Bovine β-Casein Peptide YPFPGPIH Regulates Inflammation and Macrophage Activity via TLR/NF-κB/MAPK Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
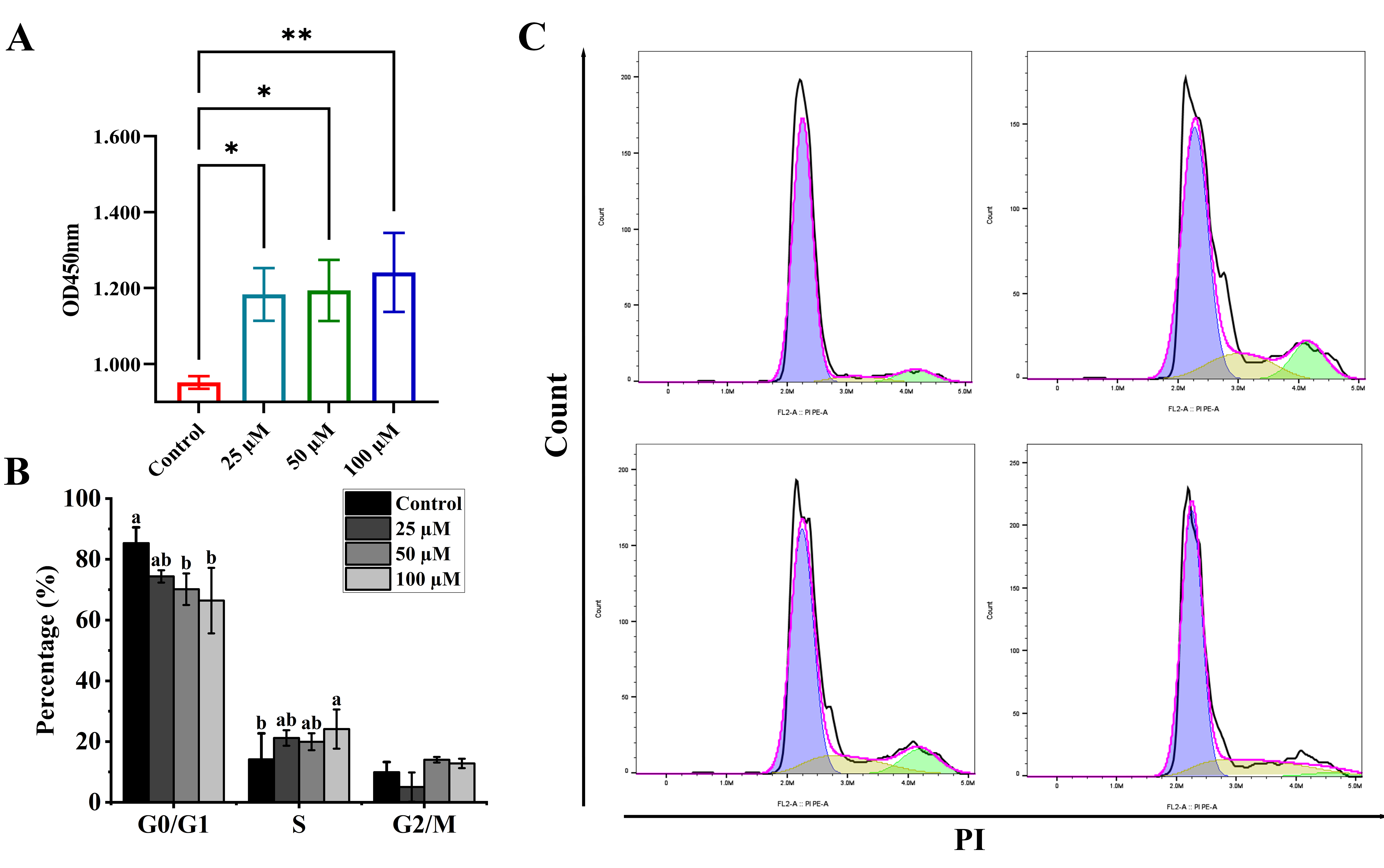
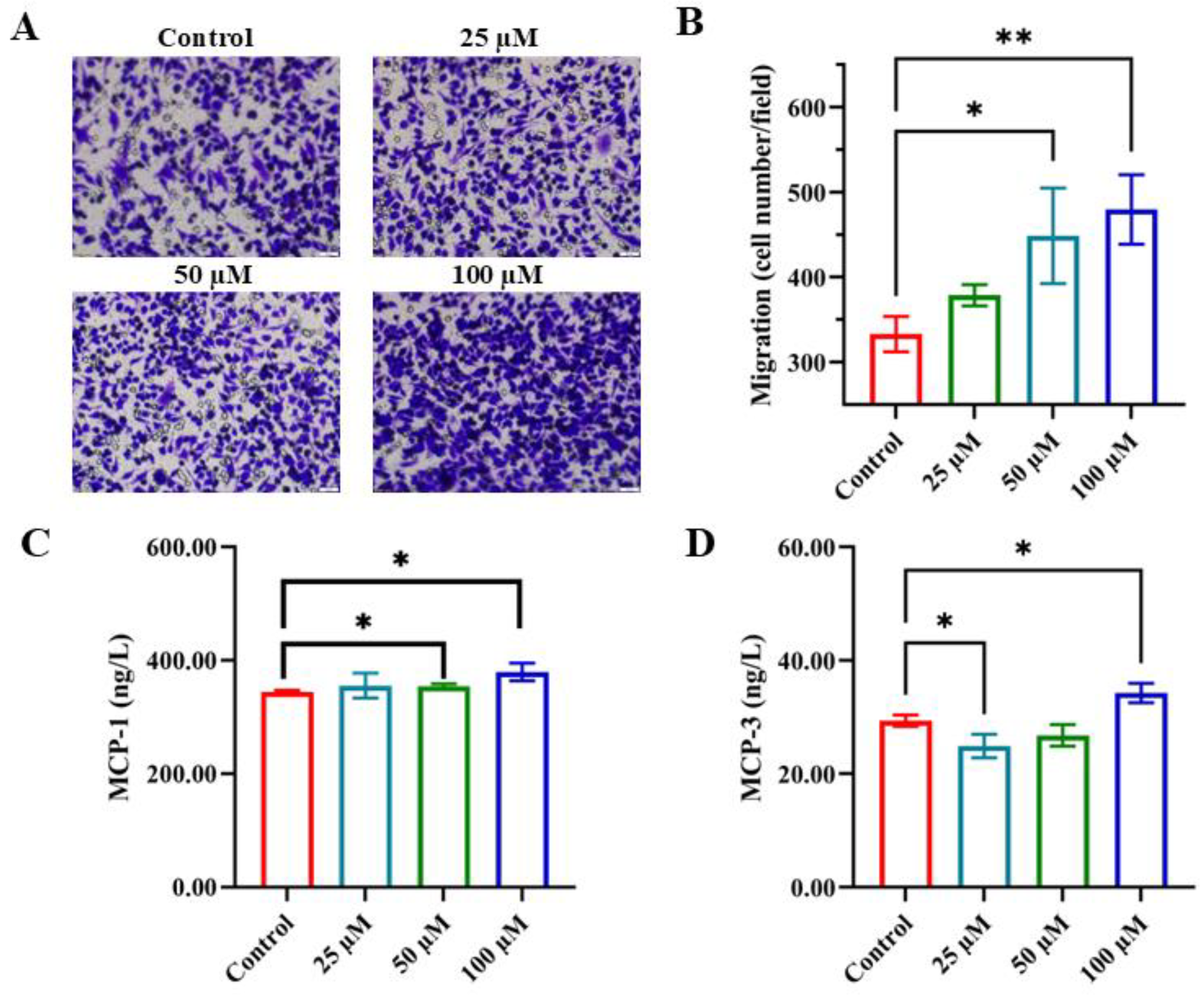
2.2. Cell Proliferation, Cycle, and Migration Assays
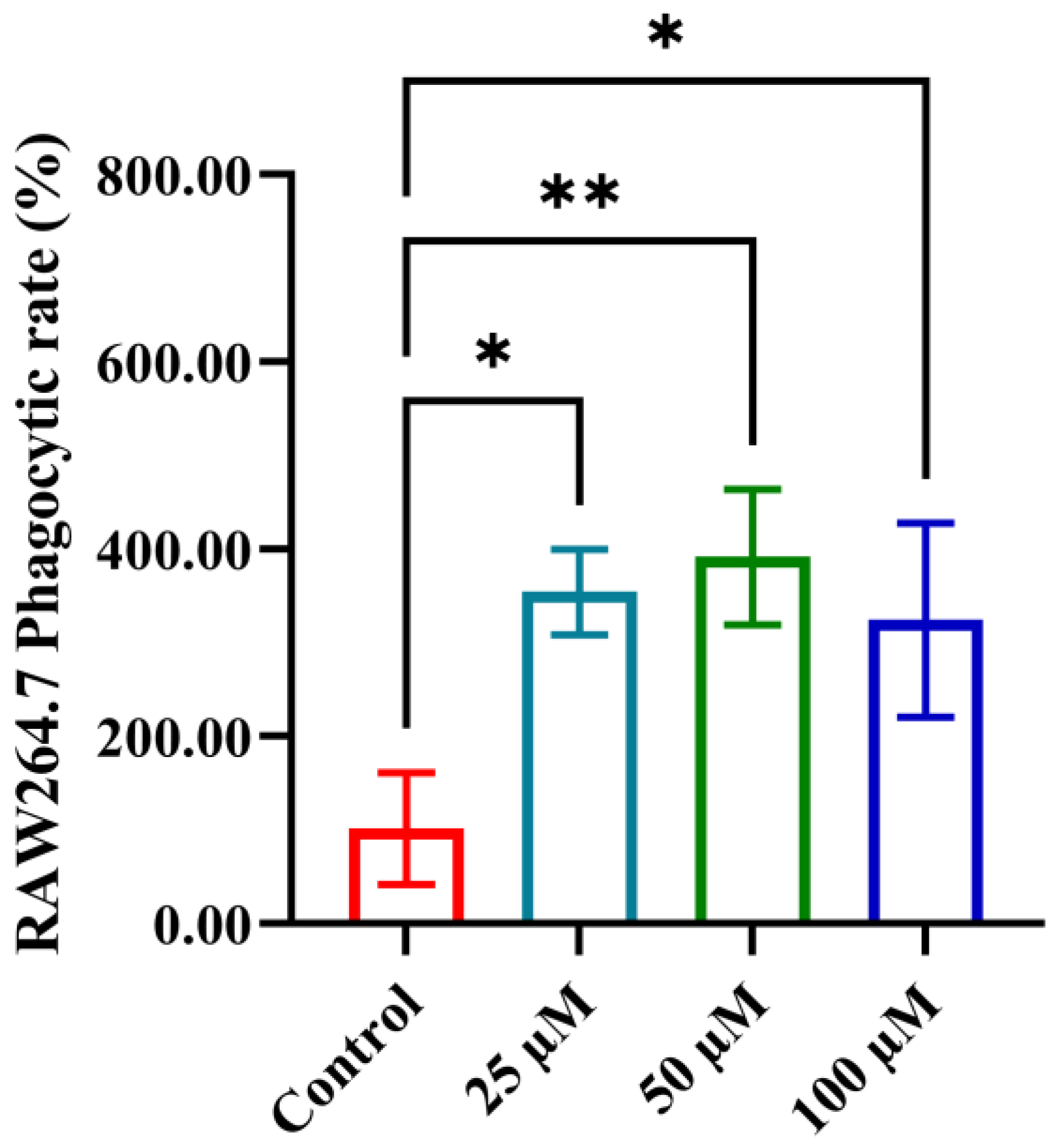
2.3. Macrophage Phagocytosis
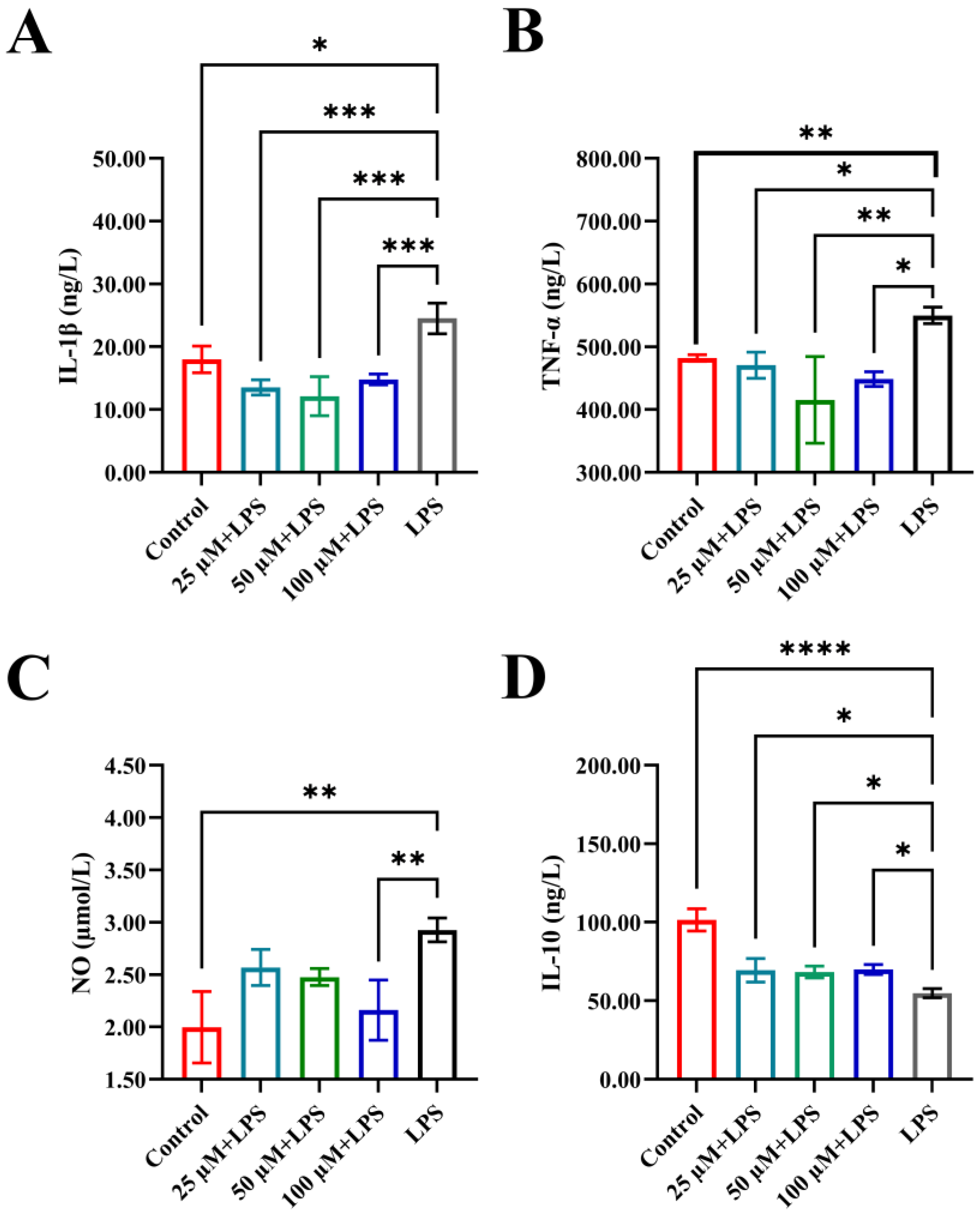
2.4. Immune Factor
2.5. Relative Expression of Immune Factor mRNA
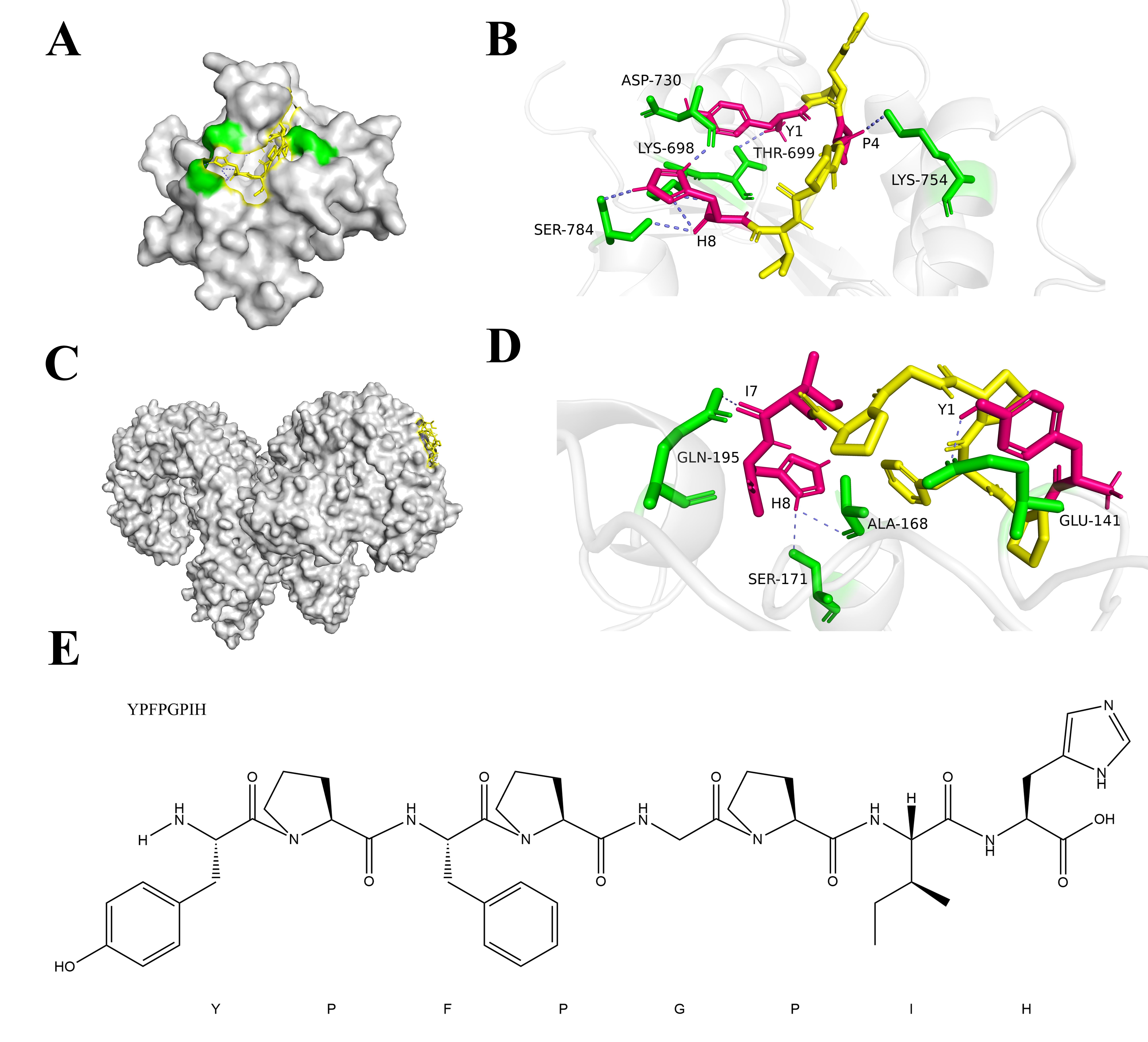
2.6. Molecular Docking
2.7. Immunofluorescence Staining
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of YPFPGPIH on Macrophage Proliferation
3.2. Enhancing Monocyte Chemotactic Migration with YPFPGPIH
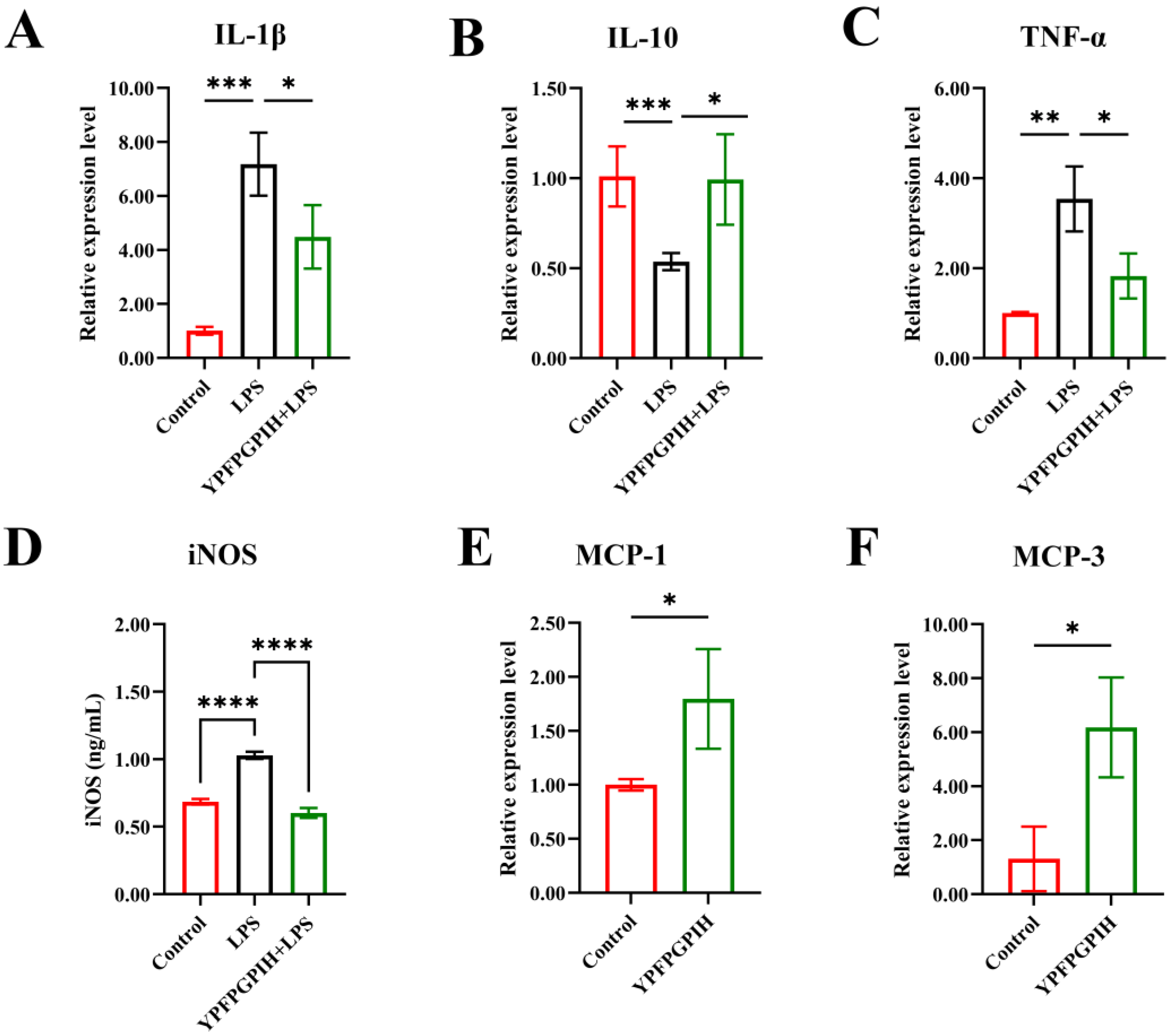
3.3. The Impact of YPFPGPIH on Cytokine Production
3.4. The Impact of YPFPGPIH on Macrophage Phagocytosis
3.5. Impact of YPFPGPIH on Relative mRNA Expression of Immune Factors
3.6. Impact of YPFPGPIH on NF-κB and MAPK Signaling Pathways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Li, Y.; Li, P.T.; Luo, Z.H.; Zhang, Z.P.; Wang, Y.L.; Zou, P.F. Novel Findings in Teleost TRAF4, a Protein Acts as an Enhancer in TRIF and TRAF6 Mediated Antiviral and Inflammatory Signaling. Front. Immunol. 2022, 13, 944528. [Google Scholar] [CrossRef]
- Fan, R.; Hao, Y.; Du, Q.; Kang, J.; Xu, M.; Li, Y. Beneficial Effects of Walnut Oligopeptides on Muscle Loss in Senescence-Accelerated Mouse Prone-8 (SAMP8) Mice: Focusing on Mitochondrial Function. Nutrients 2022, 14, 2051. [Google Scholar] [CrossRef]
- Tikhonova, M.A.; Shoeva, O.Y.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients 2020, 12, 3877. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Jauhar, S.K.; Paul, S.K. Leveraging blockchain to improve nutraceutical supply chain resilience under post-pandemic disruptions. Comput. Ind. Eng. 2023, 183, 109475. [Google Scholar] [CrossRef]
