Abstract
In recent decades, the consumption of animal proteins has been rethought by consumers. Factors such as improved health and sustainability are key aspects of this scenario. Studies have sought innovative and sustainable technologies to improve protein extraction from alternative sources to increase their competitiveness. In this sense, the aim of this work was to combine the effects of nonconventional extraction methods on the process yield and the resulting techno-functional properties extracted from alternative proteins. The literature contains significant publications regarding the use of ultrasound (US), pulsed electric fields (PEFs), microwaves (MWs) and deep eutectic solvents (DESs) for enhancing protein extraction. Re-emerged techniques such as reverse micelles and aqueous two-phase extraction have also been reported. For this reason, the present study aimed not only to present the obtained results but also to discuss how the mechanisms associated with the aforementioned technologies impact the extraction yield and modification of proteins. In general, US tends to increase protein solubility (20–30%) and emulsifying capacity (35%); MWs can increase protein yield (25%) while reducing extraction time (50–70%); DES-based extraction tends to retain more than ~40% of the native functionality, and PEFs have demonstrated up to a 20% improvement in protein recovery. Nonconventional extraction methods have varying effects on the characteristics and quality of extracted proteins, offering benefits and challenges that should be considered when choosing the most suitable technology. The specificity related to each technology can be used to make possible interesting industrial applications involving nonanimal proteins.
1. Introduction
Daily protein intake and its corresponding source are some of the subjects of major concern for studies in food science and nutrition. Studies state that the general recommended daily protein intake for healthy adults aged 20–65 years is approximately 0.66 g per kilogram of body weight, which should constitute 8–10% of total dietary energy intake. This basic information underlines the importance of including protein sources in a balanced diet to ensure proper nutrition and promote health [1,2].
Another aspect to highlight is that most of the proteins consumed by adults are currently of animal origin [3]. However, in recent decades, the consumption of animal proteins has been rethought by consumers, especially those who are more concerned with animal welfare, improving health, and sustainability in the nutritional and livestock context [4,5].
Although the industries of animal-based products tend to become increasingly concerned with the welfare of the animals, there are rudimentary processing units that still make use of overcrowded environments and carry out management practices that ignore essential care. The life cycle of these animals culminates in slaughter methods that are also ethically questionable and that are somewhere allowed by law, further aggravating their suffering. The population can also be a precursor to changes in animal welfare and, consequently, excessive consumption of animal-based proteins. The consumer perspective affects decision-makers at the industrial, political, regulatory, and market levels [6].
Additionally, diets high in animal proteins may contribute to the development of chronic kidney disease by increasing the rates of decline in kidney function, insulin resistance, and albuminuria. There is also evidence that suggests an association between diets rich in animal protein and a greater risk of cardiovascular disease [7,8,9,10].
In addition, half of the greenhouse gas (GHG) emissions produced in the agricultural sector are attributed to livestock practices, which is why investments in alternative protein sectors have increased rapidly [11]. In this sense, consumers tend to decide on alternative proteins to contribute to more environmentally sustainable consumption. With this practice, they expect to reduce GHG emissions and land and water consumption, in addition to preserving biodiversity [12].
Finally, there is a growing demand for products with alternative proteins added to their formulations to improve their nutritional and technological properties [13]. This trend has boosted the market for analogs or substitutes of animal products [14,15,16]. Among the animal protein substitutes, plant-based proteins (e.g., soybean, pea, wheat), cellular proteins (e.g., microalgae), and fungal proteins (e.g., mushrooms) are worth mentioning [17]. Although there is a clear trend toward the valorization of these alternative proteins, the enhancement of process yield and how this macronutrient is affected during extraction have yet to be studied [18]. Scaling up and designing suitable processing conditions of such processes together with the efficient management of byproducts throughout the processing chain are still the main gaps to be covered, either when using conventional or nonconventional techniques. Consequently, increasing attention has been given to the development of efficient and sustainable processes for extracting these proteins.
The most commonly applied conventional extraction methods for obtaining these alternative proteins include air classification, extraction by solubilization in alkali media [19], aqueous extraction [20], ultrafiltration [21], and micellar/salt extraction [22]. Conventional processes are generally more accessible but do not always provide high yields. Additionally, they tend to use large amounts of natural resources, such as water and chemicals, in addition to requiring long processing times. However, it may lead to changes in protein structure, leading to denaturation and possible loss of functionality [23,24,25,26]. Therefore, methods that provide higher extraction yields, functionality preservation (or even intensification), lower energy consumption, and chemical use are of great interest to the Food Industry 4.0 [27,28].
Although studies have sought innovative and sustainable technologies for improving conventional processes for extracting proteins from alternative sources, their use can positively or negatively affect the technical-functional properties of proteins, thus reinforcing the need to study products and processes specifically [29]. A promising approach to meet sustainability and intensification requirements is the use of nonconventional and/or emerging technologies. Indeed, these technologies have been reported to confer more effective and sustainable protein extraction from plants [30].
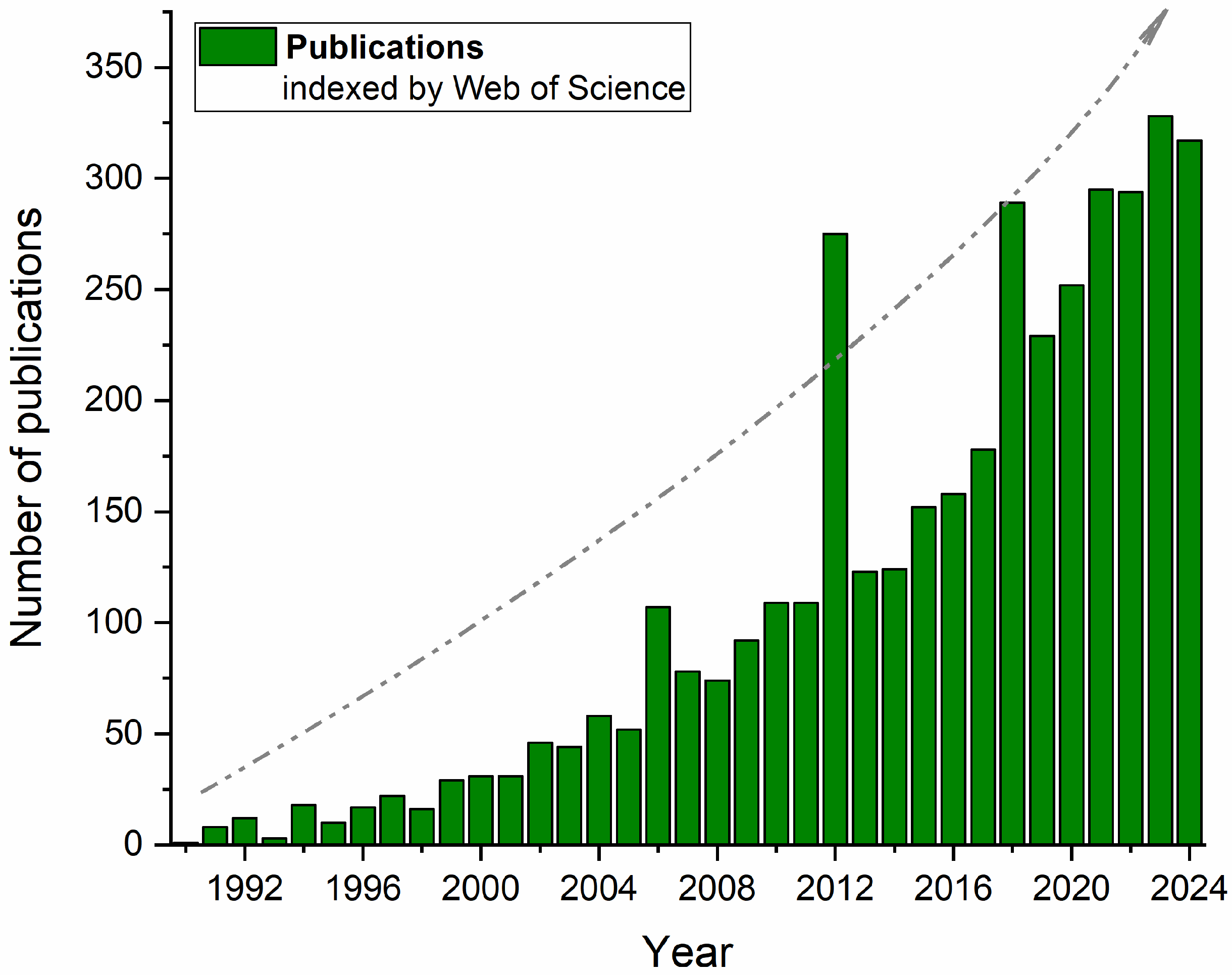
Figure 1 confirms this trend when presenting the number of publications indexed by Web of Science between 1990 and mid-2024, which include the terms “alternative proteins” and “nonconventional technologies” or “emerging technologies”. In addition to the rapid increase in publications associated with predefined keywords over the years, some dispersed peaks may also be associated with particular “booms” over the years (e.g., 2006, 2012, 2017, and 2023, after the COVID-19 pandemic), when the market of plant-based and veggie foods significantly increased with new products and new technologies, especially in Western Europe.
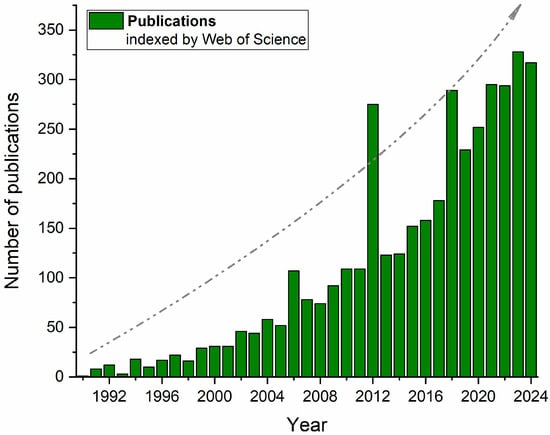
Figure 1.
Number of publications indexed by Web of Science that include the terms “alternative proteins” and “nonconventional technologies” or “emerging technologies” over the past two decades.
In addition to the growing publications of nonconventional techniques for alternative protein extraction, their combination with conventional methods is often reported to generate synergistic effects on processing and product parameters [31]. Among these methods, assisted methods, as well as pretreatment, can be used to intensify conventional extraction. Jain et al. [32] quoted the use of ultrasound (US), pulsed electric field (PEF), microwaves (MWs), and deep eutectic solvents (DESs) for protein extraction as intensifying emerging approaches, among other conventional methods that have re-emerged as reverse micelles and aqueous two-phase extraction as alternatives not only to the partitioning and/or purification of proteins.
These technologies—ultrasound (US), pulsed electric fields (PEFs), microwaves (MWs), and deep eutectic solvents (DESs) are some of the most studied techniques for extracting alternative proteins from sustainable sources in the past two decades. A consolidated state-of-the-art of these technologies made it possible for the review to address established scientific evidence and, at the same time, provided strong potential for industrial application. Other methods, such as reverse micelles or aqueous two-phase systems, on the other hand, were also discussed as (re-)emerging approaches to support extraction and partitioning, as their applications are more related to purification and separation steps than to primary removal itself. Understanding the mechanisms of each technology and reviewing processing conditions contribute to the main challenges faced by the plant protein production industry: the efficient, cost-effective, and environmentally friendly use of techniques for protein extraction. Therefore, this review focused on describing specific protein extraction mechanisms, such as cell disruption, unfolding, and solubility changes, focusing on the main effects of US, PEF, MW, and DES on the extraction of proteins from nonanimal sources. Discussions of the main effects were based on processing yields and overall functionality changes.
2. High-Intensity Ultrasound (US)
US is a versatile technique with several uses in food processing, ranging from nondestructive analysis to homogenization and compound extraction. US can be classified as low-intensity when the intensity is lower than 1 W/cm2 and when the frequency is generally above 100 kHz. On the other hand, high-intensity US exceeds 1 W/cm2, with frequencies generally between 20 kHz and 100 kHz [33,34].
Low-intensity US, also called signal ultrasound or diagnostic ultrasound, is generally used for nondestructive testing in the areas of chemistry and biomedical analysis. High-intensity ultrasound is most commonly used for diffusion, extraction, and emulsification because of the force that the mechanical wave produces in the system, which consequently promotes rupture of structures, size reduction, and conformational change [35].
The principle of the high-intensity US-assisted extraction technique is based on the application of relatively low-frequency and high-intensity sound waves to a fluid medium, which generates physical phenomena such as cavitation, microagitation, and heating, thus promoting mass transfer enhancements.
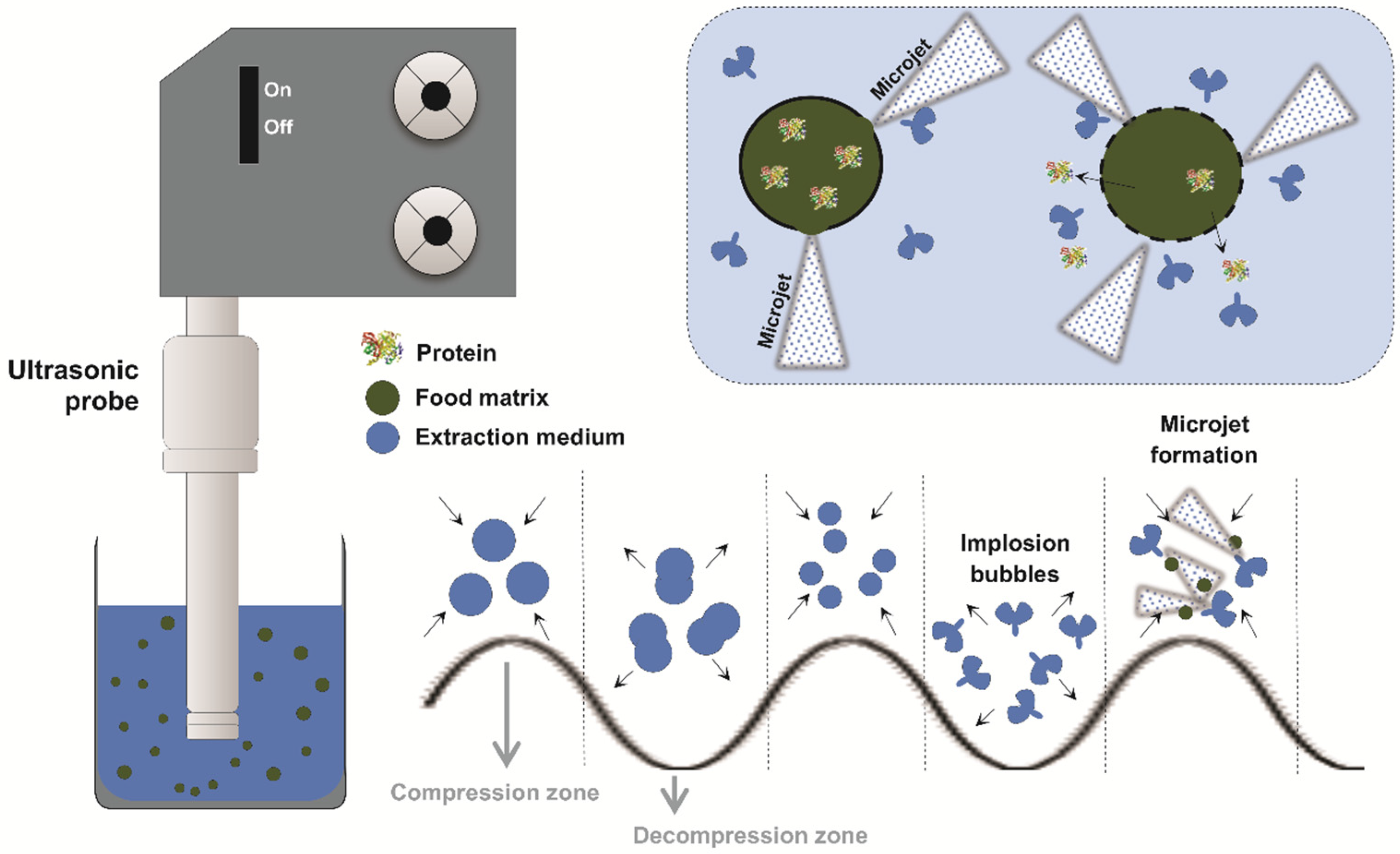
As exemplified in Figure 2, cavitation occurs when ultrasonic waves create alternating cycles of compression and rarefaction in the liquid, forming gas bubbles. After reaching a critical size, they implode violently and collapse among each other [36].
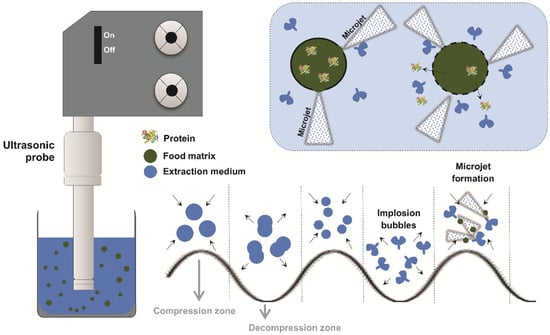
Figure 2.
Scheme of ultrasound action during protein extraction from alternative food sources. Caption: The ultrasound probe emits ultrasonic waves that propagate through the extraction medium (blue), containing the particulate food matrix (green) and the proteins of interest (colored icon). The acoustic oscillation generates compression and decompression cycles, promoting the formation of microbubbles (cavitation). During decompression, these bubbles grow, increasing until collapse that generates high-energy microjets. These microjets directly impact the solid–liquid interface, disrupting cell structures and releasing proteins towards the extraction medium.
The implosion and collapse of the bubbles generated by cavitation release highly concentrated energy at the microscopic level, resulting in extremely high localized pressures and temperatures in the form of microjets. These effects promote the disruption of plant cell structures, intensifying processes such as protein extraction. In addition to facilitating the release of compounds, the mechanical and thermal effects of US may also cause structural changes in the extracted molecules [37].
