Protective Effects of Aucubin in DSS-Induced Colitis: Modulation of Inflammatory Pathways, Intestinal Barrier Integrity, and Gut Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Animal Experiment Design
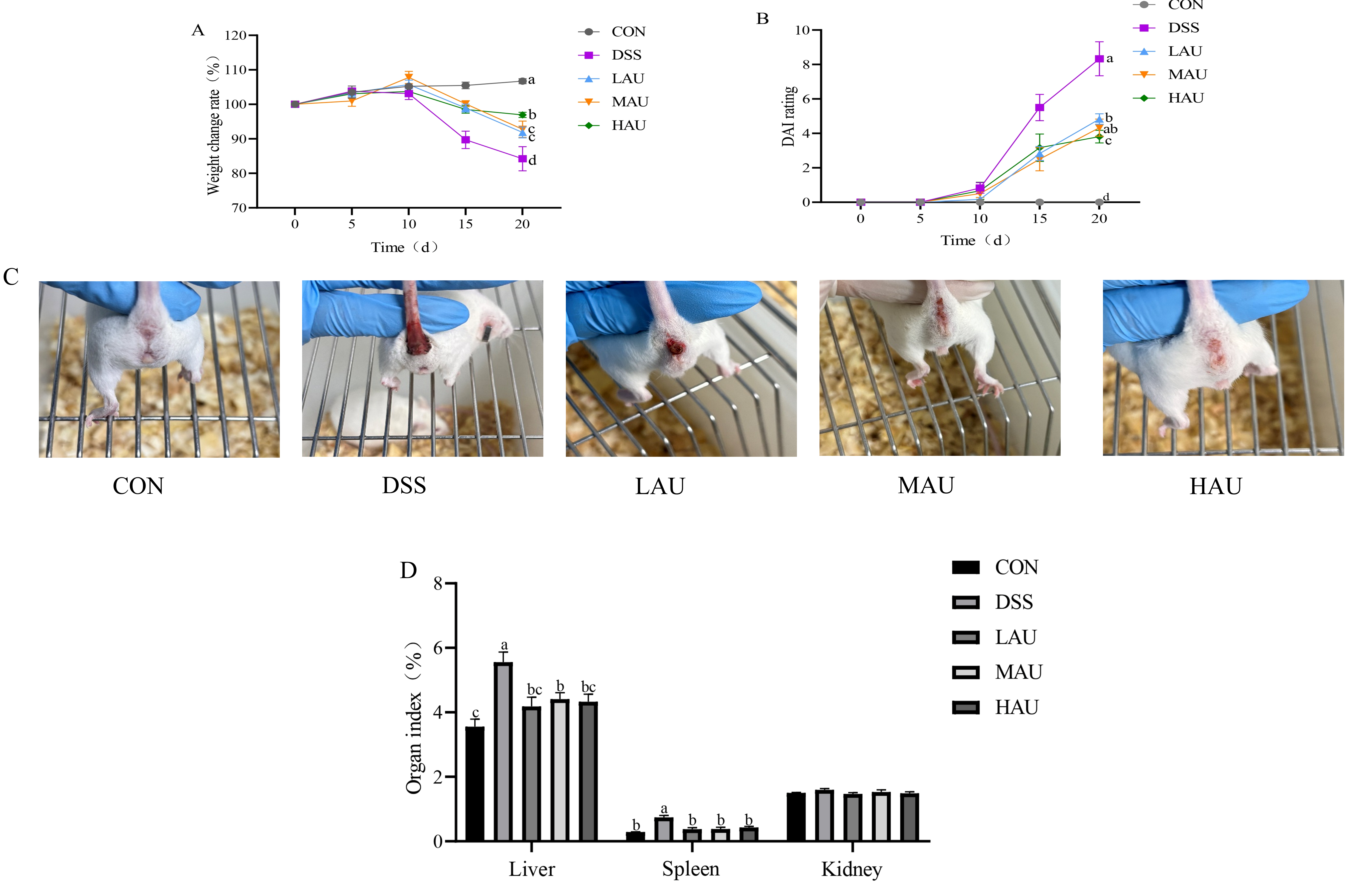
2.3. Assessment of Disease Activity Index (DAI)
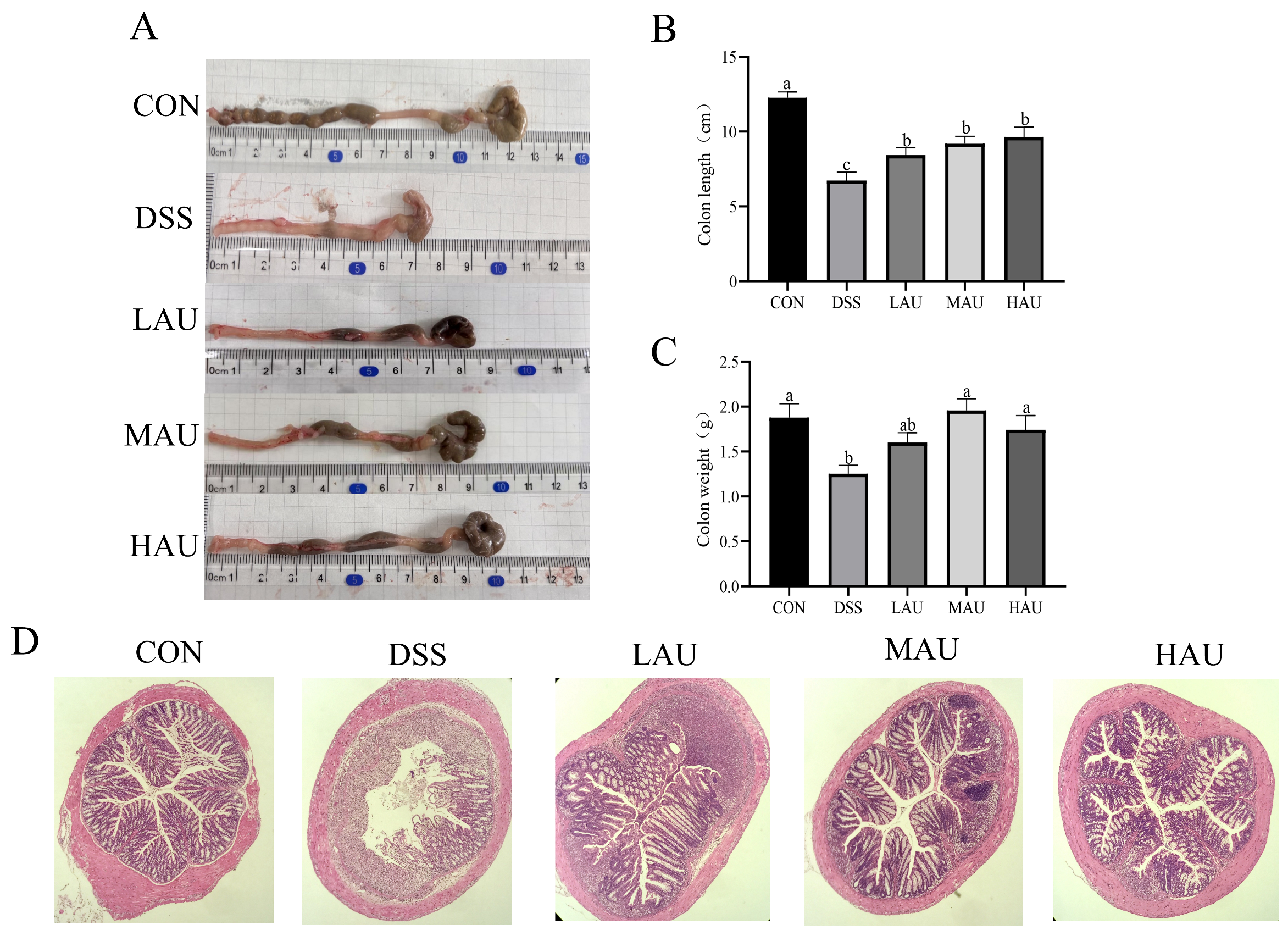
2.4. Determination of Length and Weight of Mouse Colon Tissue
2.5. HE Staining of Colon
2.6. Determination of Organ Index
2.7. Determination of Oxidative Stress-Related Indexes
2.8. Real-Time Quantitative PCR Analysis (RT-q PCR)
2.9. Determination of Microorganisms in the Cecum
2.10. Cell Culture and Treatment
Cell Resuscitation and Transfection
2.11. Cell Experimental Grouping
2.12. RT-q PCR Analysis
2.13. Statistical Analysis
3. Results
3.1. Effect of AU on the Phenotype of Colitis Mice
3.2. Effect of AU on Organ Indices in Colitis Mice
3.3. Effect of AU on the Colon of Colitis Mice
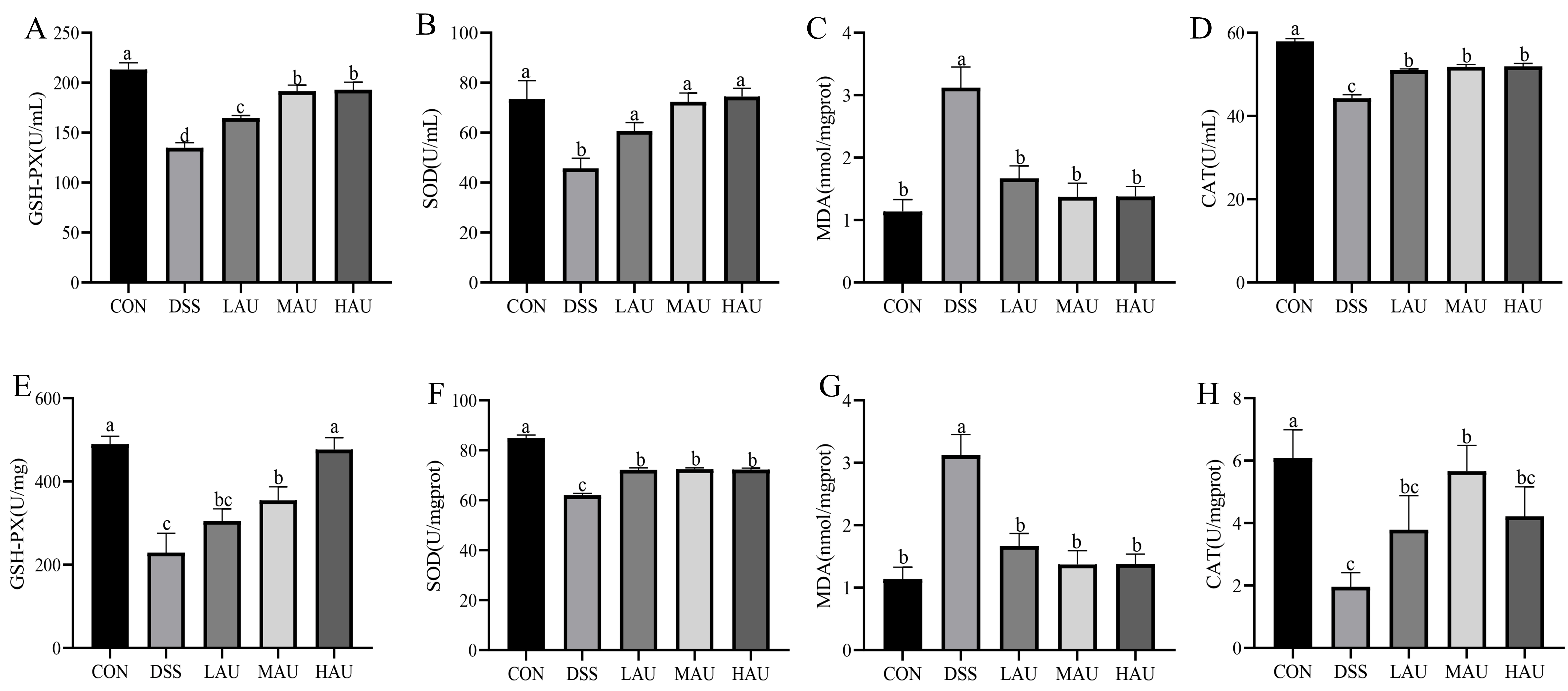
3.4. Effect of AU on Oxidative Stress in Colitis Mice
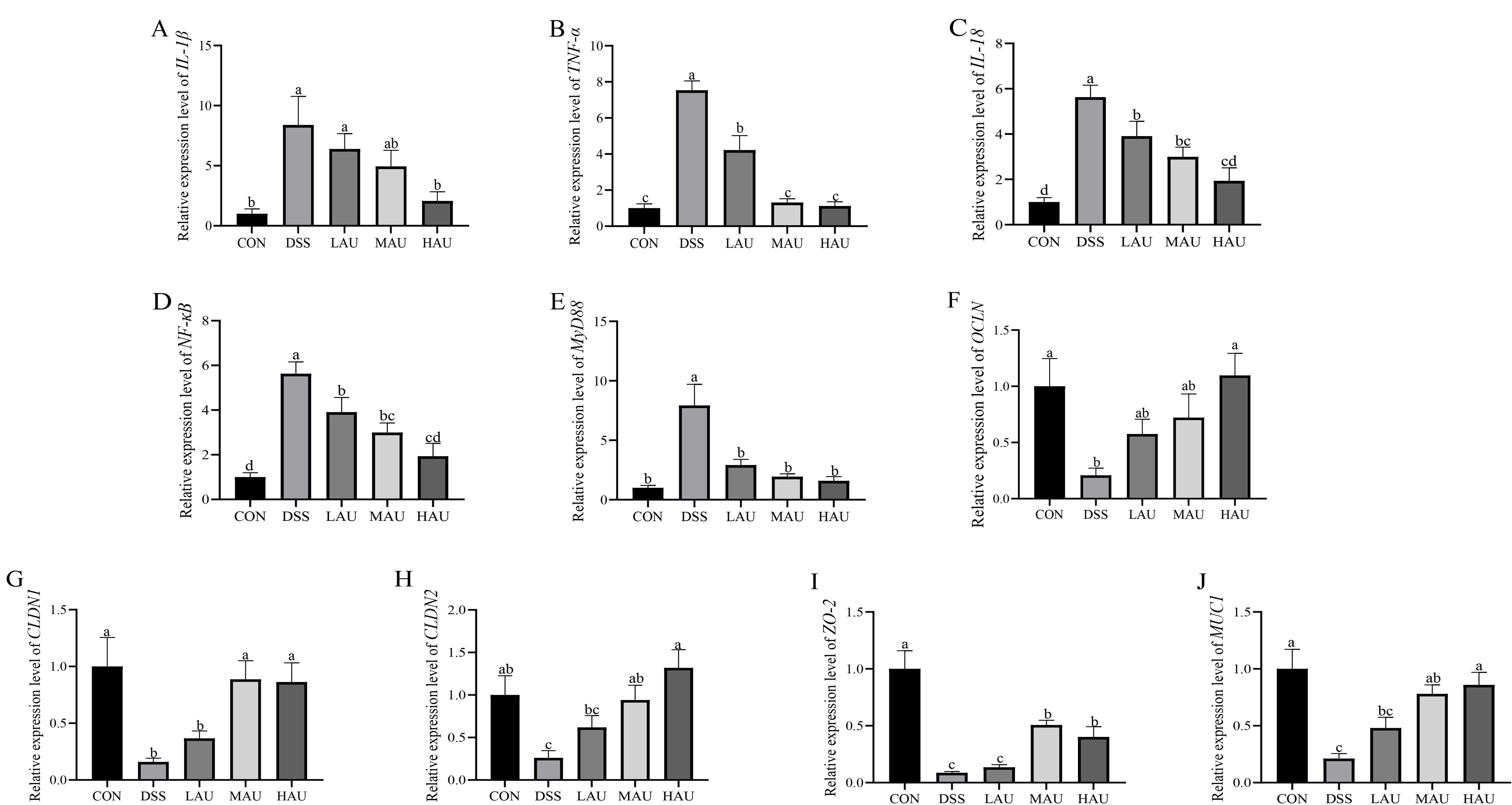
3.5. Effect of AU on Inflammatory Factors in Colitis Mice
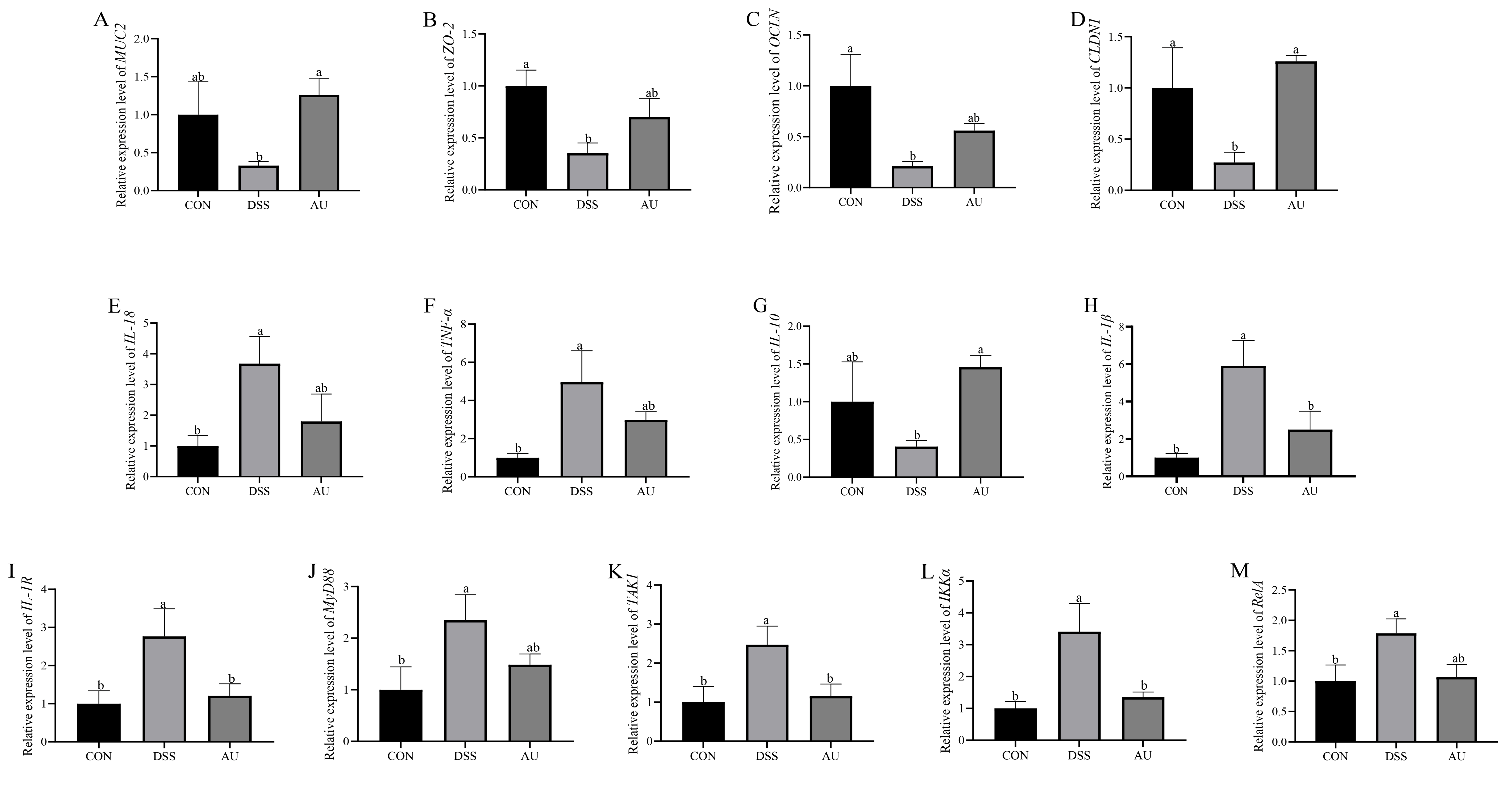
3.6. Effect of AU on the Intestinal Barrier of Colitis Mice
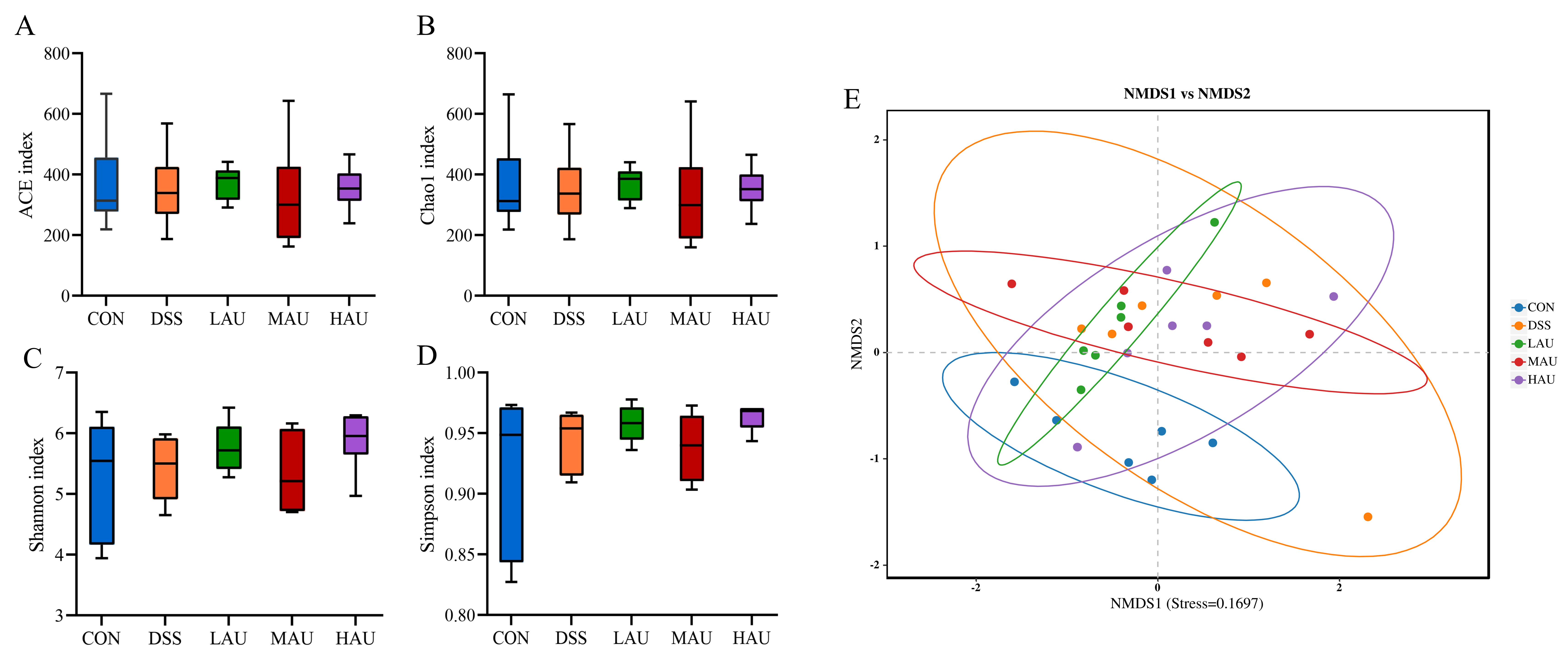
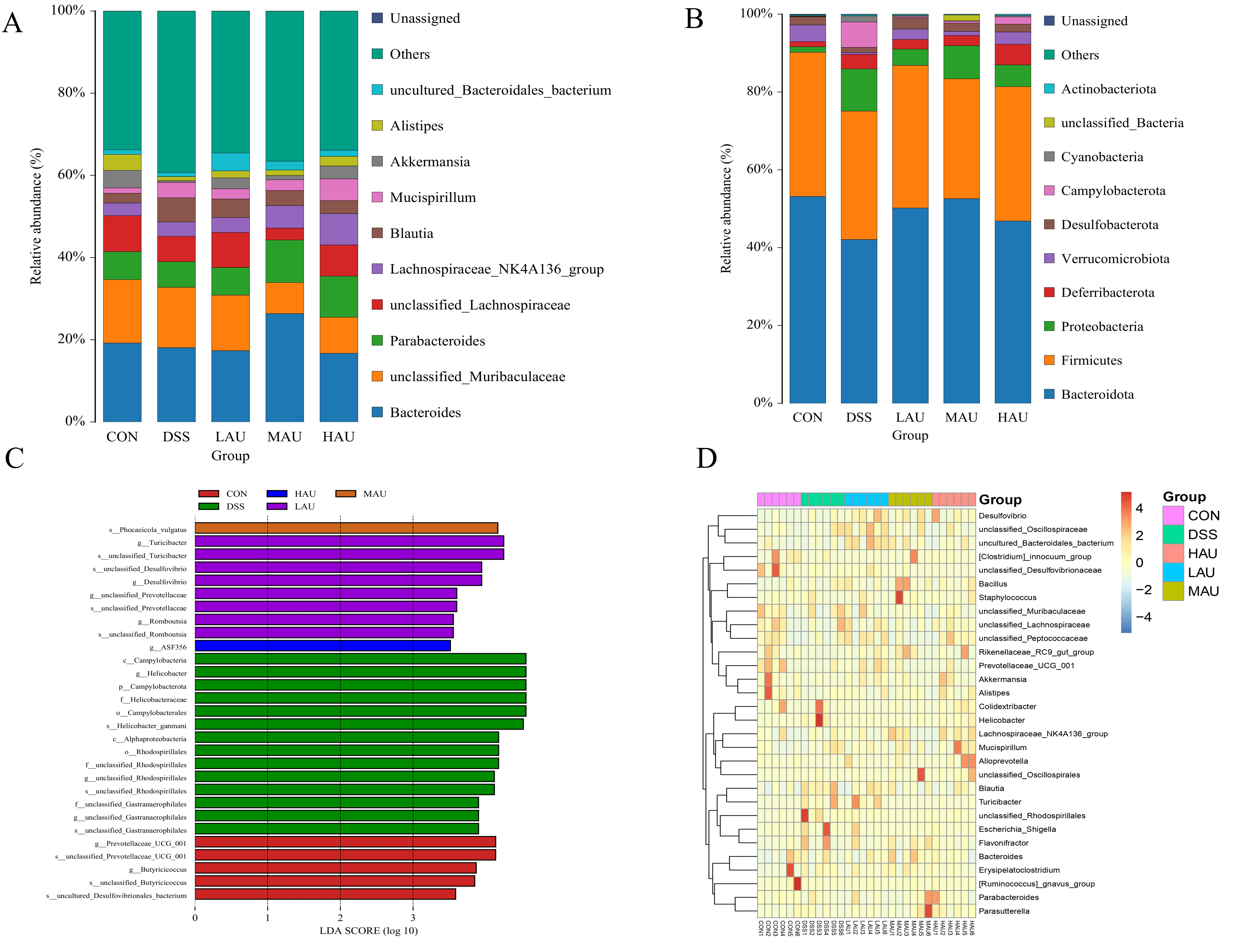
3.7. Effect of AU on the Cecum Flora of Mice with Colitis
- (1)
- Alpha Diversity Analysis
- (2)
- Beta Diversity Analysis
- (3)
- Analysis of Intestinal Flora Composition
- (4)
- Intergroup Microbial Difference Analysis
- (5)
- Genus-Level Clustering Heatmap
3.8. Effect of AU on DSS-Induced Gut Barrier in IPEC-J2 Cells
3.9. Effect of AU on DSS-Induced Inflammatory Factors in IPEC-J2 Cells
3.10. Effect of AU on Genes Related to MyD88/NF-κB Signaling Pathway in DSS-Induced IPEC-J2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Wang, Z.; Li, L.; Wang, X.; Wei, X.; Gou, S.; Ding, Z.; Cai, Z.; Ling, Q.; Hoffmann, P.R.; et al. Mannose coated selenium nanoparticles normalize intestinal homeostasis in mice and mitigate colitis by inhibiting NF-κB activation and enhancing glutathione peroxidase expression. J. Nanobiotechnol. 