- Reyes-Díaz, A.; González-Córdova, A.F.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Vallejo-Cordoba, B. Immunomodulation by hydrolysates and peptides derived from milk proteins. Int. J. Dairy Technol. 2018, 71, 1–9. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Wang, C.; Zhang, Y.; Xi, H.; Si, J.; Zhang, L.; Yan, J. Preparation, Structural Features and in vitro Immunostimulatory Activity of a Glucomannan From Fresh Dendrobium catenatum Stems. Front. Nutr. 2021, 8, 823803. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Deng, H.; Guan, Y.; Dong, Q.; An, R.; Wang, J. Chitosan-based biomaterials promote bone regeneration by regulating macrophage fate. J. Mater. Chem. B 2024, 12, 7480–7496. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Wang, X.; Jiang, C.; Shen, D.; Cui, X.; Xie, K.; Ji, C.; Cao, Y. A human β-casein-derived peptide BCCY-1 modulates the innate immune response. Food Chem. 2021, 348, 129111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Guo, J.; Zheng, Y.; Duan, H.; Liu, G.; Yan, W. Silkworm pupa protein-derived peptides alleviate LPS-induced inflammatory response in RAW264.7 macrophage cells through the NF-κB/MAPK/PI3K-AKT signaling pathway. J. Agric. Food Res. 2024, 16, 101165. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Serwin, K.; Schneider, G. Innate immunity derived factors as external modulators of the CXCL12-CXCR4 axis and their role in stem cell homing and mobilization. Theranostics 2013, 3, 3. [Google Scholar] [CrossRef]
