Processing-Induced Changes in Bioactive Compounds and Antioxidant Activity of Orange-Fleshed Sweet Potato (Ipomoea batatas L.): Steaming Versus Air-Frying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Analysis
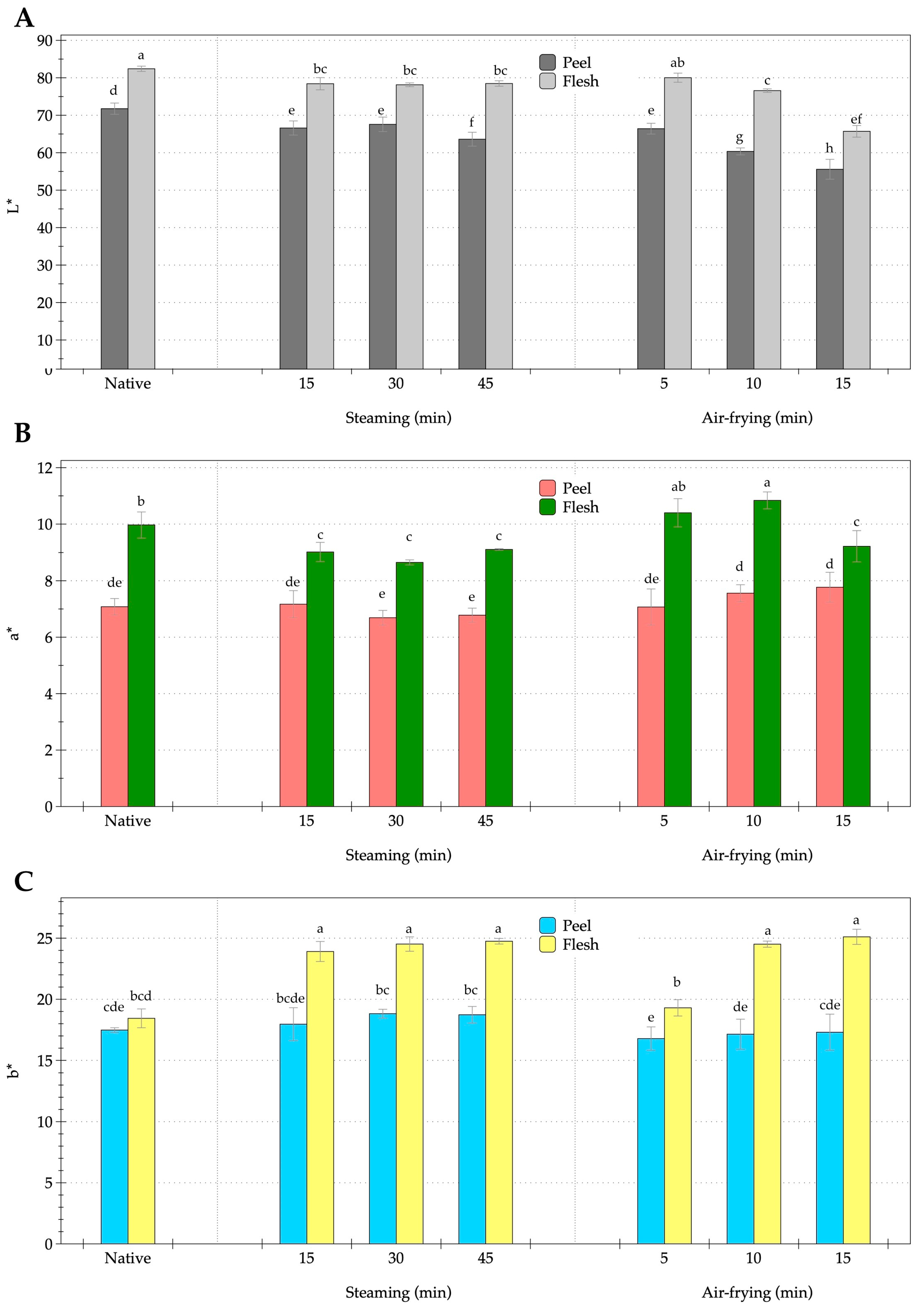
2.3.1. Color
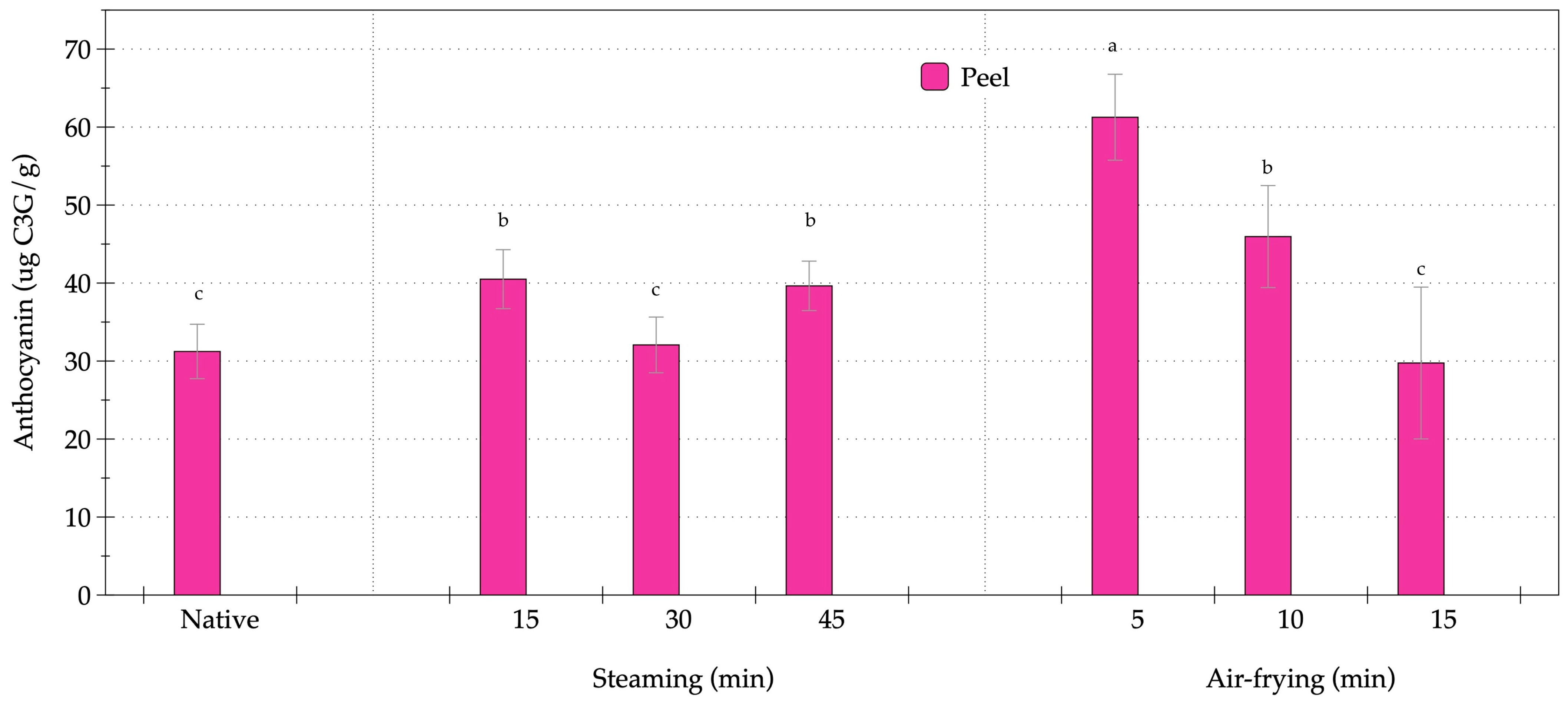
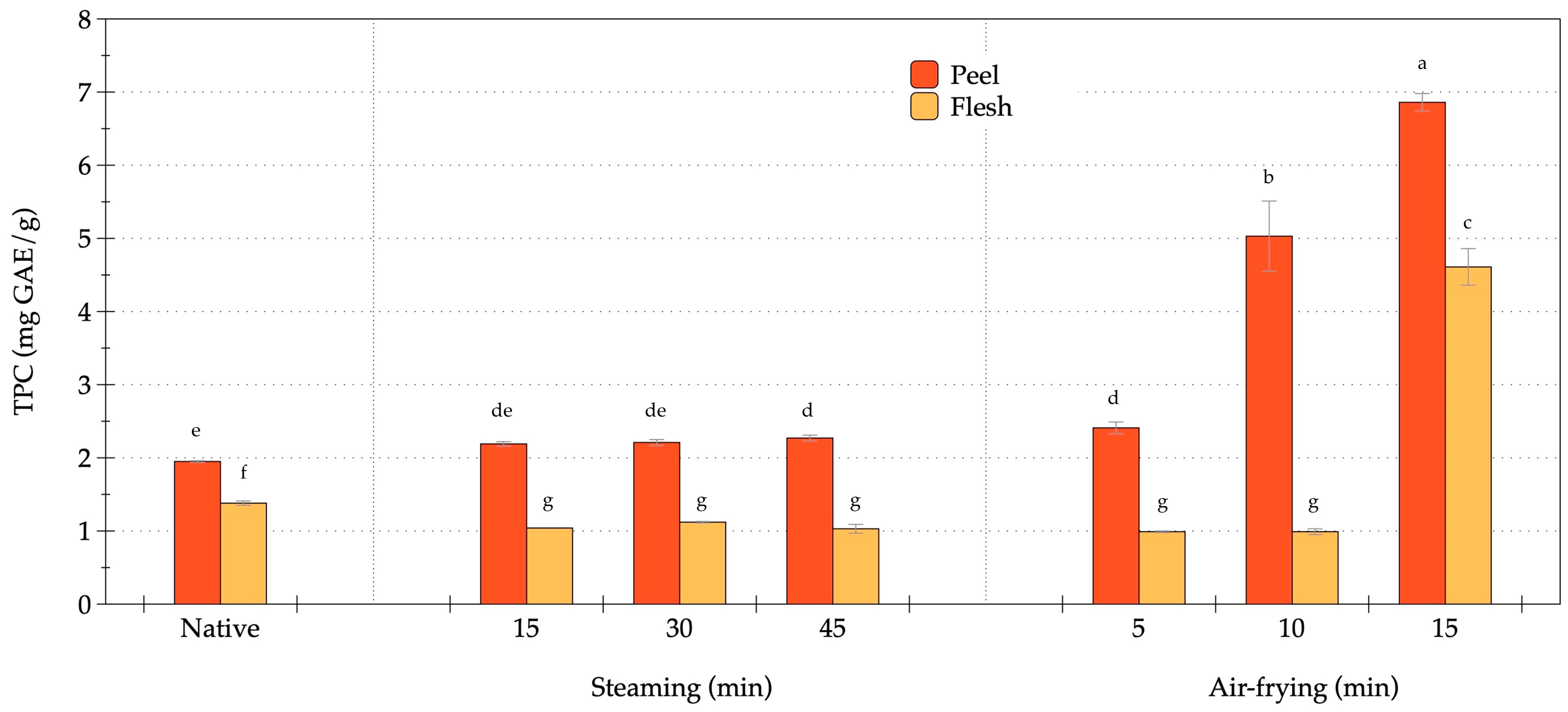
2.3.2. Bioactive Compounds
2.3.3. Starch
2.3.4. Antioxidant Activity Assessed by the DPPH Assay
2.3.5. Antioxidant Activity Assessed by the ABTS Assay
2.3.6. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4. Statistical Analysis
3. Results
3.1. Effect of Steaming and Air-Frying on Colorimetric Attributes of OFSP
3.2. Effect of Steaming and Air-Frying on Bioactive Compounds in OFSP
3.3. Effect of Steaming and Air-Frying on Starch in OFSP
3.4. Effect of Steaming and Air-Frying on Antioxidant Capacities of OFSP
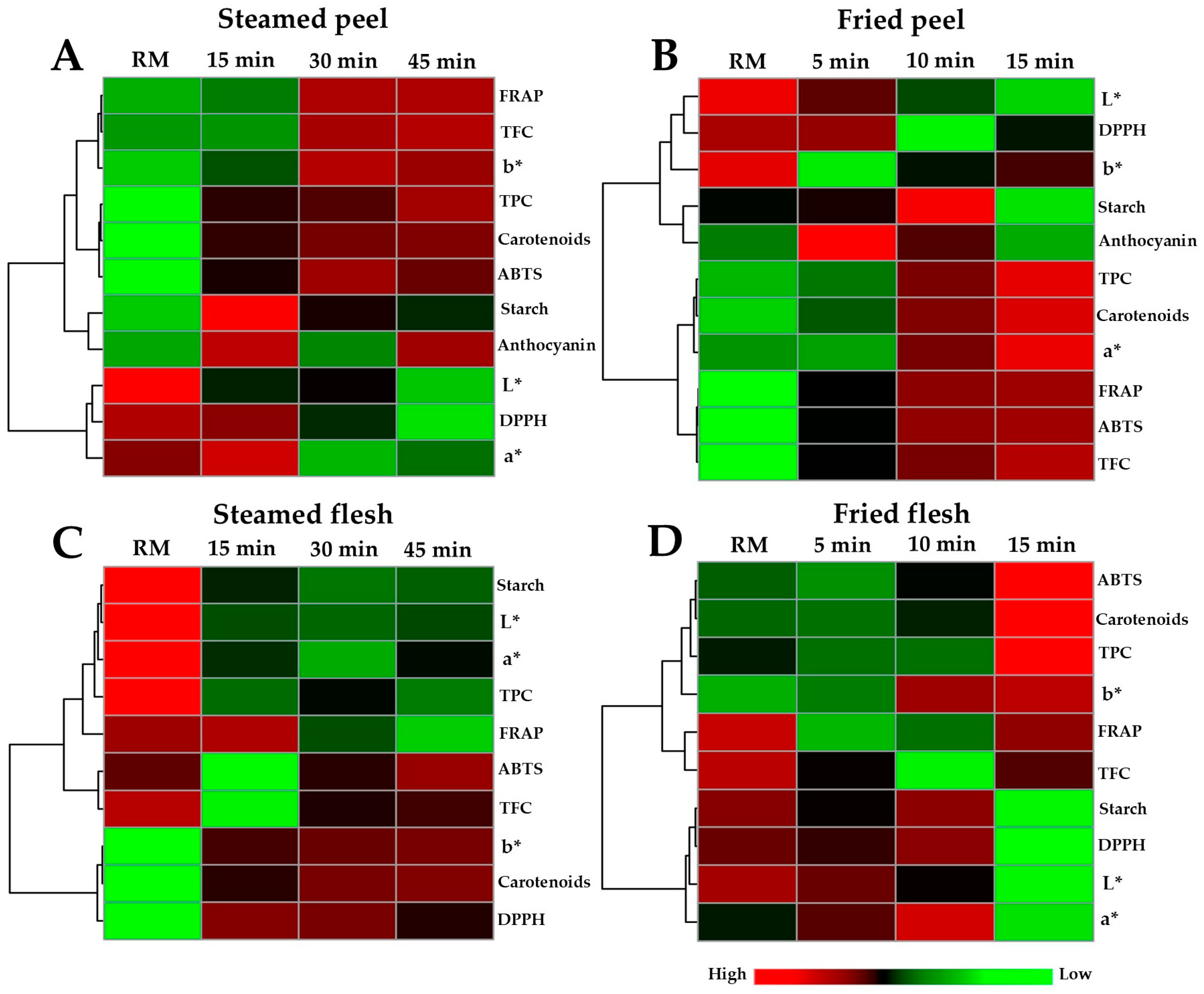
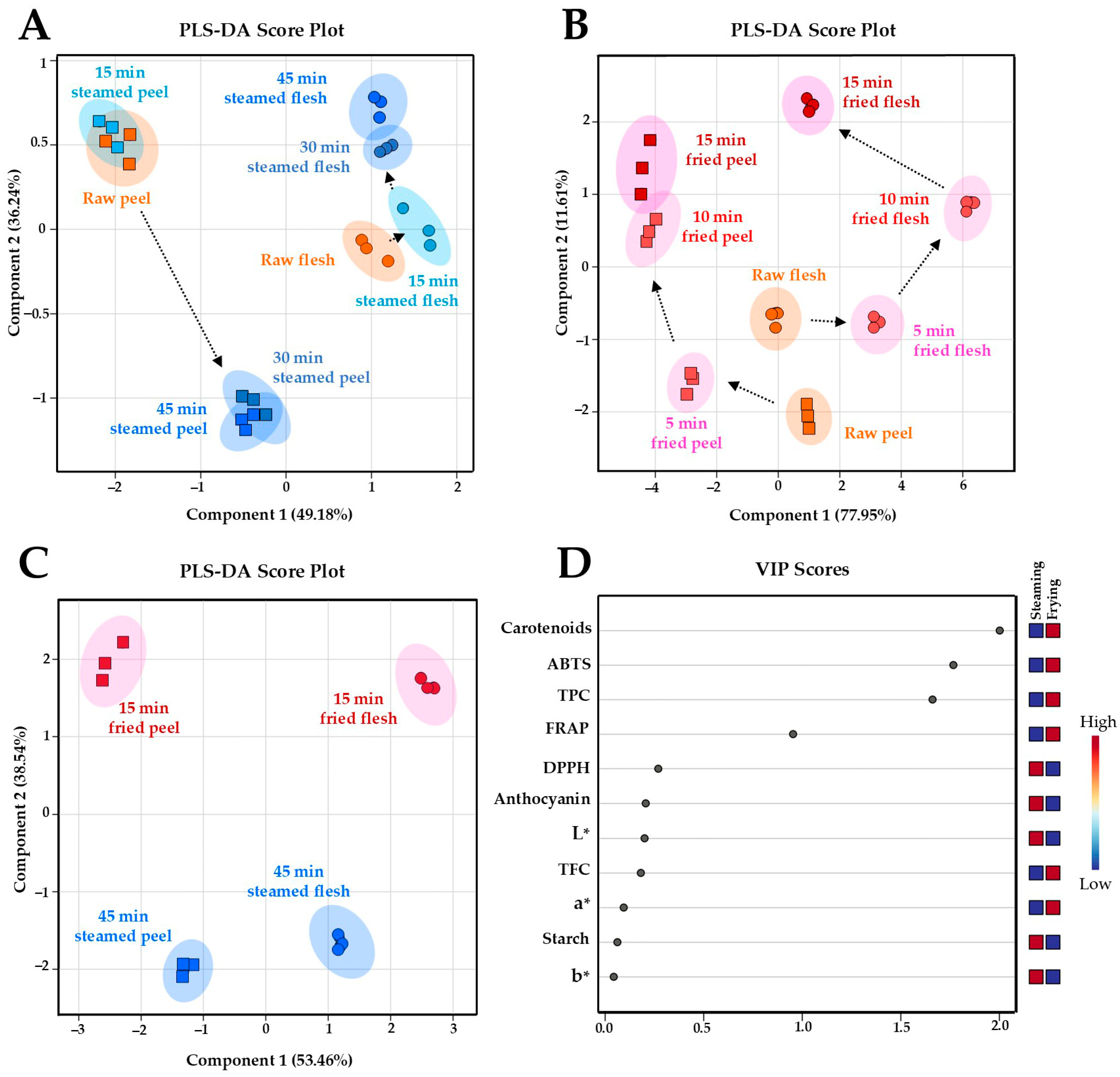
3.5. Changes in OFSP Chemical Profiles During Steaming and Air-Frying
3.6. Comparative Chemical Profiling of OFSP Across Different Processing Time Points
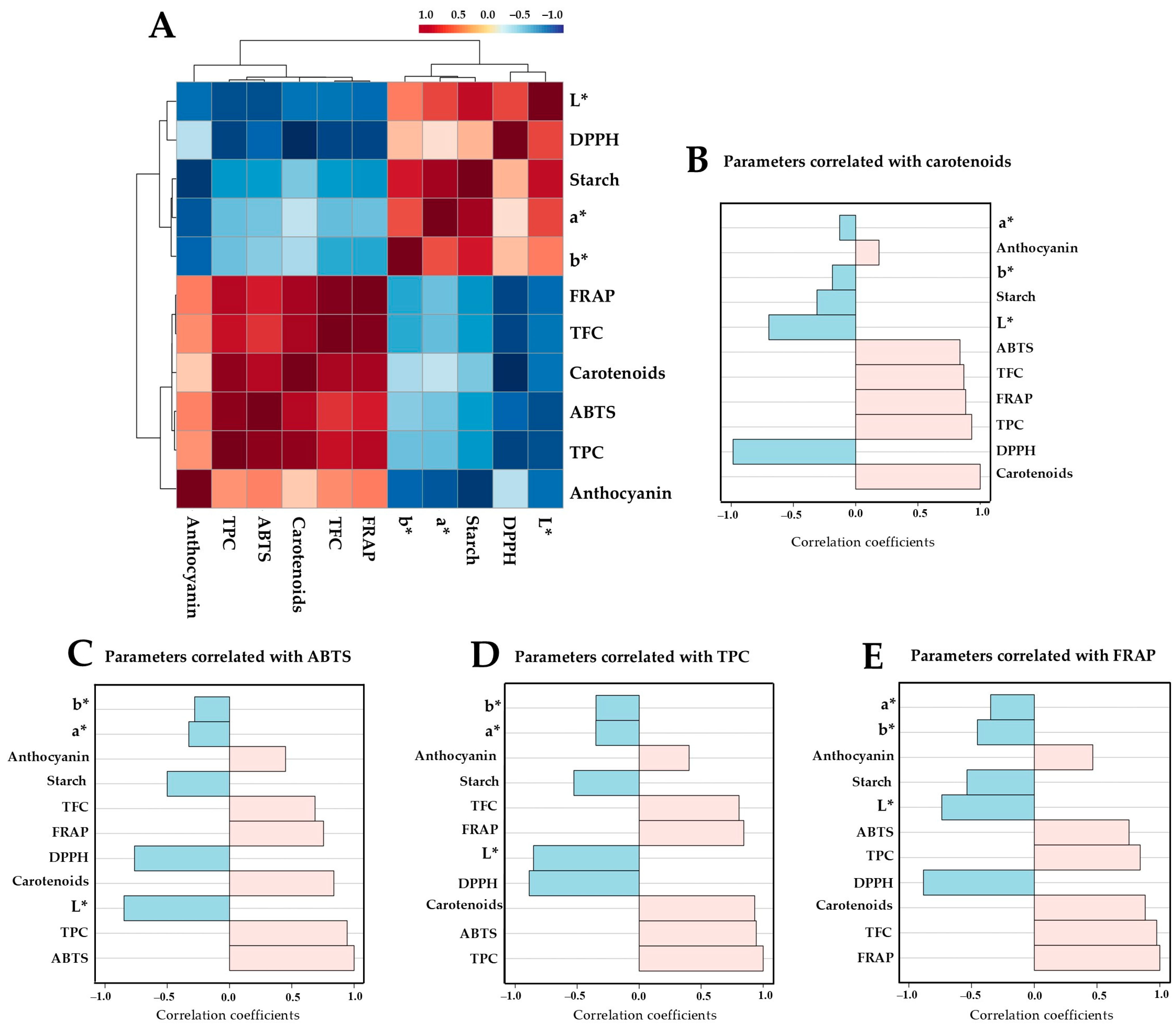
3.7. Correlation Analysis Among Chemical Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OFSP | Orange-fleshed sweet potato |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
References
- Alam, M.K. A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits. Trends Food Sci. Technol. 2021, 115, 512–529. [Google Scholar] [CrossRef]
- Gebregziabher, B.S.; Gebremeskel, H.; Debesa, B.; Ayalneh, D.; Mitiku, T.; Wendwessen, T.; Habtemariam, E.; Nur, S.; Getachew, T. Carotenoids: Dietary sources, health functions, biofortification, marketing trend and affecting factors—A review. J. Agric. Food Res. 2023, 14, 100834. [Google Scholar] [CrossRef]
- Neela, S.; Fanta, S.W. Review on nutritional composition of orange-fleshed sweet potato and its role in management of vitamin A deficiency. Food Sci. Nutr. 2019, 7, 1920–1945. [Google Scholar] [CrossRef]
- Ivane, N.M.A.; Wang, W.; Ma, Q.; Wang, J.; Sun, J. Harnessing the health benefits of purple and yellow-fleshed sweet potatoes: Phytochemical composition, stabilization methods, and industrial utilization—A review. Food Chem. X 2024, 23, 101462. [Google Scholar] [CrossRef]