2024, 22, 613. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Tahan, V.; Shen, B. Secondary causes of inflammatory bowel diseases. World J. Gastroenterol. 2020, 26, 3998–4017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, P.; Qin, D.; Zhao, Y.; Chen, X.; Ma, P. Anti-inflammatory, anti-colitis, and antioxidant effects of columbianadin against DSS-induced ulcerative colitis in rats via alteration of HO-1/Nrf2 and TLR4-NF-κB signaling pathway. Inflammopharmacology 2025, 33, 341–352. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Lee, C.; Yu, Z.; Chen, C.; Liang, C. Ulcerative colitis: Molecular insights and intervention therapy. Mol. Biomed. 2024, 5, 42. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Matsuoka, K.; Kobayashi, T.; Sawada, K.; Fujiyoshi, T.; Ando, T.; Ohnishi, Y.; Ishida, T.; Oka, M.; Yamada, M.; et al. A large-scale, prospective, observational study of leukocytapheresis for ulcerative colitis: Treatment outcomes of 847 patients in clinical practice. J. Crohns Colitis 2014, 8, 981–991. [Google Scholar] [CrossRef]
- Jädert, C.; Phillipson, M.; Holm, L.; Lundberg, J.O.; Borniquel, S. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox Biol. 2014, 2, 73–81. [Google Scholar] [CrossRef]
- Borody, T.J.; Warren, E.F.; Leis, S.; Surace, R.; Ashman, O. Treatment of ulcerative colitis using fecal bacteriotherapy. J. Clin. Gastroenterol. 2003, 37, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dong, N.; Wu, B.; Mo, Z.; Xie, J.; Lu, Q. Dihydroberberine, an isoquinoline alkaloid, exhibits protective effect against dextran sulfate sodium-induced ulcerative colitis in mice. Phytomedicine 2021, 90, 153631. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Deng, J.; Yue, T.; Hu, W.; Zeng, X.; Yang, T.; Xiao, T. Berberine promotes M2 macrophage polarisation through the IL-4-STAT6 signalling pathway in ulcerative colitis treatment. Heliyon 2023, 9, e14176. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Mao, M.; Liu, W.; Wang, Y.; Liang, Y.; Cao, W.; Zhong, X. Betaine Ameliorates Acute Sever Ulcerative Colitis by Inhibiting Oxidative Stress Induced Inflammatory Pyroptosis. Mol. Nutr. Food Res. 2022, 66, e2200341. [Google Scholar] [CrossRef]