- Behzadi, P.; García-Perdomo, H.A.; Karpiński, T.M. Toll-like receptors: General molecular and structural biology. J. Immunol. Res. 2021, 2021, 9914854. [Google Scholar] [CrossRef] [PubMed]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, S.; Jiang, L.; Mu, G.; Jiang, S. Immunomodulatory activity and molecular mechanisms of action of peptides derived from casein hydrolysate by alcalase and flavourzyme based on virtual screening. J. Dairy Sci. 2025, 108, 2152–2168. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Nicolás-Boluda, A.; Carn, F.; Gazeau, F. Cationic Polyethyleneimine (PEI)-Gold Nanocomposites Modulate Macrophage Activation and Reprogram Mouse Breast Triple-Negative MET-1 Tumor Immunological Microenvironment. Pharmaceutics 2022, 14, 2234. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Wang, S.; Zheng, S.; Chen, Z.; Song, H. Protein Binding Nanoparticles as an Integrated Platform for Cancer Diagnosis and Treatment. Adv. Sci. 2022, 9, e2202453. [Google Scholar] [CrossRef]
- Kouroshnia, A.; Zeinali, S.; Irani, S.; Sadeghi, A. Induction of apoptosis and cell cycle arrest in colorectal cancer cells by novel anticancer metabolites of Streptomyces sp. 801. Cancer Cell Int. 2022, 22, 235. [Google Scholar] [CrossRef]
- Feng, K.N.; Meng, P.; Zou, X.L.; Zhang, M.; Li, H.K.; Yang, H.L.; Li, H.T.; Zhang, T.T. IL-37 protects against airway remodeling by reversing bronchial epithelial-mesenchymal transition via IL-24 signaling pathway in chronic asthma. Respir. Res. 2022, 23, 244. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, L.; Bao, Z.; Lin, S. C-Terminal Modification on the Immunomodulatory Activity, Antioxidant Activity, and Structure-Activity Relationship of Pulsed Electric Field (PEF)-Treated Pine Nut Peptide. Foods 2022, 11, 2649. [Google Scholar] [CrossRef]