To prevent and preserve the quality of the extracted compounds, the processing time usually does not exceed 30 min, but adjustments may be necessary depending on the matrix, solvent, equipment design, etc. [35]. However, for proteins, exposure to ultrasonic waves can be beneficial or harmful, depending on the application.
In US-assisted extraction, the nominal power usually varies between 20 and 500 W with typical frequencies ranging from 20 to 40 kHz, depending on the characteristics of the material to be extracted and the solvent employed [38,39]. Controlling the processing time is also critical to prevent sample overheating, as the energy released by the implosions of the bubbles formed by cavitation can quickly increase the temperature of the medium [40]. In many situations, the process is carried out under cooling conditions, such as in an ice bath, to dissipate the heat generated during the application of US [41].
US plays an essential role in optimizing extraction processes, providing a superior yield for recovering various compounds of interest, including proteins [40]. High-intensity US has become an important way to improve the solubility of nonanimal proteins and the extraction process itself, increasing the efficiency of the interaction between the molecules and the solvent as well as the release of proteins from the food matrix. Studies indicate that the use of US can not only increase the protein extraction yield from several alternative matrices but also improve their functionality. Table 1 compiles a series of studies that have applied USs to different nonanimal sources to obtain alternative proteins to nonanimals. A compilation of the observed effects was also included in this analysis.
When revisiting the state of the art, it is possible to determine how particular the results for each studied food source and process condition are. Even though general trends can be evidenced, studying and designing adequately nonconventional processes for a given product are needed. This gap of information is even more necessary when working with proteins, which have a heterogeneous and complex structure, composition, and functional properties, which may vary not only according to the source but also according to the handling conditions.
The need to develop suitable and more efficient processes has become even more worrying due to the trend of obtaining alternative sources of proteins—not only to meet industrial interests but also to consumers expecting healthier and affordable products. Currently, there is a growing interest in “blue proteins”, which are named proteins from aquatic sources [40,41]. However, the extraction methods, intensification possibilities, modifications, and overall effects on its functionalities need to be reviewed and discussed to identify mechanisms that may favor or increase the degree of difficulty for scientific and industrial purposes [42]. Marques et al. [31] also highlighted the use of seeds as alternative protein sources to have their extraction enhanced by sonication.
Most of the studies have focused on a fixed US frequency of ~20 kHz, with other process parameters such as the nominal power and application time varying [40,43,44]. Compared with low-intensity US, the application of lower frequencies has a potential effect on cell wall permeabilization, leading to an intensification of mass transfer and process yield [45]. Several ranges of nominal power applied were found. Most of them use nominal powers above 100 W, indicating the need for a high degree of energy to displace proteins from the plant matrix. In general, the extraction of organic compounds from plants or seeds has been based on a combination of solvent type, temperature, and/or contact technique [46], which may be enhanced by the use of US at high power levels.

Table 1.
Recent studies on the improvement of ultrasound in the extraction of alternative proteins.
Table 1.
Recent studies on the improvement of ultrasound in the extraction of alternative proteins.
| Product | Extraction Conditions | Observed Effects | References |
|---|---|---|---|
| Mulberry leaf | Frequency: 40 kHz; Power: 240 W/L; Time: 25 min. | Higher protein extraction yields compared to the conventional method. The protein yield of mulberry leaf increased by 171.76%. | [47] |
| Apple seed | Frequency: 16 kHz; Power not informed; Time: 30 min. | US generated impacts on structural and techno-functional properties, where ultrasonic protein exhibited increased functional properties such as emulsification, foaming, hydrophobicity, and oil absorption properties. | [48] |
| Defatted pumpkin seeds | Frequency: 20–25 kHz; Power: 100–300 W; Time: 10–20 min. | US had a significant effect on protein yield and recovery. The optimal conditions for extraction were 193.89 W and 19.08 min, respectively, and the predicted value of protein recovery was 79.37% and the protein yield was 48.91%. | [49] |
| Fava bean (Vicia faba L.) | Frequency: 20 kHz; Power: 57.58 W/cm2; Time: 20 min. | US resulted in superior results in terms of protein purity and recovery. The highest protein content is 84.09 ± 0.75% with a corresponding extraction yield of 10.94 ± 0.71%. Water, as an extraction solvent, produced significantly higher results in protein content when ultrasound was used. | [44] |
| Cowpea | Frequency not informed; Power: 100–200 W; Time: 5 to 20 min. | US improved the yield from 31.78% to 58.96% and from 57.26% to 68.85%. Nutritional/biological, functional, and physicochemical properties were also improved. | [50] |
| Camelina seed | Frequency: 40 kHz; Power: 180 W; Time: 20 min. | Significantly improved protein extraction/content and functional properties (water holding capacity, oil absorption capacity, emulsifying foaming properties, and protein solubility). | [51] |
| Weed (Sophia, Descurainis sophia L.) | Frequency: 40 kHz; Power: 180 W; Time: 20 min. | Significantly improved protein extraction/content and functional properties (water holding capacity, oil absorption capacity, emulsifying foaming properties, and protein solubility). | [51] |
| Watermelon seeds | Frequency: 25–40 kHz; Power: 50–200 W; Time: 15 min. | In addition to the increase in extraction (82.4%), the functional properties of the extracted proteins were also superior with US compared to conventionally extracted proteins. | [39] |
| Black beans | Frequency: 37 kHz; Power: 320 W; Time: 0, 10 and 20 min. | With an increase in yield of 9.70 ± 0.10, the secondary structure of the proteins is altered by US treatment, but the primary structure remains unchanged. | [52] |
| Lentil | Frequency: 37 kHz; Power: 320 W; Time: 0, 10 and 20 min. | With an increase in yield of 7.64 ± 0.03, the secondary structure of the proteins is altered by US treatment, but the primary structure remains unchanged. | [53] |
| Quinoa | Frequency: 37 kHz; Power: 320 W; Time: 0, 10 and 20 min. | With an increase in yield of 4.10 ± 0.18, the secondary structure of the proteins is altered by US treatment, but the primary structure remains unchanged. | [53] |
| Chickpeas | Frequency: 20 kHz; Power: 315–390 W/cm2; Time: 5 min. | Increase to 33.45% of protein extraction in chickpeas, where the composition of the secondary structure for chickpea protein remained unchanged. | [34] |
| Rice bran | Frequency: 20–35 kHz; Power: 100–200 and 300 W; Time: 10, 20 min. | Increased surface hydrophobicity, improved protein emulsifying properties, great stability, and decreased tendency with increasing US power and time. | [54] |
| Soybeans | Frequency: 20 kHz; Power: 315–390 W/cm2; Time: 5 min. | Protein extraction yields from soybean flakes increased by 68.5%. The composition of the secondary structure for soybean flakes remained unchanged. | [34] |
| Beans | Frequency: 20 kHz; Power: 315–390 W/cm2; Time: 5 min. | An increase of 16.39% was obtained when HPS of 4.5 W/cm 3 was applied, as changes in the composition of the secondary structure, causing unfolding and destabilization of the native structure of the protein. | [34] |
| Pecan Nut—(Carya illinoinensis (Wangenh.)) | Frequency: 20 kHz; Power: 400 W; Time: 5 s/3 s. | The US increased the solubility of the substrate, increasing protein extraction rate (25.51%), making it easily accessible to the enzyme, thereby accelerating the chemical reaction and improving the protein yield. | [55] |
| Soy | Frequency: 20, 28, 35, 40, and 50 kHz; Power: 120 W; Time: 25 min. | The extraction rate improved significantly (p < 0.05) with the aid of US. The extraction effect was optimum at 28 kHz, and the extraction rate was improved to 73.35 g/100 g from 46.09 g/100 g control under the same condition and helps to optimize the equipment and improve efficiency. | [56] |
| Melon seed | Frequency not informed; Power: 300, 375, and 450 W; Time: 2.50, 5.00, 7.50, 10.00, 12.50, 15.00, 17.50, and 20.0 min. | The highest yield of 23.79% at 300 W, 31.05% at 375 W, and 28.93% at 450 W was obtained. An increase in water retention capacity, solubility, emulsion capacity, emulsion stability, and lower gelling capacity was observed in protein samples. | [57] |
| Turmeric powder | Frequency: 22 kHz; Power: 90 W; Time: 45, 10 min. | Maximum recovery was achieved in a minimum time of 45 min to 10 min; turmeric residue protein powder showed antidiabetic activity and maximum protein recovery (66.52%). | [58] |
| Walnut dregs (Juglans regia L.) | Frequency: 20.28 kHz; Power: 120 W; Time: 30 min. | An effective way to improve the comprehensive utilization and economic value of walnut dregs. The protein yield, purity, and CEI value of WP increased with a protein yield of 66.01%. | [49] |
| Moringa oleifera seed | Frequency: low; Power: 0, 130, 260, 390, 520, and 650 W; Time: 15 min. | Changes in secondary and tertiary structure do not significantly degrade the seed and improve thermal stability. | [59] |
| Pea | Frequency not informed; Power: 750 W; Time: 5, 15 min. | Higher levels of protein extraction (82.6%), shorter extraction times, and lower water consumption did not significantly alter amino acid composition, cause structural changes, and improve functional properties and biological activities. | [60] |
| Sesame meal | Frequency: 35 kHz; Power: 528–836 W; Time: 10, 120 min. | US increased protein yield, ranging from 41.1% to 77.7%, total phenolic content, and antioxidant capacity compared to the standard alkaline extraction method | [61] |
| Cardamina violifolia (CV) | Frequency not informed; Power: 6.5, 8.125 and 9.75 W; Time: 20, 30 and 40 min. | US treatment can increase the efficiency of protein extraction, being more effective with extract yield and purity of approximately 77%, compared to the control. | [62] |
| Peanut flour | Frequency: 24 kHz; Power not informed; Time: 15, 40 min. | US produced a protein extraction of 55%, obtaining 77% more protein compared to the control. | [63] |
| Coconut milk | Frequency: 24 kHz; Power: 6.85 W/cm2; Time: 2.5 min. | A higher protein yield was achieved in the US-treated samples compared to their control (49.6–86.1%). | [64] |
| Jackfruit leaf | Frequency: 42 kHz; Power not informed; Time: 10, 15 and 20 min. | The optimal value for protein extraction was 96.3 mg/g, exceeding the concentration by isoelectric precipitation, with the characteristic of representing a green technology. | [65] |
| Coffee bean | Frequency: 24 kHz; Power: 400 W; Time: 5, 15 min. | US provided higher extraction yields than those extracted by the control. | [66] |
| Grape seeds | Frequency: 40 kHz.; Power: 200 W; Time:15, 45 min. | US-treated albumin fractions showed higher solubilities, emulsifying properties, and in vitro digestibilities, but lower water binding capacities and thermal stability. | [67] |
| Bunch beans (GPI) | Frequency: acima de 20 kHz.; Power: 150, 250, and 350 W; Time: 5, 15 min. | US resulted in significant structural modifications and enhanced functional properties of guar protein isolate (GPI). A decrease in the particle size compared to native GPI (control) was also observed. | [68] |
| Silkworm (Bombyx mori) | Frequency: 40 kHz.; Power: 500 W; Time:15, 30 min. | US induced changes in the protein conformation that improved the emulsification and foaming properties. US also increased the antioxidant activity of this protein. | [69] |
| Sugar maple leaves (SML) | Frequency: 20 kHz.; Power: 500 W; Time:15 min. | US homogenization pretreatment improved protein extraction yield, temperature of protein denaturation was increased together with the functional and antioxidant properties. | [43] |
| Grape seeds | Frequency: 40 kHz.; Power: 200 W; Time: 60 min. | US showed an improvement in the protein extraction yield and overall stability. | [70] |
| Aquatic plant Duckweed (Lemna minor) | Frequency: 20 a 100 kHz.; Power: 400 W; Time: 20, 30 min. | US generated changes in the color, structure, and FTIR spectra of the obtained concentrates, which resulted in improvements in the solubility, emulsifying properties and foaming capacity. | [40] |
In a study of protein extraction from soybean seeds, for example, US-assisted extraction at an input power of 1280 W in 500 mL (2.56 W/mL) increased the extraction yield by approximately 46% compared with that of nonsonicated treatments [71]. The resulting sonicated soy protein isolate had an increase in solubility of 34% at pH 7.0, probably due to the reduction in the particle size of the isolate. In general, sonication slightly decreased the emulsifying and foaming capacities by up to 12% and 26%, respectively. This could be related to alterations in protein conformation that increase the difficulty of unfolding at the interface.
In Spirulina extracts, the protein extraction yield was increased by up to 136% with US compared with that of the treatments without US, mainly due to the effective disruption of microbial cell walls. This finding reinforces that US is a powerful tool for optimizing protein extraction, especially in matrices with thicker or hard-to-break cell walls [72]. Moreover, the in vitro digestibility of the obtained proteins increased 2-fold when US was applied. In terms of some techno-functionalities, the water holding capacity (WHC) and emulsifying and foaming capacities were also improved when the materials were extracted with the assistance of US.
The protein content of mulberry leaf protein concentrate extracted by US-assisted cellulase degradation was the highest, at 62.69%, in comparison with the 51.41% obtained by conventional alkaline extraction and acid precipitation and the ~59% obtained by conventional cellulase degradation. Microstructural analysis of mulberry leaf proteins demonstrated that US-assisted cellulase extraction can break down the dense structure of protein in the raw material, reducing the average size of proteins and increasing the specific surface area and roughness. These results may be a consequence of the decrease in the α-helix content of the proteins extracted when US was used. On the other hand, the β-turn and random coil contents of the proteins increased under these conditions as a consequence of the unfolding of the α-helix structure to form new β-sheet and random coil structures. The particular functional characteristics of the proteins extracted with the assistance of US were highlighted. Compared with the conventional method, it presented the highest values associated with the highest solubility, which is interesting for food applications [47].
When evaluating the changes in the structural properties of legume proteins extracted after US exposure, Quintero-Quiroz et al. [53] reported that alterations in the secondary structure of proteins, such as α-helices and β-sheets, have a direct impact on further Maillard reactions. US causes partial unfolding of the protein structure, exposing functional groups such as amino acid free radicals. For example, the exposure of ionized acidic amino acids, such as glutamic acid and aspartic acid, as well as leucine and glycine, intensifies interactions with reducing sugars. Given the large number of food applications involving the Maillard reaction, sustainable techniques to unfold proteins may serve to valorize protein ingredients and produce clean-label products. This valorization is due not only to the Maillard reaction but also to the increase in the reactivity of the amine groups, which consequently improves the solubility, foaming and emulsifying capacity, hydrophobicity, and oil-holding capacity of protein isolates [49].
In the work of Byanju et al. [34], the authors reported a significant increase in the extraction of proteins from chickpeas with the use of US. The application of US reduced the proportion of α-helix structures, whereas the β-sheet structure increased or remained unaltered in some cases. In addition, there was a significant increase in the fraction of disordered structures, indicating a loss in the native conformation. This phenomenon results in a more flexible and open configuration, exposing free sulfhydryl (–SH) groups and forming different protein conformations in the same concentration/isolate.
The resulting effects of US on the structural and techno-functional properties of proteins also impact further food applications. In general, proteins subjected to the sonication process tend to exhibit increased emulsification capacity, foaming capacity, hydrophobicity, and oil holding capacity. These observations were, in fact, evidenced by Gani et al. [48] when studying proteins from apple seeds. A similar trend was also reported when proteins extracted from camelina seeds and from bitter melon seeds were evaluated [51,57].
Therefore, the US-assisted extraction process can be used as an effective technique to intensify the extraction of proteins from alternative sources, especially those with more rigid and difficult-to-rupture cells [48]. Sonication is also interesting for the food ingredient industry because it is able to unfold the dense structure of proteins, thus reducing the average size of the protein chains and increasing their specific surface area and roughness. These results might improve the solubility and different techno-functional properties of interest for food applications.
3. Pulsed Electric Field (PEF)
Pulsed electric fields (PEFs) constitute another emerging technology in food processing that consists of applying high-intensity short-duration electrical pulses between two electrodes. The electrical current promotes electroporation, which temporarily or permanently increases the permeability of cell membranes. The creation of these pores facilitates the transport of molecules and ions through cells or tissues toward the solvent [73].
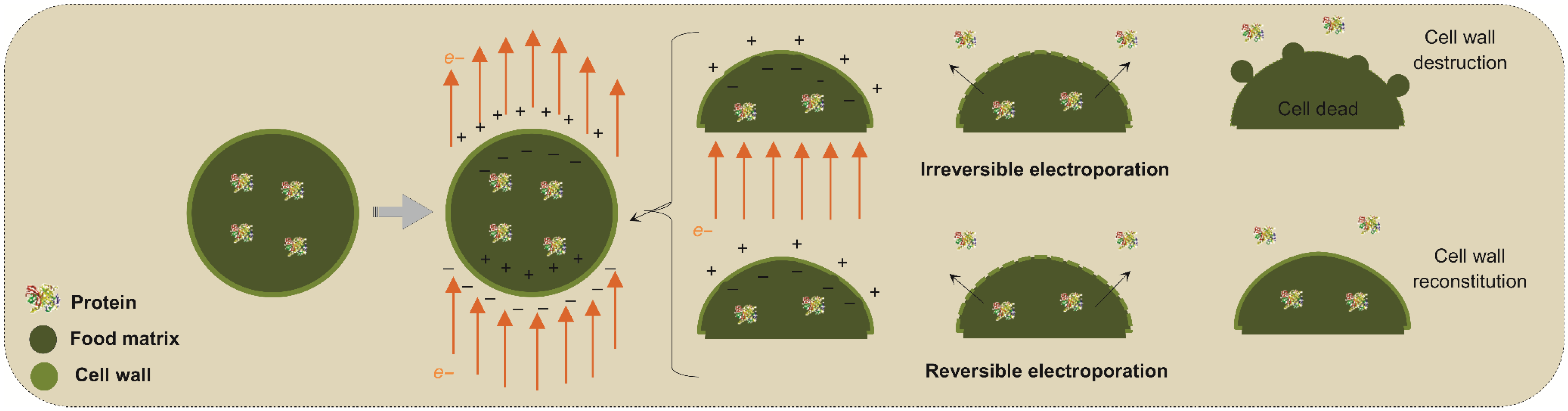
Figure 3 shows a schematic of the mechanism of actuation of PEFs on cell membranes and even in the cell wall in the case of plant-based sources. In this scheme, the PEF application through the conduction medium and cell membranes orients the charges along with the electrical field, thus creating opposite charges on the inner and outer sides of the phospholipidic bilayer membranes and wall cells. As the electrical strength becomes more intense and to promote neutralization, the opposite charges tend to compress the membrane until some ruptures occur along the surface. As a result, by opening pathways to increase the mass transfer rates of intracellular compounds and the entry of solvents into the cells, the release of cellular components such as proteins into the extracellular medium is improved, resulting in interesting extraction yields [74,75].