- Yu, P.; Wang, J.; Liu, J.; Zhou, Y.; Luo, F.; Yang, M.; Ai, X. Preparation techniques, structural features, and bioactivities of Eucommia ulmoides polysaccharides: A review. Int. J. Biol. Macromol. 2024, 275, 133686. [Google Scholar] [CrossRef]
- Wu, M.; Liu, P.; Wang, S.; Zhong, C.; Zhao, X. Ultrasonic Microwave-Assisted Micelle Combined with Fungal Pretreatment of Eucommia ulmoides Leaves Significantly Improved the Extraction Efficiency of Total Flavonoids and Gutta-Percha. Foods 2021, 10, 2399. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Tan, S.; Zhang, C.; Li, X.; Leng, X. In vitro effects of Eucommia ulmoides and its active components on the growth, lipid metabolism and collagen metabolism of grass carp (Ctenopharyngodon idellus) hepatocyte and intramuscular fibroblast. J. Fish. Biol. 2022, 101, 597–612. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, T.K.; Kim, D.W.; Ahn, J.H.; Lee, C.H.; Lim, S.S.; Kim, Y.H.; Cho, J.H.; Kang, I.J.; Won, M.H. Aucubin Exerts Neuroprotection against Forebrain Ischemia and Reperfusion Injury in Gerbils through Antioxidative and Neurotrophic Effects. Antioxidants 2023, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, T.K.; Kim, D.W.; Ahn, J.H.; Shin, M.C.; Cho, J.H.; Won, M.H.; Kang, I.J. Neuroprotective Effects of Aucubin against Cerebral Ischemia and Ischemia Injury through the Inhibition of the TLR4/NF-κB Inflammatory Signaling Pathway in Gerbils. Int. J. Mol. Sci. 2024, 25, 3461. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, H.; Wang, H.; Xue, Q.; Hu, M.; Li, Y. Aucubin produces anti-osteoporotic effects under mechanical stretch stress and orthodontic tooth movement. Chem. Biol. Interact. 2024, 393, 110955. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Wang, T.; Ferris, M.; Taylor, C.M.; Tian, X.; Luo, M.; Tran, D.Q.; Zhou, J.; Tatevian, N.; et al. Resetting microbiota by Lactobacillus reuteri inhibits T reg deficiency-induced autoimmunity via adenosine A2A receptors. J. Exp. Med. 2017, 214, 107–123. [Google Scholar] [CrossRef]