- Yin, X.Y.; Wang, C.C.; Du, P.; Wang, X.S.; Lu, Y.C.; Sun, Y.W.; Sun, Y.H.; Hu, Y.M.; Chen, X. Muse cells decrease the neuroinflammatory response by modulating the proportion of M1 and M2 microglia in vitro. Neural Regen. Res. 2023, 18, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Su, L.; Shan, P.; Ye, B.; Wu, S.; Liang, G.; Huang, W. LCZ696 Attenuated Doxorubicin-Induced Chronic Cardiomyopathy Through the TLR2-MyD88 Complex Formation. Front. Cell Dev. Biol. 2021, 9, 654051. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Jia, X.; Chen, J.; Wang, Z.; Song, B.; Li, R.; Cheong, K.-L.; Zhong, S. Sulfate glycosaminoglycan from swim bladder exerts immunomodulatory potential on macrophages via toll-like receptor 4 mediated NF-κB signaling pathways. Int. J. Biol. Macromol. 2024, 271, 132439. [Google Scholar] [CrossRef]
- Yu, G.; Yu, H.; Yang, Q.; Wang, J.; Fan, H.; Liu, G.; Wang, L.; Bello, B.K.; Zhao, P.; Zhang, H. Vibrio harveyi infections induce production of proinflammatory cytokines in murine peritoneal macrophages via activation of p38 MAPK and NF-κB pathways, but reversed by PI3K/AKT pathways. Dev. Comp. Immunol. 2022, 127, 104292. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-dependent kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef]
- Sheu, K.M.; Hoffmann, A. Functional hallmarks of healthy macrophage responses: Their regulatory basis and disease relevance. Annu. Rev. Immunol. 2022, 40, 295–321. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Tuo, Y.; Mu, G.; Jiang, S. Immunomodulatory effect, digestive stability, and action mechanism of casein peptide SPAQILQW activating macrophages via N-terminal serine. J. Dairy Sci. 2025, 108, 6617–6633. [Google Scholar] [CrossRef]
- Wang, Z. Cell cycle progression and synchronization: An overview. In Cell-Cycle Synchronization: Methods and Protocols; Humana: New York, NY, USA, 2022; pp. 3–23. [Google Scholar]