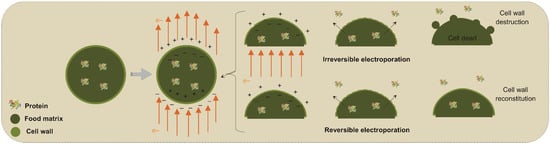
Figure 3.
Temporally and permanent electroporation during pulsed electric field application in plant cell membranes.
After these pores are created, the original structure of the membrane can recover if the applied electrical field is not intense; thus, this phenomenon is referred to as temporal electroporation. This effect is interesting for treatments in which the structure, quality and/or vigor of the material source needs to be preserved. In a nondestructive way, this technology preserves sensory and nutritional characteristics while increasing mass transfer. In some cases, even after the electric field has been disrupted, the pores (permeabilization) may remain for a few minutes, allowing the cell to return to its original state afterwards [76].
In contrast, if the electrical field is intense enough, these pores can be permanent to promote a permanent electroporation phenomenon. This latter is interesting for microbial inactivation if no commitment of the food structure is observed. On the other hand, one of the limitations of PEF for protein extraction is that, when using high electrical intensity for obtaining high cell disruption rates, the quality of the extracted fractions may be affected by also altering the technological and functional properties of the proteins while spending high input energy [77,78].
However, the extraction of compounds from the food matrix, in which the occurrence of damage in the matrix is indifferent, is also desirable. For example, Gateau et al. [79] studied the use of PEF in the extraction of proteins from microalgae as a nonconventional technology to obtain high-quality proteins from sustainable sources. Because the residue of the protein extraction may serve as a source of carbohydrates for conversion into bioproducts, it is also interesting to have a disrupted and pretreated biomass for further biorefinery applications. In fact, when alternative sources of proteins are used, the structure of the residue extracted is not highly important but rather interesting because of its circular bioeconomy.
This technology was originally studied for use against microorganisms [80], and its use continues even in the most up-to-date publications [81]. In recent years, an increasing number of studies have focused on the application of this technique for the extraction of intracellular compounds of interest, such as bioactive compounds, lipids, and proteins [74]. For this, the intensity of the electric field must be greater than a critical value to cause electroporation. Consequently, the resulting effect varies according to the cellular matrix and the main focus of the process [82,83].
In general, as reported by Chudasama et al. [84], the extraction yield of compounds of interest tends to be enhanced when working with higher electric field strengths. Shorter pulses are more effective in temporary permeabilization by reversible electroporation. As a consequence, the application of more pulses per time period (high frequency) increases the intensity of the treatment. On the other hand, enhancing the overall intensity of the treatment may result in undesirable thermal effects for heat-sensitive products. For protein extraction, an intensity between 1 and 5 kV/cm is usually reported for both plant and animal cells, although higher values can be used for more rigid matrices [79,85]. The configuration of the duration of the pulses is crucial for extraction and is used between 1 and 100 μs for sensitive proteins. In addition to pulses of shorter duration, the use of 10 to 100 pulses tends to have a better effect on the preservation of the protein structure. The repetition rate of the pulses can also vary from 1 to 10 Hz at controlled temperatures of 20 to 30 °C to avoid thermal degradation of the proteins and enhance extraction.
These parameters can be managed depending on the matrix source, the desired yield of extraction, and secondary effects on the extracted proteins [62,75,86]. In the case of plant-based sources, the application of high-intensity electric fields in the form of short-duration pulses (μs) is recommended to disrupt cell walls and increase protein extraction [87,88]. In this sense, Table 2 compiles a series of studies that used PEF for enhancing protein extraction from different sources, alternatively to animals.
When focusing on the use of PEF to extract protein from alternative sources, Kamboj et al. [86] reported an increase in the protein content and antioxidant activity of perilla seed meal (PSM) residue from the perilla oil extraction industry, a plant native to Southeast Asia. Compared with conventional alkaline extraction, PEF-assisted alkaline extraction had a positive effect on the protein content (up to 10.58%) of the concentrate obtained from PSM. On the other hand, the authors also reported that increasing the number of pulses beyond 10 is unnecessary and may also cause negative results. Increasing the pulses and electrical strength from 6 kV/cm to 9 kV/cm also decreased the yield. The authors associated this behavior with possible protein degradation and/or chemical modifications that increased the difficulty of separating soluble proteins/peptides during extraction.

Table 2.
Recent studies on the improvement of the pulsed electric field in the extraction of alternative proteins.
Table 2.
Recent studies on the improvement of the pulsed electric field in the extraction of alternative proteins.
| Product | Extraction Conditions | Observed Effects | References |
|---|---|---|---|
| Yeast S. cerevisiae | Frequency: 39.8 and 159.3 Hz; Intensity: 10, 15, and 20 kV/cm; Time: 50–200 μs. | Extraction of intracellular compounds such as mannoproteins and β-glucan. | [75] |
| Perilla Seed Meal (PSM) | Frequency not informed; Intensity: 3–15 kV/cm; Time: 10–150 μs. | From 6 kV/cm there was a decrease in the content of extracted proteins. | [86] |
| White mushrooms | Frequency: 400 Hz and 800 Hz; Intensity: 2.4, 24.8, and 38.4 kV/cm; Time: 69.4 and 136μs. | The combination of PEF with light heating resulted in a significant increase in protein extraction. | [88] |
| Cardamine violifoli | Frequency: 1.01 kHz; Intensity: 6.67 kV/cm; Time: 20 min. | Obtaining selenoproteins with 57% purity. | [62] |
| Microalgae (Chlorella vulgaris) | Frequency: 20 kV/cm; Intensity: not informed; Time: 50 μs. | Extraction of free protein up to 29% without impeding cell growth. | [89] |
| Porphyridium cruentum | Frequency: 0.5 Hz; Intensity: 1.56–7.26 kV/cm; Time: 2.2–7.2 μs. | It selectively avoided the release of the protein calmodulin (a food allergen). | [90] |
| Olive pomace | Frequency not informed; Intensity: 1.0–6.5 kV/cm; Time: 15 μs. | PEF as a pretreatment doubled the protein concentration and decreased the extraction time. | [91] |
| Sesame pie | Frequency: 0.5 Hz; Intensity: 13.3 kV/cm; Time: 10 μs. | Significant improvement in protein extraction when used as pretreatments. | [92] |
| Tomato waste | Frequency: 20 Hz; Intensity: 0.5–2.5 kV/cm; Time: 15 μs. | The concentration of protein was released as the strength and time of the electric field increased. | [82] |
| Mixtures of grasses (70%) and clover (30%) | Frequency: 305 Hz; Intensity: 1.1 kV/cm; Time: 5 μs. | When used together with alkaline pretreatment, it released insoluble chloroplast membrane proteins. | [93] |
| Oats | Frequency: 1.01 kHz; Intensity: 6.67 kV/cm; Time: 60, 90–120 min. | The results suggested that the oat protein extracted by the ChCl-1,4-butanediol/water binary mixture had higher protein content, solubility, foaming capacity, and stability. | [94] |
| Pea | Frequency: 400 Hz; Intensity: 1.65 kV/cm; Time: 5 min. | PEF was able to modify the structure of the protein by inducing unfolding, intramolecular rearrangement, and aggregate formation. These results suggest the potential of PEF to guide the structure of proteins and improve their technological functionality. | [95] |
| Rice | Frequency: 400 Hz; Intensity: 1.65 kV/cm; Time: 5 min. | PEF was able to modify the structure of the protein by inducing unfolding, intramolecular rearrangement, and aggregate formation. Structural changes were associated with negligible changes in functional properties. | [95] |
| Gluten | Frequency: 400 Hz; Intensity: 1.65 kV/cm; Time: 5 min. | PEF was able to modify the structure of the protein by inducing unfolding, intramolecular rearrangement, and aggregate formation. However, these effects were strongly dependent on the nature of the protein and pH. | [95] |
| Seaweed (C. vulgaris) | Frequency: 4.5 Hz; Intensity: 40 kV·cm −1; Time: 1 μs. | It induces irreversible permeabilization of the membrane and, consequently, programmed cell death. The release of proteins is likely facilitated by autolytic processes associated with programmed cell death. | [96] |
| Papaya peel | Frequency not informed; Intensity: 40–10 kv; Time: 2720 s ≈ 3/4 h; | The proposed two-stage PEF method allows a significant increase in the yield of high-value-added compounds and in the antioxidant capacity of papaya peels, even at neutral pH, and does not require the use of additional chemical compounds. | [97] |
| Residual brewer’s yeast | Frequency not informed; Intensity: 10 kV/cm; Time: 10 min. | The PEF method was a promising new method for extracting protein from brewer’s yeast residues that will benefit the food and agricultural industry | [98] |
| Green algae Lettuce (Ulva lactuca) | Frequency not informed; Intensity: 7.5 kV/cm; Time: 0.05 μs. | Protein yield reaches 15.1%. This study reported the highest protein (~39%) and carbohydrate (~51%) yields of the four technologies utilizing high-shear homogenization. | [99] |
| Soy | Frequency: 2, 8 and 500 Hz; Intensity: 0–40 kV/cm; Time: 0–547 μs. | Solubility and hydrophobicity increased with increasing PEF strength and treatment time at constant pulse width. | [100] |
| Nannochloropsis Microalgae | Frequency not informed; Intensity: 20 kV/cm; Time: 10.0 ± 0.1 μs. | PEF allowed for the selective extraction of a few different pure proteins. The pretreatment of PEF with the parameters used in this study was ineffective for pigment extraction. | [101] |
| Rapeseed stems and leaves | Frequency: 2.0.5 kHz; Intensity: 20 kV/cm; Time: 1 μs. | The results of this study show the efficacy of PEF treatment as a new way of valuing rapeseed stems and leaves. Polyphenol and protein extraction yields can be highly increased. | [102] |
| Large green algae Ulva ohnoi | Frequency: 3 h; Intensity: 1 kV/cm; Time: 50 μs. | In addition, PEF combined with pressing increased the protein of coextracted macroalgae by more than 4 times and the ash by 1.5 times compared to pressing alone. | [103] |
| Green marine macroalgae Ulva sp. | Frequency: 247 kJ/kg; Intensity: 50 kV/cm; Time: 50 μs. | It resulted in a ∼7-fold increase in the total yield of protein extraction compared to conventional. | [104] |
| Chickpea seeds | Frequency: 100 Hz; Intensity: 0.7–0.8–0.9–1.0 and 1.1 kV/cm; Time: 30–60–90–120 and 150 s. | PEF efficiently contributed to obtaining chickpea protein isolates with excellent functional properties, making possible the production of proteins with highlighted functional properties. | [105] |
| Wet biomass of A. platensis (Spirulina) | Frequency: 20 Hz; Intensity: 7.8 kV/cm; Time: 0.113–0.565–2.260 and 3.955 μs. | PEF demonstrated significantly accelerated protein extraction from Spirulina, while positively affecting the purity of the extract. A synergistic effect of electroporation and proteolytic enzyme activity during incubation of PEF-treated biomass was also observed. | [78] |
| Defatted yellowworm flour (T enebrio molitor). | Frequency: 20 Hz; Intensity: 1.5–3.125 and 5 kV/cm; Time: not informed. | A 27% increase in protein yield and decreased molecular weight were observed when comparing to the control extracts. Furthermore, PEF induced modification of the protein structure, as indicated by a reduction in fluorescence and β-sheet content. The observed changes had an impact on the measured foaming properties, | [106] |
| Green marine macroalgae (Ulva sp.) | Frequency: 5–10 Hz; Intensity: 0–750–1000 and 1250 Vcm−1; Time: 60 μs. | Greater efficiency and especially environmental sustainability, as no large waste streams are generated. It demonstrates superior extraction performance to the conventional alkaline extraction. In addition, the values for water and oil holding capacity showed suitability for various food and biochemical applications. | [107] |
Buchmann et al. [89] studied the use of PEF in Chlorella vulgaris cultures. The authors reported that as the intensity of the electric field increased, the rate of protein extraction also increased. However, the increase in extraction rates caused undesirable growth of microalgae due to the presence of free protein extraction, indicating that the efficiency of PEF extraction without affecting the vitality of the microalgae is probably due to temporal electroporation. Finally, the authors reported that the extraction efficiency depended on the cell growth phase, which was limited mainly by diffusion due to the biomass loading in the extraction medium.
Another study reported a permeabilization of 53.8% of the Porphyridium cruentum population (6 kV/cm by 90 μs), which resulted in the solubilization of approximately 97% of the water-soluble proteins (β-phycoerythrin) after 48 h of extraction [108]. Although most studies have shown that PEF increases the yield of protein extracted from microalgae, comparative studies suggest that other unconventional methods should be studied [109].
A study conducted by Andreou et al. [82] revealed that using PEF as a pretreatment in olive pomace (1.0 to 6.5 kV/cm, 0.9 to 51.1 kJ/kg, pulse of 15 μs) followed by conventional solid–liquid extraction (50% ethanol–water solution) doubled the concentration of protein in the dispersant, in addition to decreasing the extraction time. In another work carried out by Andreou et al. [91], the efficiency of PEF pretreatment (5 kV/1.5 ms) in tomato waste was evidenced by the increase in protein extraction from 88.9 mg/100 g of tomato waste (untreated) to 145.1 mg/100 g of tomato waste.
Sarkis et al. [92] also applied this technology as a pretreatment for sesame cake, which resulted in the best extraction under an energy input of 83 kJ/kg. The authors suggest that when a PEF is used followed by hot extraction, it is possible to reduce the diffusion temperature. This can be beneficial, as 20 min of extraction at 60 °C can decrease the protein concentration due to denaturation and coagulation.
Recent studies have diversified the use of the PEF technique. Steinbruch et al. [107] reported the intensification of aqueous extraction of protein from fresh biomass of the green alga Ulva sp. The authors also highlighted the performance of extraction together with its environmental sustainability without generating large amounts of waste. The extracted proteins presented increased water- and oil-holding capacities, suggesting interesting food and biochemical applications.
PEF application (6.67 kV/cm, 1.01 kHz) was used to increase the yield of protein extracted from C. violifoli. In addition, selenoproteins with 57% purity were obtained. They are able to incorporate selenium directly into their structure during protein synthesis, in addition to presenting antioxidant functions. Even though there was an increase in protein extraction compared with conventional extraction, PEF conditions were not optimized and, consequently, were not as efficient as ultrasound application in a comparative study. However, the quality and structure of the proteins in the PEF-based extracts were similar [62]. The application of PEF followed by ultrasonication did not result in greater results than did ultrasound-assisted extraction, probably because PEF was able to release proteins into the extraction medium and leave them exposed to ultrasound, increasing the likelihood of denaturation and decreased solubilization.
In view of that, PEF presents advantages by involving less intense heating effects than those of ultrasound application and even some conventional methods. This minimizes the risk of denaturation and maintains the secondary and tertiary structure of the proteins, which may be interesting for food applications and nutrition. Some applications can require functional proteins with excellent structural properties [105].
Indeed, PEF application also impacts the structural and techno-functional properties of proteins. Under well-designed conditions, PEF tends to improve the solubility of the extracted proteins, a crucial property for improving their interaction with other components and their extractability. The exposure of functional sites together with the preservation of the native structure can also improve emulsification capacity and gel formation. Proteins extracted by PEF often have good foaming properties, which benefits products such as milk and beverages. In general, PEF is recognized by preserving the functionality and integrity of proteins [110].
Notably, when alternative sources of proteins are used, the allergenicity of the extracted fraction should be considered. Prabhu et al. [103] applied PEF pretreatment combined with a pressing technique on Ulva ohnoi seaweed to obtain a protein concentrate, among other components, which has the potential to present protein sequences annotated to allergens. The concentration of proteins extracted was four times greater than that extracted by pressing alone. Polikovsky et al. [90] studied the individual application of PEF to Ulva ohnoi but also focused on the allergenicity of the resulting fraction. In addition, compared with osmotic shock treatment and mechanical pressing, the application of 50 PEF pulses (7.26 kV/cm, duration 2.3 μs, 0.5 Hz) was the most efficient technique for extracting proteins, and compared with the control, the application of PEFs selectively prevented the release of calmodulin (annotated to allergens) without PEFs.
The use of PEF as a pretreatment has been shown to be a potential technology to increase the yield and reduce the time of protein extraction from nonanimal sources. With respect to the impact of PEF technology on the extraction of protein from microalgae, this technology has proven to be a potential substitute for conventional methods and is faster than some nonconventional treatments. The effect of PEF on the techno-functional properties and quality of the extracted proteins is challenging and is of interest for food applications [111].
In brief, PEF has emerged as an innovative and efficient technology for enhancing protein extraction, especially from plant sources and microalgae in place of meat sources. This method has advantages such as increased extraction yield, preservation of sensory and nutritional properties, and improved techno-functional properties. In recent years, PEF has been recognized as an efficient technology for the cascade extraction of a series of compounds, which makes it possible to obtain a variety of valuable biomolecules from biomass, such as spent S. cerevisiae yeast [112]. Although the impact of processing conditions on the efficiency and quality of extraction still requires further study, agro-industrial coproducts could be valorized by encouraging PEF application in cascade applications, which would also contribute to the circular economy strategy [105,110].