- Nascimento, R.P.D.; Machado, A.; Galvez, J.; Cazarin, C.B.B.; Maróstica Junior, M.R. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef] [PubMed]
- Nunes, N.S.; Chandran, P.; Sundby, M.; Visioli, F.; da Costa Gonçalves, F.; Burks, S.R.; Paz, A.H.; Frank, J.A. Therapeutic ultrasound attenuates DSS-induced colitis through the cholinergic anti-inflammatory pathway. EBioMedicine 2019, 45, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.X.; He, J.F.; Zhang, K.Q.; Luo, X.G.; Zhang, T.C. EtOAc extract of H. attenuatum Choisy inhibits inflammation by suppressing the NF-κB and MAPK pathways and modulating the gut microbiota. Phytomedicine 2019, 57, 292–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, G.; Wang, F.; Zhang, J.; Xu, L.; Yu, C. The impact of antibiotic exposure on antibiotic resistance gene dynamics in the gut microbiota of inflammatory bowel disease patients. Front. Microbiol. 2024, 15, 1382332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Yang, X.L.; Wang, L.; Cui, M.X.; Cai, Y.Q.; Li, X.L.; Wu, Y.J. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can. J. Physiol. Pharmacol. 2010, 88, 888–898. [Google Scholar] [CrossRef]
- Xin, J.; Wang, T.; Ou, Y.; Qiu, X.; Li, M.; Zhang, Y.; Lu, S.; Xu, L.; Guan, L.; Yao, H. Structural Characterization and Protective Effects of Camellia oleifera Fruit Shell Polysaccharides against DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2025, 73, 16860–16878. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Stefanon, B.; Colitti, M. Effect of plant extracts on H2O2-induced inflammatory gene expression in macrophages. J. Inflamm. Res. 2014, 7, 103–112. [Google Scholar] [CrossRef]
