated using the maximum regulatory limits for heavy metals in food in Indonesia [
16] as the heavy metal concentration.
The exposure to Cd, Pb, and As was calculated by multiplying the concentration by the rice consumption data and then dividing by the body weight data. This heavy metal exposure calculation was performed for the age groups of infants (0–59 months), children (5–12 years), adolescents (13–18 years), adults (19–55 years), and those over 55 years old. Rice consumption and body weight data by age group were obtained from the SKMI 2014 [
22]. The formula used for the exposure calculation, based on information from FAO/WHO [
31], is as follows:
where
C (µg/kg) is the concentration of the heavy metal in rice; the resulting exposure value is expressed in µg/kg bw/day.
The exposure to Cd, Pb, and As in cooked rice was calculated by multiplying the exposure value for rice by the processing factor (Cd −76.65%; Pb 1403%; Total As −34%), which is obtained from the change in Cd, Pb, and As concentrations in rice after it has been processed into cooked rice.
2.4.2. Risk Characterization
The risk characterization for Cd is carried out by calculating the risk value by comparing the exposure value with the total daily intake (TDI) value. The TDI value is obtained by dividing the PTMI value of 30 from Codex Alimentarius Standard 193-1995 [
17]. The formula for calculating the Cd risk value is as follows [
32]:
The risk characterization for Pb is carried out by calculating the Margin of Exposure (MOE) based on the BMDL
01 for cardiovascular effects in adults (1.5 µg/kg bw/day), BMDL
10 for kidney function impairment in adults (0.63 µg/kg bw/day), and BMDL
01 for neurotoxicity in children (0.5 µg/kg bw/day). The risk value for inorganic As is calculated by determining the MOE based on the reference BMDL for a 0.5% increase in lung cancer incidence due to i-As exposure, with a BMDL
0.5 of 3.0 µg/kg bw/day. The MOE calculation formula is as follows:
High lead exposure in infants and children increases the likelihood of intelligence decline. The estimation of Pb exposure through diet related to IQ reduction in children uses the combined output from the bilinear and Hill models, where every 30 μg of lead per day is associated with a 1-point decrease in IQ [
19].
2.5. Statistical Analyses