4. Microwave (MW)
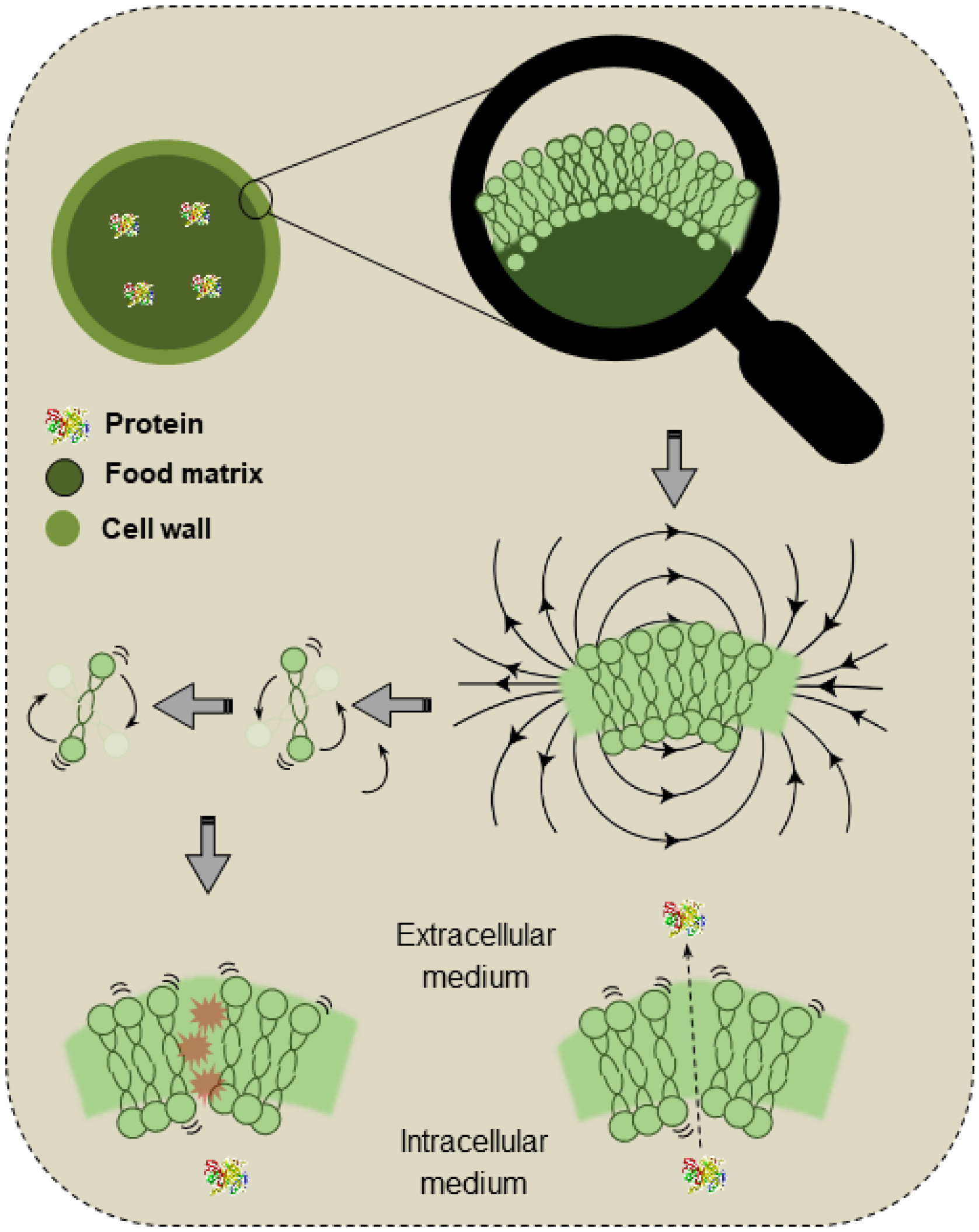
Microwaves are electromagnetic waves with frequencies varying from 300 MHz to 300 GHz, a range between radio frequency and infrared waves. These waves are generated by the action of a magnetron, a device responsible for converting electrical energy into microwaves. The generated waves are conducted to the cavity of the instrument, where the sample to be treated is placed. The sample absorbs energy due to ionic polarization and dipole interactions, which results in heating [113]. Figure 4 exemplifies the mechanisms of MW actuation on the cell walls and membranes.
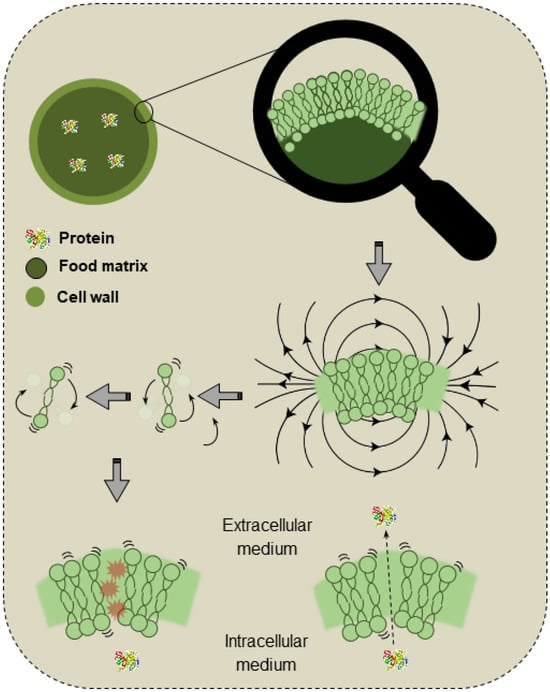
Figure 4.
Schematic demonstration of the effects of microwaves on the cell walls and membranes.
MWs selectively interact with polar molecules and induce intracellular heating, with the intensity varying according to the dielectric properties of the material. The “hot points” and localized pressure in the cell walls lead to cell rupture, improving the extraction of intracellular compounds such as proteins. As the applied power increases, a significant reduction in the extraction rate is also expected [114].
In comparison with the conventional method of protein extraction by alkaline solubilization, the extraction assisted by MW presents several advantages: the extraction process is considered a green technology, because it does not involve the use of pure solvents, causing a reduction in solvent use of 10–33% [115,116], besides demanding lower amounts of water and energy [117,118,119]. Another differential is the use of short treatments, varying between 30 s and 10 min, also resulting in high rates of extraction. When compared with other conventional and nonconventional methods, MW will achieve a 50% reduction in CO2 footprint during life cycle assessment (LCA), reinforcing the affirmation of the production of more sustainable proteins [120,121].
When extending microwave radiation, rapid heating of the sample is notorious, leading to protein unfolding or partial denaturation [122]. The study of Z. Li et al. [123] reports protein denaturation levels of more than 20 to 40%, compared to ultrasound at moderate intensity, which yields denaturation of 15 to 30%. However, PEF, under equivalent conditions, yields protein denaturation of 30 to 60%. Unlike ultrasound and PEF, which induce mechanical effects, the thermal effects of microwave radiation are pronounced even at low temperatures and treatment duration [122,123].
At both the industrial and laboratory scales, the frequencies of MW applications commonly range from 2.45 GHz to 3.00 GHz. Concerning the input power applied, the literature reports a wide range of values comprising powers from 100 to 750 W. The nominal power applied depends on the robustness of the matrices, the sensitivity of the extracted proteins, and the degree of modification/denaturation accepted. For more complex structures (which are alternative sources, such as plant-based coproducts and seeds), higher values are used to promote access to the intracellular contents and inner matrices. However, it can cause changes in the structure of proteins [124,125], highlighting the specific need to adequately study the process to balance the efficiency and quality of the resulting product.
MW extraction can generate undesirable changes in the structural conformation if it is not performed under well-designed operating conditions. With rapid heating and/or the use of too much power, modifications may occur in the secondary, tertiary, and even quaternary structures of the proteins. The loss of secondary structures, such as alpha-helices and beta sheets, can expose previously protected functional groups, such as amine or carboxyl groups. On the other hand, this structural alteration can increase the reactivity of proteins and their solubility [126].
Studies with promising results on alternative and sustainable sources of proteins have been reported with the assistance of MW technology. For the MW-assisted extraction of proteins from peanuts, a power of 725 W was applied for 8 min. As a result, 55% protein extraction resulted in recovery, which was 77% greater than that of the extraction control without MW application. For the extraction of soy protein, a frequency of 2450 MHz was applied for 30 min at 60 °C [63]. In these experiments, an increase of 58% in the extraction yield was obtained compared with that of the conventional process with hot water [127,128].
Barrios et al. [114] performed MW extractions for more efficient protein recovery from agri-food waste, such as brewer’s spent brewer grains (BSGs), spent coffee grounds (SCGs), and kale stems. The extraction yield in alkaline media varied for each source used, reaching a minimum value of 61% and a maximum value of 96%. In addition, low concentrations of NaOH (0.5 to 1.3 M) and time intervals (3 to 15 min) were required for the processes analyzed. For the specific case of kale stems, the authors highlight that hot water may provide interesting results for extracting proteins with the assistance of MW. This finding reinforces that, for a given material, MW can not only improve the extraction rate but also reduce chemical usage.
Martínez-Padilla et al. [64] reported the results of nonconventional technologies used to extract coconut oil and proteins from coconut pulp. Coconut milk was prepared, and MW (1 min, 3 pulses of 20 s; 2.5 GHz; 4.31 kW/kg by pulse) was applied to the milk prior to destabilization at pH 4 to obtain a cream and a protein-rich aqueous fraction. In addition to improved oil recovery compared with the control, up to 58% more protein could be recovered in the protein-rich fraction obtained from coconut milk.
When the protein extraction process from rice bran was studied by Bedin et al. [129], the authors obtained yields similar to those of the conventional extraction method but 30 times faster when MW technology was used as an assistive technology. The most favorable conditions for the extraction of 79.98% of the protein were 55 °C and pH 11 for 120 s [130].
Table 3 presents a compilation of studies together with the conditions used to report the use of MWs for the extraction of protein from alternative sources. In brief, the MW has been shown to be a promising alternative for the extraction of vegetable proteins because of its efficiency, intensification properties, and lower environmental impact. In addition, the MW tended to preserve the functional properties of proteins when used under controlled conditions. On the other hand, with the use of high power and long exposure times, structural changes may occur.

Table 3.
Recent studies on the improvement of the use of microwaves in the extraction of alternative proteins.
Table 3.
Recent studies on the improvement of the use of microwaves in the extraction of alternative proteins.
| Product | Extraction Conditions | Observed Effects | References |
|---|---|---|---|
| Peanut | Frequency: not informed; Power: 725 W; Time: 8 min; Temperature not informed. | It extracted 100% protein with 55% yield compared to the conventional extraction method. | [63] |
| Soy | Frequency: 2450 Hz; Power: not informed; Time: 30 min; Temperature: 60.1 °C. | 58% increase in extraction compared to hot water. | [128] |
| Rice bran | Frequency: 50–60 Hz; Power: not informed; Time: 120 s; Temperature: 55 °C. | 79.98% protein content of rice bran. 30 times faster than the conventional extraction method. | [129] |
| Rice bran | Frequency: 2450 MHz; Power: 600–1000 W; Time: 60–120 s; Temperature not informed. | The protein yield was higher than that obtained from an alkaline extraction. Protein digestibility was increased. | [131] |
| Rice bran | Frequency: 2450 MHz; Power: 800 W; Time: 20, 30, 40, 60 and 90 s; Temperature not informed. | 82.6% protein recovery. | [132] |
| Beer fermentation bagasse | Frequency 50 Hz; Power: not informed; Time: 9.98 min; Temperature: 110 °C. | 93.99% of the protein extraction yield from used beer grains. | [114] |
| Coffee Powder | Frequency 50 Hz; Power: not informed; Time: 3.33 min; Temperature: 113 °C. | 61.17% of the protein extraction yield of the spent coffee powder. | [114] |
| Kale stalk | Frequency: 50 Hz; Power: not informed; Time: 14.93 min; Temperature: 109 °C. | 96.55% of the protein extraction yield from the cabbage stalk. | [114] |
| Defatted watermelon seeds | Frequency: not informed; Power: 50 W; Time: 2 min; Temperature not informed. | 90% extraction compared to the conventional method. | [133] |
| Coconut milk | Frequency: 2.5 GHz; Power: not informed; Time: 1 min. being 3 pulses of 20 s every; Temperature: 45 °C. | 19.5% increase in protein extraction in coconut milk. | [64] |
| Soy milk | Frequency: not informed; Power: 675 W; Time: 60–120 s; Temperature: 80 °C. | 24% and 44.4% increase in extraction yield and protein content, respectively, compared to the conventional method. | [134] |
| Jackfruit leaf | Frequency: 42 kHz; Power: 1200 W; Time: 2, 3 or 4 min; Temperature: not informed. | In the extraction of proteins from jackfruit leaves, we obtained a content of 84.1 mg/g using conventional methods, but in MW extraction we obtained a higher concentration of 95.6 mg/g. | [65] |
| Cottonseed meal | Frequency not informed; Power: 900 W; Time: 6 min; Temperature: not informed. | MWs can cause conformational changes in proteins, generating free radicals or large or small molecules, thereby damaging the primary, secondary, and tertiary structure of the protein and influencing solubility, emulsification, foaming, and other functional properties. | [135] |
| Wheat germ | Frequency not informed; Power: 186 W; Time: 3.28 min; Temperature: not informed. | The results obtained revealed that the use of MW technology as a process intensifier device for protein extraction improved the amount of protein extracted and functional properties such as water/oil retention capacity, emulsifying capacity, digestibility solubility, gelling properties and foaming capacity. | [121] |
| Pineapple peel waste | Frequency not informed; Power: 300 to 600 W; Time: 40 to 50 min; Temperature: not informed. | Shorter time, higher extraction rate, lower cost), it has been found to be a captivating medium for the purpose of extraction | [136] |
| Tremor grains | Frequency not informed; Power: 200 to 2000 W; Time: 10 min; Temperature: not informed. | Increased extraction yield in MW pretreated samples with shorter processing time (10 min) compared to conventional (1 h). | [137] |
| Milk caper (L. volemus) | Frequency: 50/60 Hz; Power: not informed; Time: 10 min; Temperature: 20 °C. | Increased extraction rate and better functionality of proteins extracted with MWs compared to conventional extractions. | [138] |
| Jack bean (Canavalia ensiformis L.) | Frequency: not informed; Power: 800 W; Time: 120 s; Temperature: 50 ± 5 °C. | MW-assisted alkaline extraction showed a higher protein content of 86.34% with interesting gelation properties. | [139] |
| Silkworm (Bombyx mori) | Frequency: not informed; Power: 730 W; Time: 1–2 min; Temperature: not informed. | Changes in the formation of protein that improved the emulsification and foaming properties. | [69] |
| Red algae (Palmaria palmata) | Frequency: not informed; Power: 50, 100, 150, 200, 250, and 500 W; Time: 5, 10, 15, 20 and 30 min; Temperature: 45 °C. | The MW treatment (30 min) provided a protein extraction yield of 47.6 ± 3.4% with purities ~90% higher compared to the conventional method. | [140] |
| Foxtail millet (Setaria italica) | Frequency: not informed; Power: 480–960 W; Time: 30–90 s; Temperature: 30 °C. | MW significantly increased protein yield compared to conventional extraction methods, improving functional properties, solubility, foaming and emulsification properties and digestibility. | [141] |
| Pigeon pea (Cajanus cajan) | Frequency: not informed; Power: 400–700 and 960 W; Time: 60–120 s; Temperature: 21–23 °C. | MW increased the protein recovery and yield within 2 min of treatment with a consequent increase in the protein solubility. | [142] |
Behere et al. [133] evaluated the extraction of proteins from defatted watermelon seeds containing 50% protein. The authors used an MW-assisted extraction process with powers of 30, 50, and 70 W; a solid-to-solvent ratio of 1:10–1:40; an application time ranging from 30 s to 8 min; and a pH of the solvent in the range of 7–10, with the effects of moisture content or preleaching. The authors also compared the results with those of ultrasound-assisted and conventional extraction. A comparative study revealed 90% protein recovery by applying only 2 min of MW at a solid-to-solvent ratio of 1:30 (w/v), with a similar yield obtained with ultrasound but for 9 min with a solid-to-solvent ratio of 1:50 (w/v). Conventional extraction reached only 72% with 25 min of processing and high use of water (solid-to-solvent ratio of 1:70 (w/v)). The functional properties of the proteins obtained with the aid of MWs were superior to those of the proteins obtained via the conventional method and comparable to those obtained with the assistance of ultrasonication.
Current studies, such as those of Karki et al. [141], revealed clearer modifications in the proteins of the Foxtail millet (Setaria italica) by MW treatment, in addition to a significant increase in protein yield in comparison with conventional extraction methods. The authors obtained better results in terms of the techno-functional properties of protein concentrates, particularly their solubility, foaming capacity, foaming stability, and emulsifying activity index. The hydrolysates had better foaming properties and significantly greater protein digestibility (62.57%). When applying FTIR analysis, they confirmed the close relationship of the techno-functional properties and digestibility with the structural changes in the secondary protein structure induced by MWs.
In addition to the aforementioned advantages, the MW has been applied to products with value added with recent interest, such as red seaweed (Palmaria palmata). Compared with the conventional extraction method, the use of 500 W for 30 min was the most effective method, resulting in a final protein extraction yield of 47.6 ± 3.4% and a concentration purity of ~2-fold greater [140].
5. Deep Eutectic Solvents (DESs)
Deep eutectic solvents have emerged as alternatives to the commonly used volatile organic compounds. DESs are a class of green solvents formed by two or more components, mostly a mixture of organic halide salts, such as choline chloride or zinc chloride, and a hydrogen donor compound [143]. Among the hydrogen donors are amines, amides, alcohols and carboxylic acids, which remain liquids at room temperature. The mixture of components does not form ionic or covalent bonds; rather, they interact through hydrogen bonds. Its designation “deep” is due to its ability to form a eutectic mixture with a melting point much lower than that of its individual constituents [144].