- Mierke, C.T.; Mierke, C.T. Cell cycle, DNA replication, centrosomes, centrioles and cell division. In Cellular Mechanics and Biophysics: Structure and Function of Basic Cellular Components Regulating Cell Mechanics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 667–742. [Google Scholar]
- Zeng, Y.; Cheng, H.; Zhong, R.; Zhong, W.; Zheng, R.; Miao, J. Novel immunomodulatory peptides from hydrolysates of the Rana spinosa (Quasipaa spinosa) meat and their immunomodulatory activity mechanism. Food Chem. 2025, 465, 142024. [Google Scholar] [CrossRef]
- Ryan, A.T.; Kim, M.; Lim, K. Immune cell migration to cancer. Cells 2024, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef]
- Jerka, D.; Bonowicz, K.; Piekarska, K.; Gokyer, S.; Derici, U.S.; Hindy, O.A.; Altunay, B.B.; Yazgan, I.ı.; Steinbrink, K.; Kleszczyński, K. Unraveling endothelial cell Migration: Insights into Fundamental forces, inflammation, Biomaterial Applications, and tissue regeneration strategies. ACS Appl. Bio Mater. 2024, 7, 2054–2069. [Google Scholar] [CrossRef]
- Rath, B.; Stickler, S.; Hochmair, M.J.; Hamilton, G. Expression of cytokines in pleural effusions and corresponding cell lines of small cell lung cancer. Transl. Lung Cancer Res. 2024, 13, 5. [Google Scholar] [CrossRef]
- Pourjafar, M.; Tiwari, V.K. Plasticity in cell migration modes across development, physiology, and disease. Front. Cell Dev. Biol. 2024, 12, 1363361. [Google Scholar] [CrossRef]
- Leblanc, G.G.; Bronner-Fraser, M.E. Neural crest cell differentiation. In Development, Regeneration and Plasticity of the Autonomic Nervous System; Routledge: London, UK, 2024; pp. 95–137. [Google Scholar]