- Owade, J.; Abong, G.; Okoth, M. Production, Utilization and Nutritional Benefits of Orange Fleshed Sweetpotato (OFSP) Puree Bread: A Review Article History. Curr. Res. Nutr. Food Sci. J. 2018, 6, 644. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Effects of cooking process on carotenoids and antioxidant activity of orange-fleshed sweet potato. LWT 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Franková, H.; Musilová, J.; Árvay, J.; Šnirc, M.; Jančo, I.; Lidiková, J.; Vollmannová, A. Changes in Antioxidant Properties and Phenolics in Sweet Potatoes (Ipomoea batatas L.). Due Heat. Treatments. Mol. 2022, 27, 1884. [Google Scholar] [CrossRef]
- Amagloh, F.C.; Kaaya, A.N.; Yada, B.; Chelangat, D.M.; Katungisa, A.; Amagloh, F.K.; Tumuhimbise, G.A. Bioactive compounds and antioxidant activities in peeled and unpeeled sweetpotato roots of different varieties and clones in Uganda. Future Foods 2022, 6, 100183. [Google Scholar] [CrossRef]
- Kamal, A.; Golshany, H. Effect of Cooking Treatments on Drying Kinetics, Polyphenols Content and Antioxidant Activity of Orange-Fleshed Sweet Potato. Int. J. Environ. Agric. Biotechnol. 2024, 9, 318–325. [Google Scholar] [CrossRef]
- Téllez-Morales, J.A.; Rodríguez-Miranda, J.; Aguilar-Garay, R. Review of the influence of hot air frying on food quality. Meas. Food 2024, 14, 100153. [Google Scholar] [CrossRef]
- Nandasiri, R.; Semenko, B.; Wijekoon, C.; Suh, M. Air-Frying Is a Better Thermal Processing Choice for Improving Antioxidant Properties of Brassica Vegetables. Antioxidants 2023, 12, 490. [Google Scholar] [CrossRef]
- Fornazier, E.L.; Sant Ana, C.T.; da Silva Oliveira, D.; Costa, N.M.B.; Carneiro, J.C.S.; Silva, P.I. Biofortified Sweet Potato Submitted to Different Domestic Cooking Processes: Impact on β-Carotene Retention and Antioxidant Capacity. Plant Foods Hum. Nutr. 2025, 80, 68. [Google Scholar] [CrossRef]
- Basílio, L.; Nunes, A.; Minatel, I.O.; Diamante, M.; Lázaro, C.; Silva, A.; Vargas, P.; Vianello, F.; Maraschin, M.; Lima, G. The Phytochemical Profile and Antioxidant Activity of Thermally Processed Colorful Sweet Potatoes. Horticulturae 2023, 10, 18. [Google Scholar] [CrossRef]
- Jakkranuhwat, N.; Kunchansombat, P. Effect of Foam-Mat Drying Conditions on Antioxidant Activity, Total Phenolic Compound, Anthocyanin Content and Color of Purple-Fleshed Sweet Potato Powder. Chiang Mai Univ. J. Nat. Sci. 2021, 20, e2021045. [Google Scholar] [CrossRef]
- Phomkaivon, N.; Amilia, N.; Tanintaratan, W.; Yonekura, L.; Tamura, H. Polyphenols in Naruto Kintoki sweet potato enhanced antiallergic activity after baking and microwave cooking. Food Sci. Technol. Res. 2022, 28, 275–283. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Ooi, S.; Sukri, S.; Zakaria, N.; Harith, Z. Carotenoids, phenolics and antioxidant properties of different sweet potatoes (Ipomoea batatas) varieties. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kelantan, Malaysia, 9 March 2021; IOP Publishing: Bristol, UK, 2021; p. 012077. [Google Scholar]