- Huang, T.C.; Tsai, S.S.; Liu, L.F.; Liu, Y.L.; Liu, H.J.; Chuang, K.P. Effect of Arctium lappa L. in the dextran sulfate sodium colitis mouse model. World J. Gastroenterol. 2010, 16, 4193–4199. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Guan, K.; Liu, R.; Mao, K.; Xu, X.; Li, Q.; Wang, R. Intestinal barrier, immunity and gut microbiota-based protective effects of Lactococcus lactis HF08 and its postbiotic derivative on aging and aging colitis mice. Food Res. Int. 2024, 197, 115164. [Google Scholar] [CrossRef]
- Cai, J.; Liu, J.; Fan, P.; Dong, X.; Zhu, K.; Liu, X.; Zhang, N.; Cao, Y. Dioscin prevents DSS-induced colitis in mice with enhancing intestinal barrier function and reducing colon inflammation. Int. Immunopharmacol. 2021, 99, 108015. [Google Scholar] [CrossRef]
- Liu, K.; Yin, Y.; Shi, C.; Yan, C.; Zhang, Y.; Qiu, L.; He, S.; Li, G. Asiaticoside ameliorates DSS-induced colitis in mice by inhibiting inflammatory response, protecting intestinal barrier and regulating intestinal microecology. Phytother. Res. 2024, 38, 2023–2040. [Google Scholar] [CrossRef]
- Tian, M.; Ma, P.; Zhang, Y.; Mi, Y.; Fan, D. Ginsenoside Rk3 alleviated DSS-induced ulcerative colitis by protecting colon barrier and inhibiting NLRP3 inflammasome pathway. Int. Immunopharmacol. 2020, 85, 106645. [Google Scholar] [CrossRef]
- Song, W.; Zhang, T.; Wang, Y.; Xue, S.; Zhang, Y.; Zhang, G. Glycyrrhiza uralensis Polysaccharide Modulates Characteristic Bacteria and Metabolites, Improving the Immune Function of Healthy Mice. Nutrients 2025, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yang, R.; Qiang, J.; Xu, X.; Zhou, M.; Ji, X.; Lu, Y.; Dong, Z. Gypenoside XLIX attenuates sepsis-induced splenic injury through inhibiting inflammation and oxidative stress. Int. Immunopharmacol. 2024, 127, 111420. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.H.; Li, J.J.; Li, S.F.; Guo, J.; Yan, R.P.; Chen, T.G.; Shi, X.H.; Wang, J.D.; Zhang, L.W. Phillygenin Attenuated Colon Inflammation and Improved Intestinal Mucosal Barrier in DSS-induced Colitis Mice via TLR4/Src Mediated MAPK and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 2238. [Google Scholar] [CrossRef]