The data were analyzed using Microsoft Excel. The mean, maximum values and SD of each metal were calculated to describe the metal contents in the rice samples. The data 10 log-transformation was performed in order to reach normal distribution, and thus further proceed with parametric statistical tests. Statistical analysis using ANOVA was conducted to compare the Pb and Cd test results among the different provinces. Student’s t-tests compared the 10 log-transformed means of each metal in the soaked and rinsed grains. Differences between the treatment methods used to prepare the rice were considered significant at p-value < 0.05.
The relationship between t-As and iAs was analyzed using simple linear regression to derive a predictive equation. Statistical analysis was performed using Microsoft Excel, and the goodness-of-fit of the regression model was evaluated using the coefficient of determination (R
2). A significance level of
p < 0.05 was considered for all statistical tests. The regression equation can be seen in
Figure 1.
Although Microsoft Excel was used for statistical analysis and regression modeling, the risk characterization was conducted manually. This step followed established health risk assessment guidelines using standard equations and reference values. No statistical software was employed for the risk characterization calculations.
4. Conclusions
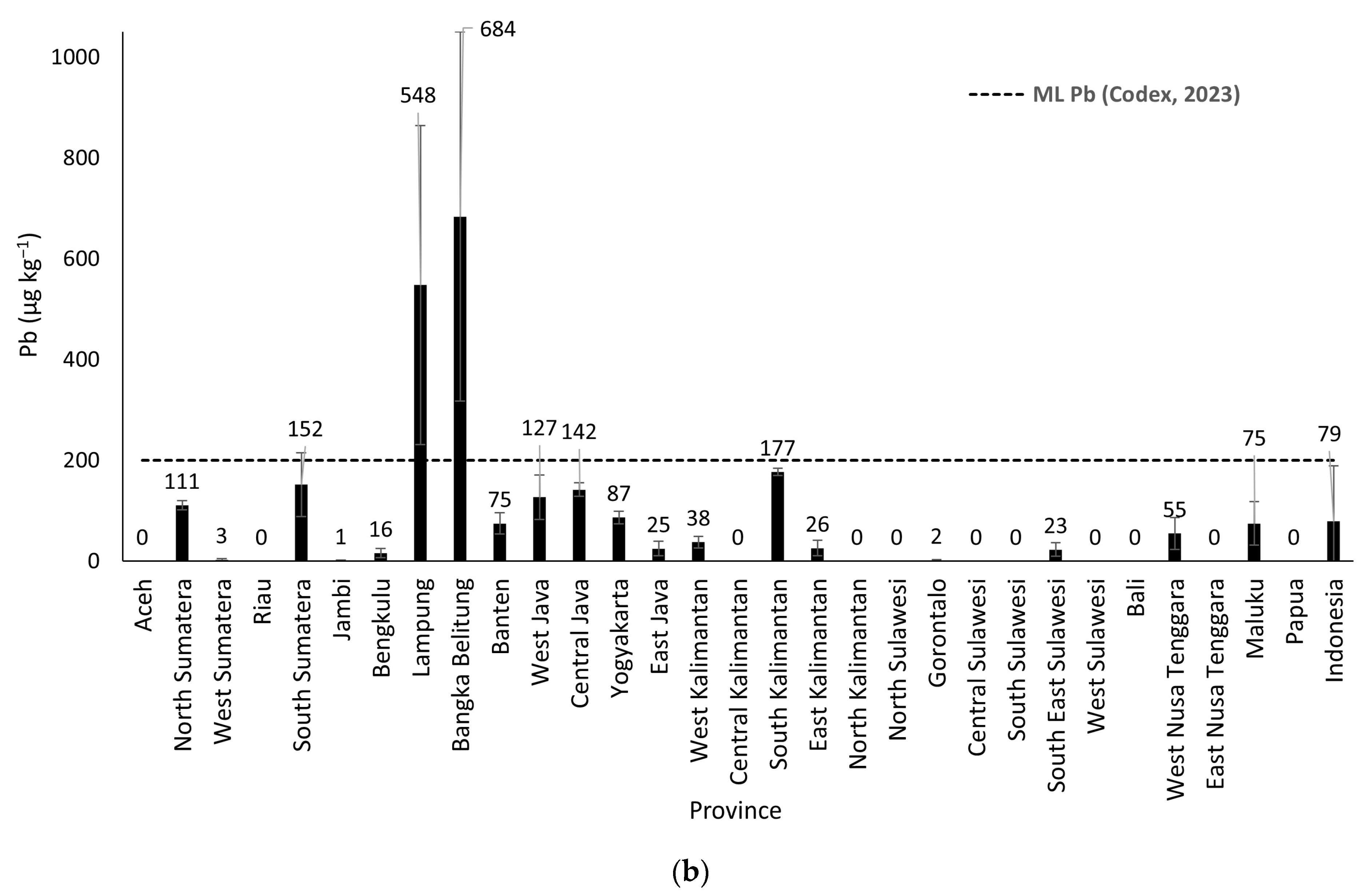
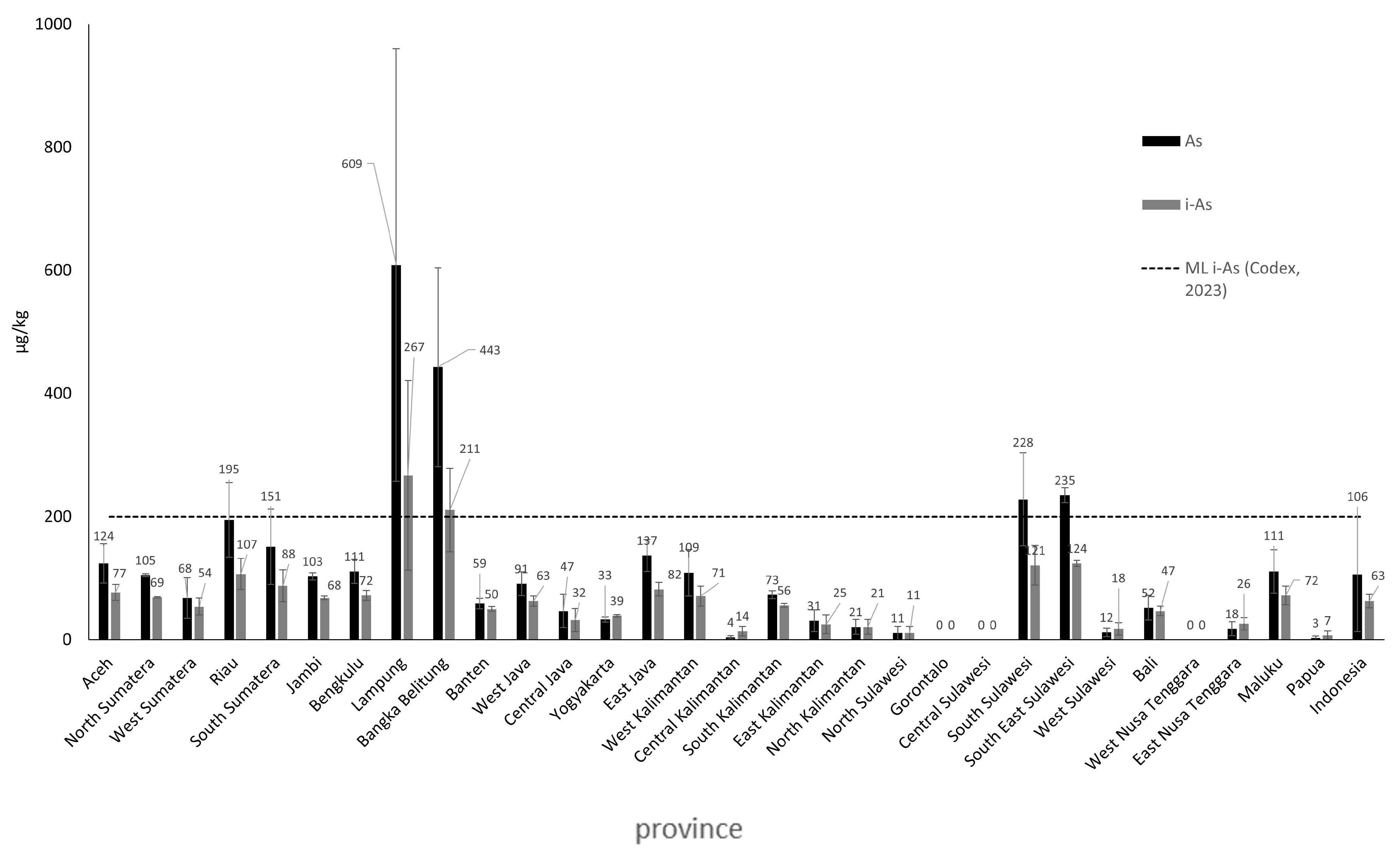
In this study, the average levels of heavy metals Cd, Pb, and As in rice in Indonesia are below the maximum contamination limits set by Codex Alimentarius. The average Cd level was 41 µg/kg, with values ranging from <LoD to 136 µg/kg. The average Pb level was 79 µg/kg, with concentrations ranging from <LoD to 684 µg/kg. In this study, the heavy metal testing for As in rice was conducted using the tAs test, with an average value of tAs of 106 µg/kg, and minimum and maximum values ranging from <LoD to 609 µg/kg. The estimated average concentration of inorganic arsenic (iAs) in rice samples from 30 provinces was 63 µg/kg, with values ranging from <LoD to 267 µg/kg. The concentrations of heavy metals in rice in Indonesia are in the following order: Pb > iAs > Cd. The Cd levels in rice from West Sumatra and Gorontalo, as well as Pb and As levels in rice from Lampung and Bangka Belitung, exceed the Codex Alimentarius maximum contamination limits. High concentrations of Cd and Pb in rice may be caused by various anthropogenic activities, including the use of chemical fertilizers and pesticides. High levels of As may be linked to mining activities and anthropogenic pollution that contaminate soil and irrigation in rice fields.