- McCleary, B.V.; Charmier, L.M.J.; McKie, V.A. Measurement of Starch: Critical Evaluation of Current Methodology. Starch-Stärke 2019, 71, 1800146. [Google Scholar] [CrossRef]
- Fang, H.; Yin, X.; He, J.; Xin, S.; Zhang, H.; Ye, X.; Yang, Y.; Tian, J. Cooking methods affected the phytochemicals and antioxidant activities of potato from different varieties. Food Chem. X 2022, 14, 100339. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Oualcadi, Y.; Aityoub, A.; Berrekhis, F. Investigation of different antioxidant capacity measurements suitable for bioactive compounds applied to medicinal plants. J. Food Meas. Charact. 2021, 15, 71–83. [Google Scholar] [CrossRef]
- Akter, M.; Anjum, N.; Roy, F.; Yasmin, S.; Sohany, M.; Mahomud, M.S. Effect of drying methods on physicochemical, antioxidant and functional properties of potato peel flour and quality evaluation of potato peel composite cake. J. Agric. Food Res. 2023, 11, 100508. [Google Scholar] [CrossRef]
- Téllez-Morales, J.A.; Arce-Ortiz, A. Advances in the quality characteristics of fried potato products with air frying technology: A mini review. Sustain. Food Technol. 2024, 2, 1228–1234. [Google Scholar] [CrossRef]
- Fadli, W.; Thow, Z. Effect of Osmotic Dehydration as a Pre-Treatment on Air Fried Sweet Potato (Ipomoea batatas) Chips. J. Agrobiotechnology 2022, 13, 64–73. [Google Scholar] [CrossRef]
- Peng, J.; Wang, K.; Ma, C.; Long, J.; Tu, K.; Pan, L. Determination of anthocyanin and moisture content of purple sweet potatoes during drying process by their optical properties in the 400–1050 nm range. Food Chem. 2021, 359, 129811. [Google Scholar] [CrossRef]
- Sanchez, P.D.; Hashim, N.; Shamsudin, R.; Nor, Z. Effects of different storage temperatures on the quality and shelf life of Malaysian sweet potato (Ipomoea batatas L.) varieties. Food Packag. Shelf Life 2021, 28, 100642. [Google Scholar] [CrossRef]
- Boruczkowska, H.; Boruczkowski, T.; Bronkowska, M.; Prajzner, M.; Rytel, E. Comparison of Colour Measurement Methods in the Food Industry. Processes 2025, 13, 1268. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Sharma, M.; Bhat, R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). Foods 2021, 10, 787. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; HarvestPlus Technical Monograph Series; Science and Education Publishing: Newark, DE, USA, 2004; Volume 2. [Google Scholar]