- Yu, S.; Sun, Y.; Shao, X.; Zhou, Y.; Yu, Y.; Kuai, X.; Zhou, C. Leaky Gut in IBD: Intestinal Barrier-Gut Microbiota Interaction. J. Microbiol. Biotechnol. 2022, 32, 825–834. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, Q. Momordica charantia polysaccharides alleviate diarrhea-predominant irritable bowel syndrome by regulating intestinal inflammation and barrier via NF-κB pathway. Allergol. Immunopathol. 2022, 50, 62–70. [Google Scholar] [CrossRef]
- Qiu, L.; Yan, C.; Yang, Y.; Liu, K.; Yin, Y.; Zhang, Y.; Lei, Y.; Jia, X.; Li, G. Morin alleviates DSS-induced ulcerative colitis in mice via inhibition of inflammation and modulation of intestinal microbiota. Int. Immunopharmacol. 2024, 140, 112846. [Google Scholar] [CrossRef]
- Yao, X.; Chen, Y.; Li, Y.; Mo, J.; Liu, X.; Wang, P.; Jia, D.; Li, H.; Guo, C. Chrysin ameliorates dextran sulfate-induced ulcerative colitis in mice by modulating inflammation and gut microbiota. Int. J. Color. Dis. 2025, 40, 57. [Google Scholar] [CrossRef]
- Wang, L.; Lu, H.; Gui, H.; Ni, Z.; Sun, Z.; Wang, Z.; Wang, Z.; Liu, X.; Yuan, Q. D-Tagatose attenuates DSS-induced ulcerative colitis by inhibiting inflammation, reducing intestinal barrier damage and modulating the intestinal flora composition. Food Funct. 2025, 16, 5556–5572. [Google Scholar] [CrossRef]
- Pan, H.; Yang, S.; Kulyar, M.F.; Ma, H.; Li, K.; Zhang, L.; Mo, Q.; Li, J. Lactobacillus fermentum 016 Alleviates Mice Colitis by Modulating Oxidative Stress, Gut Microbiota, and Microbial Metabolism. Nutrients 2025, 17, 452. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, Q.; Xue, S.; Hui, W.; Kong, W.; Zhang, M.; Gao, F. Clinical characteristics of ulcerative colitis patients with different types of Helicobacter pylori infection. Microbiol. Spectr. 2024, 12, e0355423. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lin, H.; Shen, W.; Cao, W.; Qin, X.; Gao, J.; Chen, Z.; Zheng, H.; Zhong, S.; Huang, H. The Preventive Effect of Low-Molecular Weight Oyster Peptides on Lipopolysaccharide-Induced Acute Colitis in Mice by Modulating Intestinal Microbiota Communities. Foods 2024, 13, 2391. [Google Scholar] [CrossRef]