- Gomez-Salinero, J.M.; Redmond, D.; Rafii, S. Microenvironmental determinants of endothelial cell heterogeneity. Nat. Rev. Mol. Cell Biol. 2025, 26, 476–495. [Google Scholar] [CrossRef]
- Ha, H.; Debnath, B.; Neamati, N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics 2017, 7, 1543. [Google Scholar] [CrossRef]
- Yellowley, C. CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair. BoneKEy Rep. 2013, 2, 300. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Smith, R.; Rubin, E.J.; Glassberg, M.K.; Farkouh, M.E.; Rosenson, R.S. Inflammation unites diverse acute and chronic diseases. Eur. J. Clin. Investig. 2024, 54, e14280. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-inflammatory and anti-inflammatory interleukins in infectious diseases: A comprehensive review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Beringer, A.; Miossec, P. Systemic effects of IL-17 in inflammatory arthritis. Nat. Rev. Rheumatol. 2019, 15, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, M.; Wu, H.; Lu, Q. The pathological role of B cells in systemic lupus erythematosus: From basic research to clinical. Autoimmunity 2020, 53, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- John, L.J.; Fromm, M.; Schulzke, J.-D. Epithelial barriers in intestinal inflammation. Antioxid. Redox Signal. 2011, 15, 1255–1270. [Google Scholar] [CrossRef]
- Choy, J.C.; Pober, J.S. Generation of NO by bystander human CD8 T cells augments allogeneic responses by inhibiting cytokine deprivation-induced cell death. Am. J. Transplant. 2009, 9, 2281–2291. [Google Scholar] [CrossRef][Green Version]
- Xie, Z.; Wang, Y.; Huang, J.; Qian, N.; Shen, G.; Chen, L. Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264.7 macrophages. Int. J. Biol. Macromol. 2019, 129, 61–67. [Google Scholar] [CrossRef]