- Kurek, M.A.; Aktaş, H.; Pokorski, P.; Pogorzelska-Nowicka, E.; Custodio-Mendoza, J.A. A Comprehensive Review of Analytical Approaches for Carotenoids Assessment in Plant-Based Foods: Advances, Applications, and Future Directions. Appl. Sci. 2025, 15, 3506. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Comparative study on chemical composition, polyphenols, flavonoids, carotenoids and antioxidant activities of various cultivars of sweet potato. Int. J. Food Sci. Technol. 2019, 55, 369–378. [Google Scholar] [CrossRef]
- Narra, F.; Piragine, E.; Benedetti, G.; Ceccanti, C.; Florio, M.; Spezzini, J.; Troisi, F.; Giovannoni, R.; Martelli, A.; Guidi, L. Impact of thermal processing on polyphenols, carotenoids, glucosinolates, and ascorbic acid in fruit and vegetables and their cardiovascular benefits. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13426. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Buzigi, E.; Pillay, K.; Siwela, M. Effect of cooking locally available common bean (Obwelu) on iron and zinc retention, and pumpkin (Sweet cream) on provitamin A carotenoid retention in rural Uganda. Food Sci. Nutr. 2020, 8, 5916–5925. [Google Scholar] [CrossRef]
- Shilpa, S.; Shwetha, H.J.; Raju, M.; Lakshminarayana, R. 2-Factors affecting bioaccessibility and bio-efficacy of carotenoids. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 41–73. [Google Scholar]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2022, 62, 1999–2049. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Esquivel, P.; Rodriguez-Amaya, D.B. Comprehensive review on carotenoid composition: Transformations during processing and storage of foods. Food Res. Int. 2023, 169, 112773. [Google Scholar] [CrossRef]
- Cheong, J.J.; Ahmad, F.; Tengku Mohamad, T.R. Effects of cooking methods on physicochemical properties, antioxidant properties and sensory acceptability of purple sweet potato (Ipomoea batatas). Food Res. 2022, 6, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Demiray, E.; Tulek, Y.; Yilmaz, Y. Degradation kinetics of lycopene, β-carotene and ascorbic acid in tomatoes during hot air drying. LWT-Food Sci. Technol. 2013, 50, 172–176. [Google Scholar] [CrossRef]
- Demiray, E.; Tulek, Y. Degradation kinetics of β-carotene in carrot slices during convective drying. Int. J. Food Prop. 2017, 20, 151–156. [Google Scholar] [CrossRef]