- Wu, M.; Bian, J.; Han, S.; Zhang, C.; Xu, W.; Tao, L.; Li, Z.; Zhang, Y. Characterization of hepatotoxic effects induced by pyraclostrobin in human HepG2 cells and zebrafish larvae. Chemosphere 2023, 340, 139732. [Google Scholar] [CrossRef]
- Li, M.; Tang, D.; Yang, T.; Qian, D.; Xu, R. Apoptosis Triggering, an Important Way for Natural Products From Herbal Medicines to Treat Pancreatic Cancers. Front. Pharmacol. 2021, 12, 796300. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Liu, J.; Li, M. Lipopolysaccharide promotes apoptosis and oxidative injury of porcine small intestinal epithelial cells by down-regulating the expression of glutamine transporter ASCT2. J. Anim. Sci. 2023, 101, skad229. [Google Scholar] [CrossRef]
- Goo, D.; Ko, H.; Sharma, M.K.; Choppa, V.S.R.; Paneru, D.; Shi, H.; Kim, W.K. Comparison of necrotic enteritis effects on growth performance and intestinal health in two different meat-type chicken strains Athens Canadian Random Bred and Cobb 500. Poult. Sci. 2024, 103, 103599. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Li, K.; Han, X.; Lin, W.; Qin, Y.; Cao, R.; Ren, Y. Long-term use of etomidate disrupts the intestinal homeostasis and nervous system in mice. Toxicology 2024, 504, 153802. [Google Scholar] [CrossRef]
- Juneja, P.; Sharma, A.; Shasthry, S.M.; Kumar, G.; Tripathi, D.M.; Rajan, V.; Rastogi, A.; Sarin, S.K.; Kaur, S. Podoplanin-positive dilated lymphatic vessels in duodenum associates with three-month mortality in patients with cirrhosis. Front. Physiol. 2023, 14, 1045983. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Sun, W. Research progress of probiotics and their protective strategy in the field of inflammatory bowel disease treatment: A review. Medicine 2024, 103, e40401. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Zheng, T.; Chen, X.; Xie, Z. Yellow Teas Protect against DSS-Induced Ulcerative Colitis by Inhibiting TLR4/NF-κB/NLRP3 Inflammasome in Mice. Foods 2024, 13, 2843. [Google Scholar] [CrossRef]