- Rosales, C.; Uribe-Querol, E. Phagocytosis: A fundamental process in immunity. BioMed Res. Int. 2017, 2017, 9042851. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef]
- Lim, J.J.; Grinstein, S.; Roth, Z. Diversity and versatility of phagocytosis: Roles in innate immunity, tissue remodeling, and homeostasis. Front. Cell. Infect. Microbiol. 2017, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Hakansson, A.; Orihuela, C.; Bogaert, D. Bacterial-host interactions: Physiology and pathophysiology of respiratory infection. Physiol. Rev. 2018, 98, 781–811. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Long, R.; Lu, S.; Chen, X.; Ye, W.; Wang, T.; Wang, X.; Xu, F.; Li, N. Human milk peptide MAMP-1 alleviates necrotizing enterocolitis via inhibition of the TLR4-mediated PI3K-AKT-NF-κB signaling pathway. Food Funct. 2025, 16, 3904–3917. [Google Scholar] [CrossRef]
- Jin, L.; Chen, J.; Fu, J.; Lou, J.; Guo, Y.; Liu, X.; Xu, X.; Fu, H.; Shou, Q. PARP1 Exacerbates Prostatitis by Promoting M1 Macrophages Polarization through NF-κB Pathway. Inflammation 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. et Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-κB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef]
- Qu, X.; Tang, Y.; Hua, S. Immunological approaches towards cancer and inflammation: A cross talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Li, J.; Li, S.; Lv, J.; Zhong, G.; Gao, M.; Yang, S.; Han, S.; Hao, W. Targeting TLR4 and regulating the Keap1/Nrf2 pathway with andrographolide to suppress inflammation and ferroptosis in LPS-induced acute lung injury. Chin. J. Nat. Med. 2024, 22, 914–928. [Google Scholar] [CrossRef] [PubMed]