- Vargha, S.; Igual, M.; Miraballes, M.; Gámbaro, A.; García-Segovia, P.; Martínez-Monzó, J. Influence of Cooking Technique on Bioaccessibility of Bioactive Compounds in Vegetable Lentil Soup. Foods 2024, 13, 2405. [Google Scholar] [CrossRef]
- Jati, I.; Darmoatmodjo, L.; Suseno, T.I.P.; Ristiarini, S.; Wibowo, C. Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products. Plants 2022, 11, 440. [Google Scholar] [CrossRef] [PubMed]
- Negara, B.F.S.P.; Lee, M.-J.; Tirtawijaya, G.; Cho, W.-H.; Sohn, J.-H.; Kim, J.-S.; Choi, J.-S. Application of Deep, Vacuum, and Air Frying Methods to Fry Chub Mackerel (Scomber japonicus). Processes 2021, 9, 1225. [Google Scholar] [CrossRef]
- Oancea, S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Liu, C.; Miao, Y.; Zhou, W.; Ma, Y.; Guo, W.; Li, A. Impact of Thermal Processing on the Structure, Antioxidant Properties and Hypoglycemic Activities of Sweet Potato Polysaccharides. Foods 2024, 13, 3082. [Google Scholar] [CrossRef]
- Hong, K.; Koh, E. Effects of Cooking Methods on Anthocyanins and Total Phenolics in Purple-Fleshed Sweet Potato. J. Food Process. Preserv. 2016, 40, 1054–1063. [Google Scholar] [CrossRef]
- Li, M.; Chen, X.; Deng, J.; Ouyang, D.; Wang, D.; Liang, Y.; Chen, Y.; Sun, Y. Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. 2020, 332, 127429. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, W.; Shao, P.; Wu, W.; Chen, H.; Fang, X.; Mu, H.; Xiao, J.; Gao, H. Impact of thermal processing on dietary flavonoids. Curr. Opin. Food Sci. 2022, 48, 100915. [Google Scholar] [CrossRef]
- El Hosry, L.; Elias, V.; Chamoun, V.; Halawi, M.; Cayot, P.; Nehme, A.; Bou-Maroun, E. Maillard Reaction: Mechanism, Influencing Parameters, Advantages, Disadvantages, and Food Industrial Applications: A Review. Foods 2025, 14, 1881. [Google Scholar] [CrossRef]
- Cui, Y.; Li, X.; Sun, D.; Guo, L.; Cui, B.; Zou, F.; Wang, J.; Sun, C. Retrogradation inhibition of starches in staple foods with maltotetraose-forming amylase. Food Chem. 2024, 449, 139232. [Google Scholar] [CrossRef] [PubMed]
- Allan, M.C.; Marinos, N.; Johanningsmeier, S.D.; Sato, A.; Truong, V.-D. Relationships between isolated sweetpotato starch properties and textural attributes of sweetpotato French fries. J. Food Sci. 2021, 86, 1819–1834. [Google Scholar] [CrossRef]
- Yang, S.; Hu, W.; Qiao, S.; Song, W.; Tan, W. Advances in Processing Techniques and Determinants of Sweet Potato Starch Gelatinization. Foods 2025, 14, 545. [Google Scholar] [CrossRef]