- Kulhari, U.; Rajanan, A.; Ambujakshan, A.; Verma, S.; Mugale, M.N.; Sahu, B.D. Biochanin A mitigates ulcerative colitis and intestinal inflammation in mice by inhibiting MAPK/NF-kB (p65) axis. J. Biochem. Mol. Toxicol. 2024, 38, e23738. [Google Scholar] [CrossRef]
| Score | Percentage Weight Loss % | Degree of Diarrhea | Degree of Bleeding in the Stool |
|---|---|---|---|
| 0 | 0 | Normal | Normal |
| 1 | 1~5% | Mildly soft stools, loose and shapely | Normal |
| 2 | 6~10% | Severe soft stools, loose | Small amount of blood in the stool |
| 3 | 11~20% | Mild diarrhea, loose, moist | Mild blood in stool |
| 4 | >21% | Severe diarrhea, liquid adherence to anus | Severe blood in stool |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qiao, H.; Cao, Y.; Zhang, M.; Zhang, X.; Li, P. Protective Effects of Aucubin in DSS-Induced Colitis: Modulation of Inflammatory Pathways, Intestinal Barrier Integrity, and Gut Microbiota. Foods 2025, 14, 3648. https://doi.org/10.3390/foods14213648
Zhang Y, Qiao H, Cao Y, Zhang M, Zhang X, Li P. Protective Effects of Aucubin in DSS-Induced Colitis: Modulation of Inflammatory Pathways, Intestinal Barrier Integrity, and Gut Microbiota. Foods. 2025; 14(21):3648. https://doi.org/10.3390/foods14213648
Chicago/Turabian StyleZhang, Yong, Han Qiao, Yuxin Cao, Meng Zhang, Xuelei Zhang, and Peng Li. 2025. "Protective Effects of Aucubin in DSS-Induced Colitis: Modulation of Inflammatory Pathways, Intestinal Barrier Integrity, and Gut Microbiota" Foods 14, no. 21: 3648. https://doi.org/10.3390/foods14213648
APA StyleZhang, Y., Qiao, H., Cao, Y., Zhang, M., Zhang, X., & Li, P. (2025). Protective Effects of Aucubin in DSS-Induced Colitis: Modulation of Inflammatory Pathways, Intestinal Barrier Integrity, and Gut Microbiota. Foods, 14(21), 3648. https://doi.org/10.3390/foods14213648