Some authors describe DESs as a subclass of ionic liquids [145,146]. However, there are differences in both the starting materials and the production mechanisms. In general, these two solvents tend to present several similar characteristics, such as high thermal stability, low vapor pressure, high ionic conductivity, high biodegradability, low toxicity, and low cost.
DESs are typically easy to prepare: they use affordable equipment and are able to minimize water demand. Some DES preparation methods can be performed with additional unit operations, including vacuum evaporation, grinding, and/or freeze-drying. However, the most commonly used preparation method involves only heating and stirring the constituents together under an inert atmosphere until a homogeneous liquid is formed [147]. As it is not necessary to add any other solvent, no traditional reaction takes place; thus, no purification steps are needed. Owing to their structure and wide polarity range, the resulting DESs tend to have a good ability to dissolve micro- and macromolecules of natural products, and this property increases the potential to extract compounds such as proteins from many different sources. Therefore, it has been considered a sustainable solvent for extraction and obtaining value-added molecules [148].
There are several DESs, and depending on their composition, they can act as stabilizers, protecting the protein structure against thermal or oxidative degradation. In other cases, interactions with solvent components can lead to modifications in the protein structure, leading to possible partial unfolding and denaturation [149].
For example, Chen et al. [150] used choline chloride and glycerol-based DESs for soy protein extraction. The authors obtained an increase in the hydrophobic interaction and the hydrogen binding properties in the deep eutectic solvent. Thus, the amount of protein extracted was 0.3462 ± 0.046 g, which was close to the value obtained via extraction with conventional methods (0.3518 g).
The characterization of the extracted soy protein revealed that, although irreversible denaturation was caused by DES extraction compared with conventional methods, the proteins extracted by DES had better heat resistance and greater hydrophobicity. Notably, the technofunctional properties of proteins can be altered according to the solvent used for extraction. The unfolding of proteins and the exposure of polar groups may result in improved solubility, whereas structural modification can alter the properties of emulsification and gel formation [151].
Yue et al. [152] prepared nine mixtures of choline chloride and butanediol isomers to extract protein from oats. They reported that the proteins extracted by the binary mixture of choline chloride-1,4-butanediol/water resulted in the highest percentage of protein extraction (55.72%). The presence of moisture in the DES increased the denaturation temperature of the obtained proteins, resulting in great stability of the oat protein against thermal processing, good foaming capacity, and improved water solubility.
A study carried out by Hernández-Corroto et al. [153] demonstrated that extracting proteins from pomegranate peel via choline chloride/acetic acid resulted in an ~2-fold greater protein content (19.2 mg protein/g peel) than did the pressurized liquid extraction method (9 mg protein/g peel). In addition, the proteins obtained via DES extraction had more preserved structures with higher antihypertensive capacities than those obtained via pasteurized liquid extraction.
Lin et al. [154] extracted proteins from bamboo shoots via different molar ratios of choline chloride-levulinic acid and compared the efficiency of the DES with that of the conventional solubilization process in alkaline media. The optimal conditions for extraction performance were a hydrogen acceptor (HBA) and hydrogen donor (HBD) molar ratio of 6, with a solid–liquid ratio of 30 mg/mL. The authors reported a yield of 39.16 ± 1.22 mg protein/g dry weight, which was significantly greater than that of conventional extraction using NaOH solution (23.88 mg protein/g dry weight). The results also suggest that bamboo processing byproducts could be an interesting source of alternative proteins.
Wahlström et al. [155] mixed carboxylate salts (potassium and sodium formate and acetate) with urea to synthesize DES for the extraction of proteins from barley grains. They reported that sodium/urea acetate (molar ratio of 1:2) extracted 79% of the protein content of the grains, producing an extract with more than 50% protein. Despite the high yield and protein content of the extract, the authors recommended the use of some components, such as urea, for food applications.
A study carried out by Liu et al. [156] also combined the use of microwave irradiation to assist in the extraction of proteins from pumpkin seeds via the use of choline chloride and polyethylene glycol. They obtained an extraction yield of 93.95% and the highest pumpkin seed protein precipitation rate (97.97%) via isoelectric coprecipitation with ethanol–polyethylene glycol.
Moldes et al. [157] investigated the application of DESs in the selective extraction of proteins from the brown seaweed Saccharina latissima using previously freeze-dried biomass. Several experimental plans were tested, and the highest extraction yield was obtained after 1 h of processing the biomass with the eutectic mixture of 1 betaine:2 urea at 40 °C, with the addition of 47% water to the system. They reached a protein recovery of 10.6%, comparable to that of conventional methods (bead milling), but with superior selectivity. They highlighted the sustainability of the method in recovering proteins from macroalgae efficiently.
Additionally, Ecem Bayram et al. [158] explored protein extraction from bee bread, a fermented product derived from pollen that is rich in nutrients and widely used by bees as a protein source. They studied the application of 12 different DESs with different hydrogen bond acceptor (HBA)–hydrogen bond donor (HBD) combinations. In particular, choline chloride and urea not only increased the extraction efficiency but also resulted in greater valorization of the contents of free amino acids and total phenolic compounds.
These findings reinforce the versatility of DESs in the extraction of bioactive compounds from complex natural matrices. However, some challenges are encountered when using DESs on an industrial scale. Studies have reported the complexity of recovering solvents used in extraction, thus increasing operational costs [159]. Furthermore, the regulatory agencies’ definition of acceptable solvent types for food use creates greater scalability difficulties, as the solvents must be of high purity and considered Generally Recognized As Safe (GRAS) [160,161,162]. Their application to other products is demonstrated in Table 4. In view of the previous studies presented in Table 4, the use of DES as a nonconventional technology becomes a viable solution to reduce the impact of conventional protein extraction from alternative sources. Potential intensification of the process, lower environmental impact, ease-to-prepare aspects and the ability to preserve or even improve certain techno-functional properties of the extracted proteins, such as solubility and thermal resistance, could be observed.
However, compared with ultrasonic, microwave, and pulsed-electric methods, the use of DESs presents greater challenges and limitations, such as the potential for denaturation of the extracted proteins depending on the composition of the DES and the extraction conditions [163]. In addition, the solvent components need to be carefully selected to avoid undesirable residues, especially in food applications. There is a lack of standardized methods for different matrices, which requires additional and specific studies to ensure the replicability of the results and evidence the improvement over other alternative technologies. Under suitable conditions, DESs represent a potential technology for scaling up protein extraction in industrial applications [164,165].

Table 4.
Recent studies on the improvement of deep eutectic solvents in the extraction of alternative proteins.
Table 4.
Recent studies on the improvement of deep eutectic solvents in the extraction of alternative proteins.
| Product | Extraction Conditions | Observed Effects | References | ||
|---|---|---|---|---|---|
| Solvent | Temperature (°C) | Time (min) | |||
| Oats | Choline chloride-butanediol | 80 | 90 | The presence of water from DES increased the denaturation temperature, denoting great stability of the protein against thermal processing and foaming capacity. The presence of water improved the solubility of the protein. | [152] |
| Soy | Choline-glycerol chloride | 60 | 234 | Positive hydrophobic and hydrogen bonding interaction in DES. Irreversible denaturation was caused by DES extraction during heating. Extracted protein showed better heat resistance and stronger hydrophobicity. | [150] |
| Bamboo shoots | Choline chloride-levulinic acid | 80 | 50 | Protein extraction yield of 39.16 mg/g significantly higher than the conventional sodium hydroxide extraction method (23.88 mg). | [154] |
| Brewer’s Spent Grain | Carboxylate-urea | 80 | 240 | 79% extraction yield was obtained with >50% protein. | [155] |
| Pomegranate peel | Choline chloride-acetic acid | 120 | 15 | 19.2 mg/g of protein were obtained. The hydrolysis obtained from proteins extracted by DES showed high antihypertensive capacity. | [153] |
| Pumpkin seed | Choline chloride-polyethylene glycol (PEG) | 43 | 4 | The extraction yield was 93.95% and the protein precipitation rate was 97.97, with a precipitation time of only 4 min using these conditions. | [156] |
| Cold-pressed cakes produced from linen (Linum usitatissimum) | Choline chloride and glycerol | 80 | 60 | In general, the proteins were extracted with DES and precipitated with water, obtaining higher yield. An improvement in extraction was achieved by increasing the temperature of the treatment. Different acylglyceride profiles were identified in the residual oils obtained by solvent extraction of the cakes. | [159] |
| Cold-pressed cakes produced from camelina (Camelina sativa) | Choline chloride and glycerol | 80 | 60 | An improvement in extraction was achieved by increasing the temperature of the treatment. Different acylglyceride profiles were identified in the residual oils obtained by solvent extraction of the cakes. | [159] |
| Cold-pressed cakes produced from sunflower (Helianthus annuus). | Choline chloride and glycerol | 80 | 60 | An improvement in extraction was achieved by increasing the temperature of the treatment. Different acylglyceride profiles were identified in the residual oils obtained by solvent extraction of the cakes. | [159] |
| Sesame meal flour | Choline chloride: 1,3-Propanediol 1:2 | 60 | 60 | The results showed that the purity of the protein extracted with DES was significantly higher (up to 93%) than that with the precipitation extraction method (77.1%). | [149] |
| Oats | Choline chloride: 1,3-Propanediol 1:2 | 80 | 90 | Higher protein content—62.50%; with extraction yield of 8.18% and protein recovery—35.76% | [94] |
| Oats | Choline chloride: ethylene glycol 1:2 | 55 | 45 | Overall, protein extraction and recovery yields were higher for oat proteins extracted by hydrated DESs. DESs demonstrated their great potential for biorefining oat proteins obtained from biscuit flour. | [152] |
| Rapeseed Cake | Choline chloride: glycerin 1:2 | 100 | 120 | The precipitate yield improved with the increase in the treatment temperature, reaching a maximum of 20% and 35% at 140 °C. In general, the protein content of the extracts was 40–50%, which is up to 20% more than the starting materials. Extraction yield—9%. | [162] |
| Soy | Choline chloride: glycerol | 80 | 234 | Extraction yield—0.3462 and precipitation yield—0.3192. | [162] |
| Punica granatum | Choline chloride: glucose 1:1 | 80 | 234 | Single extraction of pomegranate seed proteins using DES under alkaline conditions allowed for to extraction of up to 15.3 g protein/100 g pomegranate seeds (61% protein in pomegranate seeds). Extraction yield—4.2 g/100 g. | [153] |
| Punica granatum | Choline chloride: acetic acid 1:2 | 60 | 15 | Single extraction of pomegranate seed proteins using DESs under alkaline conditions allowed to extract up to 15.3 g protein/100 g pomegranate seeds (61% protein in pomegranate seeds). Extraction yield—4.2 g/100 g. | [64] |
| Fava bean | Choline chloride: glycerol. | 50 | 60 | DES resulted in higher rate and yield of protein extraction. The secondary structures of the obtained concentrates revealed an increase in the α-helix content (21.37%) in the proteins extracted with DES compared to proteins extracted using the conventional method (10.68%). | [166] |
| Seaweed (Saccharina latissima) | Betaine:2Urea:Water | 30 | 60 | Good protein recovery yield (11%), higher selectivity, and the suitable particle size of extracted proteins (5–10 kDa). | [157] |
| Mushroom stem (MS) | 1choline chloride:2 glycerol and choline chloride: lactic acid | 50 | 60 | DES efficiently extracted proteins from lignocellulosic biomass with higher rate of protein recovery compared to alkaline solubilization. DES also showed better preservation of the emulsion capacities and balanced foaming properties, with enhanced foaming properties. | [167] |
| Cyanobacterial biomass (Spirulina) | Choline chloride and urea | 25–30 | 15–45 | Together with US, DES solubilization achieved a high protein yield (80.62%). The techno-functional properties of the extracted proteins, including high foaming capacity, emulsifying capacity, digestibility, and antioxidant properties, were highlighted. | [168] |
| Bee bread (Perga) | Choline chloride and urea. | 35 | 25 | Extracts obtained with DES showed higher levels of total protein, total individual phenolics, and total individual amino acids in comparison to ethanol solubilization. | [158] |
| Canola/rapeseed (Brassica napus) | ratio of choline chloride/d-sorbitol, glycerol, d-glucose, or urea/water. | 35 | 25 | All studied DES extracted higher percentages of napins (small and water-soluble proteins), resulting in more preserved protein structures compared to alkaline treatments. | [169] |
Although ultrasound, pulsed electric fields, microwaves, and deep eutectic solvents represent the most studied nonconventional technologies for protein extraction, other methods have also gained attention due to their complementary roles in the production process. (Re)emerging techniques, such as reverse micelles and aqueous two-phase systems, are not primarily aimed at maximizing extraction yield but rather at improving the partitioning, purification, or selective recovery of protein fractions. Therefore, rather than competing directly with the aforementioned emerging technologies, these approaches can act as follow-up or parallel strategies, enabling greater purity, functionality, or efficiency in protein extraction processing. Therefore, it is relevant to address these alternative methods and their potential in the context of protein extraction.
6. Other Emerging Technologies
In addition to nonconventional technologies, other emerging (or reemerging) practices have shown the potential to be employed solely or in combination with nonconventional technologies to increase the viability of protein extraction from sustainable sources. Among these methods, extraction via reverse micelles has become an interesting technique for the efficient recovery of proteins from alternative sources.
The technique of reversing micelles consists of a micelle-type system but contains an aqueous inner core stabilized by nanosized surfactants, making the solubilization of hydrophilic compounds possible in nonpolar media [170]. In the case of protein extraction, the soluble proteins are initially solubilized into the aqueous inner core of the reverse micelles (forward extraction) for further recovery by breaking down the micelle structure in a second extraction (backward extraction). The most common surfactant used for reverse micelle preparation is anionic AOT (sodium bis (2-ethylhexyl) sulfosuccinate), which is generally recognized as safe (GRAS). Conventional backward extraction is performed by adding an equivalent volume of aqueous solution to the forward extraction solutions [24].
The advantages of this method over conventional extraction are the possibility of recovering both the surfactant and the solvent; it has a low associated cost; it is scalable; and the extracted protein is preserved in the micelles, and, thus, denaturation is minimized [24]. When dealing with oily but protein-rich products such as seeds, reverse micelles have advantages over conventional extraction methods for simultaneously fractioning oil and proteins, as the presence of lipids increases the difficulty of protein recovery.
Therefore, studies have aimed to use reverse micelles for proteins (either with lipids or without lipids) from sustainable sources, such as hemp seeds [170], cruciferous oilseeds [171], walnuts [172], tea byproducts [173], and plant sources in general [174]. In general, proteins extracted by reverse micelles tend to present more preserved techno-functionality, a more compact structure, and greater solubility than those extracted by conventional methods. An analysis of the previously cited studies revealed that the yield of protein extraction in the forward step was greater than 50%, with more than 80–90% recuperation from the backward solution. Moreover, the method used for backward extraction (the recovery of proteins from the inner core) plays an important role in determining their final properties. For this purpose, nonconventional technologies such as microwaves [172] and ultrasound [173] can be incorporated into the process.
Another re-emerged technique is the use of an aqueous two-phase system (ATPS), which is currently used for recovering proteins from sustainable sources and even more for purifying them. In this type of liquid–liquid extraction, two immiscible compounds are put in contact. Generally, a hydrophilic polymer (e.g., polyethylene glycol, dextran, or polypropylene glycol) and a salt (e.g., phosphate, sulfate, or citrate), or even a mixture of two polymers, are used. Owing to the low interfacial tension, the diffusion of solvents and compounds of interest is promoted between the biphasic system [175]. The factors affecting the partitioning of proteins are related to the molecular weight and concentration of the polymers, ionic strength of the salt, pH of the solution, and additives used to alter hydrophobicity [176]. It is worth noting that some limitations prevent its use on an industrial scale, mainly concerning cost, polymer/salt recovery, and purification aspects [177,178]. Like reverse micelles, ATPSs have been continuously developed in combination with nonconventional techniques, such as DESs [179] and ultrasound [180], to improve fractionation.
The main advantage of using ATPS for protein extraction is related to the fact that denaturation or loss of biological activity cannot easily occur, thus tending to preserve the functionality and structure of ATPS [179]. In addition, varying the molecular weight of polyethylene glycol and the type of saline solution can cover a wide range of proteins to be selectively concentrated and separated from other compounds [181].
Studies on the use of ATPSs for protein extraction from microalgae have reported their suitability because of their ability to separate biomolecules (sugars, pigments, and proteins) [182]. The extraction of polysaccharides and proteins from Pleurotus ostreatus was studied by Yang et al. [183], who also used ethanol/K2HPO4 as ATPS. The authors obtained more than 75% protein recovery from the fungus. Compared with alkaline solubilization, for example, ATPS resulted in greater efficiency and purity and better preservation of the physical structure.
When alternative sources with potential for large-scale production are evaluated, Gu et al. [184] reported that ATPS is suitable for protein extraction and purification from corn extracts. These authors emphasized that the concentrations of polyethylene glycol and Na2SO4, together with the ionic force (by NaCl addition), can be used to recover either hydrophobic or hydrophilic proteins. An ATPS consisting of betaine-urea as a DES together with K2HPO4 was used for extracting proteins from walnut and its corresponding cold-pressed meal. Compared with alkaline solubilization and isoelectric precipitation, the obtained proteins presented better color and lower surface hydrophobicity, with distinct characteristics of superior solubility at low pH [185].
In addition to the possibility of incorporating nonconventional technologies into these techniques, the addition of pretreatments or further steps may be a potential alternative. Saw et al. [186] used a liquid biphasic flotation (LBF) system when extracting proteins from Persicaria tenulla leaves. An antisolvent step, in which surface-active or hydrophobic compounds are adsorbed on bubble surfaces and then recovered by flotation in a superior organic solution, is added.