- Park, J.; Oh, S.-K.; Chung, H.-J.; Shin, D.S.; Choi, I.; Park, H.-J. Effect of steaming and roasting on the quality and resistant starch of brown rice flour with high amylose content. LWT 2022, 167, 113801. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; McClements, D.J.; Chen, L.; Miao, M.; Jin, Z. Effect of New Frying Technology on Starchy Food Quality. Foods 2021, 10, 1852. [Google Scholar] [CrossRef]
- Dong, L.; Qiu, C.-y.; Wang, R.-c.; Zhang, Y.; Wang, J.; Liu, J.-m.; Yu, H.-n.; Wang, S. Effects of Air Frying on French Fries: The Indication Role of Physicochemical Properties on the Formation of Maillard Hazards, and the Changes of Starch Digestibility. Front. Nutr. 2022, 9, 889901. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, S.; Giglou, M.T.; Esmaielpour, B.; Dehdar, B.; Estaji, A.; Hano, C.; Gohari, G.; Vergine, M.; Vita, F. Antioxidant Compounds of Potato Breeding Genotypes and Commercial Cultivars with Yellow, Light Yellow, and White Flesh in Iran. Plants 2023, 12, 1707. [Google Scholar] [CrossRef]
- He, S.; He, S.; Niu, L.; Sun, C.; Zeng, Z.; Xiao, J. Effects of different roasting conditions on sugars profile, volatile compounds, carotenoids and antioxidant activities of orange-fleshed sweet potato. Food Chem. X 2025, 25, 102201. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Evaluation of Spectrophotometric Methods for Assessing Antioxidant Potential in Plant Food Samples—A Critical Approach. Appl. Sci. 2025, 15, 5925. [Google Scholar] [CrossRef]
- Bolchini, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Assessing antioxidant properties of Maillard reaction products: Methods and potential applications as food preservatives. Eur. Food Res. Technol. 2025, 251, 2039–2059. [Google Scholar] [CrossRef]
- Miškec, K.; Frlin, M.; Šola, I. Impact of Different Thermal Processing Techniques on the Phytochemical Composition, Antioxidant Capacity, and DNA-Protective Properties of Broccoli. Appl. Sci. 2025, 15, 7469. [Google Scholar] [CrossRef]
- Chikpah, S.K.; Korese, J.K.; Sturm, B.; Hensel, O. Evaluation of nutrients, antioxidants and sensory characteristics of optimized wheat-orange-fleshed sweet potato and pumpkin composite bread and storage stability in three packaging materials. Discov. Food 2025, 5, 36. [Google Scholar] [CrossRef]
- García-Ríos, D.; Hernández, I.; Alvaro, J.E.; Pedreschi, F.; Campos, D.; Behn, A.; Pedreschi, R. Analysis of Maillard reaction precursors and secondary metabolites in Chilean potatoes and neoformed contaminants during frying. Food Chem. 2024, 460, 140478. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Yang, H.; Xu, Z.; Wang, X.; Zhang, Z.; Deng, J. Effects of drying methods on phytochemicals and antioxidant activity of broccoli by-products. Food Res. Int. 2025, 208, 116284. [Google Scholar] [CrossRef]