The Cd levels in rice decreased by a factor of 0.76 compared to those in raw rice. This reduction occurs due to washing and heating during the rice cooking process. Pb levels in rice increased by a factor of 14.03 compared to the levels in raw rice, likely due to Pb leaching from cooking equipment used during rice preparation. The As levels in rice showed no significant decrease compared to those in raw rice, as the cooking process with low water volumes does not significantly reduce As content.
The average risk values for Cd exposure from raw and cooked rice for all age groups are considered low (<100% PTMI). The Margin of Exposure (MOE) values for Pb exposure, based on BMDL01 (1.5 μg/kg bw/day) for cardiovascular effects in adults, BMDL10 (0.63 μg/kg bw/day) for kidney function impairment in adults, and BMDL01 (0.5 μg/kg bw/day) for neurotoxicity in children, are all below 10,000, indicating a high risk. Similarly, the MOE for As exposure, based on BMDL0.5 for lung cancer (3 µg/kg bw/day) for raw and cooked rice, is also below 10,000, indicating a high risk. The estimated IQ reduction due to Pb exposure from rice for the age group of toddlers (0–59 months) from rice is 0.18 ± 0.07 points, while for children (5–12 years), it is 0.38 ± 0.14 points. These reductions are lower than the IQ decrease observed at the maximum regulatory exposure limits. However, the IQ reduction from Pb exposure in rice for toddlers is 2.59 ± 0.95 points, and for children, it is 5.27 ± 1.93 points.
The results highlight the critical importance of strengthening food safety risk management practices in both rice production and processing. However, these results should be interpreted with caution due to several limitations, including the reliance on secondary dietary data, limited sampling scope, indirect estimation methods for inorganic arsenic, and the use of a small number of cooking scenarios. Future studies should address these limitations by incorporating direct measurements, expanding geographic and sample coverage, and exploring a wider range of cooking practices to enhance the accuracy and generalizability of the findings.