7. Comparisons and Current Industrial Challenges
After detailing each unconventional technology, it is crucial to make a critical comparison among them based on the presented studies. Ultrasound (US), for example, has been shown to be highly effective for dense plant matrices; however, its processing on an industrial scale is limited by high energy consumption and additional cooling costs. [187,188,189]. US also requires high energy and is not always industrially scalable [190]—demanding physical assessment regarding wave resonance in the reactor and probe disposal in the batch chamber. Continuous processes are also challenging in order to match the efficient exposure time with the processing needs. In addition to its costs, if not carefully controlled, it can lead to thermal degradation of sensitive compounds present in the samples [191].
On the other hand, pulsed electric fields (PEFs) operate with lower energy consumption compared to US, offering potential for microalgae and biomass, considered more fragile materials [192,193]. However, electrode durability poses a challenge for scalability, as it generates high equipment costs Differently to US, the scalability of [187,188,189] continuous PEF treatment is easier to design due to the short treatment time and lower residence time in the treatment chamber. Microwave-assisted extraction (MW) was reported to provide rapid and efficient protein recovery, but increasing the frequency leads to challenges such as heating uniformity [194,195]. The review published by [196] reinforces that studies are still incipient when evaluating high amounts of raw material (~0.5 kg/batch). They also highlight that, in contrast, the use of deep eutectic solvents (DESs) does not entail additional challenges other than the recovery and reuse of the solvent.
Deep eutectic solvents (DESs) have proven to be a sustainable alternative to conventional solvents, enabling protein recovery while preserving their functionality. However, the cost of solvents and recovery procedures limits their industrial viability. Furthermore, there are no methodologies to verify the toxicity of DES [191,196]. Overall, US and MW are closer to industrial use and are reported to be of low environmental impact [197]. However, PEF and DES remain in the early stages, requiring further study. Other aspects than the technology chosen itself are the management and application of the raw material for extraction. The use of wet sources may avoid the drying process, thus demanding low energy and water consumption. On the other hand, the extraction efficiency tends to be damaged in these applications. Therefore, the selection of technology must be specific to each case, considering the type of substrate, the desired functionality, and energy efficiency.
8. Conclusions
Nonconventional extraction methods have varying impacts on the characteristics and quality of extracted proteins, mainly when dealing with different alternative sources. These methods represent alternatives to intensify and implement extraction from alternative sources, especially for obtaining plant-based proteins and promising blue proteins (from aquatic sources) and concentrates/isolates from insects and agroindustrial byproducts.
Certainly, nonconventional technologies offer benefits and challenges that should be considered when choosing the technology and the protein source. Ultrasound-assisted extraction, for example, stands out for its efficiency in breaking up dense cell structures, reducing the average size of proteins, and increasing surface area and roughness, which improves properties such as solubility, emulsification, and the ability to favor the Maillard reaction.
On the other hand, the pulsed electric field (PEF) tends to be highly applicable in plant sources and microalgae, providing high yields but also preserving sensory and techno-functional properties according to the processing parameters. The microwave application, in turn, combines efficiency, high mass transfer rates, and lower environmental impact and is able to preserve the functionalities of proteins when used under controlled and fast conditions, even though there is still a risk of denaturation under inappropriate parameters. Deep eutectic solvents (DESs) have greater potential for innovation but also present significant challenges, such as the choice of suitable solvent composition and combination, which makes standardization for different matrices difficult. However, once the method is well established, the technology can be easily scaled up. Techniques such as reverse micelles and aqueous two-phase extraction have also been observed as complementary approaches not only for extracting proteins but also for purifying them for further use.
In conclusion, compared with conventional alkaline solubilization, all of these covered technologies have the potential to improve protein extraction and reduce the environmental impact (possible reductions in the use of chemicals, water consumption, temperature, etc.). Owing to the focused source of protein, each one may present some specificities. When the process is properly designed, nonconventional technology offers sustainable and improved methods for recovering proteins from nonanimal sources, highlighting new possibilities for future industrial applications.
Author Contributions
Conceptualization, T.C.P. and J.T.d.P.S.; formal analysis, C.d.S.C., G.X.O., M.G.P. and N.A.B.; writing—original draft preparation, C.d.S.C., G.X.O., M.G.P., N.A.B. and T.C.P.; writing—review and editing, T.C.P., J.T.d.P.S. and S.C.d.P.; supervision, T.C.P.; project administration, T.C.P., J.T.d.P.S. and S.C.d.P.; funding acquisition, T.C.P. and S.C.d.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by São Paulo Research Foundation—FAPESP—PROValue project (Grant 2024/03269-5), to the National Council for Scientific and Technological Development—CNPq (Brazil, Grant 407622/2023-3), and to the Coordination of Higher Education Personnel Improvement (Brazil, Finance Code 001). The APC was funded by the São Paulo Research Foundation—FAPESP through the PROValue project (Grant 2024/03269-5).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gardner, C.D.; Hartle, J.C.; Garrett, R.D.; Offringa, L.C.; Wasserman, A.S. Maximizing the intersection of human health and the health of the environment with regard to the amount and type of protein produced and consumed in the United States. Nutr. Rev. 2019, 77, 197–215. [Google Scholar] [CrossRef]
- Halkjær, J.; Olsen, A.; Bjerregaard, L.J.; Deharveng, G.; Tjønneland, A.; Welch, A.A.; Crowe, F.L.; Wirfält, E.; Hellstrom, V.; Niravong, M.; et al. Intake of total, animal and plant proteins, and their food sources in 10 countries in the european prospective investigation into cancer and nutrition. Eur. J. Clin. Nutr. 2009, 63, S16–S36. [Google Scholar] [CrossRef]
- Sheffield, S.; Fiorotto, M.L.; Davis, T.A. Nutritional importance of animal-sourced foods in a healthy diet. Front. Nutr. 2024, 11, 1424912. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Dias, C.; Moran, D.; Rounsevell, M.D.A. Chapter 1—Sustainable Proteins Production. Proteins Sustain. Source Process. Appl. 2019, 1–39. [Google Scholar] [CrossRef]
- Home-WOAH-World Organisation for Animal Health. Available online: https://www.woah.org/en/home/ (accessed on 13 June 2025).
- Coleman, G.J.; Hemsworth, P.H.; Hemsworth, L.M.; Hemsworth, L.M.; Munoz, C.A.; Rice, M. Differences in public and producer attitudes toward animal welfare in the red meat industries. Front. Psychol. 2022, 13, 875221. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; González-Lucán, M.; Fernández-Fernández, C.; Carneiro-Freire, N.; Seco-Filgueira, M.; Pedre-Piñeiro, A.M. Effect of diet composition on insulin sensitivity in humans. Clin. Nutr. ESPEN 2019, 33, 29–38. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Przysławski, J. Non-Pharmacological Treatments for Insulin Resistance: Effective Intervention of Plant-Based Diets—A Critical Review. Nutrients 2022, 14, 1400. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Fernández-Fernández, C.; Carneiro-Freire, N.; Vila-Altesor, M.; Ameneiros-Rodríguez, E. The differential effect of animal versus vegetable dietary protein on the clinical manifestations of diabetic kidney disease in humans. Clin. Nutr. ESPEN 2022, 48, 21–35. [Google Scholar] [CrossRef]
- Wood, P.; Tavan, M. A review of the alternative protein industry. Curr. Opin. Food Sci. 2022, 47, 100869. [Google Scholar] [CrossRef]
- Liu, Y.; Aimutis, W.R.; Drake, M.A. Dairy, Plant, and Novel Proteins: Scientific and Technological Aspects. Foods 2024, 13, 1010. [Google Scholar] [CrossRef]
- Diaz-Bustamante, M.L.; Keppler, J.K.; Reyes, L.H.; Alvarez Solano, O.A. Trends and prospects in dairy protein replacement in yogurt and cheese. Heliyon 2023, 9, e16974. [Google Scholar] [CrossRef]
- Mishal, S.; Kanchan, S.; Bhushette, P.R.; Sonawane, S.K. Development of plant-based meat analogue. Food Sci. Appl. Biotechnol. 2022, 5, 45–53. [Google Scholar] [CrossRef]
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- van Vliet, S.; Kronberg, S.L.; Provenza, F.D. Plant-Based Meats, Human Health, and Climate Change. Front. Sustain. Food Syst. 2020, 4, 555088. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Tay, W.; Quek, R.; Lim, J.; Kaur, B.; Ponnalagu, S.; Henry, C.J. Plant-based alternative proteins—are they nutritionally more advantageous? Eur. J. Clin. Nutr. 2023, 77, 1051–1060. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Enhanced alkaline extraction techniques for isolating and modifying plant-based proteins. Food Hydrocoll. 2023, 145, 109132. [Google Scholar] [CrossRef]
- Ntone, E.; Bitter, J.H.; Nikiforidis, C.V. Not sequentially but simultaneously: Facile extraction of proteins and oleosomes from oilseeds. Food Hydrocoll. 2020, 102, 105598. [Google Scholar] [CrossRef]
- Alfaro-Diaz, A.; Urías-Silvas, J.E.; Loarca-Piña, G.; Gaytan-Martínez, M.; Prado-Ramirez, R.; Mojica, L. Techno-functional properties of thermally treated black bean protein concentrate generated through ultrafiltration process. LWT 2021, 136, 110296. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Albolafio, S.; Gil, M.I.; Allende, A.; Xanthakis, E. Potential of Wastewater Valorization after Wet Extraction of Proteins from Faba Bean and Pea Flours. Recent Prog. Mater. 2021, 3, 13. [Google Scholar] [CrossRef]
- Sun, X.; Bandara, N. Applications of reverse micelles technique in food science: A comprehensive review. Trends Food Sci. Technol. 2019, 91, 106–115. [Google Scholar] [CrossRef]
- Paredes-López, O.; Ordorica-Falomir, C. Production of safflower protein isolates: Composition, yield and protein quality. J. Sci. Food Agric. 1986, 37, 1097–1103. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Udenigwe, C.C. Impact of processing on the chemistry and functionality of food proteins. In Proteins in Food Processing, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 27–45. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Hassoun, A. Food Industry 4.0: Emerging Trends and Technologies in Sustainable Food Production and Consumption; Academic Press: New York, NY, USA, 2024; pp. 1–298. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Laurindo, J.B.; Moreno, Y.M.F.; Carciofi, B.A.M. Influence of Emerging Technologies on the Utilization of Plant Proteins. Front. Nutr. 2022, 9, 809058. [Google Scholar] [CrossRef]
- Ganjeh, A.M.; Saraiva, J.A.; Pinto, C.A.; Casal, S.; Silva, A.M.S. Emergent technologies to improve protein extraction from fish and seafood by-products: An overview. Appl. Food Res. 2023, 3, 100339. [Google Scholar] [CrossRef]
- Marques, B.C.; Maniglia, B.C.; Favaro-Trindade, C.S.; Luchese, C.L.; Fai, A.E.C.; Martelli-Tosi, M.; Tiago, C.; Polachini, T.C.; Pinho, S.C. Protein Extraction From Seeds as a Strategy for Valorizing Agroindustrial Subproducts and Wastes: Challenges, Techniques, and Scaling-up Potential. Food Bioprocess Technol. 2025, 18, 6893–6913. [Google Scholar] [CrossRef]
- Jain, I.; Kaur, R.; Kumar, A.; Paul, M.; Singh, N. Emerging protein sources and novel extraction techniques: A systematic review on sustainable approaches. Int. J. Food Sci. Technol. 2024, 59, 6797–6820. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High intensity ultrasound treatment of protein isolate extracted from dephenolized sunflower meal: Effect on physicochemical and functional properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef]
- Byanju, B.; Rahman, M.M.; Hojilla-Evangelista, M.P.; Lamsal, B.P. Effect of high-power sonication pretreatment on extraction and some physicochemical properties of proteins from chickpea, kidney bean, and soybean. Int. J. Biol. Macromol. 2020, 145, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Linares, G.; Rojas, M.L. Ultrasound-Assisted Extraction of Natural Pigments From Food Processing By-Products: A Review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Velasco, A.; Lobato-Calleros, C.; Hernández-Rodríguez, B.E.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. High intensity ultrasound treatment of faba bean (Vicia faba L.) protein: Effect on surface properties, foaming ability and structural changes. Ultrason. Sonochem. 2018, 44, 97–105. [Google Scholar] [CrossRef]
- Karabulut, G.; Feng, H.; Yemiş, O. Physicochemical and Antioxidant Properties of Industrial Hemp Seed Protein Isolate Treated by High-Intensity Ultrasound. Plant Foods Hum. Nutr. 2022, 77, 577–583. [Google Scholar] [CrossRef]
- Mehta, N.S.J.; Kumar, P.; Verma, A.K.; Umaraw, P.; Khatkar, S.K.; Khatkar, A.B.; Pathak, D.; Kaka, U.; Sazili, A.Q. Ultrasound-Assisted Extraction and the Encapsulation of Bioactive Components for Food Applications. Foods 2022, 11, 2973. [Google Scholar] [CrossRef]
- Gadalkar, S.M.; Rathod, V.K. Extraction of watermelon seed proteins with enhanced functional properties using ultrasound. Prep. Biochem. Biotechnol. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Mirón-Mérida, V.A.; Soria-Hernández, C.; Richards-Chávez, A.; Ochoa-García, J.C.; Rodríguez-López, J.L.; Chuck-Hernández, C. The Effect of Ultrasound on the Extraction and Functionality of Proteins from Duckweed (Lemna minor). Molecules 2024, 29, 1122. [Google Scholar] [CrossRef]
- Prajaputra, V.; Maryam, S.; Isnaini, N.; Apriani, S.; Maqfirah, S.; Lestari, A.S.; Mulyana, W.S. Influence of extraction time on collagen yield and proximate composition from yellowfin tuna (Thunnus albacares) bones: Insights from industrial waste valorization. Ecol. Eng. Environ. Technol. 2025, 26, 1–7. [Google Scholar] [CrossRef]
- Zheng, X.; Zou, B.; Zhang, J.; Cai, W.; Na, X.; Du, M.; Zhu, B.; Wu, C. Recent advances of ultrasound-assisted technology on aquatic protein processing: Extraction, modification, and freezing/thawing-induced oxidation. Trends Food Sci. Technol. 2024, 144, 104309. [Google Scholar] [CrossRef]
- Yeasmen, N.; Orsat, V. Pulsed ultrasound assisted extraction of alternative plant protein from sugar maple leaves: Characterization of physical, structural, thermal, electrical, and techno-functional properties. Food Hydrocoll. 2024, 152, 109960. [Google Scholar] [CrossRef]
- Suchintita Das, R.; Zhu, X.; Hannon, S.; Mullins, E.; Alves, S.; Garcia-Vaquero, M.; Tiwari, K.B. Exploring Osborne fractionation and laboratory/pilot scale technologies (conventional extraction, ultrasound-assisted extraction, high-pressure processing and hydrodynamic cavitation) for protein extraction from faba bean (Vicia faba L.). Innov. Food Sci. Emerg. Technol. 2023, 89, 103487. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, K.B. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Mitrović, J.; Nikolić, N.; Karabegović, I.; Savić, S.; Petrović, S.; Pešić, M.; Šimurina, O. Evaluation of the solvent effect on the extraction and antioxidant activity of phenolic compounds from the nettle (Urtica dioica L.) seeds: Application of PCA and regression analyses. J. Food Meas. Charact. 2024, 18, 6618–6626. [Google Scholar] [CrossRef]
- Fan, W.; Duan, H.; Ren, X.; Guo, X.; Zhang, Y.; Li, J.; Zhang, F.; Chen, J.; Yang, X. Optimization of ultrasound-assisted cellulase degradation method on the extraction of mulberry leaf protein and its effect on the functional characteristics. Ultrason. Sonochem. 2023, 99, 106561. [Google Scholar] [CrossRef] [PubMed]
- Gani, A.; ul Ashraf, Z.; Noor, N.; Ahmed Wani, I. Ultrasonication as an innovative approach to tailor the apple seed proteins into nanosize: Effect on protein structural and functional properties. Ultrason. Sonochem. 2022, 86, 106010. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Devi, L.M.; Badwaik, L.S. Ultrasound-assisted extraction of pumpkin seeds protein and its physicochemical and functional characterization. Appl. Food Res. 2022, 2, 100121. [Google Scholar] [CrossRef]
- Loushigam, G.; Shanmugam, A. Modifications to functional and biological properties of proteins of cowpea pulse crop by ultrasound-assisted extraction. Ultrason. Sonochem. 2023, 97, 106448. [Google Scholar] [CrossRef]