- Sáez, L.; Blanch, G.; Morales, F.; Mesias, M. Health-related compounds and Maillard reaction products in dry and steam roasted purple carrots (Daucus carota L.). Food Chem. 2025, 483, 144296. [Google Scholar] [CrossRef]
- Toydemir, G.; Gultekin Subasi, B.; Hall, R.D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of food processing on antioxidants, their bioavailability and potential relevance to human health. Food Chem. X 2022, 14, 100334. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.S.; Viana, D.S.B.; Keller, L.M.; de Melo, M.T.T.; Mulandeza, O.F.; Barbosa, M.I.M.J.; Barbosa Júnior, J.L.; Saldanha, T. Impact of air frying on food lipids: Oxidative evidence, current research, and insights into domestic mitigation by natural antioxidants. Trends Food Sci. Technol. 2024, 147, 104465. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Chai, Y.; Huang, Y.; Jin, Y.; Ma, J.; Jiang, Y.; Tang, Y. Cutting morphology and steaming duration impact physicochemical quality, bioactive retention, and maillard reaction products in purple-fleshed sweet potatoes. Food Chem. 2025, 495, 146245. [Google Scholar] [CrossRef]
- Ospina, M.A.; Moreno, J.L.; Tran, T.; Jaramillo, A.M.; Gallego-Castillo, S.; Ospina, B.; Dufour, D. Kinetics of thermal degradation of carotenoids related to potential of mixture of wheat, cassava and sweet potato flours in baking products. J. Sci. Food Agric. 2024, 104, 4671–4679. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors affecting the stability of anthocyanins and strategies for improving their stability: A review. Food Chem. X 2024, 24, 101883. [Google Scholar] [CrossRef] [PubMed]
- Gunathilake, K.; Ranaweera, K.; Rupasinghe, H.P.V. Effect of Different Cooking Methods on Polyphenols, Carotenoids and Antioxidant Activities of Selected Edible Leaves. Antioxidants 2018, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Jia, Y.; Feng, C.H.; Zhang, H.; Ren, F.; Zhao, G. Effects of thermal processing on natural antioxidants in fruits and vegetables. Food Res. Int. 2024, 192, 114797. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.C.; Kaaya, A.N.; Tumuhimbise, G.A.; Katungisa, A.; Amagloh, F.K.; Yada, B. Household Processing Methods and Their Impact on Bioactive Compounds and Antioxidant Activities of Sweetpotato Genotypes of Varying Storage Root Flesh Colours. Antioxidants 2022, 11, 1867. [Google Scholar] [CrossRef] [PubMed]