- Remley, V.A.; Linden, J.; Bauer, T.W.; Dimastromatteo, J. Unlocking antitumor immunity with adenosine receptor blockers. Cancer Drug Resist. 2023, 6, 748–767. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence (5′ to 3′) |
|---|---|---|
| MCP-1 | Forward | GGCTCAGCCAGATGCAGTTAA |
| Reverse | CCTACTCATTGGGATCATCTTGCT | |
| MCP-3 | Forward | AAGAAGGGCATGGAAGTCTG |
| Reverse | TCAAGGCTTTGGAGTTGGG | |
| TNF-α | Forward | CTGGATGTCAATCAACAATGGGA |
| Reverse | ACTAGGGTGTGAGTGTTTTCTGT | |
| IL-1β | Forward | TGTGAAATGCCACCTTTTGA |
| Reverse | TGAGTGATACTGCCTGCCTG | |
| IL-10 | Forward | CAGAGCCACATGCTCCTAGA |
| Reverse | TGTCCAGCTGGTCCTTTGTT | |
| GAPDH | Forward | AGGTCGGTGTGAACGGATTTG |
| Reverse | GCAGCTCTAGGAGCATGTGG |
| Sequence | Affinity (kcal/mol) TLR2 | Affinity (kcal/mol) TLR4/MyDD |
|---|---|---|
| YPFPGPIH | −9.9 | −5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, X.; Mu, G.; Wu, X.; Wu, J. Bovine β-Casein Peptide YPFPGPIH Regulates Inflammation and Macrophage Activity via TLR/NF-κB/MAPK Signaling. Foods 2025, 14, 3572. https://doi.org/10.3390/foods14203572
Zhang J, Zhang X, Mu G, Wu X, Wu J. Bovine β-Casein Peptide YPFPGPIH Regulates Inflammation and Macrophage Activity via TLR/NF-κB/MAPK Signaling. Foods. 2025; 14(20):3572. https://doi.org/10.3390/foods14203572
Chicago/Turabian StyleZhang, Junpeng, Xinyu Zhang, Guangqing Mu, Xiaomeng Wu, and Jianping Wu. 2025. "Bovine β-Casein Peptide YPFPGPIH Regulates Inflammation and Macrophage Activity via TLR/NF-κB/MAPK Signaling" Foods 14, no. 20: 3572. https://doi.org/10.3390/foods14203572
APA StyleZhang, J., Zhang, X., Mu, G., Wu, X., & Wu, J. (2025). Bovine β-Casein Peptide YPFPGPIH Regulates Inflammation and Macrophage Activity via TLR/NF-κB/MAPK Signaling. Foods, 14(20), 3572. https://doi.org/10.3390/foods14203572