- Ngo, N.T.T.; Shahidi, F. Functional properties of protein isolates from camelina (Camelina sativa (L.) Crantz) and flixweed (sophia, Descurainis sophia L.) seed meals. Food Prod. Process. Nutr. 2021, 3, 31. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Quintero-Quiroz, J.; Celis-Torres, A.; Ciro-Gómez, G.; Torres, J.; Corrales-García, L.; Rojas, J. Physicochemical properties and functional characteristics of ultrasound-assisted legume-protein isolates: A comparative study. J. Food Sci. Technol. 2022, 59, 1665–1676. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Wang, W.; Wang, L.; Jiang, L.; Yu, D.; Xie, F. Effect of ultrasound on the properties of rice bran protein and its chlorogenic acid complex. Ultrason. Sonochem. 2021, 79, 105758. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Huang, M.; Hayat, K.; Kurtz, N.C.; Wu, X.; Ahmad, M.; Zheng, F. Ultrasound-assisted alkaline proteinase extraction enhances the yield of pecan protein and modifies its functional properties. Ultrason. Sonochem. 2021, 80, 105789. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, H.; Wang, K.; Azam, R.S.M.; Wang, Y.; Zhou, J.; Qu, W. Ultrasound frequency effect on soybean protein: Acoustic field simulation, extraction rate and structure. LWT 2021, 145, 111320. [Google Scholar] [CrossRef]
- Naik, M.; Natarajan, V.; Modupalli, N.; Thangaraj, S.; Rawson, A. Pulsed ultrasound assisted extraction of protein from defatted Bitter melon seeds (Momardica charantia L.) meal: Kinetics and quality measurements. LWT 2022, 155, 112997. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Simultaneous extraction and partial purification of proteins from spent turmeric powder using ultrasound intensified three phase partitioning and its potential as antidiabetic agent. Chem. Eng. Process. Process. Intensif. 2022, 172, 108788. [Google Scholar] [CrossRef]
- Tang, S.Q.; Du, Q.H.; Fu, Z. Ultrasonic treatment on physicochemical properties of water-soluble protein from Moringa oleifera seed. Ultrason. Sonochem. 2021, 71, 105357. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Xu, L.; Ma, H. An efficient ultrasound-assisted extraction method of pea protein and its effect on protein functional properties and biological activities. LWT 2020, 127, 109348. [Google Scholar] [CrossRef]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Wu, M.; Zhu, Z.; Li, S.; Cai, J.; Cong, X.; Yu, T.; Yang, W.; He, J.; Cheng, S. Green recovery of Se-rich protein and antioxidant peptides from Cardamine Violifolia: Composition and bioactivity. Food Biosci. 2020, 38, 100743. [Google Scholar] [CrossRef]
- Ochoa-Rivas, A.; Nava-Valdez, Y.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Microwave and Ultrasound to Enhance Protein Extraction from Peanut Flour under Alkaline Conditions: Effects in Yield and Functional Properties of Protein Isolates. Food Bioprocess Technol. 2017, 10, 543–555. [Google Scholar] [CrossRef]
- Martínez-Padilla, P.L.; Hernández-Rojas, F.S.; Sosa-Herrera, M.G.; Juliano, P. Novel application of ultrasound and microwave-assisted methods for aqueous extraction of coconut oil and proteins. J. Food Sci. Technol. 2022, 59, 3857–3866. [Google Scholar] [CrossRef]
- Moreno-Nájera, L.C.; Ragazzo-Sánchez, J.A.; Gastón-Peña, C.R.; Calderón-Santoyo, M. Green technologies for the extraction of proteins from jackfruit leaves (Artocarpus heterophyllus Lam). Food Sci. Biotechnol. 2020, 29, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Prandi, B.; Di Massimo, M.; Tedeschi, T.; Rodríguez-Turienzo, L.; Rodríguez, O. Ultrasound and Microwave-assisted Extraction of Proteins from Coffee Green Beans: Effects of Process Variables on the Protein Integrity. Food Bioprocess Technol. 2022, 15, 2712–2722. [Google Scholar] [CrossRef]
- Garcia, S.R.; Orellana-Palacios, J.C.; McClements, D.J.; Moreno, A.; Hadidi, M. Sustainable proteins from wine industrial by-product: Ultrasound-assisted extraction, fractionation, and characterization. Food Chem. 2024, 455, 139743. [Google Scholar] [CrossRef] [PubMed]
- Loushigam, G.L.; Sari, T.P.; Badgujar, P.C.; Dige, S.M.; Pareek, S. Extraction and functional properties modification of guar protein isolates by ultrasound: A promising alternative protein. Food Struct. 2025, 44, 100420. [Google Scholar] [CrossRef]
- Phuangjit, U.; Klinkesorn, U.; Tan, C.P.; Katekhong, W. Enhancing silkworm protein yield, extraction efficiency, structure, functionality, and antioxidant activity using ultrasound-, microwave-, and freeze–thaw-assisted methods. J. Sci. Food Agric. 2024, 104, 383–390. [Google Scholar] [CrossRef]
- Chaji, S.; Capaldi, G.; Gallina, L.; Grillo, G.; Boffa, L.; Cravotto, G. Semi-industrial ultrasound-assisted extraction of grape-seed proteins. J. Sci. Food Agric. 2024, 104, 5689–5697. [Google Scholar] [CrossRef]
- Karki, B. Use of High-Power Ultrasound During Soy Protein Production and Study of Its Effect on Functional Properties of Soy Protein Isolate; Iowa State University: Ames, IA, USA, 2009. [Google Scholar] [CrossRef]
- Purdi, T.S.; Setiowati, A.D.; Ningrum, A. Ultrasound-assisted extraction of Spirulina platensis protein: Physicochemical characteristic and techno-functional properties. J. Food Meas. Charact. 2023, 17, 5474–5486. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Shouqin, Z.; Jun, X.; Changzheng, W. High hydrostatic pressure extraction of flavonoids from propolis. J. Chem. Technol. Biotechnol. 2005, 80, 50–54. [Google Scholar] [CrossRef]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction of compounds of interest from yeast biomass assisted by pulsed electric fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef]
- Brito, I.P.C.; Silva, E.K. Pulsed electric field technology in vegetable and fruit juice processing: A review. Food Res. Int. 2024, 184, 114207. [Google Scholar] [CrossRef]
- Akaberi, S.; Krust, D.; Müller, G.; Frey, W.; Gusbeth, C. Impact of incubation conditions on protein and C-Phycocyanin recovery from Arthrospira platensis post- pulsed electric field treatment. Bioresour. Technol. 2020, 306, 123099. [Google Scholar] [CrossRef]
- Marín-Sánchez, J.; Berzosa, A.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Pulsed Electric Fields Effects on Proteins: Extraction, Structural Modification, and Enhancing Enzymatic Activity. Bioelectricity 2024, 6, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Gateau, H.; Blanckaert, V.; Veidl, B.; Burlet-Schiltz, O.; Pichereaux, C.; Gargaros, A.; Marchand, J.; Schoefs, B. Application of pulsed electric fields for the biocompatible extraction of proteins from the microalga Haematococcus pluvialis. Bioelectrochemistry 2021, 137, 107588. [Google Scholar] [CrossRef] [PubMed]
- Vega-Mercado, H.; Martín-Belloso, O.; Qin, B.L.; Chang, F.J.; Góngora-Nieto, M.M.; Barbosa-Cánovas, G.V.; Swanson, B.G. Non-thermal food preservation: Pulsed electric fields. Trends Food Sci. Technol. 1997, 8, 151–157. [Google Scholar] [CrossRef]
- Katsimichas, A.; Limnaios, A.; Dimitrakopoulos, K.; Dimopoulos, G.; Taoukis, P. Pulsed Electric Fields assisted extraction of proteins and phycocyanin from Arthrospira platensis biomass: A kinetic study. Food Bioprod. Process. 2024, 147, 304–314. [Google Scholar] [CrossRef]
- Andreou, V.; Dimopoulos, G.; Dermesonlouoglou, E.; Taoukis, P. Application of pulsed electric fields to improve product yield and waste valorization in industrial tomato processing. J. Food Eng. 2020, 270, 109778. [Google Scholar] [CrossRef]
- Ramaswamy, R.; Krishnan, S.B.; Leong, S.S.J. Pulsed Electric Field Technology for Recovery of Proteins from Waste Plant Resources and Deformed Mushrooms: A Review. Processes 2024, 12, 342. [Google Scholar] [CrossRef]
- Chudasama, M.; Singh, D.K.; Pradhan, R.C. Review on Electroporation Mechanisms for PEF-Assisted Extraction and Microbial Inactivation. Food Eng. Rev. 2025, 17, 706–726. [Google Scholar] [CrossRef]
- Zhang, C.; Lyu, X.; Arshad, R.N.; Aadil, R.M.; Tong, Y.; Zhao, W.; Yang, R. Pulsed electric field as a promising technology for solid foods processing: A review. Food Chem. 2023, 403, 134367. [Google Scholar] [CrossRef]
- Kamboj, A.; Chopra, R.; Singh, R.; Saxena, V.; Kumar, P.G.V. Effect of pulsed electric field parameters on the alkaline extraction of valuable compounds from perilla seed meal and optimization by central composite design approach. Appl. Food Res. 2022, 2, 100240. [Google Scholar] [CrossRef]
- Xue, D.; Farid, M.M. Pulsed electric field extraction of valuable compounds from white button mushroom (Agaricus bisporus). Innov. Food Sci. Emerg. Technol. 2015, 29, 178–186. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, S.; Waterhouse, G.I.N.; Zhou, T.; Xu, F.; Wang, R.; Sun-Waterhouse, D.; Wu, P. High-intensity pulsed electric field-assisted acidic extraction of pectin from citrus peel: Physicochemical characteristics and emulsifying properties. Food Hydrocoll. 2024, 146, 109291. [Google Scholar] [CrossRef]
- Buchmann, L.; Brändle, I.; Haberkorn, I.; Hiestand, M.; Mathys, A. Pulsed electric field based cyclic protein extraction of microalgae towards closed-loop biorefinery concepts. Bioresour. Technol. 2019, 291, 121870. [Google Scholar] [CrossRef]
- Polikovsky, M.; Fernand, F.; Sack, M.; Frey, W.; Müller, G.; Golberg, A. In silico food allergenic risk evaluation of proteins extracted from macroalgae Ulva sp. with pulsed electric fields. Food Chem. 2019, 276, 735–744. [Google Scholar] [CrossRef]
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of pulsed electric fields and high pressure on improved recovery of high-added-value compounds from olive pomace. J. Food Sci. 2020, 85, 1500–1512. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Blouet, C.; Tessaro, I.C.; Marczak, L.D.F.; Eugène Vorobiev, E. Effect of pulsed electric fields and high voltage electrical discharges on polyphenol and protein extraction from sesame cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar] [CrossRef]
- Guo, X.; Aganovic, K.; Bindrich, U.; Juadjur, A.; Hertel, C.; Ebert, E.; Macke, J.G.; Geil, C.; Heinz, V. Extraction of protein from juice blend of grass and clover pressed by a pilot pressing facility combined with a pulsed electric field treatment. Futur. Foods 2022, 6, 100173. [Google Scholar] [CrossRef]
- Yue, J.; Zhu, Z.; Yi, J.; Li, H.; Chen, B.; Rao, J. One-step extraction of oat protein by choline chloride-alcohol deep eutectic solvents: Role of chain length of dihydric alcohol. Food Chem. 2022, 376, 131943. [Google Scholar] [CrossRef]
- Melchior, S.; Calligaris, S.; Bisson, G.; Manzocco, L. Understanding the impact of moderate-intensity pulsed electric fields (MIPEF) on structural and functional characteristics of pea, rice and gluten concentrates. Food Bioprocess Technol. 2020, 13, 2145–2155. [Google Scholar] [CrossRef]
- Scherer, D.; Krust, D.; Frey, W.; Mueller, G.; Nick, P.; Gusbeth, C. Pulsed electric field (PEF)-assisted protein recovery from Chlorella vulgaris is mediated by an enzymatic process after cell death. Algal Res. 2019, 41, 101536. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, M.; Lin, S.; Liu, J.; Yang, Y.; Jin, Y. Optimization of extraction parameters for protein from beer waste brewing yeast treated by pulsed electric fields (PEF). Afr. J. Microbiol. Res. 2012, 6, 4739–4746. [Google Scholar] [CrossRef]
- Postma, P.R.; Cerezo-Chinarro, O.; Akkerman, R.J.; Olivieri, G.; Wijffels, R.H.; Brandenburg, W.A.; Eppink, H.M. Biorefinery of the macroalgae Ulva lactuca: Extraction of proteins and carbohydrates by mild disintegration. J. Appl. Phycol. 2017, 30, 1281–1293. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Mo, H. Effects of pulsed electric fields on physicochemical properties of soybean protein isolates. LWT Food Sci. Technol. 2007, 40, 1167–1175. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Res. 2015, 8, 128–134. [Google Scholar] [CrossRef]
- Yu, X.; Bals, O.; Grimi, N.; Vorobiev, E. A new way for the oil plant biomass valorization: Polyphenols and proteins extraction from rapeseed stems and leaves assisted by pulsed electric fields. Ind. Crop. Prod. 2015, 74, 309–318. [Google Scholar] [CrossRef]
- Prabhu, M.S.; Levkov, K.; Livney, Y.D.; Israel, A.; Golberg, A. High-Voltage Pulsed Electric Field Preprocessing Enhances Extraction of Starch, Proteins, and Ash from Marine Macroalgae Ulva ohnoi. ACS Sustain. Chem. Eng. 2019, 7, 17453–17463. [Google Scholar] [CrossRef]
- Robin, A.; Kazir, M.; Sack, M.; Israel, A.; Frey, W.; Mueller, G.; Livney, Y.D.; Golberg, A. Functional Protein Concentrates Extracted from the Green Marine Macroalga Ulva sp., by High Voltage Pulsed Electric Fields and Mechanical Press. ACS Sustain. Chem. Eng. 2018, 6, 13696–13705. [Google Scholar] [CrossRef]
- Lai, X.J.; Chen, J.Q.; Nie, J.; Guo, P.; Manzoor, M.F.; Huang, Y.; Li, J.; Lin, S.; Zeng, X.; Wang, R. Enhancement of extraction efficiency and functional properties of chickpea protein isolate using pulsed electric field combined with ultrasound treatment. Ultrason. Sonochem. 2024, 111, 107089. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.T.; Casanova, F.; Queiroz, L.S.; Petersen, H.O.; García-Moreno, P.J.; Feyissa, A.H. Protein extraction from yellow mealworm (Tenebrio molitor) assisted by pulsed electric fields: Effect on foaming properties. LWT 2024, 213, 117041. [Google Scholar] [CrossRef]
- Steinbruch, E.; Kashyap, M.; Chemodanov, A.; Levkov, K.; Golberg, A. Continuous pulsed electric field processing for intensification of aqueous extraction of protein from fresh green seaweed Ulva sp. biomass. Food Hydrocoll. 2024, 157, 110477. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed electric field permeabilization and extraction of phycoerythrin from Porphyridium cruentum. Algal Res. 2019, 37, 51–56. [Google Scholar] [CrossRef]
- Zhang, R.; Lebovka, N.; Marchal, L.; Vorobiev, E.; Grimi, N. Pulsed electric energy and ultrasonication assisted green solvent extraction of bio-molecules from different microalgal species. Innov. Food Sci. Emerg. Technol. 2020, 62, 102358. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Šimonis, P.; Stankevič, V.; Gomaa, M.A.E.; Stirkė, A. Pulsed Electric Field: Fundamentals and Effects on the Structural and Techno-Functional Properties of Dairy and Plant Proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Kang, D.C.; Zou, Y.H.; Cheng, Y.P.; Xing, L.; Zhou, G.; Zhang, W. Effects of power ultrasound on oxidation and structure of beef proteins during curing processing. Ultrason. Sonochem. 2016, 33, 47–53. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Barrios, C.; Fernández-Delgado, M.; López-Linares, J.C.; García-Cubero, M.T.; Coca, M.; Lucas, S. A techno-economic perspective on a microwave extraction process for efficient protein recovery from agri-food wastes. Ind. Crop. Prod. 2022, 186, 115166. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Li, M.; Zhou, C.; Wang, B.; Zeng, S.; Mu, R.; Li, G.; Li, B.; Lv, W. Research progress and application of ultrasonic- and microwave-assisted food processing technology. Compr. Rev. Food Sci. Food Saf. 2023, 22, 3707–3731. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kamboj, A.; Singh, R.; Chopra, R. Effect of microwave treatment on the alkaline extraction of proteins and phenolics from perilla seed meal in varying pH conditions: An optimization study using multicomponent analysis. J. Microw. Power Electromagn. Energy 2023, 57, 306–329. [Google Scholar] [CrossRef]
- Olalere, O.A.; Gan, C.Y. Extractability of defatted wheat germ protein and their functionalities in a deep eutectic solvent (DES)-Microwave extraction approach compared to conventional processing. Sustain. Chem. Pharm. 2023, 32, 101002. [Google Scholar] [CrossRef]
- Ait Amer Meziane, I.; Maizi, N.; Abatzoglou, N.; Benyoussef, E.H. Modelling and optimization of energy consumption in essential oil extraction processes. Food Bioprod. Process. 2020, 119, 373–389. [Google Scholar] [CrossRef]
- Drinić, Z.; Pljevljakušić, D.; Živković, J.; Bigović, D.; Šavikin, K. Microwave-assisted extraction of O. vulgare L. spp. hirtum essential oil: Comparison with conventional hydro-distillation. Food Bioprod. Process. 2020, 120, 158–165. [Google Scholar] [CrossRef]
- Flórez, N.; Conde, E.; Domínguez, H. Microwave assisted water extraction of plant compounds. J. Chem. Technol. Biotechnol. 2015, 90, 590–607. [Google Scholar] [CrossRef]
- Senavirathna, M.D.H.J.; Maimaiti, Z. Assessing the biochemical and genotoxic effects of low intensity 2.45GHz microwave exposure on Arabidopsis thaliana plants. Electromagn. Biol. Med. 2024, 43, 303–311. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Impact of combined ultrasound-microwave treatment on structural and functional properties of golden threadfin bream (Nemipterus virgatus) myofibrillar proteins and hydrolysates. Ultrason. Sonochem. 2020, 65, 105063. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.H.; Gamlath, C.J.; Martin, G.J.O.; Ashokkumar, M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems—A review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Su, J.; Cavaco-Paulo, A. Effect of ultrasound on protein functionality. Ultrason. Sonochem. 2021, 76, 105653. [Google Scholar] [CrossRef]