| Time (min) | DPPH (mg AAE/g) | ABTS (mg AAE/g) | FRAP (mg AAE/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peel | ||||||||||||
| Native | 0.16 | ± | 0.00 | a | 0.38 | ± | 0.01 | e | 1.05 | ± | 0.10 | fg |
| Steaming 15 min | 0.16 | ± | 0.00 | a | 0.53 | ± | 0.04 | d | 1.43 | ± | 0.04 | fg |
| Steaming 30 min | 0.16 | ± | 0.00 | a | 0.60 | ± | 0.06 | c | 7.93 | ± | 0.33 | d |
| Steaming 45 min | 0.16 | ± | 0.00 | a | 0.56 | ± | 0.01 | cd | 8.05 | ± | 0.20 | d |
| Air-frying 5 min | 0.15 | ± | 0.00 | b | 0.93 | ± | 0.07 | b | 10.55 | ± | 0.38 | c |
| Air-frying 10 min | 0.08 | ± | 0.00 | e | 1.51 | ± | 0.10 | a | 31.50 | ± | 1.08 | b |
| Air-frying 15 min | 0.00 | ± | 0.00 | f | 1.57 | ± | 0.01 | a | 36.18 | ± | 3.94 | a |
| Flesh | ||||||||||||
| Native | 0.15 | ± | 0.00 | b | 0.23 | ± | 0.03 | fg | 3.61 | ± | 0.25 | e |
| Steaming 15 min | 0.15 | ± | 0.00 | b | 0.10 | ± | 0.02 | h | 3.64 | ± | 0.06 | e |
| Steaming 30 min | 0.15 | ± | 0.00 | b | 0.21 | ± | 0.01 | fg | 3.05 | ± | 0.24 | ef |
| Steaming 45 min | 0.15 | ± | 0.00 | b | 0.26 | ± | 0.02 | f | 2.75 | ± | 0.11 | ef |
| Air-frying 5 min | 0.14 | ± | 0.00 | c | 0.18 | ± | 0.02 | g | 0.45 | ± | 0.03 | g |
| Air-frying 10 min | 0.15 | ± | 0.00 | b | 0.36 | ± | 0.01 | e | 0.69 | ± | 0.01 | g |
| Air-frying 15 min | 0.12 | ± | 0.00 | c | 1.50 | ± | 0.02 | a | 2.76 | ± | 0.35 | ef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan-utai, W.; Phomkaivon, N.; Settachaimongkon, S. Processing-Induced Changes in Bioactive Compounds and Antioxidant Activity of Orange-Fleshed Sweet Potato (Ipomoea batatas L.): Steaming Versus Air-Frying. Foods 2025, 14, 3637. https://doi.org/10.3390/foods14213637
Pan-utai W, Phomkaivon N, Settachaimongkon S. Processing-Induced Changes in Bioactive Compounds and Antioxidant Activity of Orange-Fleshed Sweet Potato (Ipomoea batatas L.): Steaming Versus Air-Frying. Foods. 2025; 14(21):3637. https://doi.org/10.3390/foods14213637
Chicago/Turabian StylePan-utai, Wanida, Naraporn Phomkaivon, and Sarn Settachaimongkon. 2025. "Processing-Induced Changes in Bioactive Compounds and Antioxidant Activity of Orange-Fleshed Sweet Potato (Ipomoea batatas L.): Steaming Versus Air-Frying" Foods 14, no. 21: 3637. https://doi.org/10.3390/foods14213637
APA StylePan-utai, W., Phomkaivon, N., & Settachaimongkon, S. (2025). Processing-Induced Changes in Bioactive Compounds and Antioxidant Activity of Orange-Fleshed Sweet Potato (Ipomoea batatas L.): Steaming Versus Air-Frying. Foods, 14(21), 3637. https://doi.org/10.3390/foods14213637