- Debenedictis, E.P.; Keten, S. Mechanical unfolding of alpha- and beta-helical protein motifs. Soft Matter 2019, 15, 1243–1252. [Google Scholar] [CrossRef]
- Khrustalev, V.V.; Khrustaleva, T.A.; Poboinev, V.V. Amino acid content of beta strands and alpha helices depends on their flanking secondary structure elements. Biosystems 2018, 168, 45–54. [Google Scholar] [CrossRef]
- Kamal, M.M.; Saifullah, M.; Karim, N.; Umair, M.; Raza, H.; Shishir, M.R.I. Sonication-microwave synergistic extraction of proteins from plant sources and its effect on protein. In Ultrasound and Microwave for Food Processing: Synergism for Preservation and Extraction; Academic Press: New York, NY, USA, 2023; pp. 291–344. [Google Scholar] [CrossRef]
- Bedin, S.; Zanella, K.; Bragagnolo, N.; Taranto, O.P. Implication of microwaves on the extraction process of rice bran protein. Braz. J. Chem. Eng. 2019, 36, 1653–1665. [Google Scholar] [CrossRef]
- Bedin, S.; Netto, F.M.; Bragagnolo, N.; Taranto, O.P. Reduction of the process time in the achieve of rice bran protein through ultrasound-assisted extraction and microwave-assisted extraction. Sep. Sci. Technol. 2019, 55, 300–312. [Google Scholar] [CrossRef]
- Phongthai, S.; Lim, S.T.; Rawdkuen, S. Optimization of microwave-assisted extraction of rice bran protein and its hydrolysates properties. J. Cereal Sci. 2016, 70, 146–154. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.; Chakraborty, C.; Kumar Barman, A. Effect of Microwave and Enzymatic Treatment on the Recovery of Protein from Indian Defatted Rice Bran Meal. J. Oleo Sci. 2012, 61, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Behere, M.; Patil, S.S.; Rathod, V.K. Rapid extraction of watermelon seed proteins using microwave and its functional properties. Prep. Biochem. Biotechnol. 2021, 51, 252–259. [Google Scholar] [CrossRef]
- Varghese, T.; Pare, A. Effect of microwave assisted extraction on yield and protein characteristics of soymilk. J. Food Eng. 2019, 262, 92–99. [Google Scholar] [CrossRef]
- Kadam, D.M.; Parab, S.S.; Kasara, A.; Dange, M.M.; Mahawar, K.M.; Kumar, M.; Arude, V.G. Effect of microwave pre-treatment on protein extraction from de-oiled cottonseed meal and its functional and antioxidant properties. Food Humanit. 2023, 1, 263–270. [Google Scholar] [CrossRef]
- Bansod, S.P.; Parikh, J.K.; Sarangi, P.K. Pineapple peel waste valorization for extraction of bio-active compounds and protein: Microwave assisted method and Box Behnken design optimization. Environ. Res. 2023, 221, 115237. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cases, L.; Alves, S.; Sun, D.; Tiwari, B.K. Protein extraction from lupin (Lupinus angustifolius L.) using combined ultrasound and microwave techniques: Impact on protein recovery, structure, and functional properties. Ultrason. Sonochem. 2025, 115, 107232. [Google Scholar] [CrossRef]
- Shi, R.; He, Y.; Wang, Q.; Cai, J.; Gantumur, M.; Jiang, Z. Insight into the physicochemical characteristics, functionalities and digestion behavior of protein isolate derived from Lactarius volemus (L. volemus): Impacts of microwave-assisted extraction. Food Chem. 2024, 431, 137070. [Google Scholar] [CrossRef]
- Ajayi, F.F.; Mudgil, P.; Maqsood, S. Molecular structural modification of jack bean protein using thermo-shearing/ultrasound/microwave treatments for improved extractability, functional and gelling properties: The underlying impacts of matrix pre-treatment versus alkaline-assisted extraction. Food Hydrocoll. 2024, 154, 110066. [Google Scholar] [CrossRef]
- Wijethunga, A.M.; He, Q.S.; Prithiviraj, B.; Sun, X. Acid hydrolysis and microwave digestion enhanced protein extraction from red seaweed Palmaria palmata. Food Chem. X 2025, 25, 102222. [Google Scholar] [CrossRef]
- Karki, S.; Prathumpai, W.; Anal, A.K. Microwave-assisted protein extraction from foxtail millet: Optimization, structural characterization, techno-functional properties, and bioactivity of peptides. Int. J. Biol. Macromol. 2025, 293, 139312. [Google Scholar] [CrossRef]
- Coutinho, G.S.M.; Prado, P.M.C.; Ribeiro, A.E.C.; Nickerson, M.T.; Caliari, M.; Júnior, S.S.M. Exploring microwave-assisted extraction on physicochemical and functional properties of pigeon pea protein for food applications. J. Food Eng. 2025, 392, 112497. [Google Scholar] [CrossRef]
- Bušić, V.; Molnar, M.; Tomičić, V.; Dalia Božanović, D.; Jerković, I.; Gašo-Sokač, D. Choline Chloride-Based Deep Eutectic Solvents as Green Effective Medium for Quaternization Reactions. Molecules 2022, 27, 7429. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; de la Guardia, M.; Andruch, V.; Vilková, M. Deep eutectic solvents vs ionic liquids: Similarities and differences. Microchem. J. 2020, 159, 105539. [Google Scholar] [CrossRef]
- Yahaya, N.; Mohamed, A.H.; Sajid, M.; Zain, N.N.M.; Liao, P.; Chew, K.W. Deep eutectic solvents as sustainable extraction media for extraction of polysaccharides from natural sources: Status, challenges and prospects. Carbohydr. Polym. 2024, 338, 122199. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sato, Y.; Sakai, Y.; Kansha, Y. A review of ionic liquids and deep eutectic solvents design for CO2 capture with machine learning. J. Clean. Prod. 2023, 414, 137695. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.-H.; Zhang, C.-X.; Ma, Y.-X.; Yu, Y.; Liu, H.; Wang, X.; Zheng, Y. Extraction of protein from sesame meal: Impact of deep eutectic solvents on protein structure and functionality. LWT 2023, 187, 115366. [Google Scholar] [CrossRef]
- Chen, Q.; Chaihu, L.; Yao, X.; Cao, X.; Bi, W.; Lin, J.; Chen, D.D.Y. Molecular Property-Tailored Soy Protein Extraction Process Using a Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2021, 9, 10083–10092. [Google Scholar] [CrossRef]
- De Angelis, D.; Latrofa, V.; Squeo, G.; Pasqualone, A.; Summo, C. Techno-functional, rheological, and chemical properties of plant-based protein ingredients obtained with dry fractionation and wet extraction. Curr. Res. Food Sci. 2024, 9, 100906. [Google Scholar] [CrossRef]
- Yue, J.; Zhu, Z.; Yi, J.; Lan, Y.; Chen, B.; Rao, J. Structure and functionality of oat protein extracted by choline chloride–dihydric alcohol deep eutectic solvent and its water binary mixtures. Food Hydrocoll. 2021, 112, 106330. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, L.; Concepción García, M. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Lin, Z.; Jiao, G.; Zhang, J.; Celli, B.G.; Brooks, M.S. Optimization of protein extraction from bamboo shoots and processing wastes using deep eutectic solvents in a biorefinery approach. Biomass Convers. Biorefinery 2020, 11, 2763–2774. [Google Scholar] [CrossRef]
- Wahlström, R.; Rommi, K.; Willberg-Keyriläinen, P.; Ercili-Cura, D.; Holopainen-Mantila, U.; Hiltunen, J.; Mäkinen, O.; Nygren, H.; Mikkelson, A.; Kuutti, L. High Yield Protein Extraction from Brewer’s Spent Grain with Novel Carboxylate Salt-Urea Aqueous Deep Eutectic Solvents. ChemistrySelect 2017, 2, 9355–9363. [Google Scholar] [CrossRef]
- Liu, R.L.; Yu, P.; Ge, X.L.; Bai, X.; Li, X.; Fu, Q. Establishment of an Aqueous PEG 200-Based Deep Eutectic Solvent Extraction and Enrichment Method for Pumpkin (Cucurbita moschata) Seed Protein. Food Anal. Methods 2017, 10, 1669–1680. [Google Scholar] [CrossRef]
- Moldes, D.; Requejo, P.F.; Vega, M.; Bolado, S.; Wijffels, R.H.; Kazbar, A. Protein extraction from seaweed Saccharina latissima with deep eutectic solvents. Microchem. J. 2024, 205, 111275. [Google Scholar] [CrossRef]
- Ecem Bayram, N.; Gerçek, Y.C.; Kutlu, N.; Çelik, S.; Bayram, S.; Baştürk, F.N.; Yıldırım, N. The application of deep eutectic solvents for protein extraction from bee bread (Perga). Microchem. J. 2024, 204, 111139. [Google Scholar] [CrossRef]
- Parodi, E.; La Nasa, J.; Ribechini, E.; Petri, A.; Piccolo, O. Extraction of proteins and residual oil from flax (Linum usitatissimum), camelina (Camelina sativa), and sunflower (Helianthus annuus) oilseed press cakes. Biomass Convers. Biorefinery 2023, 13, 1915–1926. [Google Scholar] [CrossRef]
- Grudniewska, A.; De Melo, E.M.; Chan, A.; Gniłka, R.; Boratyński, F.; Matharu, A.S. Enhanced Protein Extraction from Oilseed Cakes Using Glycerol-Choline Chloride Deep Eutectic Solvents: A Biorefinery Approach. ACS Sustain. Chem. Eng. 2018, 6, 15791–15800. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, W.; Zhang, N.; Soladoye, O.P.; Zhang, Y.; Fu, Y. Deep eutectic solvents as new media for green extraction of food proteins: Opportunity and challenges. Food Res. Int. 2022, 161, 111842. [Google Scholar] [CrossRef]
- Boateng, I.D. Evaluating the status quo of deep eutectic solvent in food chemistry. Potentials and limitations. Food Chem. 2023, 406, 135079. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Getachew, A.T.; Santana, Á.L.; Jacobsen, C. Legume Proteins in Food Products: Extraction Techniques, Functional Properties, and Current Challenges. Foods 2025, 14, 1626. [Google Scholar] [CrossRef]
- Patra, A.; Arun Prasath, V.; Pandiselvam, R. Deep eutectic solvent: An emerging trend for extraction of plant proteins. J. Mol. Liq. 2023, 389, 122887. [Google Scholar] [CrossRef]
- Thakur, R.; Gupta, V.; Ghosh, T.; Das, A.B. Effect of anthocyanin-natural deep eutectic solvent (lactic acid/fructose) on mechanical, thermal, barrier, and pH-sensitive properties of polyvinyl alcohol based edible films. Food Packag. Shelf Life 2022, 33, 100914. [Google Scholar] [CrossRef]
- Hewage, A.; Olatunde, O.O.; Nimalaratne, C.; House, J.D.; Aluko, R.E.; Bandara, N. Improved protein extraction technology using deep eutectic solvent system for producing high purity fava bean protein isolates at mild conditions. Food Hydrocoll. 2023, 147, 109283. [Google Scholar] [CrossRef]
- Karabulut, G.; Köroğlu, D.G.; Feng, H.; Karabulut, Z. Sustainable fungi-based protein extraction from agro-waste mushroom stem using deep eutectic solvents. Food Chem. X 2024, 24, 101931. [Google Scholar] [CrossRef]
- Zin, M.T.; Kaewkod, T.; Chaipoot, S.; Kanthakat, G.; Chen, Y.; Cheirsilp, B.; Srinuanpan, S. Ultrasound-Assisted Deep Eutectic Solvent Extraction of Antioxidant and Anti-Colorectal Cancer Proteins from Spirulina Biomass: Process Intensification, Characterization, and Bioactivity Evaluation. Antioxidants 2025, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Bhowmik, P.; Yang, T.C.; Samaranayaka, A.; Chen, L. Extraction of canola protein via natural deep eutectic solvents compared to alkaline treatments: Isolate characteristics and protein structural and functional properties. Food Hydrocoll. 2024, 152, 109922. [Google Scholar] [CrossRef]
- Fang, B.; Gu, Z.; Ohm, J.B.; Chen, B.; Rao, J. Reverse micelles extraction of hemp protein isolate: Impact of defatting process on protein structure, functionality, and aromatic profile. Food Hydrocoll. 2023, 135, 108158. [Google Scholar] [CrossRef]
- Ugolini, L.; De Nicola, G.; Palmieri, S. Use of reverse micelles for the simultaneous extraction of oil, proteins, and glucosinolates from cruciferous oilseeds. J. Agric. Food Chem. 2008, 56, 1595–1601. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Hu, H.; Wang, M.; Zhang, X.; Liu, H. Improved backward extraction of walnut protein using AOT reverse micelles with microwave and its characteristics. J. Food Process. Preserv. 2021, 45, e15470. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, N.; Pend, J.; Li, Y.; Li, L.; Xie, X. Ultrasound-assisted reverse micelle extraction and characterization of tea protein from tea residue. J. Sci. Food Agric. 2023, 103, 4068–4076. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Manzanilla-Valdez, M.L.; Wang, Y.; Mondor, M.; Hernández-Álvarez, A.J. Micellar Precipitation and Reverse Micelle Extraction of Plant Proteins. In Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development; Springer: Cham, Switzerland, 2023; pp. 237–263. [Google Scholar] [CrossRef]
- Segaran, A.; Chua, L.S. Review of recent applications and modifications of aqueous two-phase system for the separation of biomolecules. Int. J. Biol. Macromol. 2024, 276, 133856. [Google Scholar] [CrossRef]
- Asenjo, J.A.; Andrews, B.A. Aqueous two-phase systems for protein separation: Phase separation and applications. J. Chromatogr. A 2012, 1238, 1–10. [Google Scholar] [CrossRef]
- Liu, B.; Tan, Z. Separation and Purification of Astragalus membranaceus Polysaccharides by Deep Eutectic Solvents-Based Aqueous Two-Phase System. Molecules 2022, 27, 5288. [Google Scholar] [CrossRef]
- Iqbal, M.; Tao, Y.; Xie, S.; Zhu, Y.; Chen, D.; Wang, X.; Huang, L.; Peng, D.; Sattar, A.; Shabbir, M.A.B.; et al. Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 2016, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, M.; Han, S.; Zong, W. Aqueous two-phase system (ATPS): From basic science to applications. RSC Adv. 2025, 15, 9041–9054. [Google Scholar] [CrossRef] [PubMed]
- Otu, P.N.Y.; Osae, R.; Abdullateef, M.T.; Cunshan, Z.; Xiaojie, Y.; Azumah, B.K. Characterization of Moringa oleifera leaf polysaccharides extracted by coupling ionic liquid separation system with ultrasound irradiation. J. Food Process. Eng. 2020, 43, e13417. [Google Scholar] [CrossRef]
- Menegotto, A.L.L.; Fernandes, I.A.; Steffens, J.; Valduga, E. Protein purification of Arthrospira platensis using aqueous two-phase system composed of polyethylene glycol and potassium phosphate/sodium citrate. J. Appl. Phycol. 2022, 34, 311–320. [Google Scholar] [CrossRef]
- Suarez Ruiz, C.A.; Kwaijtaal, J.; Peinado, O.C.; Berg, C.V.D.; Wijffels, R.H.; Eppink, M.H.M. Multistep Fractionation of Microalgal Biomolecules Using Selective Aqueous Two-Phase Systems. ACS Sustain. Chem. Eng. 2020, 8, 2441–2452. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Wang, H.; Xie, H.; Zhang, Y.; Wang, M. Simultaneous extraction and purification of polysaccharides and proteins from Pleurotus ostreatus using an aqueous two-phase system. J. Food Sci. 2025, 90, e17674. [Google Scholar] [CrossRef]
- Gu, Z.; Glatz, C.E. Aqueous two-phase extraction for protein recovery from corn extracts. J. Chromatogr. B 2007, 845, 38–50. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Li, M.H.; Yang, M.; Xu, J.; Ren, M.; Zhao, Z.; Wang, L.; Yang, J.; Liu, J.; Zhang, M. Extraction of walnut protein using deep eutectic solvent aqueous two-phase system: Investigation of structural and functional properties. Food Chem. X 2025, 28, 102546. [Google Scholar] [CrossRef]
- Saw, H.S.; Sankaran, R.; Khoo, K.S.; Chew, K.W.; Phong, W.N.; Tang, M.S.Y.; Lim, S.S.; Zaid, H.F.M.; Naushad, M.; Show, P.L. Application of a Liquid Biphasic Flotation (LBF) System for Protein Extraction from Persiscaria tenulla Leaf. Processes 2020, 8, 247. [Google Scholar] [CrossRef]
- Ampofo, J.; Ngadi, M. Ultrasound-assisted processing: Science, technology and challenges for the plant-based protein industry. Ultrason. Sonochem. 2022, 84, 105955. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Liu, S. Power ultrasound and its applications: A state-of-the-art review. Ultrason. Sonochem. 2020, 62, 104722. [Google Scholar] [CrossRef]
- Mierez, J.; AlTammar, M.J.; Alruwaili, K.M.; Alfaraj, R.T. Recent advances of ultrasound applications in the oil and gas industry. Ultrason. Sonochem. 2024, 103, 106767. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, H. Enhancing tilapia fish myosin solubility using proline in low ionic strength solution. Food Chem. 2020, 320, 126665. [Google Scholar] [CrossRef] [PubMed]
- Knappert, J.; McHardy, C.; Rauh, C. Kinetic Modeling and Numerical Simulation as Tools to Scale Microalgae Cell Membrane Permeabilization by Means of Pulsed Electric Fields (PEF) From Lab to Pilot Plants. Front. Bioeng. Biotechnol. 2020, 8, 209. [Google Scholar] [CrossRef]
- Straessner, R.; Nikolausz, M.; Silve, A.; Nazarova, N.; Wuestner, R.; Papachristou, I.; Akaberi, S.; Leber, K.; Mueller, G.; Frey, W. Holistic exploitation of pulsed electric field (PEF)-treated and lipid extracted microalgae Auxenochlorella protothecoides, utilizing anaerobic digestion (AD). Algal Res. 2023, 69, 102950. [Google Scholar] [CrossRef]
- Chai, K.F.; Chen, W.N. Recovery of antioxidative protein hydrolysates with functional properties from fermented brewer’s spent grain via microwave-assisted three phase partitioning. Innov. Food Sci. Emerg. Technol. 2024, 91, 103551. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Granados, M.; Sentellas, S.; Saurina, J. Microwave-assisted extraction with natural deep eutectic solvents for polyphenol recovery from agrifood waste: Mature for scaling-up? Sci. Total Environ. 2024, 912, 168716. [Google Scholar] [CrossRef]
- Prabhune, A.; Dey, R. Green and sustainable solvents of the future: Deep eutectic solvents. J. Mol. Liq. 2023, 379, 121676. [Google Scholar] [CrossRef]
- Aslanbay Guler, B.; Tepe, U.; Imamoglu, E. Sustainable Point of View: Life Cycle Analysis for Green Extraction Technologies. ChemBioEng Rev. 2024, 11, 348–362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).